INTRODUCTION
Lipodystrophy is a rare, heterogeneous group of syndromes characterized by the complete or partial loss or absence of subcutaneous adipose tissue (1,2). Lipodystrophy is often, though not always, accompanied by metabolic derangements, including insulin resistance, diabetes mellitus, hepatic steatosis or steatohepatitis, and dyslipidemia (1,2). The metabolic derangements associated with lipodystrophy can be severe and lead to substantial comorbidities, including acute pancreatitis (due to severe hypertriglyceridemia), hepatic cirrhosis, and premature cardiovascular disease (1,2). Other manifestations of metabolic derangements can include polycystic ovarian syndrome (PCOS), acanthosis nigricans (due to severe insulin resistance), and eruptive xanthomas (due to severe hypertriglyceridemia) (1,2). Since the key characteristic of lipodystrophy is the selective absence of adipose tissue (primarily subcutaneous), the levels of adipocyte hormones can be altered (3). The best characterized of these hormones is leptin, with low leptin levels typically observed in patients with lipodystrophy (4); however, levels of other adipocytokines, such as adiponectin, are also lower in lipodystrophy (5).
Lipodystrophy can generally be classified on the basis of the extent or pattern of fat loss (generalized or partial) as well as whether the disease is genetic or acquired (1,6). This simplified classification scheme yields 4 major lipodystrophy subtypes: congenital generalized lipodystrophy (CGL), acquired generalized lipodystrophy (AGL), familial partial lipodystrophy (FPL), and acquired partial lipodystrophy (APL). Human immunodeficiency virus-associated lipodystrophy has been categorized as a type of APL (7) but, given the differences in presentation and pathophysiology, is considered separately from rare forms of acquired lipodystrophy for the purpose of this discussion. It should be noted that while this classification scheme serves as a useful general framework for understanding lipodystrophy, it is not all-inclusive of lipodystrophy syndromes given the heterogeneity of manifestations, variable patterns of fat loss, and genetic bases that have yet to be identified.
Lipodystrophy syndromes have attracted the curiosity of researchers for over 6 decades, yet have shifted further into the clinical/endocrinology spotlight in recent years, in part because adipose tissue has been found to secrete a variety of cytokines/hormones. Adipose tissue carries a distinct ability to communicate the general energy status and inflammation threat posed to the brain and other organs of an organism through adipocytokines such as leptin, adiponectin, resistin, and many others (8). Further attention was attracted to the field of lipodystrophy in 2002, when the treatment effect of recombinant human leptin (metreleptin) was first reported in several severe cases of lipodystrophy (9). Subsequent longer-term studies over the last decade have supported the salutary effect of metreleptin treatment in lipodystrophy syndromes (10-13).
Despite progress in identifying the molecular basis of many lipodystrophy syndromes and published criteria for AGL and APL (14,15), many patients still escape the attention of physicians and are diagnosed late in the course of their disease. The American Association of Clinical Endocrinologists (AACE) convened a task force, which included clinical practitioners and leaders in lipodystrophy management and research, for the development of consensus recommendations for the detection of lipodystrophy. This document attempts to educate clinicians about lipodystrophy, increase awareness of its presence, improve the detection of subtle forms of lipodystrophy that may be underdiagnosed, and to provide a framework for the development of future diagnostic criteria. The target audience of these recommendations includes endocrinology specialists, reproductive endocrinologists, cardiologists, lipidologists, internists, family physicians, and pediatricians (especially neonatologists and pediatric endocrinologists).
A MEDLINE literature search was conducted and available references were compiled by the workshop chairs and distributed to task force members to facilitate a panel discussion. Consensus recommendations were formulated on the basis of the evidence gathered as well as through expert opinion. Unlike more common metabolic disorders, such as type 2 diabetes mellitus, for which evidence-based AACE guidelines have previously been developed, lipodystrophy is such a rare condition that the evidence base is generally less robust. A greater reliance on expert experience and opinion was required to develop criteria for increasing the detection of lipodystrophy.
