Abstract
Objective. To investigate metabolic changes within the spinal cord using proton magnetic resonance spectroscopy (1H-MRS) and determine their relationship with clinical function in patients with complete brachial plexus avulsion who underwent reimplantation of the ventral roots. Methods. Single-voxel 1H-MRS of the cord between C1 and C3 was performed in 10 patients with normal spinal cord on MRI, who underwent reimplantation of C5 to T1 ventral roots on average 5.5 years earlier, and 19 healthy controls. The ratios of the concentrations of the following main metabolites, with respect to total creatine levels, were obtained: total N-acetyl-aspartate, choline-containing compounds, creatine and phosphocreatine (Cr), and myo-inositol (m-Ins). Patient disability was assessed using upper limb scales. Differences in metabolite concentration ratios and their correlations with disability were investigated. Results. Patients showed increased m-Ins/Cr ratio compared with controls, which was associated with the level of function of the affected arm and time from injury. Conclusions. The finding of increased m-Ins/Cr in patients suggests that reactive gliosis, perhaps in response to the degeneration of avulsed fibers, may occur in the spinal cord above the site of injury and be relevant to motor dysfunction. However, this pathological process appears to diminish with time. These insights underline the need to integrate metabolic imaging with structural and functional magnetic resonance imaging to obtain a complete view of spinal cord plasticity. Last, this study provides the first steps toward identifying markers to serve as outcome measures for trials comparing strategies of plexus repair following avulsion injury.
Keywords: spinal cord trauma, brachial plexus avulsion, magnetic resonance spectroscopy, brachial plexus reimplantation
Introduction
Brachial plexus avulsion is a devastating injury most commonly affecting young adults following motorcycle accidents. Excessive lateral flexion of the neck towards the contralateral side or traction of the arm can lead to interruption of the roots at the surface of the spinal cord; this is effectively a central nervous system injury and often described as “longitudinal spinal cord injury.”1 Involvement of all 5 sets of the dorsal and ventral roots (C5 to T1) leads to a completely paralyzed and anesthetic arm, with associated neuropathic pain. Pioneering reimplantation surgery has been trialed in patients with such injury.2 This procedure involves the implantation of the avulsed ventral roots back in the spinal cord through a slit of the pia mater and a small superficial incision in the ventrolateral spinal cord. Studies have shown significant reduction in the number of motor neurons lost and return of some function mainly in the proximal muscles of the upper limb.1,3 In the majority of these patients, the spinal cord appears normal, but motoneuron cell death and Wallerian degeneration have been described in experimental models of brachial plexus avulsion.4
Advanced magnetic resonance imaging (MRI) techniques have the potential to provide information on the structural and functional changes occurring in the spinal cord after injury, both at the level of the lesion and at distances from the site of injury. One of these techniques, proton magnetic resonance spectroscopy (1H-MRS), albeit technically challenging, has been applied to the spinal cord of patients with neurological diseases. Optimized protocol for quantitative (single voxel) 1H-MRS has been shown to provide reliable spectra especially for voxels placed in the upper cervical cord.5 In patients with cord tumors, multiple sclerosis, chronic cervical spondylotic myelopathy, and chronic whiplash,6-9 1H-MRS has demonstrated abnormal concentrations of metabolites that reflect specific pathological processes. The most commonly reported finding is a reduced concentration of N-acetyl-aspartate, which is considered to reflect axonal loss and/or dysfunction.10
The present study applies 1H-MRS of the upper complete cervical cord (ie, above the site of injury) to patients with complete brachial plexus root avulsion who have received re-implantation, to investigate whether metabolic abnormalities are present in this region and whether there is a relationship between metabolite levels and neurological disability.
Methods
Subjects
Ten chronic subjects (mean age, 35 years; SD, 10.8; all men) treated with re-implantation of avulsed spinal cord roots in our unit and 19 age-matched and sex-matched healthy subjects (mean age, 36.1 years; SD, 10.7; 18 men) were studied. Patients were recruited from our database of brachial plexus re-implantations. Only patients with complete brachial plexus injury confirmed with open exploration of the brachial plexus were included in the study. Traumatic meningocoeles were observed in 2 patients. Computed tomography myelogram demonstrated appearances suggestive of complete brachial plexus avulsion in 5 patients. Patients with abnormal signal within the spinal cord on conventional MRI, spinal canal stenosis, clinical signs unrelated to the brachial plexus injury (eg, leg weakness), and MRI incompatible metal implants were excluded from the study.
