Abstract
Galectin-3, a member of β-galactoside-binding gene family is a multi-functional protein, which regulates pleiotropic biological functions such as cell growth, cell adhesion, cell-cell interactions, apoptosis, angiogenesis and mRNA processing. Its unique structure enables it to interact with a plethora of ligands in a carbohydrate dependent or independent manner. Galectin-3 is mainly a cytosolic protein, but can easily traverse the intracellular and plasma membranes to translocate into the nucleus, mitochondria or get externalized. Depending on the cell type, specific experimental conditions in vitro, cancer type and stage, galectin-3 has been reported to be exclusively cytoplasmic, predominantly nuclear or distributed between the two compartments. In this review we have summarized the dynamics of galectin-3 shuttling between the nucleus and the cytoplasm, the nuclear transport mechanisms of galectin-3, how its specific interactions with the members of β-catenin signaling pathways affect tumor progression, and its implications as a therapeutic target.
Galectin-3: structure and function
Galectin-3 is a unique ~31kD lectin belonging to the evolutionarily conserved family of galectins that share a unique carbohydrate recognition domain (CRD). Galectin-3 is a chimera protein, which contains a collagen-α-like domain and an N-terminal domain in addition to the CRD [1]. Each of the three structural domains of galectin-3 is associated with at least one specific function: (a) the NH2 terminal domain contains a serine phosphorylation site, which is important in regulating its nuclear localization; (b) the proline-rich collagen-α-like sequence is cleavable by matrix metalloproteinases and this cleavage acts as a surrogate diagnostic marker for MMP-2 and MMP-9 activities in the tissue; and (c) a COOH terminal contains a single carbohydrate-recognition domain and the NWGR anti-death motif. Although the presence of galactose is essential for all galectins’ binding, its affinity for the monosaccharide ligand is rather weak, with Kd values in mM range. The binding affinity of galectins increases if galactose is attached to other saccharides e.g. N-acetylglucosamine forming N-acetyllactosamine [2]. Galectin-3 is mainly a cytosolic protein, but there is ample evidence to confirm its presence on the cell surface, in the conditioned media of some cell lines, in the extracellular matrix and in the biological fluids and sera. This suggests that galectin-3 is a shuttling protein and may have multiple functions accordingly [3-5]. Galectin-3 lacks the classical secretion signal sequence and does not pass through the standard ER/Golgi pathway [6]. Still it can be transported into the extracellular milieu via a non-classical pathway [7].
Cells differ widely in their capacity to secrete galectin-3. While J774.2 macrophage cells secrete 30–45% of their galectin pool [8], BHK and MDCK cells export 10–15% [9, 10] and WEHI-3 mouse macrophages secrete a very small percentage of galectin-3 in the conditioned medium [8]. The exact mechanism of galectin-3 secretion is not yet known. Various groups have proposed different possible mechanism for its secretion such as via vesicular release [8-11], release as a component of the exosome [12], or by mechano-transduction mechanism [13]. Once it is released into the extracellular matrix, because of its ability to bind to glycosylated proteins, it can interact with a myriad of partners such as EGFR, integrins, N-CAM, fibronectin, and laminin [14-18]. Thus, galectin-3 plays an integral role in multiple biological processes including, but not limited to, cell–cell or cell–matrix adhesion, signal transduction, inhibition of cell receptor internalization, induction of T-cell apoptosis, and induction of angiogenesis [14, 19-23].
Galectin-3 also plays significant roles when expressed intra-cellularly. For example, it binds to members of the serine (S)- and arginine (R)-rich splicing factor family (SR proteins) and form spliceosome complexes within the nucleus [24, 25]. Galectin-3 translocate to the perinuclear membrane in breast cancer cells following a variety of apoptotic stimuli such as cisplatin, staurosporine, or serum withdrawal [26-28]. Over-expression of cytoplasmic galectin-3 in the tongue cancer patients was associated with a decreased disease-free survival [29]. Expression of galectin-3 in the nucleus of human prostate cancer correlated with decreased cell proliferation, while its over-expression in the cytoplasm was reported to promote its anti- apoptotic activity as well as increased cell proliferation, tumor growth, invasion, and angiogenesis [30, 31]. Nuclear staining of galectin-3 was correlated to the lobular type of invasive carcinoma, while tumor stromal expression represented high-grade malignancy in human breast carcinoma [32, 33]. In colorectal cancer an increased cytoplasmic expression of galectin-3 was observed in more advanced stages [34].
Numerous cytosolic molecules have been identified as galectin-3 ligands, which include several molecules involved in the apoptotic pathway: Bcl-2 [35, 36], CD95 (APO-1/Fas)[37], Nucling [38], Alix/A1P1 [39]. Galectin-3 has been shown to translocate either from the cytosol or from the nucleus to the mitochondria following exposure to apoptotic stimuli [27] and to block changes in the mitochondrial membrane potential thereby preventing apoptosis [40]. The involvement of cytosolic galectin-3 in regulation of cell proliferation, differentiation, survival and cell death was additionally confirmed by the findings that it affects K-ras [41, 42] protein and Akt protein [43, 44]. Synexin (annexin VII) a Ca2+ and phospholipid binding protein mediates the translocation of galectin-3 to perinuclear mitochondrial membranes indicating its involvement in cellular trafficking [27].
In this review we will focus on the nuclear/cytoplasmic transport of galectin-3 and its implications as a therapeutic target.
The nuclear pore complex and nucleo-cytoplasmic protein transport
The nuclear envelope is mostly recognized as a diffusion barrier between the cytoplasm and the nucleoplasm. The nuclear envelope consists of two phospholipid bilayers, called the inner and the outer nuclear membrane. The inner nuclear membrane faces the nucleoplasm and contains proteins that interact with the chromatin [45, 46] The outer nuclear membrane is continuous with the endoplasmic reticulum of the cell and has partially overlapping functions in the transport, synthesis and folding of proteins, and the synthesis of lipids [47]. The inner and the outer nuclear membrane of the nuclear envelope are penetrated by a large macromolecular structure nuclear pore complex (NPC) [48, 49].
NPCs are the only gateway between nuclear contents and cytoplasm. The NPCs mediate selective bidirectional nucleocytoplasmic transport [50, 51]. Small molecules, ions and metabolites below a molecular weight of roughly 20 kDa can diffuse through NPCs; however, macromolecules greater than 40–60 kDa need to be actively transported [52]. The overall structure of the NPC has been determined by distinct electron microscopy approaches and is evolutionarily conserved from yeasts to mammals [53, 54]. Vertebrate NPCs display an eightfold rotational symmetry, have an outer diameter of ~ 120 nm, and are large, ~ 125 MDa structures embedded in the nuclear membrane. The complex can be minimally characterized as having three substructures: the cytoplasmic fibrils, a central core, and the nuclear basket [51, 52, 54]. In higher eukaryotes, NPCs are found at a similar density, ~ 4000 NPCs/nucleus in cultured human cells, although it is strongly influenced by the cell size and the level of biosynthetic activity [55].
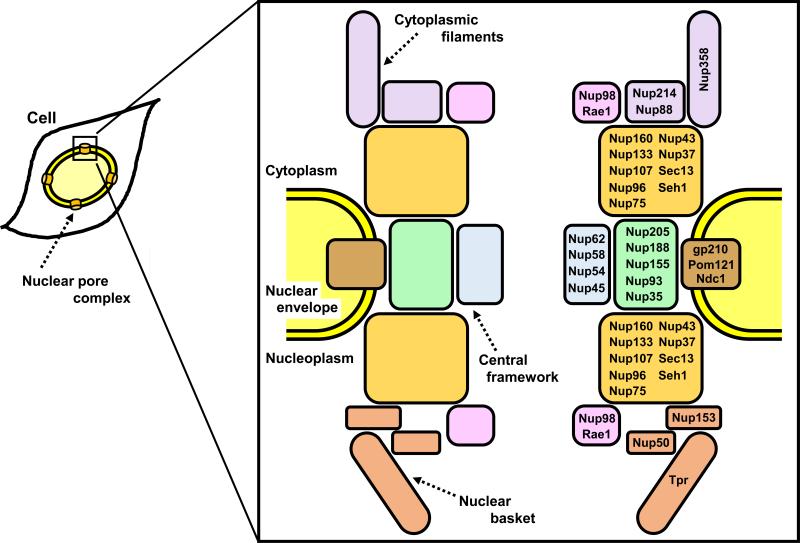
The NPC is composed of approximately 30 different proteins termed nucleoporins (Nups; Fig. 1) [56]. Nucleoporins have a very limited set of domains, restricted to β-propellers, α-solenoids, phenylalanine-glycine (FG) repeats, coiled-coil and transmembrane domains, and can be subdivided into three groups [57-59]. The first group comprises the transmembrane proteins that anchor the NPC to the nuclear envelope and reside at the boundary between the central framework and the pore membrane, including gp210, Ndc1 and POM121. The second group represents the structural scaffold of the NPC embedded into the nuclear envelope, including the Nup107-160 complex and the Nup93-205 complex. The third group includes the nucleoporins that form peripheral components of the NPC and contain repetitive FG motifs and/or coiled-coil motifs, which facilitate nucleocytoplasmic transport through the nuclear pore. This third group can be further subdivided into the cytoplasmic filaments including Nup358 and Nup214, the central framework including Nup98 and the Nup62 complex, and the nuclear basket including Nup153, Nup50 and Tpr [57-59]. The nucleocytoplasmic transport is mediated by factors that belong to nuclear transport receptors termed karyopherins (importins/exportins; Fig. 2) [60, 61]. Karyopherins bind their protein cargoes in the cytoplasm by binding specific nuclear localization signal (NLS). The karyopherin-cargo complex translocates into nucleus through the NPC via interactions with nucleoporins. Once in the nucleus, RanGTP binds to karyopherin which triggers the dissociation of the import complex, whereby the karyopherin is recycled back to the cytoplasm. Conversely, a protein containing a nuclear export signal (NES) forms a trimeric complex with an exporting karyopherin and RanGTP. The export complex passes through the NPC and is dissociated in the cytoplasm by hydrolysis of Ran-bound GTP [50, 60, 61]. All karyopherins bind directly to nucleoporins containing FG repeats as they pass through the NPC [54]. Several studies have demonstrated that various karyopherins have different affinities for specific nucleoporins [54, 62].
