Abstract
This study was performed to review studies carried out in Korea reporting toxic reactions to traditional Chinese medicines (TCMs) as a result of heavy metal contamination. PubMed (1966-August 2013) and International Pharmaceutical Abstracts (1965-August 2013) were searched using the medical subject heading terms of "Medicine, Chinese Traditional," "Medicine, Korean Traditional," "Medicine, Traditional," "Metals, Heavy," and "Drug Contamination". For Korean literature, Korea Med (http://www.koreamed.org), the Korean Medical Database (http://kmbase.medric.or.kr), National Discovery for Science Leaders (www.ndsl.kr), Research Information Sharing Service (http://www.riss.kr), and Google Scholar were searched using the terms "Chinese medicine," "Korean medicine," "herbal medicine," and "metallic contamination" in Korean. Bibliographies of case reports and case series, identified using secondary resources, were also utilized. Only literature describing cases or studies performed in Korea were included. Case reports identified clear issues with heavy metal, particularly lead, contamination of TCMs utilized in Korea. No international standardization guidelines for processing, manufacturing and marketing of herbal products exist. Unacceptably high levels of toxic metals can be present in TCM preparations. Health care providers and patients should be educated on the potential risks associated with TCMs. International advocacy for stricter standardization procedures for production of TCMs is warranted.
Keywords: Medicine, Korean traditional, medicine, chinese traditional, metals, heavy
INTRODUCTION
The terms "oriental medicine," "eastern medicine," or "traditional Chinese medicine" (TCM) refer to a comprehensive medical practice developed in Asian countries. This practice, which can be traced back 3000 years, employs a philosophical and holistic approach to treating humans, based on the yin-yang, the five elements (wood, fire, earth, metal, and water), and employing massage, acupuncture, clinical diagnosis, and herbology.1,2 TCM makes use of both crude Chinese preparations of plant matter, animal parts, or minerals and manufactured Chinese proprietary medicines (CPMs), defined as "a medicinal product used for a therapeutic purpose".3
Globally, Eastern medicine has become a popular alternative therapy for preventing or curing disease. The World Health Organization (WHO) has reported its use by 50-90% of the population in some Asian countries in 2008.4 This has led to a significant increase in annual expenditure on traditional herbal medicine in some Asian countries. In Korea, annual expenditure on traditional medicine increased from US$ 4.4 billion in 2004 to US$ 7.4 billion in 2009.4
The high prevalence of Eastern medicine use has led to safety concerns, especially in relation to herbal medicines. These include poor quality control and adulteration of herbal preparations, which can expose patients to unexpected risk. TCM may contain toxic contaminants such as heavy metals, pesticides or other harmful materials that become part of the pharmaceutical preparation during the process of growing, collecting, and manufacturing. Contamination of soil, water, and air directly leads to contamination of plants and herbal preparations, and high levels of heavy metals such as lead, mercury, and arsenic, which have been observed in some TCM and Indian herbal medicine preparations.3,5,6,7
This article reviews studies carried out in Korea in which adverse events due to metal contamination of TCMs were reported. Because Koreans consume large amounts of TCMs, with an estimated 86% having consumed some form of TCM according to a National survey in 2008,4,8 this has led to numerous safety concerns. This review aims to provide metal contamination risks in TCMs used in Korea and to offer a perspective on regulation of these products, both in Korea and in the United States of America (USA).
LITERATURE REVIEW METHODS
PubMed (1966-July 2013) and International Pharmaceutical Abstracts (1965-July 2013) were searched using the medical subject heading terms "Medicine, Chinese Traditional," "Medicine, Korean Traditional," "Medicine, Traditional", "Metals, Heavy," and "Drug Contamination" For Korean literature, Korea Med (http://www.koreamed.org), the Korean Medical Database (http://kmbase.medric.or.kr), National Discovery for Science Leaders (www.ndsl.kr), Research Information Sharing Service (http://www.riss.kr), and Google Scholar were used to search the terms "Chinese medicine," "Korean medicine," "herbal medicine," and "metallic contamination" in Korean. Bibliographies of case reports and case series, identified using secondary resources, were also utilized. We only included literature describing adverse events in humans associated with metal contamination of TCM and studies performed in Korea.
RESULTS
In total, 10 publications were retrieved and included in this review. The publications identified are described in more detail below.
