Abstract
Dentatorubral-pallidoluysian atrophy (DRPLA) is a progressive neurodegenerative disorder that currently has no curative treatments. DRPLA is caused by an expansion of a CAG trinucleotide repeat region within the protein-encoding sequence of the atrophin-1 (ATN-1) gene. Inhibition of mutant ATN-1 protein expression is one strategy for treating DRPLA, and allele-selective gene silencing agents that block mutant expression over wild-type expression would be lead compounds for therapeutic development. Here we develop an assay for distinguishing mutant from wild-type ATN-1 protein by gel electrophoresis. We use this assay to evaluate duplex RNAs and single-stranded silencing RNAs (ss-siRNAs) for allele-selective inhibition of ATN-1 protein expression. We observed potent and allele-selective inhibition by RNA duplexes that contain mismatched bases relative to the CAG target and have the potential to form miRNA-like complexes. ss-siRNAs that contained mismatches were as selective as mismatch-containing duplexes. We also report allele-selective inhibition by duplex RNAs containing unlocked nucleic acids or abasic substitutions, although selectivities are not as high. Five compounds that showed >8-fold allele selectivity for mutant ATN-1 were also selective for inhibiting the expression of two other trinucleotide repeat disease genes, ataxin-3 (ATXN-3) and huntingtin (HTT). These data demonstrate that the expanded trinucleotide repeat within ATN-1 mRNA is a potential target for compounds designed to achieve allele-selective inhibition of ATN-1 protein, and one agent may allow the targeting of multiple disease genes.
Dentatorubral-pallidoluysian atrophy (DRPLA) is an inherited autosomal dominant neurodegenerative disease with clinical manifestations that include dementia, ataxia, epilepsy, chorea, and psychological disturbances.1 DRPLA is caused by an expansion of the CAG repeat region within the atrophin-1 (ATN-1)2 gene from a normal value of fewer than 34 repeats to up to 90 in DRPLA patients.3 The mean repeat number in patients is estimated to be 63–68 repeats.2 DRPLA affects between 2 and 7 per million of the Japanese population4 but is also found in European and North American families.5 Model mice with 129 repeats within an introduced ATN-1 transgene exhibit a severe phenotype reminiscent of juvenile onset DRPLA.6
Because the expression of the mutant ATN-1 protein causes DRPLA, reducing the level of expression of mutant ATN-1 may delay the onset or slow progression of the disease. Huntington disease (HD) is caused by a similar CAG expansion within the gene encoding huntingtin (HTT) protein.7 Pharmacological inhibition of HTT protein expression with an antisense oligonucleotide that targets both normal and mutant alleles has been shown to alleviate disease and reverse some symptoms in a mouse HD model.8 This report, taken together with other studies, suggests that nucleic acids may be a promising approach for developing agents for the treatment of intractable neurological disease.9−11 For ATN-1, one report indicates that a 2′-O-methyl-substituted phosphorothioate DNA oligonucleotide that targets the expanded CAG repeat reduces levels of the mutant ATN-1 transcript.12
Mice that lack the ATN-1 gene show no phenotype,13 suggesting that allele-selective silencing may not be necessary. ATN-1 protein is a potent transcriptional regulator,13 and in the more complex human central nervous system, ATN-1 may have more critical functions. Because of this uncertainty about the role of ATN-1 in humans, effective allele-selective silencing agents would be a useful option for clinical development.
We began investigations into allele-selective targeting of trinucleotide repeat genes using peptide nucleic acid (PNA) and locked nucleic acid (LNA) oligomers complementary to the expanded CAG repeat within HTT mRNA.14 We achieved allele-selective inhibition of HTT protein expression, but allele selectivities for the inhibition of wild-type (wt) HTT versus mutant HTT were rarely greater than 6-fold. We reasoned that we might achieve more robust and selective inhibition using duplex RNAs that were also complementary to the CAG repeat. When we performed this experiment, we found that, while inhibition by these compounds was potent, little or no selectivity was observed.
RNA interference by standard fully complementary siRNAs involves cleavage of target mRNA and is a powerful mechanism for gene silencing, possibly too powerful to allow discrimination between mutant and wild-type alleles that differ only in the number of CAG repeats. Micro RNAs (miRNAs) supply an endogenous gene silencing mechanism that typically involves duplex RNAs that are mismatched relative to their mRNA targets. Argonaute 2 (AGO2) is the catalytic engine of RNAi15 that allows cleavage of target RNAs when sequences are fully complementary. The introduction of mismatches within the central region of the duplex eliminates the potential for substrate cleavage by AGO2.16
To test whether redesigned RNAs might offer better results, we introduced mismatches into the center of the anti-CAG duplex RNAs. These mismatch-containing RNAs proved to be powerful allele-selective repressors of HTT expression, with several compounds achieving selectivities of >25-fold.17,18 These results are consistent with the hypothesis that duplexes functioning through a non-cleavage-dependent miRNA-like mechanism are better able to discriminate between normal and extended CAG repeat targets. Krzyzosiak and co-workers achieved similar results.19
Subsequent studies have demonstrated that mismatched bases were not the only design element capable of promoting allele-selective inhibition of HTT. Introducing unlocked nucleic acid (UNA) nucleotides20−22 or abasic substitutions23 into the central region of duplex RNAs also yielded highly selective inhibition of HTT expression. These data demonstrate that removing a nucleobase (abasic) or opening the ribose ring (UNA) at central positions within the RNA duplex is sufficient to manipulate AGO activity and make gene silencing become sensitive to the number of CAG trinucleotides within a repeat region.
Allele-selective inhibition through RNAi can also be achieved with single-stranded RNA oligomers. Unmodified RNA, however, is rapidly degraded and is not an effective gene silencing agent inside cells. It is possible to introduce chemical modifications into single-stranded RNA that stabilize the single strands and allow them to be potent modulators of RNAi activity in cell culture and in animals.24 When we designed these single-stranded silencing RNAs (ss-siRNAs) to target CAG repeats and introduced them into patient-derived cells, the RNAs also proved to be potent and allele-selective agents for inhibiting mutant HTT protein.25,26 Inhibition by ss-siRNAs occurred through the RNAi pathway and could be observed upon intraventricular administration in a mouse HD model.