All patients underwent ventral root re-implantation within 1 month from the day of their injury. The mean duration from injury to assessment for this study was 5.5 years (SD, 4.3).
The study was approved by the regional ethics committee, and informed (and written) consent was obtained from all patients and healthy volunteers participating in the study.
Clinical Assessment
On the day of the scan, patient disability was assessed using the following scales: (a) Disability for Arm, Shoulder and Hand (DASH), which is a 30-item self-report questionnaire designed to measure physical function and symptoms in people with musculoskeletal disorders of the upper limb. A higher score indicates greater disability;11 (b) Visual Analogue Pain Scale (VAS), a widely used tool for measuring pain by asking the patient to indicate his/her perceived pain intensity by marking a point along a 100-mm horizontal line;12 (c) Michigan Hand Outcomes Questionnaire (MHQ), which is a hand-specific questionnaire including 6 subscales: overall hand function, activities of daily living (ADL), pain, work performance, and patient satisfaction with hand function and aesthetics. Higher scores indicate better hand function, except for the pain subscale on which higher scores correspond to more pain;13 (d) Medical Research Council (MRC) muscle strength scale for the upper limb, which included 7 muscles scored from 0 to 5.
Magnetic Resonance Protocol and Spectroscopy
All magnetic resonance data were collected on a Magnetom Tim Trio 3T system (Siemens AG, Erlangen, Germany), using the posterior half of a 12-channel head coil, the posterior part of a neck array coil, and the upper element of the spine array coil.
All subjects underwent conventional T2 sagittal and coronal images (sagittal-oblique: turbo spin echo with effective TE = 96 ms; TR = 3 s; parallel imaging with acceleration factor 2; field of view [FoV] 220 mm2, in-plane resolution 0.69 × 0.92 mm2, slice thickness 1.5 mm, gap 0.15 mm; coronal-oblique: 3D-HASTE with TE = 247 ms; TR = 3 s; parallel imaging with acceleration factor 2, FoV 200 mm2, in-plane resolution 0.78 × 0.78 mm2, 52 partitions, slice thickness 0.8 mm), which confirmed the normal radiological appearance of the spinal cord in all patients. These scans were acquired aligned with the main axis of the spinal cord, essential for correctly positioning the spectroscopy voxel.
Single-voxel spectra were obtained by cardiac-gated point resolved spectroscopy (PRESS) sequence with TR ≅ 3000 ms (depending on the cardiac cycle), TE = 30 ms, and 160 averages, with chemical-shift-selective (CHESS) water suppression. 14 We used the PRESS sequence provided by the manufacturer, and no additional saturation bands were used. The excitation frequency was −2.3 ppm, with respect to the water reference frequency, that is, 2.4 ppm. Readout bandwidth was 1200 Hz (1024 points collected). Our longer TR compared with previous studies reduced sensitivity to metabolites T1 changes. A non–water-suppressed spectrum (2 averages) was also acquired for eddy-current correction. As suggested by Cooke et al,5 the spectroscopic voxel (mean volume of all subjects including patients and healthy volunteers of 2.4 mL, SD 0.6 mL) was placed along the main axis of the cord, centered at C2 spinal level with its prescription boundaries completely contained within the cord on the T2-weighted images in all 3 planes (Figure 1). To increase the spectroscopy signal, we increased the length of spectroscopy voxel to maximum possible length so that the voxel spanned between C2 and C3 spinal levels. Optimal shim currents for all first-order (X, Y, and Z) and second-order (Z 2, ZX, ZY, X 2-Y 2, and XY) shim gradients were calculated off-line with in-house software based on (a) acquisition of field maps (gradient-recalled sequence generating 2 images with TE = 4.92 ms and TE = 7.38 ms, respectively, 2 mm isotropic resolution, 1 minute acquisition time) and (b) minimization of the magnetic field variation within the prescribed MRS voxel using calibrated field maps for each shim coil.15,16 Data from 2 healthy controls and 1 patient were not used because of incorrect/failed shimming procedures.