OVERVIEW OF LIPODYSTROPHY SUBTYPES
Congenital Generalized Lipodystrophy
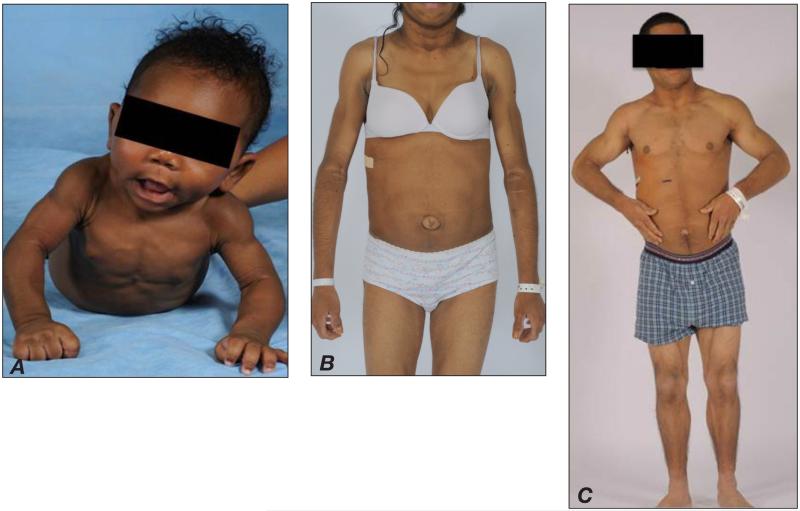
CGL, or Berardinelli-Seip syndrome, is an autosomal recessive disorder characterized by a generalized lack of adipose tissue at birth or shortly thereafter (within the first year of life), and is accompanied by prominent muscularity and subcutaneous veins (16,17). In addition, patients may have umbilical prominence, hepatomegaly, and splenomegaly (Figs. 1 A and B). In early childhood, patients with CGL may exhibit hyperphagia (possibly a manifestation of underlying leptin deficiency), accelerated linear growth, advanced bone age, or acromegaloid features (enlarged hands, feet, and mandible), while later in childhood, acanthosis nigricans can develop and become widespread (18). Hyperinsulinemia and hypertriglyceridemia can occur at an early age, with ketosis-resistant diabetes mellitus usually developing later in adolescence. Hepatomegaly from severe hepatic steatosis is common and can progress to steatohepatitis, cirrhosis, and liver failure. Finally, females with CGL may have hirsutism, clitoromegaly, irregular menstrual periods, polycystic ovaries, and/or infertility (7). It should be noted that most cases of CGL are usually diagnosed in early childhood; however, a few patients without access to regular medical care may reach early adulthood without a clear-cut diagnosis (Fig. 1 C). The key clinical findings in CGL and the 3 other major lipodystrophy subtypes are summarized in Table 1.
Fig. 1.
Congenital generalized lipodystrophy in A, a 6-month-old infant with prominent muscularity and veins, B, a 16-year-old girl with acanthosis nigricans and umbilical prominence, and C, a 15-year-old boy with umbilical prominence and otherwise normal appearing muscular habitus.
Table 1.
Clinical Findings of the Major Lipodystrophy Subtypes
| CGL | FPL | AGL | APL | |
|---|---|---|---|---|
| Age range of onset | Infancy to early childhood |
Childhood through adulthood |
Childhood through adulthood |
Childhood through adulthood |
| Approximate gender distribution (male:female) |
1:1-2 | 1:1-2a | 1:3 | 1:4 |
| Fat loss locations | ||||
| Face and neck | X | X | X | |
| Chest/trunk | X | + | X | X |
| Upper extremities | X | X | X | X |
| Lower extremities | X | X | X | |
| Intra-abdominal | X | + | ||
| Fat accumulation/sparing | ||||
| Face and neck | + | |||
| Hips and buttocks | + | |||
| Lower extremities | + | |||
| Intra-abdominal | + | + | + | |
| Other findings | ||||
| Accelerated linear growth | + | |||
| Acromegaloid features | + | |||
| Umbilical prominence | + | |||
| Panniculitis | + | |||
| Hepatomegaly | + | + | + | |
| Splenomegaly | + | + | ||
| Acanthosis nigricans | + | + | + | |
| Hirsutism | + | + | + | |
| Hyperphagia | + | + | + | |
| Hypogonadism | + | + | ||
| Hyperandrogenism in females | + | + | ||
Abbreviations: AGL = acquired generalized lipodystrophy; APL = acquired partial lipodystrophy; CGL = congenital generalized lipodystrophy; FPL = familial partial lipodystrophy; X = essential finding; + = finding may be present.
FPL is typically more readily recognized in females and affects females more severely.