CAG expansions that occur within different genes cause several diseases. For example, Machado Joseph disease (MJD) is caused by a CAG expansion within the ataxin-3 (ATXN-3) gene.27 We found that duplex RNAs and ss-siRNAs targeting the CAG repeat were allele-selective inhibitors of ATXN-3 protein expression.23,28,29
Here we further test the generality of anti-CAG oligomers by testing the hypothesis that allele-selective inhibition can be extended to ATN-1. We identify several duplex RNAs and ss-siRNAs that target CAG repeats and inhibit expression of mutant ATN-1 protein with good selectivity relative to the selectivity of inhibition of wild-type protein expression. Our results suggest a potential approach to therapeutic development for DRPLA.
Materials and Methods
RNA Synthesis
UNA-modified antisense RNAs and unmodified sense RNAs were synthesized by Sigma Custom Products (The Woodlands, TX). ss-siRNAs were synthesized at ISIS Pharmaceuticals.24 Chemically modified and abasic RNA oligonucleotides were synthesized at Alnylam Pharmaceuticals.23 Double-stranded RNAs were prepared by annealing the two RNA strands in 2.5× PBS solutions. Stock solutions (20 μM) were used for transfection in cell cultures.
Cell Culture and Transfection
DRPLA patient-derived fibroblast cell line GM13716 was obtained from the Coriell Institute (Camden, NJ). The fibroblasts were maintained at 37 °C and 5% CO2 in Minimal Essential Media Eagle (MEM) (Sigma, M4655) supplemented with 15% heat-inactivated fetal bovine serum (Sigma) and 0.5% MEM nonessential amino acids (Sigma). Cells were plated at a density of 80000 per well of a six-well 35 mm diameter plate 48 h before transfection. siRNAs or ss-siRNAs were transfected into cells with lipid RNAiMAX (Life Technologies) as previously described.17,25 Cells were typically harvested 2 days after transfection. The siRNA/siATN1 is a positive control siRNA targeting the ATN1 mRNA, 5′-UCGAUCUCAGUUCUUCCCGdTdT-3′ (AS). It was tested at a concentration of 50 nM in fibroblast cells.
Western Blot Analysis
Mutant and wild-type ATN-1 from GM13716 cells were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and analyzed by Western blot analysis. Tris-HCl SDS–PAGE was used to separate ATN-1 isoforms [separating gel consisting of a 5% acrylamide/bisacrylamide mixture (37.5:1), 450 mM Tris-HCl (pH 8.8), and 0.1% SDS; stacking gel consisting of a 4% acrylamide/bisacrylamide mixture (37.5:1) and 150 mM Tris-HCl (pH 6.8); running buffer consisting of Tris, glycine, and SDS buffer (Bio-Rad)]. Gels were run at 75 V for 15 min and then at 120 V for 60 min. The electrophoresis tank was kept in an ice–water bath to keep the temperature of the inner part of the tank containing the gel at approximately 25 °C. Maintaining the temperature was necessary to maximize the separation between mutant and wild-type bands and to ensure that the bands that were due to ATN-1 were not obscured by the detection of other proteins. The primary antibodies used included anti-ATN-1 (A300–753A, Bethyl laboratories, 1:1000) and anti-β-actin (Sigma, 1:10000). Protein bands were quantified using ImageJ. The percentage of inhibition was calculated relative to a control sample. Dose fitting curves were generated using GraphPad Prism 6 with the equation y = 100[1 – xm/(nm + xm)], where y is the percentage of inhibition, x is the siRNA concentration, n is the IC50 value, and m is the Hill coefficient.
Results
Assay for Mutant versus Wild-Type ATN-1 Expression
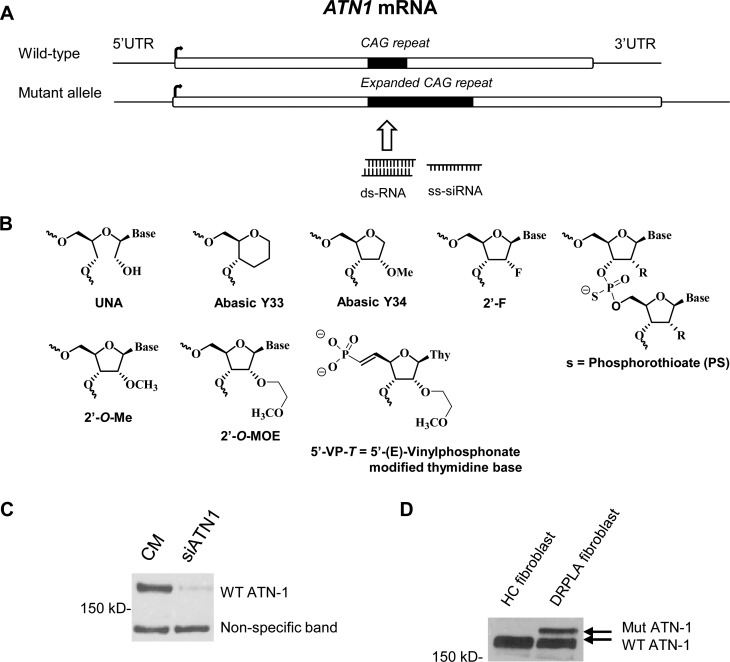
To evaluate allele-selective silencing of ATN-1, we used a fibroblast cell line derived from DRPLA patient cells (GM13716). GM13716 cells are heterozygous for mutant ATN-1 and contain 16 CAG repeats in the wild-type allele and 68 repeats within the mutant mRNA. The calculated molecular mass of the ATN-1 protein is approximately 125 kDa, and the CAG repeats are located within the protein-encoding region (Figure 1A). Chemical modifications (Figure 1B) were introduced into duplex RNAs and ss-siRNAs to improve RNA stability and enhance allele-selective inhibition of mutant ATN-1 expression.
Figure 1.
ATN-1 target mRNA, chemical modifications, and assay development. (A) Diagram of ATN-1 mutant and wild-type mRNA showing the CAG repeat region that is the target for duplex and single-stranded RNAs. (B) Chemical structures of modifications used in this study. (C) Use of an siRNA, siATN1, targeting ATN-1 mRNA to identify the ATN-1 protein band in a control fibroblast cell line. CM is a control duplex RNA that is not complementary to ATN-1 mRNA. (D) Western analysis showing separation of mutant and wild-type ATN-1 protein in DRPLA patient-derived cells compared with a healthy control fibroblast cell line.