Figure 1.
Nuclear pore complex (NPC) structure and nucleoporins. In the box, schematic representation of the main structural components of NPC (left) and subcomplexes of nucleoprins within NPC (right) are shown. NPC essentially consists of cytoplasmic filaments, a central framework and a nuclear basket. Nucleoporins can be subdivided into different subcomplexes; Nup214 complex (Nup214, Nup88), Nup107-160 complex (Nup160, Nup133, Nup107, Nup96, Nup75, Nup43, Nup37, Sec13, Seh1), Nup62 complex (Nup62, Nup58, Nup54, Nup45), Nup93-205 complex (Nup205, Nup188, Nup155, Nup93, Nup35) and Nup98 complex (Nup98, Rae1).
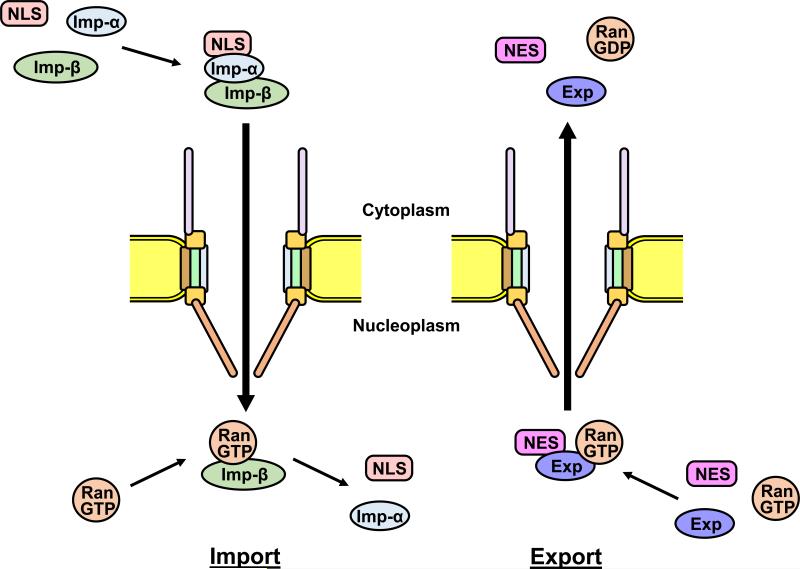
Figure 2.
Nucleocytoplasmic transport pathways through NPC. Karyopherin functions in nuclear import (importins) or nuclear export (exportins) are shown. Left: Importin-α recognizes and binds nuclear localization signal (NLS)-containing cargos. The importin-α forms a heterodimer with importin-β. The import complex docks at the NPC, and mediates import of cargos through NPC. In the nucleus the import complex encounters RanGTP, is disassembled and releases the cargos. Right: Exportin also recognizes and binds nuclear export signal (NES)-containing cargos in the nucleus in the presence of RanGTP. The export complex docks at the NPC, translocates to the cytoplasm and releases the cargos. Imp-α: importin-α; Imp-ß: importin-ß; Exp: exportin.
As described above, the main function of NPCs is to help transport of molecules between the cytoplasm and the nucleus. A tight regulation of nucleocytoplasmic transport is essential for cell homeostasis. In addition to its role in normal physiology, loss of NPC or nucleoporins function has been implicated in several diseases including cancer and autoimmune disease [59, 63, 64]. Recently gp210 has been demonstrated to be unregulated and to play a crucial role during myogenic and neuronal differentiation [65]. Nup133, a component of the Nup107-160 complex, is required for neuronal differentiation during mouse development [66]. Nup155 is shown to have a role in the physiology of the heart; a Nup155 homozygous missense mutation causes atrial fibrillation which causes sudden death, and loss of Nup155 function appears to disrupt nuclear pore function and develop cardiac arrhythmia [67]. Nup62 mutation causes autosomal recessive infantile bilateral striatal necrosis [68]. The expression of Nup98 is induced by interferons and Nup98 plays important roles in mRNA export from the nucleus, which is targeted by viruses and regulated by interferon [69]. Deletion of Nup98 in mice leads to lethality at embryonic day 6.5 to 7.5, and suggested a role for Nup98 in gastrulation [70]. Nup358 is implicated in myogenesis [71] and knockout of Nup358 leads to increased aneuploidy in mice [72]. Interestingly, Nup358 is an active E3 ligase in the SUMOylation reaction [73], thus, NPCs are indirectly involved in the regulation of numerous cellular processes through the SUMO pathway. Nup153 is also shown to bind to SUMO protease SENP2 [74]. In cancer patients, elevated levels of several nucleoporins were observed; Nup88 was found to be overexpressed in ovarian, breast, colorectal cancer, etc. [75-77]. Rae1 was elevated in breast and lung cancer [78, 79]. Chromosomal translocations between Nup98 and HOX (homeobox transcription factor) gene family have been reported in acute myelogenous leukemia [80, 81]. Nup98-Hox fusions are identified as oncogenes and display strong transcriptional activity and leukemogenic potential. Nup214 and DEK (DNA-binding protein) fusion gene also contributes to leukemia development [81, 82]. It has recently been demonstrated that Tpr interacts with tumor suppressor p53 and regulates autophagy in cancer cells [83]. Moreover, nucleoporins have been described as having important roles during mitosis, which is involved in cellular functions and cancer development [84-86]. Although recent studies have demonstrated nucleoporins are implicated in many biological and physiological functions, the detailed mechanism of nucleoporins in diseases such as cancer still remains unclear.
Galectin-3 shuttling between the nucleus and the cytoplasm
Galectin-3 exhibits pleiotropic biological functions; extracellular galectin-3 mediates cell migration and cell adhesion by interacting with cell surface and extracellular matrix glycoproteins and glycolipids, and intracellular galectin-3 regulates signaling pathways by interacting with cytoplasmic and nuclear proteins [87-89]. Interestingly, galectin-3 shuttles between the cytoplasm and the nucleus [3]. Nuclear and cytoplasmic galectin-3 is likely to be linked with proliferation and differentiation, respectively [87-89]. Nuclear galectin-3 has been associated with pre-mRNA splicing [90] and gene expression of cyclin D1 and c-myc [91, 92]. Thus, understanding the mechanism of galectin-3 transportation between the nucleus and the cytoplasm might provide a therapeutic modality to modulate gene expression related to cancer.
There have been many studies reported on the correlation between galectin-3 subcellular distribution and prognosis in various cancers. In normal cells, nuclear galectin-3 is presumably associated with cell proliferation [90]. Galectin-3 localization in the nucleus promotes cell proliferation by the induction of cyclin D1 expression in human breast epithelial cells [91]. In lung carcinoma, the expression of nuclear galectin-3 is a predictive factor of recurrence and/or a worse clinical outcome [93, 94]. In papillary thyroid cancer cells, nuclear galectin-3 interacts with the thyroid-specific TTF-1 transcription factor and enhances the transcriptional activity to promote the proliferation of the cells [95]. The elevated expression of galectin-3 in the nucleus is a significant pathological parameter related to histological differentiation and vascular invasion in esophageal squamous cell carcinoma patients [96]. In contrast, galectin-3 expression in the nucleus is greatly decreased in colon and prostate carcinomas [30, 97, 98]. In an experimental prostate cancer cell, nuclear galectin-3 suppresses malignancy whereas cytoplasmic galectin-3 promotes tumorigenicity [30, 98]. The levels of nuclear galectin-3 are markedly decreased during the progression from normal to cancerous states in tongue carcinomas [29]. Cytoplasmic galectin-3 expression translocated from the nucleus also exhibits anti-apoptotic activity by interacting with Bcl-2 [99]. It remains still unclear why galectin-3 expression is regulated differently among organs during cancer progression.
It is important to understand the transport mechanism of galectin-3 between the nucleus and the cytoplasm for the development of regulation of galectin-3 function. Although galectin-3 lacks a typical NLS, some reports have mentioned the essential sequences of galectin-3 for nuclear localization. There are several reports which have described the specific sequence responsible for galectin-3 nuclear transport ; the first 11 amino acids of human galectin-3, which contains a Ser6 phosphorylation site, is required for nuclear distribution [100]; deletion of the 103 N-terminal amino acid residues of hamster galectin-3 resulted in the nuclear localization of galectin-3 [101]; deletion of C-terminal domain of mouse galectin-3 lost the nuclear localization pattern, suggesting that nuclear translocation of galectin-3 is dependent on the IXLT type NLS in the end of C-terminal domain, the critical residues include I253, L255 and T256 fitting the IXLT motif identified to be important for the nuclear localization of the Drosophila protein Dsh [3]; incomplete forms of carbohydrate recognition domain (CRD) region of galectin-3 abrogate the nuclear accumulation, whereas N-terminal domain of galectin-3 promotes the nuclear transportation [102, 103]. There seems to be an inconsistency in these reports, probably because of the use of different species of galectin-3 or different cells, but also suggesting that mechanism of galectin-3 nuclear import is complicated and very specific. The presence of both phosphorylated and non-phosphorylated endogenous galectin-3 has been reported. The phosphorylated form of galectin-3 was found in both cytosolic and nuclear fractions, whereas the non-phosphorylated form was presented exclusively in the nucleus [104]. The phosphorylation of galectin-3 seems to be necessary and essential for its functions, and galectin-3 has been shown to be phosphorylated at the residue of N-terminal Ser6 by casein kinase 1 [105]. The galectin-3 phosphorylation is reportedly important for its nuclear export into the cytoplasm [99, 106].