In one study investigating 45 cases of lead poisoning in Korea from 1973 to 2002, TCM was the most frequent cause of non-occupational lead poisoning.9 In this study, the average duration of TCM intake was 7.3±3.8 months (ranging from 3 days to 4 years). When TCMs were analyzed for lead content, the average daily lead intake from TCMs was found to be in the range of 5 mg to 3 g. According to the Joint Food and Agriculture Organization and WHO Expert Committee on Food Additives in 1986, the Provisional Tolerable Weekly Intake for lead from all sources is 25 mcg per kilogram of body weight.9 Therefore, lead intake from TCM in this study was 3-4000 times higher than the recommended amount. Most patients (82.2%) in this study complained of abdominal pain as a result of lead intoxication in TCMs. Other symptoms included headache, vomiting, and constipation. The most frequent laboratory finding was mild anemia. Elevated reticulocyte count and bilirubin, aspartate aminotransferase, and alanine transaminase levels were also observed. Levels of 24-h urinary lead were available in 31 patients, and the mean value was 547.0±183.8 mcg/L (normal, <150 mcg/L). Levels of 24-h urinary δ-aminolevulinic acid (ALA) were available in 22 patients, and the mean value was 44.2±10.9 mg/L (normal, <19 mg/L). ALA is involved in porphyrin synthesis, and 24-h urinary δ-ALA level is generally elevated in patients with lead poisoning.10
A total of 9 Korean case reports or case series (including descriptions of 22 cases in total) of metal poisoning by TCM were identified. These are listed in chronological order in Table 1. Twenty patients had lead poisoning, one had arsenic poisoning, and one had cadmium poisoning. All patients with lead poisoning were admitted to a hospital because of complaints of abdominal pain and gastrointestinal (GI) symptoms. Laboratory results indicated anemia with basophilic stippling of red blood cells (RBCs), which is most commonly associated with lead poisoning. Metal levels in blood and urine were measured, or 24-h urinary δ-ALA levels in cases of lead intoxication, identifying levels that were higher than the reference range.
Table 1.
Cases of Toxic Heavy Metal Ingestion Associated with Traditional Chinese Medicines (TCM) Use in Korean Literature
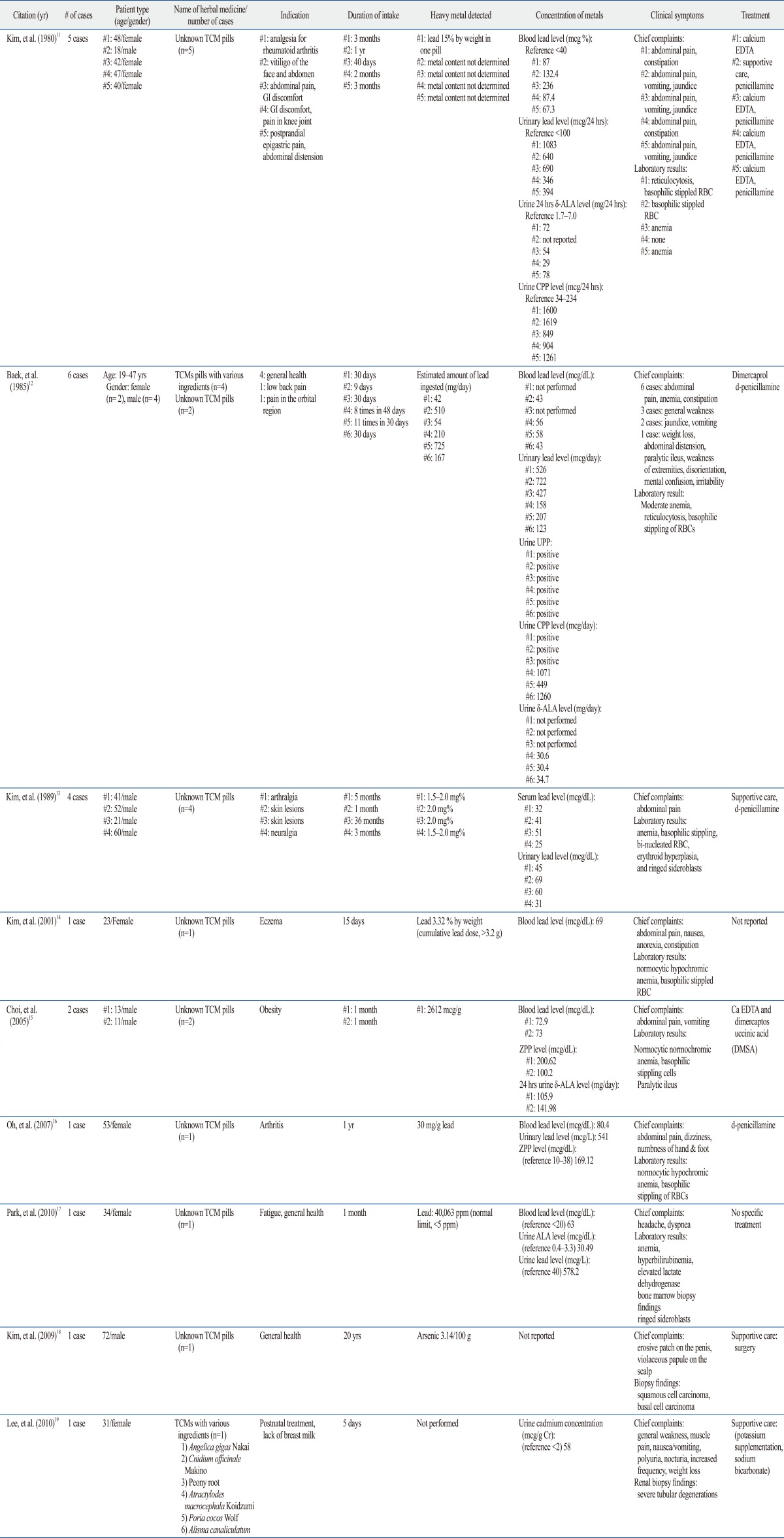