To assay the inhibition of mutant ATN-1 protein expression, it is necessary to separate mutant and wild-type variants. Separating the mutant and wild-type forms of proteins derived from trinucleotide repeat genes is relatively challenging because the mutant repeats encode expanded glutamine tracts that add only a few thousand daltons in molecular mass. For ATN-1, we faced additional challenges. The best available antibodies for examining ATN-1 were not optimal because cross reactivity caused the appearance of many bands in addition to the one caused by ATN-1. Difficulty achieving clear results led us to develop protocols for improving the identification of ATN-1 protein.
The ATN-1 protein migrates more slowly via SDS–PAGE than would be predicted by its molecular mass,30 complicating identification. To confirm the identity of the ATN-1 protein band, we designed a duplex RNA targeting the ATN-1 exon region downstream of the CAG repeat and tested the RNA in a control fibroblast cell line, which exhibits only wild-type ATN-1 expression. We found the ATN-1 protein, located at 170 kDa, disappeared after treatment with siATN1 (Figure 1C). Next we tried to separate the mutant band from the wild-type ATN-1 band by SDS–PAGE. The Tris-acetate gel system used to separate HTT14 was inadequate, producing a blurred mutant band. Switching to Tris-HCl-buffered gels produced sharper bands with good separation of wild-type and mutant protein (Figure 1D). Tris-HCl gels were constructed manually because similar commercial precast gels did not yield a useful separation of the mutant and wild-type bands.
Mismatch-Containing Duplexes Are Allele-Selective Inhibitors of ATN-1 Expression
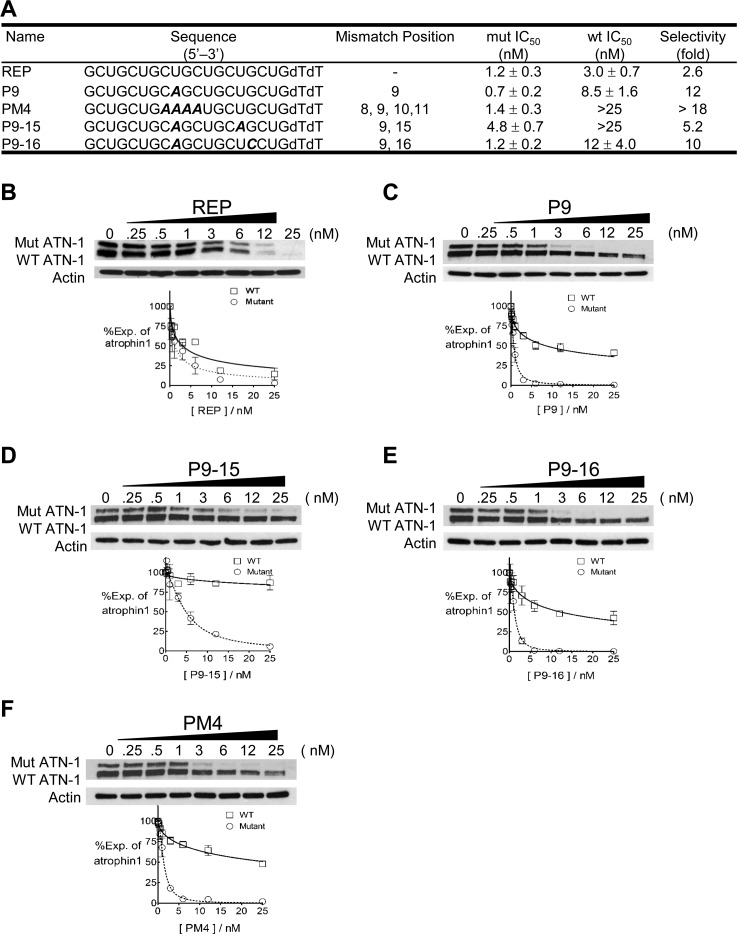
We began our investigation of selective inhibition by testing RNA duplexes containing one or more mismatches relative to the expanded CAG repeat within ATN-1 mRNA (Figure 2A). We found that fully complementary RNA REP showed only a 2.6-fold selectivity for inhibition of mutant versus wild-type ATN-1 expression (Figure 2B). The poor selectivity of the fully complementary duplex is consistent with earlier studies that investigated the inhibition of mutant HTT or ATXN-3.14,17
Figure 2.
Duplex RNAs are allele-selective inhibitors of ATN-1 expression. Gels are Western analyses of ATN-1 protein levels. Graphs are based on triplicate determinations. (A) Sequences, IC50 values, and selectivities of duplex RNAs used for panels B–F. Only the strand complementary to ATN-1 mRNA is shown. Both strands of the duplex contain two DNA/T at the 3′ end. Mismatched bases that are relative to the CAG repeat region are shown in bold italics. (B–F) Gel and dose response graphs for duplex RNAs REP, P9, P9-15, P9-16, and PM4, respectively.
By contrast, duplex RNA P9 that contained a single mismatched base at position 9 showed 12-fold selectivity (Figure 2C). The IC50 values for inhibiting the expression of mutant ATN-1 were similar for REP and P9, 1.2 and 0.7 nM, respectively, demonstrating that duplex P9 was more selective because it better preserved the expression of wild-type ATN-1. We also examined duplexes that combined a mismatch at the central P9 position with secondary mismatches and found that they were also allele-selective. Duplex P9-15 possessed a selectivity of 5-fold (Figure 2D), while duplex P9-16 (Figure 2E) was 10-fold selective.