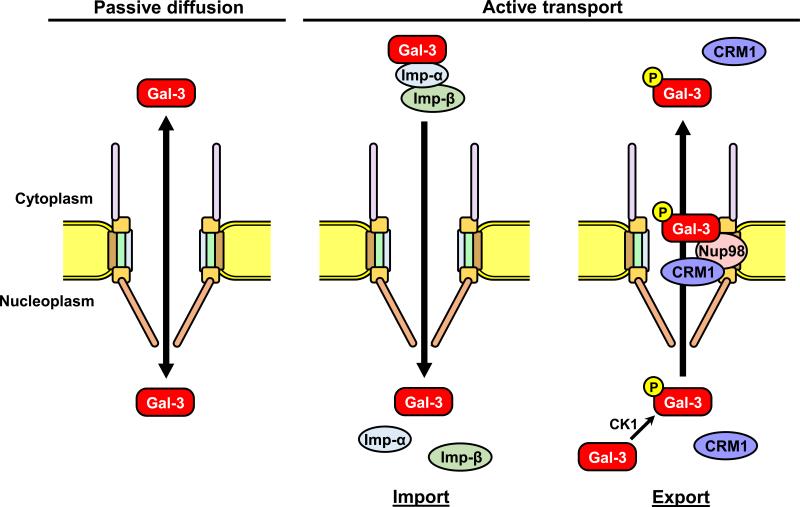
There are at least two pathways for the translocation of galectin-3 into the nucleus; a passive diffusion and an active transport through the NPC (Fig. 3). Galectin-3 is synthesized as a monomer, and partially forms a dimer and even a pentamer in some situation [107, 108]. Therefore, galectin-3 might be imported into the nucleus by passive diffusion as a monomer, or by active transport as a dimer or a pentamer form due to its size. In active transport into the nucleus, galectin-3 translocates by the importins-dependent transport system [102]. Although there is no classical NLS in galectin-3 sequence, an NLS-like sequence 223HRVKKL228 in the C-terminal region of galectin-3, which resembles sequences of p53 and c-Myc NLSs has been identified [109-111]. Importin-α is the receptor subunit that recognizes the NLS, and associates with importin-β via the importin-β binding domain [60, 61]. Interactions between importin-β and the nucleoporins containing FG repeats are essential in translocation through the NPC [54]. Galectin-3 directly binds to importin-α but not to importin-β, although the functional interaction of importin-β is required for galectin-3 nuclear translocation, and imported into the nucleus via the importin-α/β complex [102]. Furthermore, galectin-3 export mechanism has been also demonstrated. The galectin-3 NES exists in C-terminal region, and regulates the export of galectin-3 into the cytoplasm [103, 106]. During the export, nucleoporin Nup98 specifically regulates the galectin-3 transportation through NPC [112]. The N-terminal domain of Nup98 (1-505aa) interacts with CRD region of galectin-3. Galectin-3 directly binds to Nup98 but not to a nuclear export receptor CRM1 (exportin 1), although Nup98 is a cofactor for CRM1-mediated nuclear export. As described above, a few mechanisms of the interaction between NPC and galectin-3 have been established to date. Further studies are required to examine how galectin-3 shuttles between the nucleus and cytoplasm. Understanding the mechanism and control of galectin-3 transportation would provide new therapeutic target/modality for treating disease such as cancer.
Figure 3.
Schematic diagram of galectin-3 nucleocytoplasmic transport pathways. Galectin-3 is possibly translocated between nucleus and cytoplasm by both passive diffusion and active transport. During active nuclear transport, galectin-3 NLS binds to importin-α, followed by binding to importin-ß in the cytoplasm. The galectin-3–importin-α/ß complex docks at nucleoporins and enters the nucleus. The complex dissociates in the nucleus, releasing the galectin-3. In active transport into the cytoplasm, Nup98 plays an essential role in the galectin-3 export, with nuclear export protein CRM1 (exportin 1). Phosphorylation of galectin-3 by casein kinase 1 is required for the export. Gal-3: galectin-3; CK1: casein kinase 1.
Galectin-3 and β-catenin signaling through nuclear translocation
Interactions of galetin-3 with a plethora of ligands both in the intracellular and extracellular compartments have been reviewed in details previously [88, 89, 113]. In the nucleus, galectin-3 acts as a pre-mRNA splicing factor and is involved in the spliceosome assembly (reviewed in [114]). In the current review, we will focus on its interactions with β-catenin pathway resulting in the regulation of β-catenin regulated transcriptional activity. β-catenin is a downstream component of the Wnt signaling pathway. In the absence of Wnt stimulation, the levels of cytoplasmic β-catenin are low since it is ubiquitinated and constantly degraded in the proteasome [115]. It is phosphorylated by glycogen synthase kinase-3β (GSK-3β) and casein kinase 1α (CKIα) in a multi-protein complex that also contains adenomatous polyposis coli (APC) and scaffold protein axin [116-120]. Phosphorylated β-catenin is recognized by E3 ubiquitin ligase complex, ubiquitinated and degraded [121, 122]. In activation of the Wnt signaling pathway, binding of Wnt ligand to frizzled receptor and co-receptor LRP5/6 triggers the association of destruction complex with phosphorylated LRP inhibiting the degradation pathway consequently leading to stabilization of cytoplasmic β-catenin and its translocation into the nucleus, where it binds to the transcriptional factor Tcf/Lef and serves as a co-activator of Tcf/Lef to stimulate transcription of the Wnt target genes including c-myc, cyclin D1, cyclooxygenase-2, matrix metalloproteinase-7, gastrin, and ITF-2 [123-128]. Activating mutations in Wnt pathway components, including loss-of-function mutations of APC or less frequently in CTNNB1 (which encodes β-catenin) and AXIN, increase β-catenin protein levels and have been found in numerous human cancers including colorectal, gastric, and ovarian cancer [129]. However, mutations of Wnt pathway proteins are not the only factors that contribute to β-catenin activation [130]. Shimura et al [92, 131] reported that in breast cancer cell line BT-459, galectin-3 forms a complex with β-catenin independent of either APC or β-catenin mutations and it forms a ternary complex with Tcf-4. NH2 terminus of β-catenin was reported to interact with COOH terminus of galectin-3 and could be inhibited by lactose. These authors also showed that galectin-3 is phosphorylated, like β-catenin, by CKI [131] and GSK-3β and like β-catenin, its nuclear import-export is phosphorylation dependent [131]. While phosphorylation of galectin-3 at S6 by CKI serves as a molecular switch for sugar binding [132], and regulation of nuclear export [99], the phosphorylation of β-catenin by CKIα and GSK-3β promotes its proteasomal degradation. Shi and colleagues [133] showed that inhibition of both Wnt-2 and galectin-3 in colorectal cancer cells had synergistic effects on suppressing Wnt signaling and inducing apoptosis. Song et al [134] showed that in colon cancer cells, galectin-3 mediates β-catenin expression and Tcf-4 activity by regulation of GSK-3β phosphorylation and activity via the PI3K/Akt pathway. It was shown that down-regulation of galectin-3 resulted in Akt and GSK-3β dephosphorylation and increased GSK-3β activity, which resulted in increased β-catenin phosphorylation and degradation [134]. Using galectin-3 knockout mice Mendonca et al [135] recently showed that galectin-3 was an important partner for GSK-3β inactive form (phosphorylated at Ser9) to drive oncogenic transformation. We recently showed that Nup98 promotes nuclear export of galectin-3[112], while its nuclear import is regulated by karyopherins [102, 103]. Depletion of Nup98 resulted in nuclear translocation of galectin-3, where it interacts with β-catenin and reduces its transcriptional activity [112]. Ferrazzo et al [136] however showed that nuclear localization of galectin-3 in adenocarcinoma of salivary gland may be related to a more aggressive behavior and did not seem to affect cyclin D1 expression by β-catenin. Thus, there seems to be a discrepancy on the relationship of nuclear galectin-3 to β-catenin activity, which could be explained by the observation of Kim et al [137]. A germline variation in galectin-3 gene at position 191 resulting in H64 or P64 was reported by us earlier [138]. Kim et al analyzed this variation in gastric cancer patients. Presence of H64 and not P64 galectin-3 enhanced nuclear accumulation of β-catenin as well as increased expression of Tcf-4 target genes such as fascin-1 and c-myc through augmented promoter binding activity of Tcf-4 [137]. It is therefore important to first check the H/P64 status of the cells before analyzing its collaboration with β-catenin.
A more detailed understanding of the mechanisms by which galectin-3 augments Wnt signaling may facilitate the development of chemo-preventive and therapeutic strategies for various cancers such as colorectal, prostate, breast, multiple myeloma and acute myeloid leukemia, where its regulatory roles are well established.
Galectin-3: a therapeutic target?