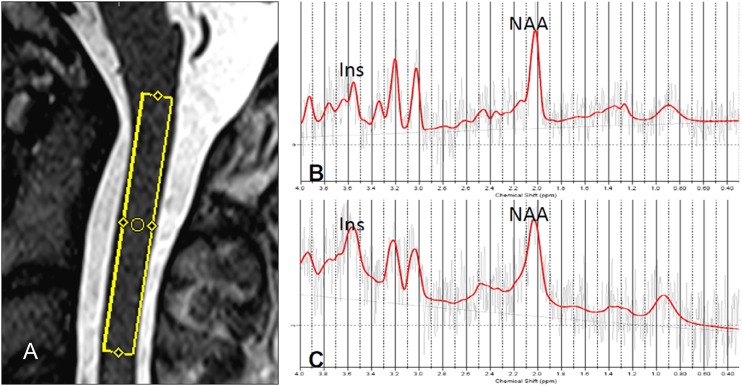
Figure 1.
(A) Location of the spectroscopic voxel between C1 and C3 on the T2-weighted sagittal image of one control. (B) Spectrum derived from voxel in (A) that shows reduced myo-inositol (m-Ins)/creatine and phosphocreatine (Cr) ratio (1.37 [%SD = 11]) in comparison with a spectrum obtained in a patient (m-Ins/Cr = 2.11 [%SD = 10]) (C).
All MRS spectra were analyzed by means of the user-independent fitting LCModel 6.117 within the 0.2 to 4.0 ppm range. Examples of patient spectra are shown in Figure 2. Ratios of the following metabolite concentrations were calculated with respect to total creatine (Cr) concentration (creatine plus phosphocreatine): N-acetyl-aspartate (NAA = NAA plus NAAG), total choline (Cho = GPC plus phosphocholine), and myo-inositol (m-Ins). LCModel standard error estimates (%SD, Cramer-Rao lower bounds) were used to assess the confidence of the concentration estimates. Spectra for 3 controls and 1 patient with %SD of (Cr) >21% were excluded. Thus, the final MRS data came from 14 healthy controls (13 men and 1 woman; mean age, 36.1 years [SD, 10.7]) and 8 patients (all men; mean age, 35 years [SD, 10.8]). The FWHM and SNR values (mean ± SD) estimated by LCModel were 14 ± 2 Hz and 2.4 ± 0.8, respectively, for the healthy subjects and 12 ± 4 Hz and 2.8 ± 1.0, respectively, for the patient group (2.4 ± 0.8 for all patients). There was no statistical difference in FWHM between the 2 groups, t(21) = 0.12, P = .91. M-Ins/Cr ratio was excluded from the analysis in 3 controls because of %SD of m-Ins >21%. NAA/Cr was excluded in 3 different controls and 1 patient because of %SD for tNAA >21%. The procedure of excluding single values of metabolite concentrations when associated with high %SD values has been performed in previous MRS studies.7 Moreover, the analysis was repeated without the spectra for subjects with %SD values greater than 21, and results did not change significantly (results not shown). The group mean %SD values obtained by LCModel analysis for the main metabolites in the patient group were 12, 15, 12, and 12 for Cr, NAA, m-Ins, and Cho, respectively. The corresponding values for healthy volunteers were 11 for Cr, 13 for NAA, 16 for m-Ins, and 14 for Cho. Finally, the overall chemical shift was fairly small in terms of volume shift and mispositioning of the VOI.
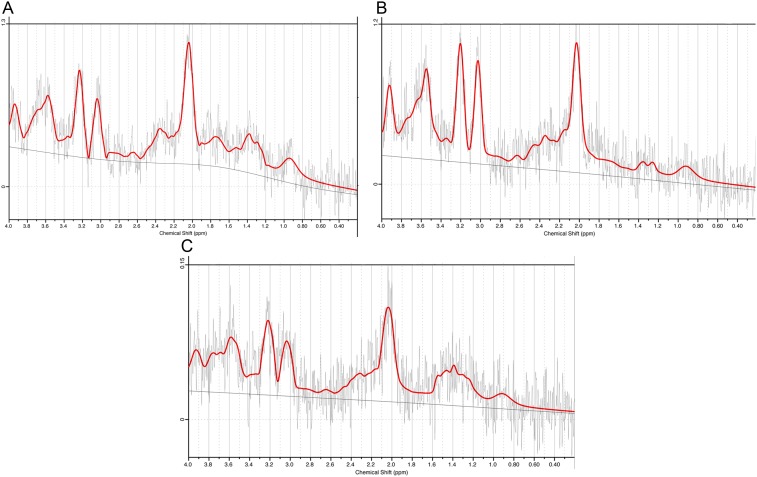
Figure 2.