Up to four mismatches were tolerated within allele-selective RNA duplexes. A duplex RNA with four contiguous mismatches, duplex PM4, possessed a potency of 1.4 nM for inhibition of mutant ATN-1 (Figure 2F). This potency was similar to that for REP or P9. The selectivity of PM4 was greater than 18-fold, also similar to that achieved by duplex P9. For allele-selective inhibition of HTT expression, our mechanistic studies had suggested that binding of mismatch-containing duplex RNAs was cooperative and that cooperative binding of multiple duplexes weakened any detrimental impact of mismatches on potency.18
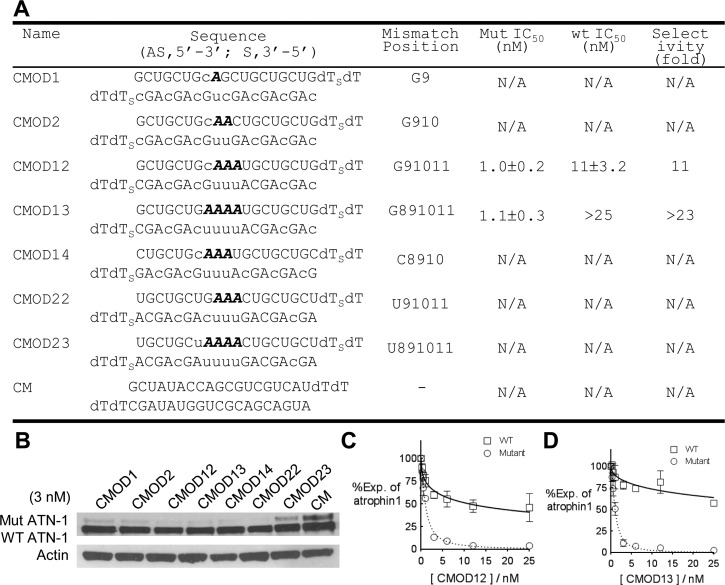
Effect of Chemically Modified Nucleotides within Mismatched RNAs
Chemical modifications are necessary to achieve the stability needed to consider in vivo applications,31 and we examined whether modifications would be compatible with discrimination between mutant and wild-type alleles (Figure 3A and Figure S1 of the Supporting Information). Chemically modified duplexes containing multiple 2′-O-methyl (Figure 1B) modifications with one to four mismatches were allele-selective inhibitors of ATN-1 expression (Figure 3B). Duplexes CMOD12 (three mismatches) and CMOD13 (four mismatches) were chosen for closer examination and achieved 11- and 23-fold selectivities, respectively (panels C and D of Figure 3, respectively). IC50 values for inhibiting expression of mutant ATN-1 were approximately 1 nM, similar to potencies for the unmodified duplexes (Figure 2).
Figure 3.
Chemically modified siRNAs are allele-selective inhibitors of ATN-1 expression. Gels are Western analyses of ATN-1 levels. Graphs are based on triplicate determinations. CM is a control duplex RNA that is not complementary to ATN-1 mRNA. (A) Sequences, IC50 values, and selectivities of chemically modified duplex RNAs used for panels B–D. The bases shown in bold italics are mismatched relative to the CAG repeat region. Lowercase denotes 2′-O-methyl base substitutions. (B) Western analysis showing inhibition of ATN-1 protein expression when RNAs CMOD1, -2, -12, -13, -14, -22, and -23 and CM are added at a concentration of 3 nM. (C and D) Dose–response curves related to IC50 value determination for duplexes for CMOD12 and CMOD13, respectively.
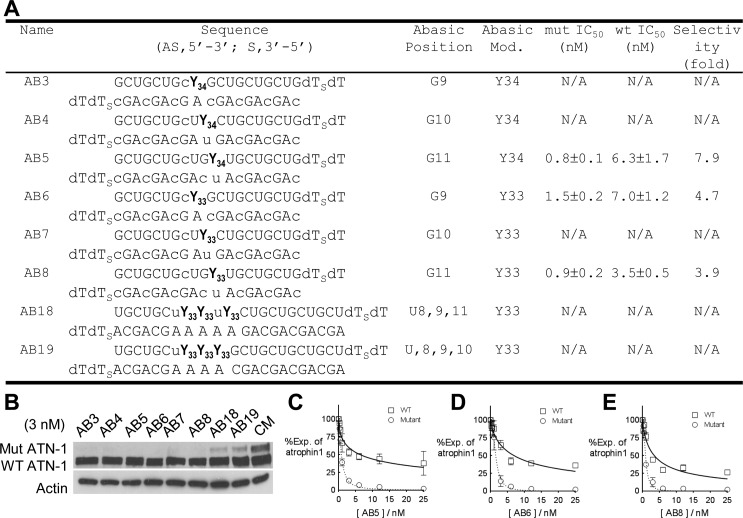
Effect of Abasic Substitutions on Allele-Selective Inhibition of ATN-1
Another strategy for disrupting the cleavage function of AGO2 is to remove the potential for base interactions entirely by using abasic substitutions (Figure 1B). We have previously shown that duplexes containing abasic substitutions can be allele-selective inhibitors of ATXN-3 or HTT expression.23 For ATN-1, abasic-modified duplexes with one to four substitutions (Figure 4A and Figure S2 of the Supporting Information) were allele-selective when they were tested at 3 nM (Figure 4B). Duplexes containing abasic substitutions at different positions were chosen for further analysis and were shown to possess selectivities ranging from 3.9- to 7.9-fold (Figure 4C–E). As observed for unmodified and chemically modified duplex RNAs, abasic RNAs possessed IC50 values for the inhibition of the expression of mutant ATN-1 of ∼1 nM.
Figure 4.
Abasic-substituted duplex RNAs are allele-selective inhibitors of ATN-1 expression. Gels are Western analyses of ATN-1 levels. Graphs are based on triplicate determinations. CM is a control duplex RNA that is not complementary to ATN-1 mRNA. (A) Sequences, IC50 values, and selectivities of duplex RNA containing abasic substitutions that were used for panels B–E. Y34 denotes (2R,3S,4S)-2-(hydroxymethyl)-4-methoxytetrahydrofuran-3-ol and Y33 (2R,3S)-2-(hydroxymethyl)tetrahydrofuran-3-ol. Lowercase denotes 2′-O-methyl RNA substitutions. (B) Western analysis for inhibition of ATN-1 expression by abasic-substituted duplexes tested at 3 nM. (C–E) Dose–response curves related to IC50 value determination for duplexes AB5, AB6, and AB8, respectively.
Effect of UNA Substitutions on Allele-Selective Inhibition of ATN-1
UNA nucleosides are acyclic and lack a connection between the C2′ and C3′ atoms (Figure 1).20,21 In contrast to abasic substitutions that preserve the ribose backbone but lose the potential for base pairing, UNA substitutions preserve the potential for base pairing but have increased backbone flexibility.