Numerous studies have focused on the molecular mechanisms of galectin-3 involved in cancer cell chemo-resistance, which have been reviewed in details [139-142]. A number of investigators have used forced expression of galectin-3 or it's down regulation or by anti-sense treatment to study its effects on drug resistance. While some other groups have used a more direct approach to show the role of galectin-3 in chemo-resistance. Cheng et al [143] reported an up-regulation of galectin-3 in cisplatin and LY294002 (a phosphatidylinositol 3-kinase inhibitor) surviving chronic myeloid leukemia cells. These authors also reported a direct correlation between GSK-3β and galectin-3. Mazurek et al [144] showed an up-regulation of galectin-3 in TRAIL resistant sub-population of metastatic colon cancer cell line LS-LiM6. Silencing of galectin-3 restored TRAIL sensitivity. It was reported that galectin-3 impedes trafficking of death receptor by anchoring them in glycan nano-clusters, blocking the execution of the apoptosis signal caused by TRAIL [144]. Galectin-3 silencing was also reported to augment gemcitabine and cisplatin-induced apoptosis in pancreatic cancer cell lines and inhibited migration and invasion through degradation of beta-catenin [145, 146]. Lin et al [147, 148] used a highly specific small molecule inhibitor of galectin-3 (Td131_1) and showed a synergistic activity with doxorubicin in papillary thyroid cancer cell lines 8505-C and TPC-1. In chronic myelogenous leukemia cells enforced expression of galectin-3 activated Akt and Erk, induced accumulation of Mcl-1 and promoted multi-drug resistance to tyrosine kinase inhibitors, doxorubicin, cytarabine, etoposide, and vincristine as a result of impaired apoptosis induction [149].
The vast majority of anti-cancer drugs currently used act by inducing apoptosis via the intrinsic pathway. Numerous mechanisms underlie cancer chemo-resistance [150]: galectin-3 appears to suppress cell apoptosis and hence, decreases sensitivity of cancer cells to chemotherapeutic drugs [151]. Several investigators have attempted to re-sensitize the cells to chemotherapeutic drugs by targeting galectin-3. Modified citrus pectin (MCP) was reported to inhibit galectin-3 functions by inhibiting its interactions with its glyco-conjugate ligands [152-154]. Johnson et al [155] showed that galectin-3 targeting via MCP or via lactosyl-L-leucine (LL) decreased malignant endothelial cell proliferation by themselves and sensitized these cells to the cytotoxic effect of doxorubicin. Treatment of metastatic cells MDA-MB-435 with MCP and LL also increased their sensitivity to taxol [151]. MCP/GCS100 also induced calpain activation in prostate cancer cells that led to their sensitization to cisplatin treatment [156]. In multiple myeloma cells an increased efficacy of bortezomide and dexamethasone on apoptosis in the presence of MCP/GCS100 was observed [157]. In a recent study, Lee et al demonstrated that inhibition of galectin-3 enhanced the efficacy of anticancer drug epirubicin in colon cancer caco-2 cells. Galectin-3 knockdown increased the intracellular accumulation of epirubicin, suppressed the mRNA and protein expression of b catenin, cyclin d1, c-myc, p-glycoprotein, MDR-associated proteins 1 and 2 and increased the mRNA levels of GSK-3β, Bax, caspase3 and caspase9 indicating that silencing of galectin-3 sensitizes the MDR cells to epirubicin by inhibiting ABC transporters and activating the mitochondrial pathway of apoptosis through modulation of b catenin/GSK-3β pathway in human colon cancer cells [158].
Concluding remarks
There is ample evidence now to indicate the efficacy of galectin-3 as a therapeutic target. Due to its unique chimeric structure, two main types of interactions of galectin-3 have been reported. Some of the interactions occur via its carbohydrate binding domain, which can be interrupted by specific sugar competitive molecules such as MCP/GSC100 or small sugar analogs. A majority of its intracellular interactions occur via protein-protein interactions and are not inhibited by lactose.
Nuclear transport of galectin-3 is phosphorylation dependent, although, the domain responsible for the nuclear transport is debatable. However, it has been confirmed by various investigators that the phosphorylated galectin-3 is instantly exported to the cytoplasm, where it protects the cells from drug induced apoptosis [99]. The galectin-3 nuclear export proceeds via leptomycin-inhibitable pathway (155). Although no studies as yet have targeted the nuclear transport of galectin-3 as a cancer preventive mechanism, it would be an interesting approach. Prevention of galectin-3 phosphorylation will prevent its nuclear export, but it will not be an easy task because of lack of a specific inhibitor. Furthermore, disruption of β-catenin and galectin-3 interactions may also prove useful. Another important aspect to investigate while using galectin-3 as a therapeutic target is the germline mutations in galectin-3. Presence of H64 in galectin-3 is associated with a more malignant phenotype. This variant is sensitive to cleavage by MMP-2 and -9, is associated with increased breast cancer incidence and also shows nuclear localization and increased β-catenin activity compared to the P64 variant. In summary the precise regulatory mechanisms of galectin-3 expression in different cell types under different physiological and pathophysiological conditions need to be analyzed, which may help in development of new strategies to fight cancer.
Acknowledgment
This work was supported by the National Institute of Health R37CA46120 (To A.R)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994;269(33):20807–10. [PubMed] [Google Scholar]
- 2.Hirabayashi J, Hashidate T, Arata Y, Nishi N, Nakamura T, Hirashima M, et al. Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochimica et biophysica acta. 2002;1572(2-3):232–54. doi: 10.1016/s0304-4165(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 3.Davidson PJ, Li SY, Lohse AG, Vandergaast R, Verde E, Pearson A, et al. Transport of galectin-3 between the nucleus and cytoplasm. I. Conditions and signals for nuclear import. Glycobiology. 2006;16(7):602–11. doi: 10.1093/glycob/cwj088. [DOI] [PubMed] [Google Scholar]
- 4.Li SY, Davidson PJ, Lin NY, Patterson RJ, Wang JL, Arnoys EJ. Transport of galectin-3 between the nucleus and cytoplasm. II. Identification of the signal for nuclear export. Glycobiology. 2006;16(7):612–22. doi: 10.1093/glycob/cwj089. [DOI] [PubMed] [Google Scholar]
- 5.Davidson PJ, Davis MJ, Patterson RJ, Ripoche MA, Poirier F, Wang JL. Shuttling of galectin-3 between the nucleus and cytoplasm. Glycobiology. 2002;12(5):329–37. doi: 10.1093/glycob/12.5.329. [DOI] [PubMed] [Google Scholar]
- 6.Hughes RC. Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim Biophys Acta. 1999;1473(1):172–85. doi: 10.1016/s0304-4165(99)00177-4. [DOI] [PubMed] [Google Scholar]
- 7.Nickel W. Unconventional secretory routes: direct protein export across the plasma membrane of mammalian cells. Traffic. 2005;6(8):607–14. doi: 10.1111/j.1600-0854.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 8.Sato S, Hughes RC. Control of Mac-2 surface expression on murine macrophage cell lines. Eur J Immunol. 1994;24(1):216–21. doi: 10.1002/eji.1830240134. [DOI] [PubMed] [Google Scholar]
- 9.Lindstedt R, Apodaca G, Barondes SH, Mostov KE, Leffler H. Apical secretion of a cytosolic protein by Madin-Darby canine kidney cells. Evidence for polarized release of an endogenous lectin by a nonclassical secretory pathway. J Biol Chem. 1993;268(16):11750–7. [PubMed] [Google Scholar]
- 10.Sato S, Burdett I, Hughes RC. Secretion of the baby hamster kidney 30-kDa galactose-binding lectin from polarized and nonpolarized cells: a pathway independent of the endoplasmic reticulum-Golgi complex. Exp Cell Res. 1993;207(1):8–18. doi: 10.1006/excr.1993.1157. [DOI] [PubMed] [Google Scholar]
- 11.Mehul B, Hughes RC. Plasma membrane targetting, vesicular budding and release of galectin 3 from the cytoplasm of mammalian cells during secretion. J Cell Sci. 1997;110(Pt 10):1169–78. doi: 10.1242/jcs.110.10.1169. [DOI] [PubMed] [Google Scholar]
- 12.Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166(12):7309–18. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 13.Baptiste TA, James A, Saria M, Ochieng J. Mechano-transduction mediated secretion and uptake of galectin-3 in breast carcinoma cells: implications in the extracellular functions of the lectin. Exp Cell Res. 2007;313(4):652–64. doi: 10.1016/j.yexcr.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boscher C, Dennis JW, Nabi IR. Glycosylation, galectins and cellular signaling. Current opinion in cell biology. 2011;23(4):383–92. doi: 10.1016/j.ceb.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Di Lella S, Sundblad V, Cerliani JP, Guardia CM, Estrin DA, Vasta GR, et al. When galectins recognize glycans: from biochemistry to physiology and back again. Biochemistry. 2011;50(37):7842–57. doi: 10.1021/bi201121m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lau KS, Dennis JW. N-Glycans in cancer progression. Glycobiology. 2008;18(10):750–60. doi: 10.1093/glycob/cwn071. [DOI] [PubMed] [Google Scholar]