Acquired Generalized Lipodystrophy
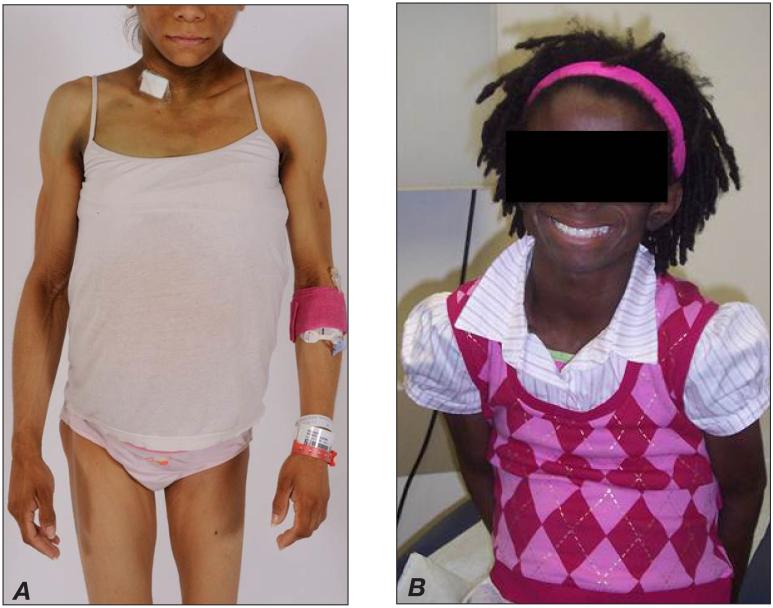
AGL, or Lawrence syndrome, is also characterized by a generalized loss of subcutaneous fat tissue; however, in contrast to CGL, patients with AGL are born with normal fat distribution but lose fat in a generalized fashion, typically starting in childhood or adolescence (rarely beginning after 30 years of age) (14). Progressive fat loss usually occurs over a period of months to years, or as rapid as a few weeks for some patients, and affects large areas of the body, especially the face and extremities (including the palms and soles) (Fig. 2). Intra-abdominal fat loss is variable, and there may be sparing of bone marrow and retro-orbital fat. In some patients, the onset of AGL is heralded by the development of subcutaneous inflammatory nodules (panniculitis); these lesions heal with localized loss of fat, but fat is subsequently lost from almost all subcutaneous regions. AGL occurs in approximately 3 times as many women as men (14). In a case series of 79 patients (14), AGL was classified into panniculitis-associated (~25% of cases), autoimmune (~25%), and idiopathic types (~50%) on the basis of clinical findings, with an overlap between panniculitis and autoimmune types. Autoimmune diseases, especially juvenile dermatomyositis and autoimmune hepatitis, occur commonly with AGL (Fig. 2 B), suggesting that AGL could represent an autoimmune disease itself, but the inciting factors (autoantigens or effector mechanisms) remain to be elucidated.
Fig. 2.
Acquired generalized lipodystrophy (AGL) in A, a 19-year-old woman, and B, a 9-year-old girl with juvenile dermatomyositis. Some common features among the 2 cases include lack of body fat and acanthosis nigricans, as well as abdominal protuberance. Note the absence of acromegaloid features in these cases in contrast to those in Fig. 1.
For both CGL and AGL, the presentation of diabetes in association with clinical evidence of insulin resistance (e.g., high triglyceride levels) in a nonobese pediatric patient should serve as key distinguishing features from type 1 diabetes. Although some experts have suggested ketosis resistance as a distinguishing feature, it is important to recognize that patients with all forms of generalized lipodystrophy can develop ketoacidosis, especially under severe metabolic stress.
Familial Partial Lipodystrophy
FPL is predominantly inherited in an autosomal dominant fashion (19), although a single case of FPL was reported to be inherited in an autosomal recessive fashion (20). Patients with FPL usually have normal body fat distribution during infancy and early childhood, but, beginning around or after puberty, typically develop variable and progressive loss of subcutaneous fat in the arms and legs resulting in a peripheral muscular appearance with variable loss of fat in the anterior abdomen and chest (21,22). Many patients (especially women) have fat accumulation in the face, neck, and intra-abdominal region (Fig. 3), which may lead to a Cushingoid appearance. Thus, a patient with FPL can have a physical appearance that resembles that typically associated with the metabolic syndrome and/or type 2 diabetes mellitus (Fig. 4), requiring astute clinical acumen and careful physical examination to recognize when lipodystrophy should be considered as a potential diagnosis or in the differential diagnosis. The identification of FPL in men can be especially difficult because of the muscular habitus of many average males, and may only come to light after diagnosis of a female relative with the condition (21).
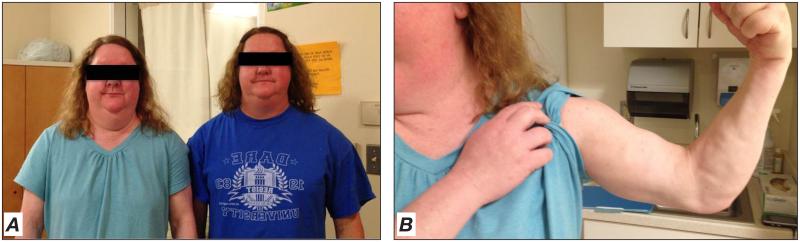
Fig. 3.
Familial partial lipodystrophy in 2 sisters. Both patients are in their early thirties. The patient on the left has diabetes mellitus, while the patient on the right is nondiabetic. Note increased fat accumulation in the face and neck (A) with subcutaneous fat loss and muscularity in the arm (B).