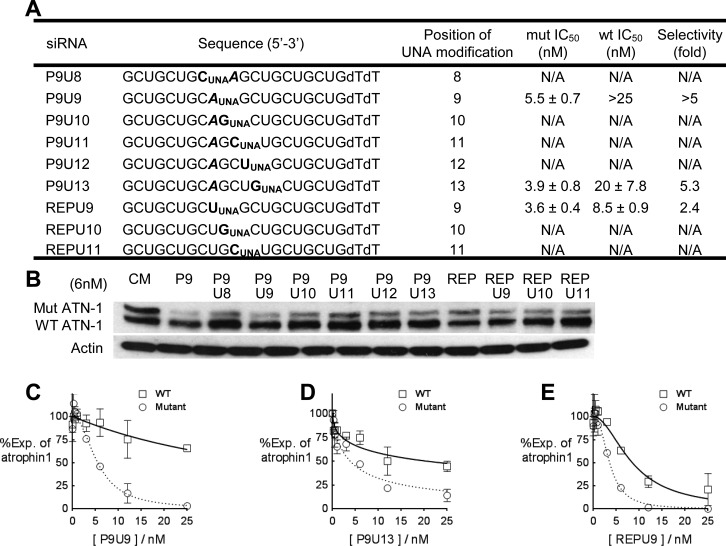
We tested UNA-substituted duplexes (Figure 5A and Figure S3 of the Supporting Information) and observed that several duplexes inhibited ATN-1 protein expression when they were tested at 6 nM (Figure 5B). Three duplexes were chosen for closer examination, including P9U9 that contained a mismatched and UNA base at position 9, P9U13 that contained a mismatched base at position 9 and a UNA base at position 13, and REPU9 that was fully complementary to the CAG repeat within ATN-1 mRNA but contained a UNA substitution at position 9. These UNA-substituted RNAs had selectivities ranging from 2.4- to 5.3-fold (Figure 5C–E). In contrast to the similar IC50 values (approximately 1 nM) for inhibiting expression of mutant HTT by mismatch-containing or abasic-containing duplexes, the IC50 values of the UNA-substituted form were 3–5 nM.
Figure 5.
Allele-selective inhibition of ATN-1 expression by UNA-substituted duplex RNAs. Gels are Western analyses of ATN-1 protein levels. Graphs are based on triplicate determinations. CM is a control duplex RNA that is not complementary to ATN-1 mRNA and lacks full complementarity to any mRNA sequence. (A) Sequences, IC50 values, and selectivities of UNA-substituted duplex RNAs used for panels B–E. Only the antisense strand is shown. Mismatched bases are shown in bold italics. (B) Western analysis for inhibition of ATN-1 expression by UNA-substituted duplexes tested at 6 nM. (C–E) Dose–response graphs for P9U9, P9U13, and REPU9, respectively.
Allele-Selective Inhibition of ATN-1 Expression by ss-siRNAs
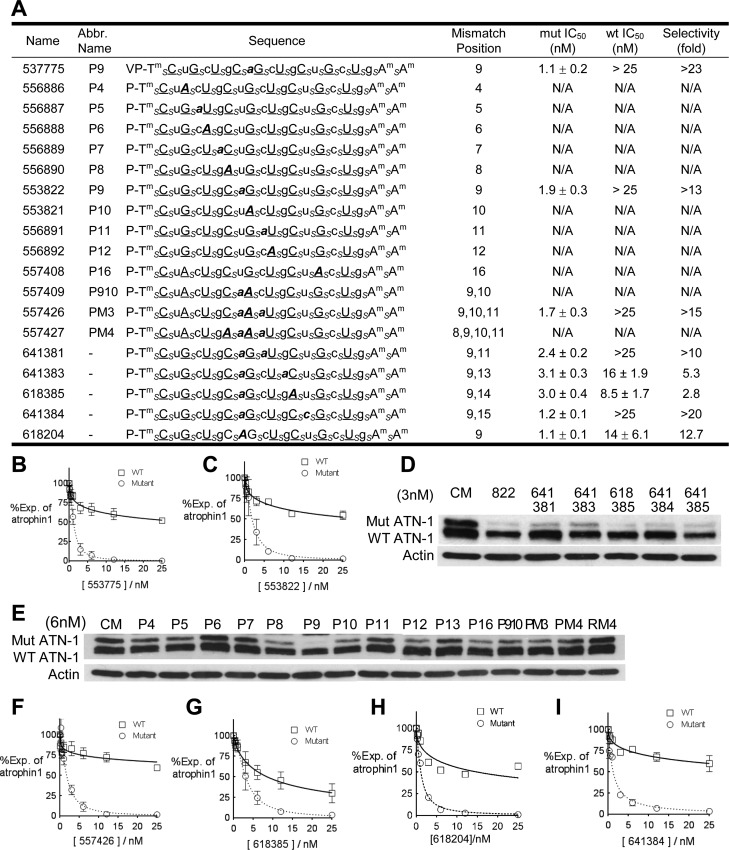
ss-siRNAs represent a new approach to gene silencing that uses single-stranded modified RNAs that retain the ability to function through the RNAi pathway.24 Previous work has shown that ss-siRNAs can be allele-selective inhibitors of HTT25,26 and ATXN-328 expression. To determine whether ss-siRNAs could also allele-selectively inhibit the expression of ATN-1, we tested a series of ss-siRNAs that varied in the position and number of mismatched bases (Figure 6A and Figure S4 of the Supporting Information).
Figure 6.