- 17.van Kooyk Y, Rabinovich GA. Protein-glycan interactions in the control of innate and adaptive immune responses. Nature immunology. 2008;9(6):593–601. doi: 10.1038/ni.f.203. [DOI] [PubMed] [Google Scholar]
- 18.Rabinovich GA, Ilarregui JM. Conveying glycan information into T-cell homeostatic programs: a challenging role for galectin-1 in inflammatory and tumor microenvironments. Immunological reviews. 2009;230(1):144–59. doi: 10.1111/j.1600-065X.2009.00787.x. [DOI] [PubMed] [Google Scholar]
- 19.Cedeno-Laurent F, Opperman MJ, Barthel SR, Hays D, Schatton T, Zhan Q, et al. Metabolic inhibition of galectin-1-binding carbohydrates accentuates antitumor immunity. The Journal of investigative dermatology. 2012;132(2):410–20. doi: 10.1038/jid.2011.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lagana A, Goetz JG, Cheung P, Raz A, Dennis JW, Nabi IR. Galectin binding to Mgat5-modified N-glycans regulates fibronectin matrix remodeling in tumor cells. Molecular and cellular biology. 2006;26(8):3181–93. doi: 10.1128/MCB.26.8.3181-3193.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markowska AI, Liu FT, Panjwani N. Galectin-3 is an important mediator of VEGF- and bFGF-mediated angiogenic response. The Journal of experimental medicine. 2010;207(9):1981–93. doi: 10.1084/jem.20090121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merlin J, Stechly L, de Beauce S, Monte D, Leteurtre E, van Seuningen I, et al. Galectin-3 regulates MUC1 and EGFR cellular distribution and EGFR downstream pathways in pancreatic cancer cells. Oncogene. 2011;30(22):2514–25. doi: 10.1038/onc.2010.631. [DOI] [PubMed] [Google Scholar]
- 23.Nangia-Makker P, Honjo Y, Sarvis R, Akahani S, Hogan V, Pienta KJ, et al. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am J Pathol. 2000;156(3):899–909. doi: 10.1016/S0002-9440(10)64959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haudek KC, Patterson RJ, Wang JL. SR proteins and galectins: what's in a name? Glycobiology. 2010;20(10):1199–207. doi: 10.1093/glycob/cwq097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park JW, Voss PG, Grabski S, Wang JL, Patterson RJ. Association of galectin-1 and galectin-3 with Gemin4 in complexes containing the SMN protein. Nucleic acids research. 2001;29(17):3595–602. doi: 10.1093/nar/29.17.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van den Brule FA, Waltregny D, Liu FT, Castronovo V. Alteration of the cytoplasmic/nuclear expression pattern of galectin-3 correlates with prostate carcinoma progression. Int J Cancer. 2000;89(4):361–7. doi: 10.1002/1097-0215(20000720)89:4<361::aid-ijc8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 27.Yu F, Finley RL, Jr., Raz A, Kim HR. Galectin-3 translocates to the perinuclear membranes and inhibits cytochrome c release from the mitochondria. A role for synexin in galectin-3 translocation. J Biol Chem. 2002;277(18):15819–27. doi: 10.1074/jbc.M200154200. [DOI] [PubMed] [Google Scholar]
- 28.Yoshii T, Fukumori T, Honjo Y, Inohara H, Kim HR, Raz A. Galectin-3 phosphorylation is required for its anti-apoptotic function and cell cycle arrest. J Biol Chem. 2002;277(9):6852–7. doi: 10.1074/jbc.M107668200. [DOI] [PubMed] [Google Scholar]
- 29.Honjo Y, Inohara H, Akahani S, Yoshii T, Takenaka Y, Yoshida J, et al. Expression of cytoplasmic galectin-3 as a prognostic marker in tongue carcinoma. Clin Cancer Res. 2000;6(12):4635–40. [PubMed] [Google Scholar]
- 30.Califice S, Castronovo V, Bracke M, van den Brule F. Dual activities of galectin-3 in human prostate cancer: tumor suppression of nuclear galectin-3 vs tumor promotion of cytoplasmic galectin-3. Oncogene. 2004;23(45):7527–36. doi: 10.1038/sj.onc.1207997. [DOI] [PubMed] [Google Scholar]
- 31.Califice S, Castronovo V, Van Den Brule F. Galectin-3 and cancer (Review). Int J Oncol. 2004;25(4):983–92. [PubMed] [Google Scholar]
- 32.Moisa A, Fritz P, Eck A, Wehner HD, Murdter T, Simon W, et al. Growth/adhesion-regulatory tissue lectin galectin-3: stromal presence but not cytoplasmic/nuclear expression in tumor cells as a negative prognostic factor in breast cancer. Anticancer Res. 2007;27(4B):2131–9. [PubMed] [Google Scholar]
- 33.Shekhar MP, Nangia-Makker P, Tait L, Miller F, Raz A. Alterations in galectin-3 expression and distribution correlate with breast cancer progression: functional analysis of galectin-3 in breast epithelial-endothelial interactions. Am J Pathol. 2004 doi: 10.1016/S0002-9440(10)63245-2. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanjuan X, Fernandez PL, Castells A, Castronovo V, van den Brule F, Liu FT, et al. Differential expression of galectin 3 and galectin 1 in colorectal cancer progression [see comments]. Gastroenterology. 1997;113(6):1906–15. doi: 10.1016/s0016-5085(97)70010-6. [DOI] [PubMed] [Google Scholar]
- 35.Akahani S, Nangia-Makker P, Inohara H, Kim HR, Raz A. Galectin-3: a novel antiapoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family. Cancer Res. 1997;57(23):5272–6. [PubMed] [Google Scholar]
- 36.Yang RY, Hsu DK, Liu FT. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci U S A. 1996;93(13):6737–42. doi: 10.1073/pnas.93.13.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fukumori T, Takenaka Y, Oka N, Yoshii T, Hogan V, Inohara H, et al. Endogenous galectin-3 determines the routing of CD95 apoptotic signaling pathways. Cancer Res. 2004;64(10):3376–9. doi: 10.1158/0008-5472.CAN-04-0336. [DOI] [PubMed] [Google Scholar]
- 38.Liu L, Sakai T, Sano N, Fukui K. Nucling mediates apoptosis by inhibiting expression of galectin-3 through interference with nuclear factor kappaB signalling. Biochem J. 2004;380(Pt 1):31–41. doi: 10.1042/BJ20031300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu FT, Patterson RJ, Wang JL. Intracellular functions of galectins. Biochim Biophys Acta. 2002;1572(2-3):263–73. doi: 10.1016/s0304-4165(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 40.Matarrese P, Fusco O, Tinari N, Natoli C, Liu FT, Semeraro ML, et al. Galectin-3 overexpression protects from apoptosis by improving cell adhesion properties. Int J Cancer. 2000;85(4):545–54. [PubMed] [Google Scholar]
- 41.Elad-Sfadia G, Haklai R, Balan E, Kloog Y. Galectin-3 augments K-Ras activation and triggers a Ras signal that attenuates ERK but not phosphoinositide 3-kinase activity. J Biol Chem. 2004;279(33):34922–30. doi: 10.1074/jbc.M312697200. [DOI] [PubMed] [Google Scholar]
- 42.Shalom-Feuerstein R, Cooks T, Raz A, Kloog Y. Galectin-3 regulates a molecular switch from N-Ras to K-Ras usage in human breast carcinoma cells. Cancer Res. 2005;65(16):7292–300. doi: 10.1158/0008-5472.CAN-05-0775. [DOI] [PubMed] [Google Scholar]
- 43.Lee YJ, Song YK, Song JJ, Siervo-Sassi RR, Kim HR, Li L, et al. Reconstitution of galectin-3 alters glutathione content and potentiates TRAIL-induced cytotoxicity by dephosphorylation of Akt. Exp Cell Res. 2003;288(1):21–34. doi: 10.1016/s0014-4827(03)00211-8. [DOI] [PubMed] [Google Scholar]
- 44.Oka N, Nakahara S, Takenaka Y, Fukumori T, Hogan V, Kanayama HO, et al. Galectin-3 inhibits tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by activating Akt in human bladder carcinoma cells. Cancer Res. 2005;65(17):7546–53. doi: 10.1158/0008-5472.CAN-05-1197. [DOI] [PubMed] [Google Scholar]
- 45.Gruenbaum Y, Margalit A, Goldman RD, Shumaker DK, Wilson KL. The nuclear lamina comes of age. Nature reviews Molecular cell biology. 2005;6(1):21–31. doi: 10.1038/nrm1550. [DOI] [PubMed] [Google Scholar]
- 46.Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L, et al. Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes & development. 2008;22(7):832–53. doi: 10.1101/gad.1652708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Voeltz GK, Rolls MM, Rapoport TA. Structural organization of the endoplasmic reticulum. EMBO reports. 2002;3(10):944–50. doi: 10.1093/embo-reports/kvf202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vasu SK, Forbes DJ. Nuclear pores and nuclear assembly. Current opinion in cell biology. 2001;13(3):363–75. doi: 10.1016/s0955-0674(00)00221-0. [DOI] [PubMed] [Google Scholar]
- 49.Meinema AC, Poolman B, Veenhoff LM. The transport of integral membrane proteins across the nuclear pore complex. Nucleus. 2012;3(4):322–9. doi: 10.4161/nucl.20439. [DOI] [PubMed] [Google Scholar]
- 50.Gorlich D, Kutay U. Transport between the cell nucleus and the cytoplasm. Annual review of cell and developmental biology. 1999;15:607–60. doi: 10.1146/annurev.cellbio.15.1.607. [DOI] [PubMed] [Google Scholar]