Examples of 3 spectra (gender, years of age): (A) male, 21; (B) male, 47; (C) male, 43.
Statistical Analysis
STATA statistical software version 10.1 (StataCorp LP, College Station, Texas) was used for statistical analysis. Data for each metabolite were tested for normal distribution and for homogeneity of variances. Differences in metabolite ratios between groups were estimated using the 2-sample t test. The relationship between disability and metabolite ratios was tested in patients using multiple linear regression, using the clinical scores, in turn, as the dependent variable and the metabolite ratios as independent variables, correcting for age. Statistical significance was accepted at the 5% level (P < .05).
Results
Patients showed an increase in m-Ins/Cr values when compared with healthy controls, t(21) = −3.73; 95% confidence interval [CI] = −1.05 to −0.29; P = .001 (Table 1 and Figure 1). NAA/Cr and Cho/Cr ratios did not differ between groups (Table 1).
Table 1.
Mean and Standard Deviation of the Ratios of the Main Metabolites With Respect to Creatine (plus Phosphocreatine) Obtained in Patients With Brachial Plexus Reimplantation and Healthy Controls
| Ratio | Controls, Mean (SD) | Patients, Mean (SD) | P Value |
|---|---|---|---|
| NAA/Cr | 1.19 (0.29) | 1.21 (0.38) | .85 |
| m-Ins/Cr | 1.18 (0.38) | 1.86 (0.41)a | .01 |
| Cho/Cr | 0.30 (0.08) | 0.33 (0.45) | .45 |
Abbreviations: NAA, N-acetyl-aspartate; m-Ins, myo-inositol; Cho, choline-containing compounds.
Statistical difference between patients and controls.
The multiple linear regression showed that m-Ins/Cr ratio was independently associated with both upper limb disability and time from injury (R 2 = 84%; F = 13.56; P = .01). In particular, a higher m-Ins/Cr ratio correlated with greater disability, as measured by the DASH scale, t(7) = 2.86; 95% CI = −0.001 to −0.02; P = .035 (Figure 3), and with shorter time from injury, t(7) = −5.15; 95% CI = −0.02 to −0.006; P = .004 (Figure 4). No statistical correlation was found between MHQ, VAS, and MRC muscle strength scale for the affected upper limb and metabolite concentrations. The time difference from injury to scan did not alter the regression models of NAA/Cr and Cho/Cr.
Figure 3.
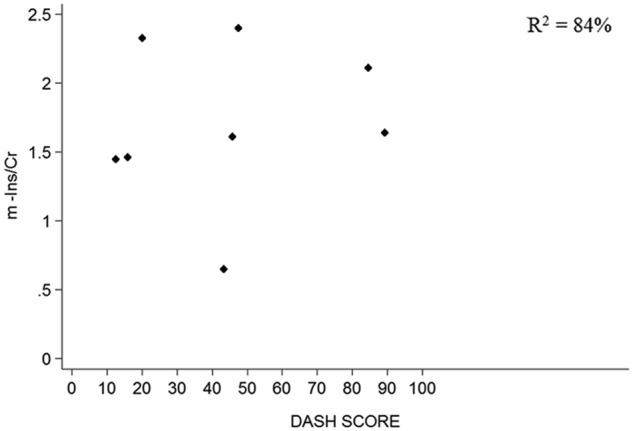
Scatter plots showing the relationship between m-Ins and DASH score. Abbreviations: m-Ins, myo-inositol; Cr, total creatine; DASH, Disability for Arm, Shoulder and Hand score.
Figure 4.
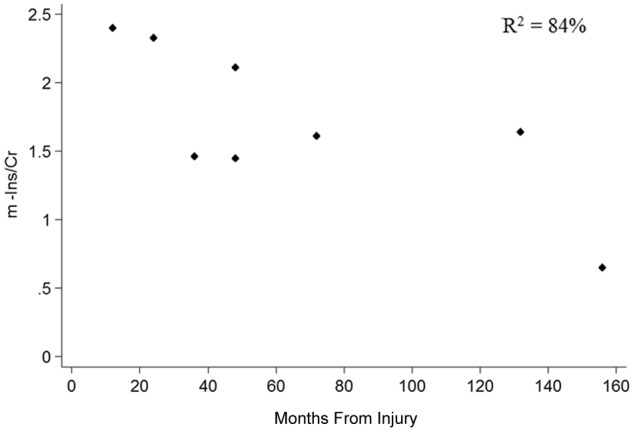
Scatter plot showing the relationship between m-Ins and months from injury. Abbreviations: m-Ins, myo-inositol; Cr, total creatine.