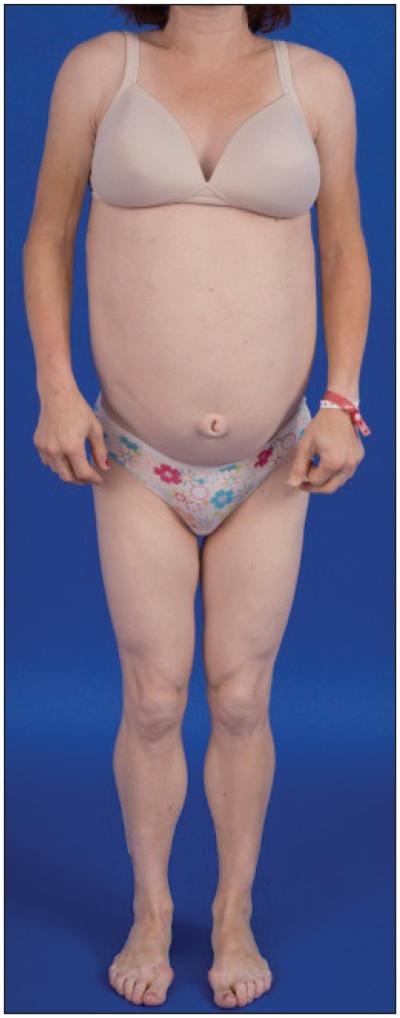
Fig. 4.
Familial partial lipodystrophy (FPL) in a mother and daughter pair A) before and B) after liposuction at the neck in the younger patient. Note the more pronounced facial and central fat accumulation in the daughter compared with the mother. FPL can be commonly mistaken for obesity associated with metabolic syndrome and or diabetes.
Metabolic abnormalities associated with FPL typically manifest in early adulthood (21). Diabetes is more common and more severe in women than in men, particularly among multiparous women with excessive intra-abdominal fat deposition. Most affected women are able to reproduce normally, although some may develop hirsutism and menstrual irregularities suggestive of PCOS at an earlier age. Hypertriglyceridemia is a common finding in FPL and can be severe, potentially leading to acute pancreatitis, while hepatic steatosis and acanthosis nigricans may be less clinically impressive than that occurring in patients with generalized forms of lipodystrophy. Finally, some patients with FPL may develop myopathy, cardiomyopathy, and/or conduction system abnormalities (23).
Acquired Partial Lipodystrophy
In APL, or Barraquer-Simons syndrome, patients typically develop loss of subcutaneous fat during childhood or adolescence, though onset as late as the fourth or fifth decade of life has been reported (15). APL is characterized by a progressive loss of subcutaneous fat over months to years from the face, neck, arms, thorax, and upper abdomen. This fat loss typically progresses in a cephalocaudal fashion with sparing of the lower extremities, although the exact pattern of fat loss can vary (Fig. 5). Some patients may have excess fat accumulation over the lower abdomen, gluteal region, and legs. Metabolic complications are less common with APL than with other lipodystrophy subtypes. The main cause of morbidity appears to be chronic renal disease (especially membranoproliferative glomerulonephritis). APL has also been associated with a number of autoimmune diseases, including dermatomyositis and systemic lupus erythematosus. Most patients with APL have low levels of serum complement 3 (C3) accompanied by detectable levels of a circulating autoantibody, C3 nephritic factor. APL is also more common in women than in men (estimated 4:1 ratio) (15).
Fig. 5.
A 36-year-old patient with acquired partial lipodystrophy secondary to juvenile dermatomyositis exhibiting loss of fat in the face (not shown) and extremities, while abdominal fat deposits are hypertrophied.
CLINICAL CHARACTERISTICS THAT INCREASE THE SUSPICION OF LIPODYSTROPHY
The cardinal feature of lipodystrophy is the selective loss of subcutaneous adipose tissue. However, the exact distribution of fat loss differs across the various forms of lipodystrophy, as described above, with sparing of fat loss or even excess accumulation of body fat in regions of the body, such as the face and the neck (FPL) or lower extremities (APL). In some cases, loss of adipose tissue can be sufficiently prominent and almost instantly recognizable if one is aware of the condition, or it can be more subtle, requiring a clinician to have considered it in the differential diagnosis of a patient presenting with common metabolic abnormalities (insulin resistance, diabetes, or hypertriglyceridemia). The conventional 2 × 2 diagnostic scheme, namely generalized versus partial and congenital versus acquired lipodystrophy, emanated largely from the identification of patients with pronounced phenotypes. However, the clinician should keep in mind that partial lipodystrophies may have a greater prevalence than previously thought due to the lack of detection of subtle forms. In one study of over 5000 Dutch patients with diabetes from 3 outpatient clinics where 2 screening criteria were applied (body mass index ≤27 kg/m2 and use of >100 units of insulin/day), 12 out of 24 patients meeting these criteria had further characterization, 5 of whom were eventually diagnosed with FPL (3 with confirmed genetic mutations) (24). Thus, with a more comprehensive understanding of the range of lipodystrophy manifestations and the relationship between gene mutations and clinical presentations, the conventional diagnostic scheme may require modification and expansion, particularly with respect to forms of partial lipodystrophy.
Although lipodystrophy is often accompanied by metabolic abnormalities, not all patients manifest them on presentation. Thus, lipodystrophy can be identified in the absence of metabolic abnormalities. Clinical laboratory testing (i.e., blood glucose, glycated hemoglobin [HbA1c], triglyceride level, liver function studies, etc.) on initial evaluation of the patient with suspected lipodystrophy may still be useful for providing a baseline from which to monitor development of future metabolic abnormalities (if not already present), and should be considered the standard of care.