Allele-selective inhibition of ATN-1 expression by ss-siRNAs. Gels are Western analyses of ATN-1 levels. Graphs are based on triplicate determinations. CM is a control duplex RNA that is not complementary to ATN-1 mRNA. (A) Sequences, IC50 values, and selectivities of ss-siRNAs used for panels B–I. A subscript s indicates a phosphorothioate (PS) linkage. The 2′-O-methyl (2′-O-Me)-modified base is denoted in lowercase. The 2′-fluoro (2′-F) group is shown in underlined uppercase. Tm means 2′-methoxyethyl (2′-MOE)-modified RNA T. Mismatched bases are shown in bold italics. The terminal thymidine has a 5′-phosphate or vinyl phosphonate. All other linkages are phosphates. (B and C) Dose–response curves related to ss-siRNAs 553775 and 553822, respectively. (D and E) ss-siRNAs tested at 3 and 6 nM, respectively. (F–I) Dose–response curves for ss-siRNAs 557426, 618385, 618204, and 641384, respectively.
ss-siRNAs require 5′-vinyl phosphonate modification for action in vivo, and an ss-siRNA containing a vinyl phosphonate and a position 9 mismatch was a potent (IC50 value of 1.1 nM) and allele-selective (>23-fold) compound (Figure 6B). For cell culture testing, ss-siRNAs with 5′-phosphate groups are active and are preferred for testing because the synthesis of large numbers of compounds is more straightforward. ss-siRNA 553822 with a 5′-phosphate terminus behaved like 537775, with an IC50 value of 1.9 and a selectivity of >13-fold (Figure 6C). We then assayed a series of 5′-phosphate ss-siRNAs at 3 and 6 nM (panels D and E of Figure 6, respectively). ss-siRNA 557426 with three central mismatches was both potent and selective, showing that multiple mismatches are tolerated with the ss-siRNA framework (Figure 6F).
One goal for anti-CAG nucleic acids is the development of compounds that are selective for allele-selective inhibition of expression of multiple trinucleotide repeat disease genes. Both ss-siRNAs with position 9 mismatches, 537775 and 553822, were allele-selective for the inhibition of the expression of all three genes (Table 1). We continued this analysis by examining inhibition of ATN-1 by two other compounds, 618385 (Figure 6G) and 618204 (Figure 6H), that were allele-selective for both HTT and ATXN-3. Of the two compounds, only ISIS 618204 was allele-selective for all three trinucleotide repeat genes, with a 12.7-fold selectivity for mutant ATN-1. For comparison, we also examined the inhibition by ISIS 641384 (Figure 6I) that had been allele-selective for HTT but not ATXN-3. ISIS 641384 was highly selective for inhibition of ATN-1 (>20-fold), even though it had not been selective for ATXN-3.
Table 1. Inhibition of Expression of ATN-1, ATXN-3, and HTT by Duplex RNAs and ss-siRNAs.
| effect
on HTT |
effect on ATX-3 |
effect
on ATN-1 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| name | mutant IC50 (nM) | wt IC50 (nM) | selectivity (x-fold) | mutant IC50 (nM) | wt IC50 (nM) | selectivity (x-fold) | mutant IC50 (nM) | wt IC50 (nM) | selectivity (x-fold) |
| dsRNAs | |||||||||
| P9 | 3.2 ± 0.3 | >100 | >31 | 4 | 50 | 11 | 0.7 ± 0.2 | 8.5 ± 1.6 | 12 |
| PM4 | 4.8 ± 1.6 | >100 | >21 | 6.5 ± 2.8 | 50 | 8 | 1.4 ± 0.3 | >25 | >18 |
| AB8 | 3.3 ± 0.6 | >100 | >30 | 3.3 ± 0.6 | >100 | >30 | 0.9 ± 0.2 | 3.5 ± 0.5 | 3.9 |
| P9U13 | 22 ± 0.4 | >100 | >43 | 13 ± 2.8 | 43 ± 7.9 | 3.2 | 3.9 ± 0.8 | 21 ± 7.8 | 5.3 |
| ss-siRNAs | |||||||||
| 537775 | 3.5 ± 0.3 | >100 | >29 | 2.9 ± 0.3 | 24 ± 6 | 8 | 1.1 ± 0.2 | >25 | >23 |
| 557426 | 3.3 ± 0.5 | >100 | >30 | 28 ± 2.2 | 67 ± 11 | 2.4 | 1.7 ± 0.3 | >25 | >15 |
| 553822 | 4.9 ± 0.8 | 90 ± 9.7 | 18 | 8.4 ± 2.5 | 99 ± 28 | 12 | 1.9 ± 0.3 | >25 | >13 |
| 618385 | 3.6 ± 0.4 | 53 | 14 | 1.4 ± 0.2 | >50 | >37 | 3.0 ± 0.4 | 8.5 ± 1.7 | 2.8 |
| 641384 | 5.1 ± 0.4 | >100 | >19 | 5.1 ± 0.6 | 17 ± 2.9 | 3.4 | 1.2 ± 0.1 | >25 | >21 |
| 618204 | 7.2 ± 1.2 | >100 | >14 | 3.1 ± 0.7 | 71 ± 26 | 23 | 1.1 ± 0.1 | 14 ± 6.1 | 13 |
Discussion
DRPLA is a severe neurological disease with no known cure. Recent advances in the delivery of active nucleic acids within the central nervous system8−11,32 and other tissues33 suggest that gene silencing is a feasible strategy for therapeutic development and might be used to silence the root cause of DRPLA, expression of mutant ATN-1 protein. The allele-selective duplex RNAs and ss-siRNAs described in this report provide several options for developing therapeutic agents for the treatment of DRPLA.
DPRLA has a relatively small patient population. Drug discovery would be facilitated if compounds being developed for other diseases could also be applied to treat DPRLA. The anti-CAG nucleic acids we describe here fit this paradigm. Both duplex RNAs and ss-siRNAs achieve good potencies and greater than 10-fold allele selectivities for three disease genes, ATN1, ATXN-3, and HTT. An anti-CAG development program may address multiple diseases, a conclusion previously reached by van Roon-Mon and colleagues.12
During our studies of anti-CAG nucleic acids that act through the RNAi pathway, we have tested more than 160 different compounds for allele-selective inhibition of at least one trinucleotide repeat gene. These compounds are derived from different classes of anti-CAG nucleic acids, including mismatch-containing duplex RNA, UNA-containing duplex RNA, abasic duplex RNA, and ss-siRNA. In broad terms, for inhibiting ATN-1 protein expression, compounds containing mismatched bases achieved allele selectivities higher than those of compounds with UNA or abasic substitutions.
For 10 compounds, we obtained potency and selectivity data for the inhibition of expression of all three different trinucleotide repeat genes, ATN1, ATXN-3, and HTT (Table 1).18,22,23 All 10 compounds had selectivities of >14-fold for inhibition of HTT protein expression. For ATN-1 and ATXN-3 protein expression, most compounds were also >8-fold selective, demonstrating that it is possible to obtain compounds with good selectivities for inhibiting all three disease genes. This generality might allow the eventual development of a single therapeutic for multiple trinucleotide repeat diseases.