Ca EDTA, calcium disodium edetate; δ-ALA, δ-aminolevulinic acid; CPP, coproporphyrin; UPP, uroporphyrin; ZPP, zinc protoporphyrin; GI, gastrointestinal; RBC, red blood cells.
Kim, et al.11 reported 5 cases of lead poisoning due to ingestion of unidentified TCM. In these cases, the patients had taken pills to relieve epigastric pain (3 cases), joint pain (1 case), and vitiligo (1 case). All patients presented with abdominal pain, vomiting, and constipation. Their peripheral blood test results showed mild anemia, reticulocytosis, and basophilic stippling. In one medicine, lead was present at a level of 15% by weight. Furthermore, all patients had elevated blood and urinary lead levels, with elevation of δ-ALA and coproporphyrin (CPP) in 24-h urine. Porphyrins such as CPP, uroporphyrin (UPP), and protoporphyrin are a group of compounds involved in hemoglobin formation that are usually elevated in lead poisoning.10
Baek, et al.12 reported 6 cases of lead poisoning after ingestion of TCM. In these cases, the patients had received the medicines for general health care or analgesia. All 6 patients complained of abdominal pain and constipation. Their laboratory and bone marrow examinations revealed anemia with basophilic stippling, binucleated RBC, erythroid hyperplasia, and ringed sideroblasts. Lead was detected at a level of 9.2-256 mg per pill, and the average estimated amount of lead ingested through TCM was 285 mg/day (range, 42-725 mg/day). Elevated lead levels were observed in blood and urine. Additionally, urinary CPP, UPP, and 24-h urine δ-ALA were elevated, reflecting lead intoxication.
Kim, et al.13 also reported 4 cases of lead poisoning caused by TCM. All patients complained of acute colicky abdominal pain and exhibited anemia with basophilic stippling of RBC. Serum lead levels were elevated and ranged from 25-51 mcg/dL (normal, <20 mcg/dL). Urinary lead levels were also elevated and ranged from 31-69 mcg/dL (normal, <4 mcg/dL).
Kim, et al.14 reported a case of a 23-year-old woman admitted to a hospital with complaints of abdominal pain and constipation. This patient reported taking unknown TCM pills for 15 days to treat eczema. Laboratory examinations showed normocytic, hypochromic anemia with basophilic stippling. Blood lead levels were elevated to 69 mcg/dL, and the herbal pills contained 3.32% lead, indicating that the patient had ingested a cumulative lead dose of over 3.2 g.