Given that certain types of lipodystrophy, especially FPL, can bear some resemblance to common metabolic conditions (i.e., obesity, metabolic syndrome, and diabetes mellitus), the AACE task force recommends considering a group of simple clinical characteristics that should raise the clinician’s suspicion of lipodystrophy in a patient. These criteria appear in Table 2. As most cases of generalized lipodystrophy (CGL and AGL) are more likely to be diagnosed earlier in life due to their characteristic and often striking physical appearance, often profound severity of metabolic derangements, and/or earlier age of onset, these clinical characteristics focus on improving the detection of more subtle manifestations of lipodystrophy (i.e., FPL) as well as detecting patients with other forms who may not have been diagnosed at an earlier age. These characteristics are believed to be applicable to most patients ≥2 years of age and can be readily measured/observed in most clinical settings.
Table 2.
Clinical Characteristics That Increase the Suspicion of Lipodystrophy
Core clinical characteristic for lipodystrophy
|
|
|
Core clinical characteristic for familial partial lipodystrophy:
|
|
|
Supportive clinical characteristics for lipodystrophy:
|
|
|
|
|
|
|
|
Abbreviations: CT = computed tomography; PCOS = polycystic ovarian syndrome.
Patients with definitive evidence of the core characteristic, or with possible evidence of the core characteristic plus at least one supportive characteristic, should be considered to have a high likelihood of lipodystrophy and should be considered for referral to a research center specializing in lipodystrophy diagnosis and management (for more information, see http://www.mylipodystrophy.com/).
CONSIDERATIONS IN THE DETECTION AND DIAGNOSIS OF LIPODYSTROPHY
Although not included in the clinical characteristics above, caliper measurements of skinfold thickness may be helpful to quantify or characterize fat loss. Approximately 90% of adult men and women will have skinfold thickness values ≥10 mm and ≥22 mm, respectively, at the anterior thigh (15,25); lower thickness values are supportive information for the diagnosis of lipodystrophy.
When fat loss is not visibly evident by physical manifestations, hyperglycemia and hypertriglyceridemia that are resistant or unresponsive to conventional treatment may serve as the only indication to the clinician that a patient may have lipodystrophy. The possibility of a lipodystrophy diagnosis should be considered in patients requiring ≥200 units/day (≥2 units/kg/day) of insulin to overcome insulin resistance or displaying triglyceride levels that remain persistently elevated (≥250 mg/dL) despite fully optimized therapy or diet modifications.
Lipodystrophy is typically accompanied by low (or relatively low) levels of the adipocyte-secreted hormone leptin (4). Thus, leptin levels may provide useful supportive information, but are not necessary or specific for the diagnosis of lipodystrophy, as low leptin levels may be observed in other conditions (e.g., hypothalamic amenorrhea and malnutrition). Similarly, while a “high” leptin level may make the diagnosis of lipodystrophy less likely, limited data exist regarding the range of leptin levels in patients with a confirmed diagnosis of lipodystrophy. In addition, while leptin levels may be ordered as a clinical laboratory test, leptin assays have not been standardized, and normative ranges have not been well established.
Finally, it is important to note that patients with the most severely affected forms of lipodystrophy may also present with associated neuroendocrine and immunological abnormalities (e.g., amenorrhea and a relative deficiency of T lymphocyte populations) as well as hyperphagia (26).
POTENTIAL MANAGEMENT MODALITIES
Current therapeutic options for the metabolic management of lipodystrophy consist of lifestyle modifications (diet and exercise) and conventional antihyperglycemic and lipid-lowering medications. Metformin, sulfonylureas, thiazolidinediones, and insulin can be used to manage hyperglycemia, while fibrates and statins can be used to manage hypertriglyceridemia. Out of the oral antihyperglycemics, thiazolidinediones have been studied the most extensively with small clinical trials or case series with variable results (27,28). Where metabolic abnormalities associated with lipodystrophy are particularly severe, conventional treatments, alone or in combination, are likely to be inadequate at re-establishing metabolic control. Plasmapheresis has been an option for lowering dangerously high triglyceride levels to control painful xanthoma and prevent pancreatitis (29). Studies employing a very low-fat diet are underway. In the presence of hyperglycemia with severe insulin resistance, patients are likely to require very high doses of insulin and will benefit from highly concentrated insulin, such as U-500 insulin. Leptin replacement therapy has been investigated and was associated with sustained reductions in triglyceride, total cholesterol, and HbA1c levels (9-11,30). Metreleptin, a human leptin analog, is currently under review by the U.S. Food and Drug Administration for the treatment of certain metabolic abnormalities associated with lipodystrophy, which underscores the need to properly identify patients with this condition, including those with more subtle clinical presentations. While not required by all patients, consideration should also be given for the cosmetic management of disfigurements (fat loss, fat redistribution, and outward manifestations of insulin resistance) that can severely impact patient self-image and sense of well-being. Finally, metabolic improvement in a few patients with FPL were reported following Roux-en-Y gastric bypass (31), but most patients with FPL do not meet the current weight thresholds for third-party reimbursement guidelines.