The three model cell lines used to examine inhibition of HTT, ATXN-1, and ATN-1 have similar normal and wild-type repeat numbers. For monitoring HTT inhibition, we used GM04281 cells (69 repeats in the mutant allele and 17 repeats in the normal allele); for monitoring ATXN-1 inhibition, we used GM06151 cells (74 repeats in the mutant allele and 24 repeats in the normal allele), and for monitoring ATN-1 inhibition, we used GM13716 cells (68 repeats in the mutant allele and 16 CAG repeats in the normal allele). Thus, the differences we observed are likely dominated by the context of the surrounding gene, not the number of expanded repeats.
Not every compound was a good inhibitor for each of the three disease genes. We found that some compounds were poorly (<4-fold) selective for inhibition of either mutant ATN-1 or ATXN-3 protein expression. For example, abasic duplex AB8 was >30-fold selective for HTT and ATXN-3 and only 3.9-fold selective for inhibition of ATN-1 protein expression. Conversely, ss-siRNA 618385 was >37-fold selective for ATXN-3 and was poorly selective for ATN-1. These differences occur even though the CAG repeat target is the same in all three expanded mRNAs. Sequences surrounding the CAG repeat can influence recognition by anti-CAG nucleic acids, but this influence is subtle and does not impact the recognition of many compounds.
Our data demonstrate that it is possible to achieve allele-selective inhibition of ATN-1 protein expression, providing a starting point for developing nucleic acids as a treatment for DRPLA. Several compounds are allele-selective inhibitors of multiple trinucleotide repeat genes, suggesting that it might be possible to identify a single compound for the treatment of multiple diseases.
Glossary
Abbreviations
- ATN-1
atrophin-1
- HTT
huntingtin
- ATXN-3
ataxin-3
- DRPLA
dentatorubral-pallidoluysian atrophy
- UNA
unlocked nucleic acid
- AGO2
argonaute 2
- ss-siRNA
single-stranded silencing RNA.
Supporting Information Available
Representative gel images of the–dose response curves for inhibition of ATN-1 expression by UNA-modified P9 duplexes (Figures S1 and S2). This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
J.H. and J.L. contributed equally to this work. J.H. and J.L. planned and executed experiments for testing the inhibition of HTT. C.M. and X.Q. designed and synthesized UNAs. T.P.P. designed and synthesized ss-siRNAs. M.M. designed and synthesized chemically modified duplex RNAs. D.R.C. planned experiments and wrote the manuscript.
Work in the Corey Laboratory was supported by the National Institutes of Health (National Institute of General Medical Sciences Grant 73042) and the Robert A. Welch Foundation (I-1244). J.H. was supported by a Young Investigator Award from the National Ataxia Foundation.
The authors declare the following competing financial interest(s): ISIS Pharmaceuticals has filed patent applications related to the use of single-stranded silencing RNAs. The University of Texas Southwestern Medical Center has filed a patent application related to use of duplex RNAs for allele-selective gene silencing.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Yamada M.; Shimohata M.; Sato T.; Tsuji S.; Takahashi H. (2006) Polyglutamine disease: Recent advances in the neuropathology of dentatorubral-pallidolusian atrophy. Neuropathology 26, 346–351. [DOI] [PubMed] [Google Scholar]
- Koide R.; Ikeuchi T.; Onodera O.; Tanaka H.; Igarashi S.; Endo K.; Takahashi H.; Kondo R.; Ishikawa A.; Hayashi T.; Saito M.; Tomoda A.; Miike T.; Naito H.; Ikuta F.; Tsuji S. (1994) Unstable expansion of CAG repeat in hereditary dentatorubral-pallidoluysian atrophy (DRPLA). Nat. Genet. 6, 9–13. [DOI] [PubMed] [Google Scholar]
- Nagafuchi S.; Yanagisawa H.; Ohsaki E.; Shirayama T.; Tadokoro K.; Inoue T.; Yamada M. (1994) Structure and expression of the gene responsible for the triplet repeat disorder, dentatorubral and pallidoluysian atrophy (DRPLA). Nat. Genet. 8, 177–181. [DOI] [PubMed] [Google Scholar]
- Tsuji S.; Onodera O.; Goto J.; Nishizawa M. (2008) Study group on ataxic diseases: Sporadic ataxias in Japan: A population-based epidemiological study. Cerebellum 7, 189–197. [DOI] [PubMed] [Google Scholar]
- Wardle M.; Morris H. R.; Robertson N. P. (2009) Clinical and genetic characteristics of non-asian dentatotrubral-pallidolyusian atrophy: A systematic review. Mov. Disord. 24, 1636–1640. [DOI] [PubMed] [Google Scholar]
- Sata T.; Miura M.; Yamada M.; Yoshida T.; Wood J. D.; Yazawa I.; Masuda M.; Suzuki T.; Shin R.-M.; Yau H.-J.; Liu F.-C.; Shimohata T.; Onodera O.; Ross C. A.; Katsuki M.; Takahashi H.; Kano M.; Aosaki T.; Tsuji S. (2009) Severe neurological phenotypes of Q129 DRPLA transgenic mice serendipitously created by en masse expansion of CAG repeats in Q76 DRPLA mice. Hum. Mol. Genet. 18, 723–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker F. O. (2007) Huntington’s disease. Lancet 369, 218–228. [DOI] [PubMed] [Google Scholar]
- Kordasiewicz H. B.; Stanek L. M.; Wancewicz E. V.; Mazur C.; McAlonis M. M.; Pytel K. A.; Artates J. W.; Weiss A.; Cheng S. H.; Shihabuddin L. S.; Hung G.; Bennett C. F.; Cleveland D. W. (2012) Sustained Therapeutic Reversal of Huntington’s Disease by Transient Repression of Huntingtin Synthesis. Neuron 74, 1031–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers M. M.; Pepers B. A.; van Deutekom J. C.; Mulders S. A.; den Dunnen J. T.; Aartsma-Rus A.; van Ommen G. J.; van Roon-Mom W. M. (2011) Targeting several CAG expansion diseases by a single antisense oligonucleotide. PLoS One 6, e24308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwell A. L.; Skotte N. H.; Bennett C. F.; Hayden M. R. (2012) Antisense oligonucleotide therapeutics for inherited neurodegenerative diseases. Trends Mol. Biol. 18, 634–643. [DOI] [PubMed] [Google Scholar]