Choi, et al.15 reported 2 cases of pediatric lead poisoning. Two children aged 11 and 13 years took unidentified TCM for weight loss. They took 30 pills a day for one month, which resulted in 13 kg of weight loss. The children experienced severe epigastric and abdominal pain with vomiting for 3 weeks (child 1) and for 1 week (child 2). Blood smears revealed normocytic, normochromic anemia, and basophilic stippling. Blood lead levels were elevated to 73 mcg/dL in both patients. Furthermore, urinary zinc protoporphyrin (ZPP) and δ-ALA levels were elevated in both cases. ZPP levels are directly associated with blood lead concentrations and have been used both as a screening and diagnostic test for lead poisoning.10 When the amount of lead in the children's TCM was assayed, the lead concentration was found to be 2612 mcg/g. This is a very high amount, as the tolerance limit for lead in such preparations is 30 mcg/g.
Oh, et al.16 reported a case of a 53-year-old woman admitted to the hospital complaining of abdominal pain, dizziness, and numbness of her hands and feet. She had taken unidentified TCM for 1 year to treat arthritis. Laboratory examinations showed normocytic hypochromic anemia with basophilic stippling of RBC. Blood and urinary lead levels were elevated to 80 mcg/dL and 541 mcg/dL, respectively. Urinary ZPP was also elevated. Analysis of the pills involved revealed that they contained 30 mg/g lead, even though regulations in Korea do not allow more than 5 mg/kg lead in herbal medicines.
Park, et al.17 reported a case of a 34-year-old woman with sideroblastic anemia. The patient was admitted to a hospital with abdominal pain and headache. She was found to have been taking unidentified TCM pills for 1 month. A peripheral blood smear showed polychromasia and RBCs with basophilic stippling. Bone marrow biopsy showed ring sideroblasts. The patient's blood lead level was elevated to 63 mcg/dL. Urinary δ-ALA and lead levels were also elevated to 30.49 mg/L (normal, 0.4-3.3 mg/L) and 578.2 g/L, respectively. The lead content of the herbal pills was 40063 ppm, 8000 times higher than that permitted by regulations in Korea (<5 ppm).17
There were 2 cases of poisoning with metals other than lead, namely arsenic and cadmium. In the case involving arsenic, a 72-year-old man was admitted to a hospital with a painful, erosive patch on his penis and a violaceous papule on his scalp.18 The patient had a medical history of prostate cancer for which he had received chemotherapy and radiotherapy 3 years prior to this admission, but he did not have any scars or abnormal signs on his penis or scalp at the time of his cancer treatment. Other skin regions appeared normal. The patient had been taking unidentified TCM for 20 years. Analysis of these pills revealed 31.4 mg of arsenic/kg, although Korean regulations do not allow more than 3 mg/kg arsenic in herbal medicines. An excisional biopsy specimen from the patient's penis showed squamous cell carcinoma in situ. Biopsy of his occipital scalp showed basal cell carcinoma.
Lee, et al.19 reported a case of a 31-year-old woman with cadmium-induced acute renal failure. The patient had been taking TCM for 5 days before developing general weakness and muscle pain. Laboratory results revealed hypophosphatemia, hypouricemia, hypokalemia, and metabolic acidosis with an increased anion gap. Serum creatinine level increased from 1.2 to 3.2 mg/dL over 8 months. Renal ultrasonography showed enlarged kidneys with a highly echogenic cortex and prominent medullary pyramids. Renal biopsy showed severe tubular degeneration, confirming a diagnosis of Fanconi syndrome. The patient's urinary cadmium concentration was 58 mcg/g, 29 times higher than the normal reference range. The authors concluded that an herbal medication containing cadmium could have caused this renal damage.
