Abstract
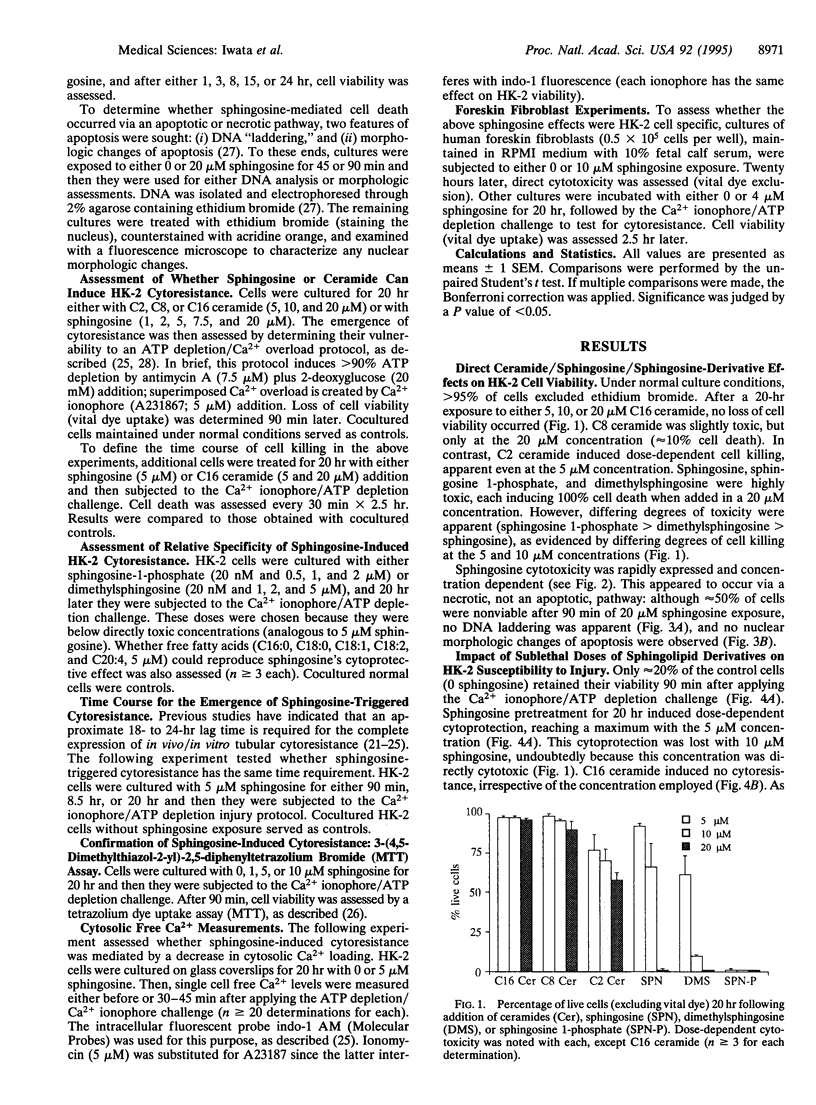
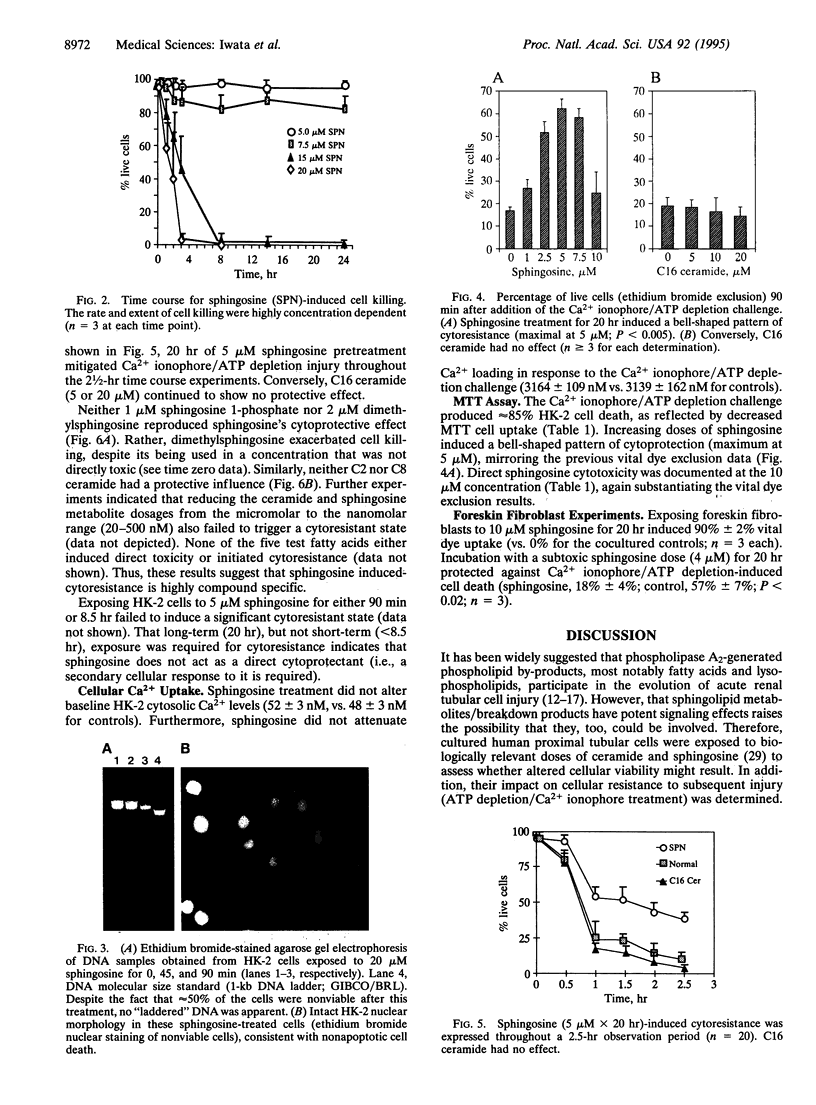
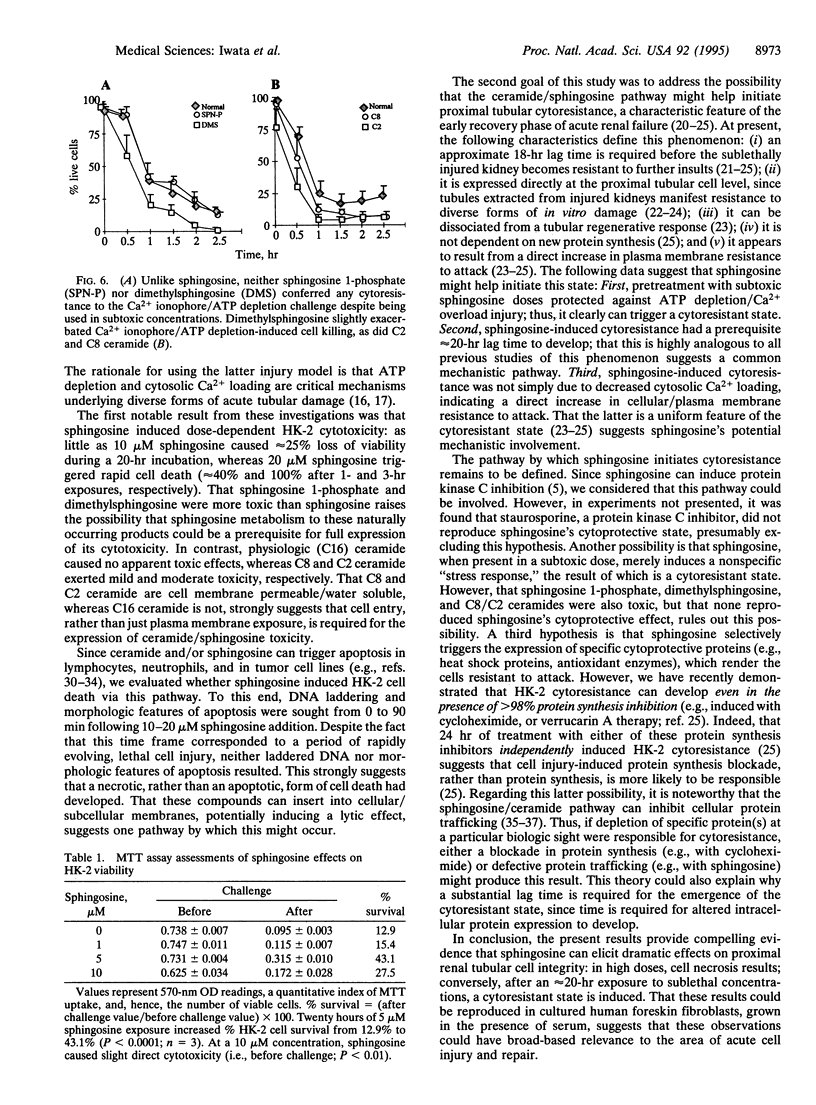
The goal of this study was to determine whether sphingosine and ceramide, second messengers derived from sphingolipid breakdown, alter kidney proximal tubular cell viability and their adaptive responses to further damage. Adult human kidney proximal tubular (HK-2) cells were cultured for 0-20 hr in the presence or absence of sphingosine, sphingosine metabolites (sphingosine 1-phosphate, dimethylsphingosine), or C2, C8, or C16 ceramide. Acute cell injury was assessed by vital dye exclusion and tetrazolium dye transport. Their subsequent impact on superimposed ATP depletion/Ca2+ ionophore-induced damage was also assessed. Sphingosine (> or = 10 microM), sphingosine 1-phosphate, dimethylsphingosine, and selected ceramides (C2 and C8, but not C16) each induced rapid, dose-dependent cytotoxicity. This occurred in the absence of DNA laddering