CONCLUSION
Lipodystrophy is a condition characterized by regional or total selective loss or absence of subcutaneous fat. This can occur either in the presence or absence of metabolic abnormalities, and with diverse clinical presentations. While generalized forms of lipodystrophy are often diagnosed during childhood or adolescence, some forms of lipodystrophy, particularly FPL, may bear some resemblance to common metabolic disorders managed by adult endocrinologists. Hyperglycemia and hypertriglyceridemia that are resistant to treatment or the use of very high doses of insulin may be important clues of lipodystrophy in the clinical setting. This consensus statement aims to improve the detection of all types of lipodystrophy and will likely be updated and refined as the knowledge base of lipodystrophy grows through continued research efforts. It is important to acknowledge that these criteria have not been tested prospectively, but efforts to bring these criteria to various clinical settings (private, academic, government, and others) may be important for understanding the true prevalence of these disorders. Improving the detection of lipodystrophy and increasing general awareness of the disease will help to ensure that patients with lipodystrophy receive appropriate treatment. Therapeutic management for patients with lipodystrophy focuses on attaining metabolic control and managing cosmetic problems associated with fat loss.
ACKNOWLEDGEMENT
Medical writing support has been provided by Robert Schupp, PharmD, of inScience Communications, Springer Healthcare, and has been funded by the American Association of Clinical Endocrinologists (AACE).
Financial support of the AACE lipodystrophy workshop was provided by Amylin Pharmaceuticals, LLC, which is developing metreleptin as a potential treatment for certain metabolic disorders associated with rare inherited and acquired forms of lipodystrophy.
Abbreviations
- AACE
American Association of Clinical Endocrinologists
- AGL
acquired generalized lipodystrophy
- APL
acquired partial lipodystrophy
- C3
complement component 3
- CGL
congenital generalized lipodystrophy
- FPL
familial partial lipodystrophy
- PCOS
polycystic ovarian syndrome
Footnotes
DISCLOSURE
Co-chairpersons:
Dr. Yehuda Handelsman reports that he has received research grant support from Boehringer Ingelheim Pharmaceuticals, Inc, Daiichi Sankyo, Inc, GlaxoSmithKline, Novo Nordisk A/S, Takeda Pharmaceutical Company Limited, sanofi-aventis U.S., XOMA, Tolerx, Inc; consultant fees from Daiichi Sankyo, Inc, Gilead, Genentech, Inc, GlaxoSmithKline, Merck & Co, Inc, XOMA, and Tolerx, Inc; and speakers’ bureau honoraria from AstraZeneca, Boehringer Ingelheim Pharmaceuticals, Inc, Bristol-Myers Squibb, Daiichi Sankyo, Inc, GlaxoSmithKline, Merck & Co, Inc, and Novo Nordisk A/S.
Dr. Elif A. Oral has received research support from the National Institute of Health (NIH), grant 5RO1-DK088114 and Amylin Pharmaceuticals, Inc, and served as an advisor to Amylin in the past; and research support received from Bristol-Myers Squibb, Novo Nordisk A/S, and GlaxoSmithKline.
Task Force Members:
Dr. Zachary T. Bloomgarden reports that he has received speaker honoraria from GlaxoSmithKline, Merck & Co, Inc, and Novo Nordisk A/S; advisory board/consultant honoraria from Bristol-Myers Squibb/AstraZeneca, Boehringer Ingelheim Pharmaceuticals, Inc, Merck & Co, Inc, Novartis AG, and Novo Nordisk A/S; and stockholder dividends from CR Bard Inc, CVS Caremark, F. Hoffmann-La Roche Ltd, and St. Jude Medical, Inc.
Dr. Rebecca J. Brown has no multiplicity of interest to disclose.
Dr. Jean L. Chan reports that she is employed by and is a stockholder of Amylin Pharmaceuticals, LLC.
Dr. Daniel Einhorn reports that he has received shares for his role as an advisor from Halozyme Therapeutics, MannKind Corporation, and Freedom Meditech, Inc; consulting fees for his role as chair of the data management committee from Eli Lilly and Company; and consulting fees for his role as executive committee member on the NAVIGATOR Clinical Trial from Novartis AG.
Dr. Alan J. Garber reports that he has received consultant honoraria from F. Hoffmann-La Roche Ltd; speakers’ bureau and advisory board honoraria from GlaxoSmithKline, Merck & Co, Inc, Daiichi Sankyo, Inc, and Novo Nordisk A/S; and clinical research support from Bristol-Myers Squibb, GlaxoSmithKline, Merck & Co, Inc, and Novo Nordisk A/S.
Dr. Abhimanyu Garg reports that he receives research support from and has served as a consultant to Amylin Pharmaceuticals, Inc. He has received speaker honoraria from Merck & Co, Inc.