Discussion
Our findings suggest that quantification of metabolites through 1H-MRS of the upper cervical cord above the site of injury provides insights into the underlying pathological processes, suggesting that 1H-MRS may prove to be a useful tool in predicting outcome in this group of patients. In this study, patients showed a significantly greater m-Ins/Cr ratio when compared with controls. This is a novel finding, which has not been observed in previous 1H-MRS studies of patients with spinal cord lesions.6,8 Only a marginally increased m-Ins has been detected in the spinal cord of patients with multiple sclerosis with acute7 and chronic lesions18 when compared with controls. Increased m-Ins suggests that reactive gliosis, including proliferation and astrocytic hypertrophy, may occur in the spinal cord above the site of injury, possibly in response to the Wallerian degeneration of avulsed neuronal fibers. This is in agreement with (a) increase in vimentin (VIM) glial fibrillary acidic protein (GFAP) immunoreactivity, which is a marker of proliferation of astrocytes and gliosis, described in animal models of acute and chronic stage after spinal cord injury19,20 and (b) proliferation of astrocytes forming a nonpermissive glial scar surrounding the transitional zone, found in models of nerve root avulsion injury.4,21,22 In this later experimental model, up to 50% motoneurons are lost in the relevant spinal cord segment and further neuronal loss occurs over time.4,21,22
In patients, a higher m-Ins/Cr ratio was associated with greater disability of the arm, indicated by a higher DASH score, suggesting that metabolic damage in the spinal cord of chronic patients treated with brachial plexus re-implantation may be relevant to clinical function of the corresponding arm. It is interesting to note that the DASH scale is a patient-reported outcome measure (PROM), which measures severity of symptoms by taking into account the patient’s perspective.23 Therefore, it may be useful in the future to integrate this PROM into assessment of patients with brachial plexus avulsion and spinal cord injury, since it may complement more direct measures of upper limb strength. We also found that a greater m-Ins/Cr ratio correlated with shorter time from injury, suggesting that gliotic changes may normalize over time, possibly reflecting reorganization in the spinal cord. To confirm the hypotheses above, we are planning studies that will obtain pre-repair MRS along with further prospective MRS examinations during the rehabilitation period.
Reduced NAA levels indicate both axonal loss and metabolic dysfunction.9,10 The aim of re-implantation surgery is to achieve regeneration of new axon fibers from the avulsed root into the spinal cord. Hence, if the surgery were successful, one would expect an increase in NAA/Cr ratio with time. In this group of patients, there was no statistical relationship between NAA/Cr ratio and time from injury. The NAA/Cr ratio was similar in patients and controls, suggesting a recovery of axonal numbers either by direct axonal regeneration or sprouting. In previous studies of patients with chronic whiplash and cervical spondylotic myelopathy,6,8 and in patients with multiple sclerosis,18,24 a lower NAA/Cr ratio was seen when compared with controls. In addition, patients studied 0.5 to 2 years after spinal cord injury showed higher NAA levels in the ipsilateral occipital cortex and motor cortex when compared with controls.25 In patients with multiple sclerosis, the acute reduction in NAA at the onset of an acute relapse was followed by a sustained, albeit partial, increase over time.7 The lack of difference in NAA/Cr ratio in our study may be due to the small number of patients and low statistical power, or a true increase in nerve fibers, which perhaps have not made functional synapses. Therefore, future studies will investigate whether NAA is reduced in the spinal cord after brachial plexus injury in the acute phase and then normalizes over time. A key question is whether patients who did not undergo re-implantation show significantly reduced levels of NAA and increased m-Ins compared with patients who underwent re-implantation. Unfortunately, untreated patients are extremely rare. Future and collaborative work, perhaps with a multicenter setting, may be able to address this question, which is relevant for forthcoming clinical trials with stem cell transplantation.26