Management of lead poisoning related to TCM includes discontinuation of the medicine and supportive care based on the patient's symptoms. Chelation therapy can be used to eliminate some specific metals. Dimercaprol (also known as British anti-Lewisite), calcium disodium edetate (CaNa2 EDTA), and oral chelators such as d-penicillamine and succimer can be used in cases of lead or arsenic poisoning. However, there is no evidence for beneficial effects of chelating therapy in cases of cadmium poisoning.20,21,22
DISCUSSION
The present study identified reports of Korean individuals with serious symptoms of heavy metal toxicity following TCM usage. A total of 9 case reports with 22 cases in total could be directly attributed to the medications ingested, since analyses showed unacceptably high levels of heavy metal in these products. GI symptoms such as abdominal pain, anorexia, nausea, constipation, and anemia with basophilic stippling are common signs and symptoms of lead intoxication, and were also present in patients using TCMs in this review. Because these signs and symptoms of metal contamination associated with TCMs were non-specific,22,23 assessment was delayed and sometimes involved unnecessary testing, such as bone marrow biopsies, as shown in this review. This finding indicated the importance of obtaining a careful medical history of patients, including TCM administration. Patients were treated with supportive care and most symptoms disappeared upon discontinuation of TCM. However, one individual with TCM-associated arsenic intoxication developed squamous cell carcinoma in his penis and basal cell carcinoma in his scalp, which required surgery.
Consistent with Korean literature, studies published in other countries have also identified lead as a major contaminant of TCMs.3,4,24,25,26 One review of reports of excessive toxic heavy metals in CPM in Singapore over 8 years found 42 out of 2080 CPM contained mercury, arsenic, lead, and copper in excessive amounts of the legal limits.3 Mercury is the most commonly detected excessive heavy metal, representing 66.7% of the 42 CPMs. Lead (19%), arsenic (16.7%), and copper (2.4%) were also detected as excessive heavy metals. Garvey, et al.24 acquired a random sample of traditional Asian medicines from China, Vietnam, and the USA and evaluated the products for lead, arsenic, and mercury content. In total, 54 products were acquired and prepared for analysis. A 0.5 g sample of each product was analyzed and the resulting heavy metal concentrations were mathematically projected to calculate the daily dose. These results indicated that 49% to 75% of the TCMs contained heavy metals. Lead was the most commonly identified heavy metal in the sample collected, as 60% of the tested products delivered a daily dose of 300 mg. Moreover, 15% of the sampled products were found to contain toxic doses of multiple heavy metals. In a study conducted by the California Department of Health Services to screen 2609 samples of 260 imported CPMs, 17 products contained undeclared pharmaceuticals and 34 contained at least 10 ppm lead.5
Although these articles present clear evidence that processed herbs, including CPM products, are at risk for heavy metal contamination, very little is known about the risk of such contamination in raw, and unprocessed Chinese herbs. Harris, et al.27 collaborated with Chinese investigators to conduct federally funded research to analyze heavy metals and pesticides in commonly prescribed raw herbs from a wide range of geographical areas in China. In this study, 334 samples of raw herbs (representing 126 individual species) were collected and examined in duplicate for arsenic, cadmium, chromium, lead, and mercury. Additionally, 294 samples (representing 112 species) were tested for pesticides, including the organophosphate insecticide, chlorpyrifos. Investigators found that 100% of these samples contained at least one reference metal and 34% of the samples contained all the metals tested. Furthermore, 42 different pesticides, half of which are not registered chemicals in the US, were detected in 108 of the 294 samples (36.7%). The number of pesticide contaminants identified per sample ranged from 1 to 9. If these preparations were ingested chronically, 231 samples (69.2%) with heavy metals and 81 samples (28%) with pesticides could contribute to elevated levels of exposure. Herbs may become adulterated with pesticides via growth in contaminated soils, storage in contaminated containers, or irrigation with contaminated water.5 Wild plants harvested for this study (representing 37.1% of the samples) had higher contaminant concentrations than cultivated (farmed) plants. Furthermore, geographical variations in the natural mineral content of the plant sources did not contribute to variations in heavy metal or pesticide concentrations in the samples (r2 <0.1).
Koreans widely consider TCM treatments to be harmless since they are of a natural origin and have been used historically without any serious adverse effects. Some patients also view them as nutritional supplements, which they are willing to take for several months, regardless of their disease state, believing they can improve energy or boost immunity. Patients believe that any adverse effects of TCM are because of a mismatch between one's body type and the herbal material used, instead of a direct result of herbal preparation ingestion. For this reason, harmful effects of TCMs are often under-recognized and under-reported. The present study identified reports of serious heavy metal toxicity in only a very low percentage of the large number of people regularly taking TCMs in Korea. However, considering the under-detection and under-reporting of adverse TCM effects, along with the frequency of TCM contamination identified in the studies described above, the prevalence of heavy metal toxicity may be greater than what is reflected in these 9 identified Korean studies. In the absence of adequate product quality, purity, or standardization assurances prior to marketing TCM, there is no guarantee of safety or efficacy.