Dr. Timothy Garvey reports that he has received speaker honoraria from Merck & Co., Inc. that he has served on advisory boards for Vivus, Daiichi Sankyo, Inc, Liposcience, and Alkermes, and that he has received research support from Amylin Pharmaceuticals, Inc and Merck & Co., Inc,
Dr. George Grunberger reports that he has received speaker honoraria from Eli Lilly and Company, Novo Nordisk A/S, Merck & Co, Inc, sanofi-aventis US, LLC, AstraZeneca, Bristol-Myers Squibb, Takeda Pharmaceuticals North America, Inc, and GlaxoSmithKline, and research grant support for his role as investigator from Eli Lilly and Company, Novo Nordisk A/S, GlaxoSmithKline, and Johnson & Johnson Services, Inc.
Dr. Robert Henry reports that he has received grant support managed by the University of California San Diego and/or San Diego Veterans Medical Research Foundation from Amylin Pharmaceuticals, Inc., AstraZeneca Pharmaceuticals LP, Boehringer Ingelheim Pharmaceuticals, Inc, Bristol-Myers Squibb Company, Johnson & Johnson, Eli Lilly and Company, Novartis Pharmaceuticals Corporation, and sanofi-aventis. He has served as a consultant and/or advisory board member to Amgen, Inc., Amylin Pharmaceuticals, Inc., Boehringer Ingelheim Pharmaceuticals Inc., Eli Lilly and Company, Genentech-Roche Pharmaceuticals, Gilead, Intarcia, Isis Pharmaceuticals, Inc., Merck, Novo Nordisk Pharmaceuticals, Inc., sanofi-aventis, Versartis, Inc. and Vivus, Inc.
Dr. Norman Lavin reports that he has received speaker honoraria from Genentech, Inc, Ipsen, Pfizer, and Endo Pharmaceuticals.
Dr. Carmen D. Tapiador has no multiplicity of interest to disclose.
Dr. Christian Weyer reports that he was a full-time employee and shareholder of Amylin Pharmaceuticals, Inc, at the time of the workshop and subsequent manuscript development.
REFERENCES
- 1.Chan JL, Oral EA. Clinical classification and treatment of congenital and acquired lipodystrophy. Endocr Pract. 2010;16:310–323. doi: 10.4158/EP09154.RA. [DOI] [PubMed] [Google Scholar]
- 2.Garg A. Lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab. 2011;96:3313–3325. doi: 10.1210/jc.2011-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oral EA, Chan JL. Rationale for leptin-replacement therapy for severe lipodystrophy. Endoc Pract. 2010;16:324–333. doi: 10.4158/EP09155.RA. [DOI] [PubMed] [Google Scholar]
- 4.Haque WA, Shimomura I, Matsuzawa Y, Garg A. Serum adiponectin and leptin levels in patients with lipodystrophies. J Clin Endocrinol Metab. 2002;87:2395–2398. doi: 10.1210/jcem.87.5.8624. [DOI] [PubMed] [Google Scholar]
- 5.Antuna-Puente B, Boutet E, Vigouroux C, et al. Higher adiponectin levels in patients with Berardinelli-Seip congenital lipodystrophy due to seipin as compared with 1-acylglycerol-3-phosphate-o-acyltransferase-2 deficiency. J Clin Endocrinol Metab. 2010;95:1463–1468. doi: 10.1210/jc.2009-1824. [DOI] [PubMed] [Google Scholar]
- 6.Garg A. Lipodystrophies. Am J Med. 2000;108:143–152. doi: 10.1016/s0002-9343(99)00414-3. [DOI] [PubMed] [Google Scholar]
- 7.Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350:1220–1234. doi: 10.1056/NEJMra025261. [DOI] [PubMed] [Google Scholar]
- 8.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 9.Oral EA, Simha V, Ruiz E, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 10.Chong AY, Lupsa BC, Cochran EK, Gorden P. Efficacy of leptin therapy in the different forms of human lipodystrophy. Diabetologia. 2010;53:27–35. doi: 10.1007/s00125-009-1502-9. [DOI] [PubMed] [Google Scholar]
- 11.Javor ED, Cochran EK, Musso C, Young JR, DePaoli AM, Gorden P. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes. 2005;54:1994–2002. doi: 10.2337/diabetes.54.7.1994. [DOI] [PubMed] [Google Scholar]
- 12.Park JY, Javor ED, Cochran EK, DePaoli AM, Gorden P. Long-term efficacy of leptin replacement in patients with Dunnigan-type familial partial lipodystrophy. Metab Clin Exp. 2007;56:508–516. doi: 10.1016/j.metabol.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simha V, Subramanyam L, Szczepaniak L, et al. Comparison of efficacy and safety of leptin replacement therapy in moderately and severely hypoleptinemic patients with familial partial lipodystrophy of the Dunnigan variety. J Clin Endocrinol Metab. 2012;97:785–792. doi: 10.1210/jc.2011-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Misra A, Garg A. Clinical features and metabolic derangements in acquired generalized lipodystrophy: case reports and review of the literature. Medicine. 