- 51.Tran EJ, Wente SR. Dynamic nuclear pore complexes: life on the edge. Cell. 2006;125(6):1041–53. doi: 10.1016/j.cell.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 52.Mohr D, Frey S, Fischer T, Guttler T, Gorlich D. Characterisation of the passive permeability barrier of nuclear pore complexes. The EMBO journal. 2009;28(17):2541–53. doi: 10.1038/emboj.2009.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Q, Rout MP, Akey CW. Three-dimensional architecture of the isolated yeast nuclear pore complex: functional and evolutionary implications. Molecular cell. 1998;1(2):223–34. doi: 10.1016/s1097-2765(00)80023-4. [DOI] [PubMed] [Google Scholar]
- 54.Suntharalingam M, Wente SR. Peering through the pore: nuclear pore complex structure, assembly, and function. Developmental cell. 2003;4(6):775–89. doi: 10.1016/s1534-5807(03)00162-x. [DOI] [PubMed] [Google Scholar]
- 55.Schermelleh L, Carlton PM, Haase S, Shao L, Winoto L, Kner P, et al. Subdiffraction multicolor imaging of the nuclear periphery with 3D structured illumination microscopy. Science. 2008;320(5881):1332–6. doi: 10.1126/science.1156947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cronshaw JM, Krutchinsky AN, Zhang W, Chait BT, Matunis MJ. Proteomic analysis of the mammalian nuclear pore complex. The Journal of cell biology. 2002;158(5):915–27. doi: 10.1083/jcb.200206106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwartz TU. Modularity within the architecture of the nuclear pore complex. Current opinion in structural biology. 2005;15(2):221–6. doi: 10.1016/j.sbi.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 58.Alber F, Dokudovskaya S, Veenhoff LM, Zhang W, Kipper J, Devos D, et al. The molecular architecture of the nuclear pore complex. Nature. 2007;450(7170):695–701. doi: 10.1038/nature06405. [DOI] [PubMed] [Google Scholar]
- 59.Capelson M, Hetzer MW. The role of nuclear pores in gene regulation, development and disease. EMBO reports. 2009;10(7):697–705. doi: 10.1038/embor.2009.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chook YM, Blobel G. Karyopherins and nuclear import. Current opinion in structural biology. 2001;11(6):703–15. doi: 10.1016/s0959-440x(01)00264-0. [DOI] [PubMed] [Google Scholar]
- 61.Mosammaparast N, Pemberton LF. Karyopherins: from nuclear-transport mediators to nuclear-function regulators. Trends in cell biology. 2004;14(10):547–56. doi: 10.1016/j.tcb.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 62.Pemberton LF, Paschal BM. Mechanisms of receptor-mediated nuclear import and nuclear export. Traffic. 2005;6(3):187–98. doi: 10.1111/j.1600-0854.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- 63.Kau TR, Way JC, Silver PA. Nuclear transport and cancer: from mechanism to intervention. Nature reviews Cancer. 2004;4(2):106–17. doi: 10.1038/nrc1274. [DOI] [PubMed] [Google Scholar]
- 64.Dauer WT, Worman HJ. The nuclear envelope as a signaling node in development and disease. Developmental cell. 2009;17(5):626–38. doi: 10.1016/j.devcel.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 65.D'Angelo MA, Gomez-Cavazos JS, Mei A, Lackner DH, Hetzer MW. A change in nuclear pore complex composition regulates cell differentiation. Developmental cell. 2012;22(2):446–58. doi: 10.1016/j.devcel.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lupu F, Alves A, Anderson K, Doye V, Lacy E. Nuclear pore composition regulates neural stem/progenitor cell differentiation in the mouse embryo. Developmental cell. 2008;14(6):831–42. doi: 10.1016/j.devcel.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang X, Chen S, Yoo S, Chakrabarti S, Zhang T, Ke T, et al. Mutation in nuclear pore component NUP155 leads to atrial fibrillation and early sudden cardiac death. Cell. 2008;135(6):1017–27. doi: 10.1016/j.cell.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 68.Basel-Vanagaite L, Muncher L, Straussberg R, Pasmanik-Chor M, Yahav M, Rainshtein L, et al. Mutated nup62 causes autosomal recessive infantile bilateral striatal necrosis. Annals of neurology. 2006;60(2):214–22. doi: 10.1002/ana.20902. [DOI] [PubMed] [Google Scholar]
- 69.Enninga J, Levy DE, Blobel G, Fontoura BM. Role of nucleoporin induction in releasing an mRNA nuclear export block. Science. 2002;295(5559):1523–5. doi: 10.1126/science.1067861. [DOI] [PubMed] [Google Scholar]
- 70.Wu X, Kasper LH, Mantcheva RT, Mantchev GT, Springett MJ, van Deursen JM. Disruption of the FG nucleoporin NUP98 causes selective changes in nuclear pore complex stoichiometry and function. Proc Natl Acad Sci U S A. 2001;98(6):3191–6. doi: 10.1073/pnas.051631598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Asally M, Yasuda Y, Oka M, Otsuka S, Yoshimura SH, Takeyasu K, et al. Nup358, a nucleoporin, functions as a key determinant of the nuclear pore complex structure remodeling during skeletal myogenesis. The FEBS journal. 2011;278(4):610–21. doi: 10.1111/j.1742-4658.2010.07982.x. [DOI] [PubMed] [Google Scholar]
- 72.Dawlaty MM, Malureanu L, Jeganathan KB, Kao E, Sustmann C, Tahk S, et al. Resolution of sister centromeres requires RanBP2-mediated SUMOylation of topoisomerase IIalpha. Cell. 2008;133(1):103–15. doi: 10.1016/j.cell.2008.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pichler A, Gast A, Seeler JS, Dejean A, Melchior F. The nucleoporin RanBP2 has SUMO1 E3 ligase activity. Cell. 2002;108(1):109–20. doi: 10.1016/s0092-8674(01)00633-x. [DOI] [PubMed] [Google Scholar]
- 74.Zhang H, Saitoh H, Matunis MJ. Enzymes of the SUMO modification pathway localize to filaments of the nuclear pore complex. Molecular and cellular biology. 2002;22(18):6498–508. doi: 10.1128/MCB.22.18.6498-6508.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martinez N, Alonso A, Moragues MD, Ponton J, Schneider J. The nuclear pore complex protein Nup88 is overexpressed in tumor cells. Cancer Res. 1999;59(21):5408–11. [PubMed] [Google Scholar]
- 76.Gould VE, Martinez N, Orucevic A, Schneider J, Alonso A. A novel, nuclear pore-associated, widely distributed molecule overexpressed in oncogenesis and development. Am J Pathol. 2000;157(5):1605–13. doi: 10.1016/S0002-9440(10)64798-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Agudo D, Gomez-Esquer F, Martinez-Arribas F, Nunez-Villar MJ, Pollan M, Schneider J. Nup88 mRNA overexpression is associated with high aggressiveness of breast cancer. International journal of cancer Journal international du cancer. 2004;109(5):717–20. doi: 10.1002/ijc.20034. [DOI] [PubMed] [Google Scholar]
- 78.Lu Y, Lemon W, Liu PY, Yi Y, Morrison C, Yang P, et al. A gene expression signature predicts survival of patients with stage I non-small cell lung cancer. PLoS medicine. 2006;3(12):e467. doi: 10.1371/journal.pmed.0030467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kao J, Salari K, Bocanegra M, Choi YL, Girard L, Gandhi J, et al. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PloS one. 2009;4(7):e6146. doi: 10.1371/journal.pone.0006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gough SM, Slape CI, Aplan PD. NUP98 gene fusions and hematopoietic malignancies: common themes and new biologic insights. Blood. 2011;118(24):6247–57. doi: 10.1182/blood-2011-07-328880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Funasaka T, Wong RW. The role of nuclear pore complex in tumor microenvironment and metastasis. Cancer Metastasis Rev. 2011;30(2):239–51. doi: 10.1007/s10555-011-9287-y. [DOI] [PubMed] [Google Scholar]
- 82.Graux C, Cools J, Melotte C, Quentmeier H, Ferrando A, Levine R, et al. Fusion of NUP214 to ABL1 on amplified episomes in T-cell acute lymphoblastic leukemia. Nature genetics. 2004;36(10):1084–9. doi: 10.1038/ng1425. [DOI] [PubMed] [Google Scholar]
- 83.Funasaka T, Tsuka E, Wong RW. Regulation of autophagy by nucleoporin Tpr. Scientific reports. 2012;2:878. doi: 10.1038/srep00878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nakano H, Funasaka T, Hashizume C, Wong RW. Nucleoporin translocated promoter region (Tpr) associates with dynein complex, preventing chromosome lagging formation during mitosis. J Biol Chem. 2010;285(14):10841–9. doi: 10.1074/jbc.M110.105890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Funasaka T, Nakano H, Wu Y, Hashizume C, Gu L, Nakamura T, et al. RNA export factor RAE1 contributes to NUP98-HOXA9-mediated leukemogenesis. Cell cycle. 2011;10(9):1456–67. doi: 10.4161/cc.10.9.15494. [DOI] [PubMed] [Google Scholar]
- 86.Imamoto N, Funakoshi T. Nuclear pore dynamics during the cell cycle. Current opinion in cell biology. 2012;24(4):453–9. doi: 10.1016/j.ceb.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 87.Takenaka Y, Fukumori T, Raz A. Galectin-3 and metastasis. Glycoconj J. 2004;19(7-9):543–9. doi: 10.1023/B:GLYC.0000014084.01324.15. [DOI] [PubMed] [Google Scholar]