Recognition of the potential dangers of TCMs has led to the establishment of supervision and regulation of quality control by the Korean government, enforced by the Korean Food and Drug Administration (KFDA). Korean regulations issued in 1995 stated that the total heavy metal content of medicinal plants should not exceed 30 ppm.28 This guidance was modified in 2008 to account for the toxicity of individual heavy metals. For example, TCMs should not contain more than 5 mg/kg lead, 3 mg/kg arsenic, 0.2 mg/kg mercury, or 0.3 mg/kg cadmium. Also, The Korean Herbal Pharmacopoeia specifies each limit for heavy metals and pesticides in individual herbal medicines. Because of these strict regulations on quality control of TCMs, the frequency of adverse events from contamination in TCMs has been reduced. However, as seen in this review, adverse events arising from contaminated TCMs have been reported recently, emphasizing the importance of continuous and careful monitoring of TCM safety. Like the MedWatch program in the USA, the KFDA disseminates information regarding TCM recalls or withdrawals, both in Korea and in other countries via its website (http://www.kfda.go.kr).28 Over the last 5 years from 2007 to 2011, 631 TCMs have been banned from the Korean market because of safety concerns. The absence of a labeled active ingredient (i.e., misbranding) led to withdrawal of 103 (16.3%) of these TCMs. Furthermore, contamination with sulfur dioxide (SO2), heavy metals, pesticides, or biological growth (such as fungi or bacteria) led to the withdrawal of 282 (44.7%) of the TCMs. Fifty-five percent (n=154) were contaminated with SO2, 32.4% (n=66) with cadmium, and 7.4% (n=21) with lead, which may have the possibility of causing adverse events of TCM in future.
Guidelines issued in the USA by the FDA Office of Compliance (updated in 2010) offer recommendations regarding the marketing and sale of homeopathic drugs, a category which includes TCMs.29 The FDA stated that drugs or preparations recognized in the Homeopathic Pharmacopeia of the United States (HPUS) would be subject to regulation in accordance with the Dietary Supplement Health and Education Act of 1994, which ensures that Current Good Manufacturing Practices and United States Pharmacopeia (USP) standards are followed.29 The USP, based upon FDA, Canadian and European Medicines Agency standards, has further proposed the limitation of the permissible daily exposure to heavy metals from all ingestible sources to be 10 mcg/day, 2 mcg/day or 15 mcg/day for each 50 kg of body weight for lead, methyl mercury, and arsenic, respectively.30 The HPUS contains approximately 1300 drugs and all ingredients plus manufacturing standards for all recognized drugs and dosage forms.31 The FDA is prompt to issue consumer and practitioner guidance through the MedWatch system regarding any problems with prescription or non-prescription drug supply, including contaminated products from overseas. Unfortunately, illegal and unregistered products could potentially penetrate US borders from foreign nations.
Unacceptably high levels of toxic metals or pesticides can be present in TCM preparations. Addressing these issues by using standardized processes and product validation by an appropriate third-party agency may optimize patient safety and improve clinical outcomes for patients seeking treatment with herbal medicines. Other regulatory approaches have been proposed by Genuis, et al.,32 who conclude that regulatory oversight would be heavily contested by the TCM and nutraceutical industries. Rather, a system of self-regulation with adjunctive governmental regulatory oversight could be more effective to meet the goal of providing safe and effective products for public consumption. Transparent reporting of ingredient origin, country of manufacture, as well as contaminant test results would provide an environment of transparency that would allow both practitioners and patients to select the most appropriate therapies.32 Internal controls including batch-to-batch comparisons for consistency and purity as well as a system of accreditation for sources of botanical- or animal-based ingredients are just a few of several ideas to promote increased product quality. In this spirit, urgent engagement from governments and regulatory authorities to ensure that TCMs and other natural products are correctly labeled and are free from harmful contaminants is warranted. If jurisdiction of authority becomes problematic for some regulatory bodies, importation of finished products from foreign sources could be banned while still allowing for regulated importation of raw ingredients (if domestic cultivation cannot occur) with final production to be conducted domestically.