Our findings suggest that metabolic changes occur in patients after brachial plexus root re-implantation at sites that are distant to the site of primary damage and that these changes may be clinically relevant. The small number of reimplanted patients recruited in this study, which was due to both the unique group of patients and local constraints in scanning patients with metal implants, is a limitation of our study. In addition, spectroscopic analysis has been limited to NAA, choline, and myo-inositol, whereas other potentially measurable metabolites such as lactate (Lac) and glutamine/glutamate (Gln/Glu) have not been studied. Lac resonance is at the very limit of detectability in most brain MRS studies because of the low concentration of Lac under normal conditions but is often detectable in pathological conditions where anaerobic metabolism ensues. However, our patients are well into the chronic stage of their injury and such elevations would not persist years after the injury. Second, Lac resonance is difficult to distinguish because of overlapping lipid resonances and requires long echo time (~140 ms) or other spectral editing techniques. To our knowledge, Lac spectra from the spinal cord have been acquired in one study of cervical spondylotic myelopathy patients in the acute stage, where Lac peaks were demonstrated in 7 out of 21 patients, 6 of which had T2 signal abnormality in the same region of the spinal cord.8 Furthermore, Gln/Glu quantification is complex and challenging and to our knowledge has not been reported in the spinal cord to date. We aim to perform pretreatment MRS and prospective longitudinal follow-up MRS studies during the rehabilitation period to demonstrate temporal changes in the spinal cord and correlate to clinical recovery, which will also enable us to obtain Lac spectra during the acute stage.
Despite the above limitations, the study opens promising perspectives for further applications of spinal cord MRS to patients with spinal cord injuries. Future studies are needed to combine functional MRI with metabolic and structural MRI and correlate pretreatment MRS to longitudinal follow-up MRS to better understand recovery after spinal cord injury and fully characterize central nervous system plasticity,27 which is the ultimate goal of multimodal imaging. Last, the current study provides the first steps toward identifying markers to serve as outcome measures for trials comparing strategies of brachial plexus repair following avulsion injury.
Acknowledgments
We thank all the patients and the healthy volunteers for their collaboration and time.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: OC has received honoraria from GE, Bayer, and CML-Multiple Sclerosis and is on the editorial board of Neurology. AJT has received honoraria and support for travel from Eisai Ltd, Novartis, BTG, and Serono Symposia International Foundation, and honoraria as editor-in-chief of Multiple Sclerosis Journal. CK, EDV, DLT, CAMWK, EB, TC, and DC report no disclosures.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the UCLH/UCL Biomedical Research Centre.
References
- 1. Carlstedt T. Root repair review: basic science background and clinical outcome. Restor Neurol Neurosci. 2008;26:225-241 [PubMed] [Google Scholar]
- 2. Carlstedt T, Anand P, Hallin R, Misra PV, Noren G, Seferlis T. Spinal nerve root repair and reimplantation of avulsed ventral roots into the spinal cord after brachial plexus injury. J Neurosurg. 2000;93:237-247 [DOI] [PubMed] [Google Scholar]
- 3. Carlstedt T, Anand P, Htut M, Misra P, Svensson M. Restoration of hand function and so called “breathing arm” after intraspinal repair of C5-T1 brachial plexus avulsion injury. Case report. Neurosurg Focus. 2004;16:E7. [DOI] [PubMed] [Google Scholar]
- 4. Koliatsos VE, Price WL, Pardo CA, Price DL. Ventral root avulsion: an experimental model of death of adult motor neurons. J Comp Neurol. 1994;342:35-44 [DOI] [PubMed] [Google Scholar]
- 5. Cooke FJ, Blamire AM, Manners DN, Styles P, Rajagopalan B. Quantitative proton magnetic resonance spectroscopy of the cervical spinal cord. Magn Reson Med. 2004;51:1122-1128 [DOI] [PubMed] [Google Scholar]