- 88.Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nature reviews Cancer. 2005;5(1):29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 89.Dumic J, Dabelic S, Flogel M. Galectin-3: an open-ended story. Biochim Biophys Acta. 2006;1760(4):616–35. doi: 10.1016/j.bbagen.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 90.Dagher SF, Wang JL, Patterson RJ. Identification of galectin-3 as a factor in pre-mRNA splicing. Proc Natl Acad Sci U S A. 1995;92(4):1213–7. doi: 10.1073/pnas.92.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin HM, Pestell RG, Raz A, Kim HR. Galectin-3 enhances cyclin D(1) promoter activity through SP1 and a cAMP-responsive element in human breast epithelial cells. Oncogene. 2002;21(52):8001–10. doi: 10.1038/sj.onc.1205820. [DOI] [PubMed] [Google Scholar]
- 92.Shimura T, Takenaka Y, Tsutsumi S, Hogan V, Kikuchi A, Raz A. Galectin-3, a novel binding partner of beta-catenin. Cancer Res. 2004;64(18):6363–7. doi: 10.1158/0008-5472.CAN-04-1816. [DOI] [PubMed] [Google Scholar]
- 93.Puglisi F, Minisini AM, Barbone F, Intersimone D, Aprile G, Puppin C, et al. Galectin-3 expression in non-small cell lung carcinoma. Cancer letters. 2004;212(2):233–9. doi: 10.1016/j.canlet.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 94.Mathieu A, Saal I, Vuckovic A, Ransy V, Vereerstraten P, Kaltner H, et al. Nuclear galectin-3 expression is an independent predictive factor of recurrence for adenocarcinoma and squamous cell carcinoma of the lung. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2005;18(9):1264–71. doi: 10.1038/modpathol.3800416. [DOI] [PubMed] [Google Scholar]
- 95.Paron I, Scaloni A, Pines A, Bachi A, Liu FT, Puppin C, et al. Nuclear localization of Galectin-3 in transformed thyroid cells: a role in transcriptional regulation. Biochemical and biophysical research communications. 2003;302(3):545–53. doi: 10.1016/s0006-291x(03)00151-7. [DOI] [PubMed] [Google Scholar]
- 96.Shibata T, Noguchi T, Takeno S, Takahashi Y, Fumoto S, Kawahara K. Impact of nuclear galectin-3 expression on histological differentiation and vascular invasion in patients with esophageal squamous cell carcinoma. Oncology reports. 2005;13(2):235–9. [PubMed] [Google Scholar]
- 97.Lotz MM, Andrews CW, Jr., Korzelius CA, Lee EC, Steele GD, Jr., Clarke A, et al. Decreased expression of Mac-2 (carbohydrate binding protein 35) and loss of its nuclear localization are associated with the neoplastic progression of colon carcinoma. Proc Natl Acad Sci U S A. 1993;90(8):3466–70. doi: 10.1073/pnas.90.8.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ellerhorst JA, Stephens LC, Nguyen T, Xu XC. Effects of galectin-3 expression on growth and tumorigenicity of the prostate cancer cell line LNCaP. The Prostate. 2002;50(1):64–70. doi: 10.1002/pros.10033. [DOI] [PubMed] [Google Scholar]
- 99.Takenaka Y, Fukumori T, Yoshii T, Oka N, Inohara H, Kim HR, et al. Nuclear export of phosphorylated galectin-3 regulates its antiapoptotic activity in response to chemotherapeutic drugs. Mol Cell Biol. 2004;24(10):4395–406. doi: 10.1128/MCB.24.10.4395-4406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gong HC, Honjo Y, Nangia-Makker P, Hogan V, Mazurak N, Bresalier RS, et al. The NH2 terminus of galectin-3 governs cellular compartmentalization and functions in cancer cells. Cancer Res. 1999;59(24):6239–45. [PubMed] [Google Scholar]
- 101.Gaudin JC, Mehul B, Hughes RC. Nuclear localisation of wild type and mutant galectin-3 in transfected cells. Biology of the cell / under the auspices of the European Cell Biology Organization. 2000;92(1):49–58. doi: 10.1016/S0248-4900(00)88763-8. [DOI] [PubMed] [Google Scholar]
- 102.Nakahara S, Hogan V, Inohara H, Raz A. Importin-mediated nuclear translocation of galectin-3. J Biol Chem. 2006;281(51):39649–59. doi: 10.1074/jbc.M608069200. [DOI] [PubMed] [Google Scholar]
- 103.Nakahara S, Oka N, Wang Y, Hogan V, Inohara H, Raz A. Characterization of the nuclear import pathways of galectin-3. Cancer Res. 2006;66(20):9995–10006. doi: 10.1158/0008-5472.CAN-06-1772. [DOI] [PubMed] [Google Scholar]
- 104.Cowles EA, Agrwal N, Anderson RL, Wang JL. Carbohydrate-binding protein 35. Isoelectric points of the polypeptide and a phosphorylated derivative. J Biol Chem. 1990;265(29):17706–12. [PubMed] [Google Scholar]
- 105.Huflejt ME, Turck CW, Lindstedt R, Barondes SH, Leffler H. L-29, a soluble lactose-binding lectin, is phosphorylated on serine 6 and serine 12 in vivo and by casein kinase I. J Biol Chem. 1993;268(35):26712–8. [PubMed] [Google Scholar]
- 106.Tsay YG, Lin NY, Voss PG, Patterson RJ, Wang JL. Export of galectin-3 from nuclei of digitonin-permeabilized mouse 3T3 fibroblasts. Experimental cell research. 1999;252(2):250–61. doi: 10.1006/excr.1999.4643. [DOI] [PubMed] [Google Scholar]
- 107.Yang RY, Hill PN, Hsu DK, Liu FT. Role of the carboxyl-terminal lectin domain in self-association of galectin-3. Biochemistry. 1998;37(12):4086–92. doi: 10.1021/bi971409c. [DOI] [PubMed] [Google Scholar]
- 108.Ahmad N, Gabius HJ, Andre S, Kaltner H, Sabesan S, Roy R, et al. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J Biol Chem. 2004;279(12):10841–7. doi: 10.1074/jbc.M312834200. [DOI] [PubMed] [Google Scholar]
- 109.Shaulsky G, Goldfinger N, Ben-Ze'ev A, Rotter V. Nuclear accumulation of p53 protein is mediated by several nuclear localization signals and plays a role in tumorigenesis. Molecular and cellular biology. 1990;10(12):6565–77. doi: 10.1128/mcb.10.12.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fabbro M, Henderson BR. Regulation of tumor suppressors by nuclear-cytoplasmic shuttling. Experimental cell research. 2003;282(2):59–69. doi: 10.1016/s0014-4827(02)00019-8. [DOI] [PubMed] [Google Scholar]
- 111.Chen MH, Ben-Efraim I, Mitrousis G, Walker-Kopp N, Sims PJ, Cingolani G. Phospholipid scramblase 1 contains a nonclassical nuclear localization signal with unique binding site in importin alpha. J Biol Chem. 2005;280(11):10599–606. doi: 10.1074/jbc.M413194200. [DOI] [PubMed] [Google Scholar]
- 112.Funasaka T, Balan V, Raz A, Wong RW. Nucleoporin Nup98 mediates galectin-3 nuclear-cytoplasmic trafficking. Biochemical and biophysical research communications. 2013;434(1):155–61. doi: 10.1016/j.bbrc.2013.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nangia-Makker P, Balan V, Raz A. Regulation of tumor progression by extracellular galectin-3. Cancer Microenviron. 2008;1(1):43–51. doi: 10.1007/s12307-008-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Haudek KC, Spronk KJ, Voss PG, Patterson RJ, Wang JL, Arnoys EJ. Dynamics of galectin-3 in the nucleus and cytoplasm. Biochimica et biophysica acta. 2010;1800(2):181–9. doi: 10.1016/j.bbagen.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. The EMBO journal. 1997;16(13):3797–804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. The EMBO journal. 1998;17(5):1371–84. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kishida S, Yamamoto H, Ikeda S, Kishida M, Sakamoto I, Koyama S, et al. Axin, a negative regulator of the wnt signaling pathway, directly interacts with adenomatous polyposis coli and regulates the stabilization of beta-catenin. J Biol Chem. 1998;273(18):10823–6. doi: 10.1074/jbc.273.18.10823. [DOI] [PubMed] [Google Scholar]
- 118.Hart MJ, de los Santos R, Albert IN, Rubinfeld B, Polakis P. Downregulation of beta-catenin by human Axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Current biology : CB. 1998;8(10):573–81. doi: 10.1016/s0960-9822(98)70226-x. [DOI] [PubMed] [Google Scholar]
- 119.Itoh K, Krupnik VE, Sokol SY. Axis determination in Xenopus involves biochemical interactions of axin, glycogen synthase kinase 3 and beta-catenin. Current biology : CB. 1998;8(10):591–4. doi: 10.1016/s0960-9822(98)70229-5. [DOI] [PubMed] [Google Scholar]
- 120.Sakanaka C, Weiss JB, Williams LT. Bridging of beta-catenin and glycogen synthase kinase-3beta by axin and inhibition of beta-catenin-mediated transcription. Proc Natl Acad Sci U S A. 1998;95(6):3020–3. doi: 10.1073/pnas.95.6.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Polakis P. Wnt signaling and cancer. Genes & development. 2000;14(15):1837–51. [PubMed] [Google Scholar]
- 122.Kitagawa M, Hatakeyama S, Shirane M, Matsumoto M, Ishida N, Hattori K, et al. An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of beta-catenin. The EMBO journal. 1999;18(9):2401–10. doi: 10.1093/emboj/18.9.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281(5382):1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 124.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96(10):5522–7. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Crawford HC, Fingleton BM, Rudolph-Owen LA, Goss KJ, Rubinfeld B, Polakis P, et al. The metalloproteinase matrilysin is a target of beta-catenin transactivation in intestinal tumors. Oncogene. 1999;18(18):2883–91. doi: 10.1038/sj.onc.1202627. [DOI] [PubMed] [Google Scholar]