LIMITATION
The Korean reports of heavy metal contamination in TCM identified in this study were case reports, not randomized clinical trials. In this context, case reports may not account for chronic exposure to heavy metals due to one's occupation, living environment or dietary intake.
CONCLUSIONS
Unacceptably high levels of toxic metals can be present in TCM preparations. The global TCM industry needs to address a host of regulatory and production issues that are negatively affecting patient safety. Health care providers should be educated on the potential risks associated with TCMs and patients should also be made aware of the potential safety concerns and encouraged to speak with their healthcare providers before starting any supplement. Further research is warranted to evaluate TCM and CPM production from cultivation and harvest to the finished product, in concert with an increased emphasis on producing a global alliance for quality assurance and surveillance of contaminants in products containing herbal and other natural materials.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Xu J, Yang Y. Traditional Chinese medicine in the Chinese health care system. Health Policy. 2009;90:133–139. doi: 10.1016/j.healthpol.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu CX. Development of Chinese medicine based on pharmacology and therapeutics. J Ethnopharmacol. 1987;19:119–123. doi: 10.1016/0378-8741(87)90035-3. [DOI] [PubMed] [Google Scholar]
- 3.Koh HL, Woo SO. Chinese proprietary medicine in Singapore: regulatory control of toxic heavy metals and undeclared drugs. Drug Saf. 2000;23:351–362. doi: 10.2165/00002018-200023050-00001. [DOI] [PubMed] [Google Scholar]
- 4.The regional strategy for traditional medicine in the Western Pacific (2011-2020) Manila: World Health Organization, Regional Office for the Western Pacific; 2002. [accessed on 2013 December 20]. Available at: http://www.wpro.who.int/publications/2012/regionalstrategyfortraditionalmedicine_2012.pdf. [Google Scholar]
- 5.Ernst E. Toxic heavy metals and undeclared drugs in Asian herbal medicines. Trends Pharmacol Sci. 2002;23:136–139. doi: 10.1016/S0165-6147(00)01972-6. [DOI] [PubMed] [Google Scholar]
- 6.Tomlinson B, Chan TY, Chan JC, Critchley JA, But PP. Toxicity of complementary therapies: an eastern perspective. J Clin Pharmacol. 2000;40:451–456. doi: 10.1177/00912700022009206. [DOI] [PubMed] [Google Scholar]
- 7.Winslow LC, Kroll DJ. Herbs as medicines. Arch Intern Med. 1998;158:2192–2199. doi: 10.1001/archinte.158.20.2192. [DOI] [PubMed] [Google Scholar]
- 8.Shin HK, Jeong SJ, Lee MS, Ernst E. Adverse events attributed to traditional Korean medical practices: 1999-2010. Bull World Health Organ. 2013;91:569–575. doi: 10.2471/BLT.12.111609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JY, Kim JH, Kim HW, Roh JH, Lee KH, Cheon BC, et al. A review of lead poisoning cases reported for recent 30 years in Korea. Korean J Med. 2004;66:617–624. [Google Scholar]
- 10.Sakai T. Biomarkers of lead exposure. Ind Health. 2000;38:127–142. doi: 10.2486/indhealth.38.127. [DOI] [PubMed] [Google Scholar]
- 11.Kim YS, Park YG, Shu WJ, Kim MJ, Bae JH, Chi HS. Clinical study on lead poisoning. Korean J Intern Med. 1980;23:707–713. In Korean. [Google Scholar]
- 12.Baek IK, Kim WT, Choi MR, Kwon SO, Yu SI, Shin KC, et al. Acute lead intoxication caused by Chinese Traditional Pills. Korean J Intern Med. 1985;29:763–770. In Korean. [Google Scholar]
- 13.Kim SS, Park SK, Han YB, Han DS, Huh MH. Lead poisoning in Korean adults caused by herbal medicine-report of 4 cares. Korean J Hematol. 1989;24:203–209. [Google Scholar]