- Porensky P. N.; Burghes A. H. (2013) Antisense oligonucleotides for the treatment of spinal muscular atrophy. Hum. Gene Ther. 24, 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalachoras I.; Evers M. M.; van Roon-Mom W. M.; Aartsma-Rus A. M.; Meijer O. C. (2011) Antisense-mediated RNA targeting: Versatile and expedient genetic manipulation in the brain. Front. Mol. Neurosci. 4, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y.; Lee G.; Choe Y.; Zoltewicz J. S.; Peterson A. S. (2007) Functional architecture of atrophins. J. Biol. Chem. 282, 5037–5044. [DOI] [PubMed] [Google Scholar]
- Hu J.; Matsui M.; Gagnon K. T.; Schwartz J. C.; Gabillet S.; Arar K.; Wu J.; Bezprozvanny I.; Corey D. R. (2009) Allele-specific silencing of mutant huntingtin and ataxin-3 genes by targeting expanded CAG repeats in mRNAs. Nat. Biotechnol. 27, 478–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Carmell M. A.; Rivas F. V.; Marsden C. G.; Thomson J. M.; Song J. J.; Hammond S. M.; Joshua-Tor L.; Hannon G. J. (2004) Argonaute2 is the catalytic engine of mammalian RNAi. Science 305, 1437–1441. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Juranek S.; Li H.; Sheng G.; Tuschl T.; Patel D. J. (2008) Structure of an argonaute silencing complex with a seed-containing guide DNA and target RNA duplex. Nature 456, 921–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J.; Liu J.; Corey D. R. (2010) Allele-Selective Inhibition of Huntingtin Expression by Switching to an miRNA-like RNAi Mechanism. Chem. Biol. 17, 1183–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J.; Liu J.; Yu D.; Chu Y.; Corey D. R. (2012) Mechanism of allele-selective inhibition of huntingtin expression by duplex RNAs that target CAG repeats: Function through the RNAi pathway. Nucleic Acids Res. 40, 11270–11280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiszer A.; Mykowska A.; Krzyzosiak W. J. (2011) Inhibition of mutant huntingtin expression by RNA duplex targeting expanded CAG repeats. Nucleic Acids Res. 39, 5578–5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak A.; Wengel J. (2011) Unlocked nucleic acid: An RNA modification with broad potential. Org. Biomol. Chem. 9, 3591–3597. [DOI] [PubMed] [Google Scholar]
- Campbell M. A.; Wengel J. (2011) Locked vs. unlocked nucleic acids (LNA vs. UNA): Contrasting structures work towards common therapeutic goals. Chem. Soc. Rev. 40, 5680–5689. [DOI] [PubMed] [Google Scholar]
- Aiba Y.; Hu J.; Liu J.; Xiang Q.; Martinez C.; Corey D. R. (2013) Effect of unlocked nucleic acids (UNA) modifications on RNAi and allele-selective inhibition of huntingtin and ataxin-3. Biochemistry 52, 9329–9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Pendergraff H.; Narayanannair K. J.; Lackey J. G.; Kuchimanchi S.; Rajeev K. G.; Manoharan M.; Hu J.; Corey D. R. (2013) RNA duplexes with abasic substitutions are potent and allele-selective inhibitors of huntingtin and ataxin-3 expression. Nucleic Acids Res. 41, 8788–8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima W. F.; Prakash T. P.; Murray H. M.; Kinberger G. A.; Li W.; Chappell A. E.; Li C. S.; Murray S. F.; Gaus H.; Seth P. P.; et al. (2012) Single-stranded siRNAs activate RNAi in animals. Cell 150, 883–894. [DOI] [PubMed] [Google Scholar]
- Yu D.; Pendergraff H.; Liu J.; Kordasiewicz H. B.; Cleveland D. W.; Swayze E. E.; Lima W. F.; Crooke S. T.; Prakash T. P.; Corey D. R. (2012) Single-Stranded RNAs Use RNAi to Potently and Allele-Selectively Inhibit Mutant Huntingtin Expression. Cell 150, 895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J.; Liu J.; Yu D.; Aiba Y.; Pendergraff H.; Lagier-Tourenne C.; Swayze E. E.; Lima W. F.; Prakash T. P.; Corey D. R. (2014) Allele-selective inhibition of mutant huntingtin expression by single-stranded silencing RNAs. Nucleic Acid Ther. 24, 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa Mdo C.; Paulson H. L. (2012) Toward understanding Machado-Joseph disease. Prog. Neurobiol. 97, 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Yu D.; Aiba Y.; Hannah P.; Swayze E. E.; Lima W. F.; Hu J.; Prakash T. P.; Corey D. R. (2013) ss-siRNAs allele selectively inhibit ataxin-3 expression: Multiple mechanisms for an alternative gene silencing strategy. Nucleic Acids Res. 41, 9570–9583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J.; Gagnon K. T.; Liu J.; Watts J. K.; Syeda-Nawaz J.; Bennett C. F.; Swayze E. E.; Randolph J.; Chattopadhyaya J.; Corey D. R. (2011) Allele-selective inhibition of ataxin-3 (ATXN3) expression by antisense oligomers and duplex RNAs. Biol. Chem. 392, 315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa I.; Nukina N.; Hashida H.; Goto J.; Yamada M.; Kanazawa I. (1995) Abnormal gene product identified in hereditary dentatorubral-pallidoluysian atrophy (DRPLA) brain. Nat. Genet. 10, 99–103. [DOI] [PubMed] [Google Scholar]
- Deleavey G. F.; Damha M. J. (2112) Designing chemically modified oligonucleotides for targeted gene silencing. Chem. Biol. 19, 937–954. [DOI] [PubMed] [Google Scholar]
- Rungta R. L.; Choi H. B.; Lin P. J. C.; Ko R. W. Y.; Ashby D.; Nair J.; Manoharan M.; Cullis P. R.; Macvicar B. A. (2013) Lipid nanoparticle delivery of siRNA to silencing neuronal gene expression in the brain. Mol. Ther.—Nucleic Acids 2, e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts J. K.; Corey D. R. (2012) Gene silencing by synthetic nucleic acids in the laboratory and the clinic. J. Pathol. 226, 365–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.