- 126.Koh TJ, Bulitta CJ, Fleming JV, Dockray GJ, Varro A, Wang TC. Gastrin is a target of the beta-catenin/TCF-4 growth-signaling pathway in a model of intestinal polyposis. The Journal of clinical investigation. 2000;106(4):533–9. doi: 10.1172/JCI9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kolligs FT, Nieman MT, Winer I, Hu G, Van Mater D, Feng Y, et al. ITF-2, a downstream target of the Wnt/TCF pathway, is activated in human cancers with beta-catenin defects and promotes neoplastic transformation. Cancer cell. 2002;1(2):145–55. doi: 10.1016/s1535-6108(02)00035-1. [DOI] [PubMed] [Google Scholar]
- 128.Araki Y, Okamura S, Hussain SP, Nagashima M, He P, Shiseki M, et al. Regulation of cyclooxygenase-2 expression by the Wnt and ras pathways. Cancer Res. 2003;63(3):728–34. [PubMed] [Google Scholar]
- 129.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochimica et biophysica acta. 2003;1653(1):1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 130.Lu Z, Hunter T. Wnt-independent beta-catenin transactivation in tumor development. Cell cycle. 2004;3(5):571–3. [PubMed] [Google Scholar]
- 131.Shimura T, Takenaka Y, Fukumori T, Tsutsumi S, Okada K, Hogan V, et al. Implication of galectin-3 in Wnt signaling. Cancer Res. 2005;65(9):3535–7. doi: 10.1158/0008-5472.CAN-05-0104. [DOI] [PubMed] [Google Scholar]
- 132.Mazurek N, Conklin J, Byrd JC, Raz A, Bresalier RS. Phosphorylation of the beta-galactoside-binding protein galectin-3 modulates binding to its ligands. J Biol Chem. 2000;275(46):36311–5. doi: 10.1074/jbc.M003831200. [DOI] [PubMed] [Google Scholar]
- 133.Shi Y, He B, Kuchenbecker KM, You L, Xu Z, Mikami I, et al. Inhibition of Wnt-2 and galectin-3 synergistically destabilizes beta-catenin and induces apoptosis in human colorectal cancer cells. International journal of cancer Journal international du cancer. 2007;121(6):1175–81. doi: 10.1002/ijc.22848. [DOI] [PubMed] [Google Scholar]
- 134.Song S, Mazurek N, Liu C, Sun Y, Ding QQ, Liu K, et al. Galectin-3 mediates nuclear beta-catenin accumulation and Wnt signaling in human colon cancer cells by regulation of glycogen synthase kinase-3beta activity. Cancer Res. 2009;69(4):1343–9. doi: 10.1158/0008-5472.CAN-08-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mendonca DF, Chammas R, Liu FT, Nonogaki S, Cardoso SV, Loyola AM, et al. The inactive form of glycogen synthase kinase-3beta is associated with the development of carcinomas in galectin-3 wild-type mice, but not in galectin-3-deficient mice. International journal of clinical and experimental pathology. 2012;5(6):547–54. [PMC free article] [PubMed] [Google Scholar]
- 136.Ferrazzo KL, Neto MM, dos Santos E, dos Santos Pinto D, de Sousa SO. Differential expression of galectin-3, beta-catenin, and cyclin D1 in adenoid cystic carcinoma and polymorphous low-grade adenocarcinoma of salivary glands. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2009;38(9):701–7. doi: 10.1111/j.1600-0714.2009.00776.x. [DOI] [PubMed] [Google Scholar]
- 137.Kim SJ, Shin JY, Cheong TC, Choi IJ, Lee YS, Park SH, et al. Galectin-3 germline variant at position 191 enhances nuclear accumulation and activation of beta-catenin in gastric cancer. Clinical & experimental metastasis. 2011;28(8):743–50. doi: 10.1007/s10585-011-9406-8. [DOI] [PubMed] [Google Scholar]
- 138.Balan V, Nangia-Makker P, Schwartz AG, Jung YS, Tait L, Hogan V, et al. Racial disparity in breast cancer and functional germ line mutation in galectin-3 (rs4644): a pilot study. Cancer Res. 2008;68(24):10045–50. doi: 10.1158/0008-5472.CAN-08-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Braeuer RR, Shoshan E, Kamiya T, Bar-Eli M. The sweet and bitter sides of galectins in melanoma progression. Pigment cell & melanoma research. 2012;25(5):592–601. doi: 10.1111/j.1755-148X.2012.01026.x. [DOI] [PubMed] [Google Scholar]
- 140.Fukumori T, Kanayama HO, Raz A. The role of galectin-3 in cancer drug resistance. Drug Resist Updat. 2007;10(3):101–8. doi: 10.1016/j.drup.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Harazono Y, Nakajima K, Raz A. Why anti-Bcl-2 clinical trials fail: a solution. Cancer Metastasis Rev. 2013 doi: 10.1007/s10555-013-9450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Turner JG, Dawson J, Sullivan DM. Nuclear export of proteins and drug resistance in cancer. Biochemical pharmacology. 2012;83(8):1021–32. doi: 10.1016/j.bcp.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cheng YL, Huang WC, Chen CL, Tsai CC, Wang CY, Chiu WH, et al. Increased galectin-3 facilitates leukemia cell survival from apoptotic stimuli. Biochemical and biophysical research communications. 2011;412(2):334–40. doi: 10.1016/j.bbrc.2011.07.099. [DOI] [PubMed] [Google Scholar]
- 144.Mazurek N, Byrd JC, Sun Y, Hafley M, Ramirez K, Burks J, et al. Cell-surface galectin-3 confers resistance to TRAIL by impeding trafficking of death receptors in metastatic colon adenocarcinoma cells. Cell death and differentiation. 2012;19(3):523–33. doi: 10.1038/cdd.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kobayashi T, Shimura T, Yajima T, Kubo N, Araki K, Tsutsumi S, et al. Transient gene silencing of galectin-3 suppresses pancreatic cancer cell migration and invasion through degradation of beta-catenin. International journal of cancer Journal international du cancer. 2011;129(12):2775–86. doi: 10.1002/ijc.25946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kobayashi T, Shimura T, Yajima T, Kubo N, Araki K, Wada W, et al. Transient silencing of galectin-3 expression promotes both in vitro and in vivo drug-induced apoptosis of human pancreatic carcinoma cells. Clinical & experimental metastasis. 2011;28(4):367–76. doi: 10.1007/s10585-011-9376-x. [DOI] [PubMed] [Google Scholar]
- 147.Lin CI, Whang EE, Abramson MA, Donner DB, Bertagnolli MM, Moore FD, Jr., et al. Galectin-3 regulates apoptosis and doxorubicin chemoresistance in papillary thyroid cancer cells. Biochemical and biophysical research communications. 2009;379(2):626–31. doi: 10.1016/j.bbrc.2008.12.153. [DOI] [PubMed] [Google Scholar]
- 148.Lin CI, Whang EE, Donner DB, Jiang X, Price BD, Carothers AM, et al. Galectin-3 targeted therapy with a small molecule inhibitor activates apoptosis and enhances both chemosensitivity and radiosensitivity in papillary thyroid cancer. Molecular cancer research : MCR. 2009;7(10):1655–62. doi: 10.1158/1541-7786.MCR-09-0274. [DOI] [PubMed] [Google Scholar]
- 149.Yamamoto-Sugitani M, Kuroda J, Ashihara E, Nagoshi H, Kobayashi T, Matsumoto Y, et al. Galectin-3 (Gal-3) induced by leukemia microenvironment promotes drug resistance and bone marrow lodgment in chronic myelogenous leukemia. Proc Natl Acad Sci U S A. 2011:17468–73. doi: 10.1073/pnas.1111138108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rebucci M, Michiels C. Molecular aspects of cancer cell resistance to chemotherapy. Biochemical pharmacology. 2013;85(9):1219–26. doi: 10.1016/j.bcp.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 151.Glinsky VV, Raz A. Modified citrus pectin anti-metastatic properties: one bullet, multiple targets. Carbohydrate research. 2009;344(14):1788–91. doi: 10.1016/j.carres.2008.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Inohara H, Raz A. Effects of natural complex carbohydrate (citrus pectin) on murine melanoma cell properties related to galectin-3 functions. Glycoconj J. 1994;11(6):527–32. doi: 10.1007/BF00731303. [DOI] [PubMed] [Google Scholar]
- 153.Nangia-Makker P, Hogan V, Honjo Y, Baccarini S, Tait L, Bresalier R, et al. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J Natl Cancer Inst. 2002;94(24):1854–62. doi: 10.1093/jnci/94.24.1854. [DOI] [PubMed] [Google Scholar]
- 154.Platt D, Raz A. Modulation of the lung colonization of B16-F1 melanoma cells by citrus pectin. J Natl Cancer Inst. 1992;84(6):438–42. doi: 10.1093/jnci/84.6.438. [DOI] [PubMed] [Google Scholar]
- 155.Johnson KD, Glinskii OV, Mossine VV, Turk JR, Mawhinney TP, Anthony DC, et al. Galectin-3 as a potential therapeutic target in tumors arising from malignant endothelia. Neoplasia. 2007;9(8):662–70. doi: 10.1593/neo.07433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang Y, Nangia-Makker P, Balan V, Hogan V, Raz A. Calpain activation through galectin-3 inhibition sensitizes prostate cancer cells to cisplatin treatment. Cell death & disease. 2010;1:e101. doi: 10.1038/cddis.2010.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chauhan D, Li G, Podar K, Hideshima T, Neri P, He D, et al. A novel carbohydrate-based therapeutic GCS-100 overcomes bortezomib resistance and enhances dexamethasone-induced apoptosis in multiple myeloma cells. Cancer Res. 2005;65(18):8350–8. doi: 10.1158/0008-5472.CAN-05-0163. [DOI] [PubMed] [Google Scholar]
- 158.Lee YK, Lin TH, Chang CF, Lo YL. Galectin-3 silencing inhibits epirubicin-induced ATP binding cassette transporters and activates the mitochondrial apoptosis pathway via beta-catenin/GSK-3beta modulation in colorectal carcinoma. PloS one. 2013;8(11):e82478. doi: 10.1371/journal.pone.0082478. [DOI] [PMC free article] [PubMed] [Google Scholar]