- 14.Kim DS, Lim HS, Yang CH. Case report of a lead poisoning by home-made herb pills. Korean J Rural Med. 2001;26:57–64. [Google Scholar]
- 15.Choi SH, Park EY, Shim JY, Kim DS, Shim JW, Jung HL, et al. Two cases of lead poisoning due to herb medicinal pills. Korean J Pediatr. 2005;48:1009–1015. [Google Scholar]
- 16.Oh SW, Lee HJ, Chae HJ, Lee SK, Moon JD, Cho D. A case of lead poisoning after ingestion of herb pills. Korean J Occup Environ Med. 2007;19:231–237. [Google Scholar]
- 17.Park HS, Kim SY, Cho JH, Moon HW, Yoon SY, Cho YH, et al. A case of sideroblastic anemia caused by lead-containing herbal medication. Korean J Med. 2010;79:448–452. [Google Scholar]
- 18.Kim BJ, Kim SY, Kim GM. A case of multiple SCCs in situ and BCC induced probably by chronic exposure to arsenic-containing pills (Hwan-Yak) Korean J Dermatol. 2009;47:1071–1073. [Google Scholar]
- 19.Lee EY, Shin HS, Jung YS, Chun BK, Rim H. A case of rapidly progressive renal failure induced by cadmium intoxication. Korean J Med. 2010;78:761–765. [Google Scholar]
- 20.Klaassen CD. Heavy metals and heavy metal antagonists. In: Hardman JG, Limbird LE, editors. The Pharmacological Basis of Therapeutics. 10th ed. New York: Macmillan; 2001. pp. 1851–1875. [Google Scholar]
- 21.American Academy of Pediatrics Committee on Drugs. Treatment guidelines for lead exposure in children. Pediatrics. 1995;96(1 Pt 1):155–160. [PubMed] [Google Scholar]
- 22.Wang EE, Mahajan N, Wills B, Leikin J. Successful treatment of potentially fatal heavy metal poisonings. J Emerg Med. 2007;32:289–294. doi: 10.1016/j.jemermed.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 23.Brodkin E, Copes R, Mattman A, Kennedy J, Kling R, Yassi A. Lead and mercury exposures: interpretation and action. CMAJ. 2007;176:59–63. doi: 10.1503/cmaj.060790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garvey GJ, Hahn G, Lee RV, Harbison RD. Heavy metal hazards of Asian traditional remedies. Int J Environ Health Res. 2001;11:63–71. doi: 10.1080/09603120020019656. [DOI] [PubMed] [Google Scholar]
- 25.Ernst E. Heavy metals in traditional Indian remedies. Eur J Clin Pharmacol. 2002;57:891–896. doi: 10.1007/s00228-001-0400-y. [DOI] [PubMed] [Google Scholar]
- 26.Lynch E, Braithwaite R. A review of the clinical and toxicological aspects of 'traditional' (herbal) medicines adulterated with heavy metals. Expert Opin Drug Saf. 2005;4:769–778. doi: 10.1517/14740338.4.4.769. [DOI] [PubMed] [Google Scholar]
- 27.Harris ES, Cao S, Littlefield BA, Craycroft JA, Scholten R, Kaptchuk T, et al. Heavy metal and pesticide content in commonly prescribed individual raw Chinese Herbal Medicines. Sci Total Environ. 2011;409:4297–4305. doi: 10.1016/j.scitotenv.2011.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korean Food and Drug Administration (KFDA) website. [accessed on 2013 December 11]. Available at: http://www.kfda.go.kr.
- 29.Food and Drug Administration Office of Compliance Website. Inspections, Compliance, Enforcement, and Criminal Investigations. [accessed on 2013 December 11]. Available at: http://www.fda.gov/iceci/compliancemanuals/compliancepolicyguidancemanual/ucm074360.htm.
- 30.United States Pharmacopeia Advisory Panel on Metal Impurities. [accessed on 2013 December 11]. Available at: http://www.usp.org/sites/default/files/usp_pdf/EN/USPNF/key-issues/2009-04-22MetalImpuritiesToxChart.pdf.
- 31.Homeopathic Pharmacopeia of the United States website. [accessed on 2013 December 11]. Available at: http://www.hpus.com/overview5.php.
- 32.Genuis SJ, Schwalfenberg G, Siy AK, Rodushkin I. Toxic element contamination of natural health products and pharmaceutical preparations. PLoS One. 2012;7:e49676. doi: 10.1371/journal.pone.0049676. [DOI] [PMC free article] [PubMed] [Google Scholar]