2003;82:129–146. doi: 10.1097/00005792-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Misra A, Peethambaram A, Garg A. Clinical features and metabolic and autoimmune derangements in acquired partial lipodystrophy: report of 35 cases and review of the literature. Medicine. 2004;83:18–34. doi: 10.1097/01.md.0000111061.69212.59. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal AK, Simha V, Oral EA, et al. Phenotypic and genetic heterogeneity in congenital generalized lipodystrophy. J Clin Endocrinol Metab. 2003;88:4840–4847. doi: 10.1210/jc.2003-030855. [DOI] [PubMed] [Google Scholar]
- 17.Van Maldergem L, Magre J, Khallouf TE, et al. Genotype-phenotype relationships in Berardinelli-Seip congenital lipodystrophy. J Med Genetics. 2002;39:722–733. doi: 10.1136/jmg.39.10.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seip M, Trygstad O. Generalized lipodystrophy, congenital and acquired (lipoatrophy) Acta Paediatr. 1996;413:2–28. doi: 10.1111/j.1651-2227.1996.tb14262.x. [DOI] [PubMed] [Google Scholar]
- 19.Peters JM, Barnes R, Bennett L, Gitomer WM, Bowcock AM, Garg A. Localization of the gene for familial partial lipodystrophy (Dunnigan variety) to chromosome 1q21-22. Nat Genetics. 1998;18:292–295. doi: 10.1038/ng0398-292. [DOI] [PubMed] [Google Scholar]
- 20.Rubio-Cabezas O, Puri V, Murano I, et al. Partial lipodystrophy and insulin resistant diabetes in a patient with a homozygous nonsense mutation in CIDEC. EMBO Mol Med. 2009;1:280–287. doi: 10.1002/emmm.200900037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garg A. Gender differences in the prevalence of metabolic complications in familial partial lipodystrophy (Dunnigan variety) J Clin Endocrinol Metab. 2000;85:1776–1782. doi: 10.1210/jcem.85.5.6605. [DOI] [PubMed] [Google Scholar]
- 22.Garg A, Peshock RM, Fleckenstein JL. Adipose tissue distribution pattern in patients with familial partial lipodystrophy (Dunnigan variety) J Clin Endocrinol Metab. 1999;84:170–174. doi: 10.1210/jcem.84.1.5383. [DOI] [PubMed] [Google Scholar]
- 23.Subramanyam L, Simha V, Garg A. Overlapping syndrome with familial partial lipodystrophy, Dunnigan variety and cardiomyopathy due to amino-terminal heterozygous missense lamin A/C mutations. Clin Genetics. 2010;78:66–73. doi: 10.1111/j.1399-0004.2009.01350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Visser ME, Kropman E, Kranendonk ME, et al. Characterisation of non-obese diabetic patients with marked insulin resistance identifies a novel familial partial lipodystrophy-associated PPARgamma mutation (Y151C) Diabetologia. 2011;54:1639–1644. doi: 10.1007/s00125-011-2142-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson AS, Pollock ML, Ward A. Generalized equations for predicting body density of women. Med Sci Sports Exerc. 1980;12:175–181. [PubMed] [Google Scholar]
- 26.Bluher S, Mantzoros CS. Leptin in humans: lessons from translational research. Am J Clin Nutr. 2009;89:991S–997S. doi: 10.3945/ajcn.2008.26788E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arioglu E, Duncan-Morin J, Sebring N, et al. Efficacy and safety of troglitazone in the treatment of lipodystrophy syndromes. Ann Intern Med. 2000;133:263–274. doi: 10.7326/0003-4819-133-4-200008150-00009. [DOI] [PubMed] [Google Scholar]
- 28.Luedtke A, Boschmann M, Colpe C, et al. Thiazolidinedione response in familial lipodystrophy patients with LMNA mutations: a case series. Hormone Metab Res. 2012;44:306–311. doi: 10.1055/s-0031-1301284. [DOI] [PubMed] [Google Scholar]
- 29.Bolan C, Oral EA, Gorden P, Taylor S, Leitman SF. Intensive, long-term plasma exchange therapy for severe hypertriglyceridemia in acquired generalized lipoatrophy. J Clin Endocrinol Metab. 2002;87:380–384. doi: 10.1210/jcem.87.1.8176. [DOI] [PubMed] [Google Scholar]
- 30.Chan JL, Lutz K, Cochran E, et al. Clinical effects of long-term metreleptin treatment in patients with lipodystrophy. Endocr Pract. 2011;17:922–932. doi: 10.4158/EP11229.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ciudin A, Baena-Fustegueras JA, Fort JM, Encabo G, Mesa J, Lecube A. Successful treatment for the Dunnigantype familial partial lipodystrophy with Roux-en-Y gastric bypass. Clin Endocrinol (Oxf) 2011;75:403–404. doi: 10.1111/j.1365-2265.2011.04057.x. [DOI] [PubMed] [Google Scholar]