- 6. Elliott JM, Pedler AR, Cowin G, Sterling M, McMahon K. Spinal cord metabolism and muscle water diffusion in whiplash. Spinal Cord. 2012;50:474-476 [DOI] [PubMed] [Google Scholar]
- 7. Ciccarelli O, Wheeler-Kingshott CA, McLean MA, et al. Spinal cord spectroscopy and diffusion-based tractography to assess acute disability in multiple sclerosis. Brain. 2007;130:2220-2231 [DOI] [PubMed] [Google Scholar]
- 8. Holly LT, Freitas B, McArthur DL, Salamon N. Proton magnetic resonance spectroscopy to evaluate spinal cord axonal injury in cervical spondylotic myelopathy. J Neurosurg Spine. 2009;10:194-200 [DOI] [PubMed] [Google Scholar]
- 9. Sajja BR, Wolinsky JS, Narayana PA. Proton magnetic resonance spectroscopy in multiple sclerosis. Neuroimaging Clin N Am. 2009;19:45-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beaton DE, Katz JN, Fossel AH, Wright JG, Tarasuk V, Bombardier C. Measuring the whole or the parts? Validity, reliability, and responsiveness of the Disabilities of the Arm, Shoulder and Hand outcome measure in different regions of the upper extremity. J Hand Ther. 2001;14:128-146 [PubMed] [Google Scholar]
- 12. Flaherty SA. Pain measurement tools for clinical practice and research. AANA J. 1996;64:133-140 [PubMed] [Google Scholar]
- 13. Chung KC, Pillsbury MS, Walters MR, Hayward RA. Reliability and validity testing of the Michigan Hand Outcomes Questionnaire. J Hand Surg Am. 1998;23:575-587 [DOI] [PubMed] [Google Scholar]
- 14. Haase A, Frahm J, Hanicke W, Matthaei D. 1H NMR chemical shift selective (CHESS) imaging. Phys Med Biol. 1985;30:341-344 [DOI] [PubMed] [Google Scholar]
- 15. Kim DH, Adalsteinsson E, Glover GH, Spielman DM. Regularized higher-order in vivo shimming. Magn Reson Med. 2002;48:715-722 [DOI] [PubMed] [Google Scholar]
- 16. Webb P, Macovski A. Rapid, fully automatic, arbitrary- volume in vivo shimming. Magn Reson Med. 1991;20:113-122 [DOI] [PubMed] [Google Scholar]
- 17. Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672-679 [DOI] [PubMed] [Google Scholar]
- 18. Marliani AF, Clementi V, Albini RL, et al. Quantitative cervical spinal cord 3T proton MR spectroscopy in multiple sclerosis. AJNR Am J Neuroradiol. 2010;31:180-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Verma P, Garcia-Alias G, Fawcett JW. Spinal cord repair: bridging the divide. Neurorehabil Neural Repair. 2008;22:429-437 [DOI] [PubMed] [Google Scholar]
- 20. Erschbamer M, Oberg J, Westman E, Sitnikov R, Olson L, Spenger C. 1H-MRS in spinal cord injury: acute and chronic metabolite alterations in rat brain and lumbar spinal cord. Eur J Neurosci. 2011;33:678-688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Risling M, Aldskogius H, Hildebrand C, Remahl S. Effects of sciatic nerve resection on L7 spinal roots and dorsal root ganglia in adult cats. Exp Neurol. 1983;82:568-580 [DOI] [PubMed] [Google Scholar]
- 22. Risling M, Cullheim S, Hildebrand C. Reinnervation of the ventral root L7 from ventral horn neurons following intramedullary axotomy in adult cats. Brain Res. 1983;280: 15-23 [DOI] [PubMed] [Google Scholar]
- 23. Carr AJ, Gibson B, Robinson PG. Measuring quality of life: is quality of life determined by expectations or experience? BMJ. 2001;322:1240-1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blamire AM, Cader S, Lee M, Palace J, Matthews PM. Axonal damage in the spinal cord of multiple sclerosis patients detected by magnetic resonance spectroscopy. Magn Reson Med. 2007;58:880-885 [DOI] [PubMed] [Google Scholar]
- 25. Puri BK, Smith HC, Cox IJ, et al. The human motor cortex after incomplete spinal cord injury: an investigation using proton magnetic resonance spectroscopy. J Neurol Neurosurg Psychiatry. 1998;65:748-754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kachramanoglou C, Li D, Andrews P, et al. Novel strategies in brachial plexus repair after traumatic avulsion. Br J Neurosurg. 2011;25:16-27 [DOI] [PubMed] [Google Scholar]
- 27. Cadotte D, Wilson J, Mikulis D, Stroman PW, Brady S, Fehlings M. Conventional MRI as a diagnostic and prognostic tool in spinal cord injury: a systemic review of its application to date and an overview on emerging MRI methods. Exper Opin Med Diagn. 2011;5:121-133 [DOI] [PubMed] [Google Scholar]