or morphologic changes of apoptosis, suggesting a necrotic form of cell death. Prolonged exposure (20 hr) to subtoxic sphingosine doses (< or = 7.5 microM) induced substantial cytoresistance to superimposed ATP depletion/Ca2+ ionophore-mediated damage. Conversely, neither short-term sphingosine treatment (< or = 8.5 hr) nor 20-hr exposures to any of the above sphingosine/ceramide derivatives/metabolites or various free fatty acids reproduced this effect. Sphingosine-induced cytoresistance was dissociated from the extent of cytosolic Ca2+ loading (indo-1 fluorescence), indicating a direct increase in cell resistance to attack. We conclude that sphingosine can exert dual effects on proximal renal tubular viability: in high concentrations it induces cell necrosis, whereas in low doses it initiates a cytoresistant state. These results could be reproduced in human foreskin fibroblasts, suggesting broad-based relevance to the area of acute cell injury and repair.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dobrowsky R. T., Kamibayashi C., Mumby M. C., Hannun Y. A. Ceramide activates heterotrimeric protein phosphatase 2A. J Biol Chem. 1993 Jul 25;268(21):15523–15530. [PubMed] [Google Scholar]
- Dressler K. A., Mathias S., Kolesnick R. N. Tumor necrosis factor-alpha activates the sphingomyelin signal transduction pathway in a cell-free system. Science. 1992 Mar 27;255(5052):1715–1718. doi: 10.1126/science.1313189. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Bifunctional role of glycosphingolipids. Modulators for transmembrane signaling and mediators for cellular interactions. J Biol Chem. 1990 Nov 5;265(31):18713–18716. [PubMed] [Google Scholar]
- Hakomori S., Igarashi Y. Gangliosides and glycosphingolipids as modulators of cell growth, adhesion, and transmembrane signaling. Adv Lipid Res. 1993;25:147–162. [PubMed] [Google Scholar]
- Hannun Y. A., Bell R. M. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science. 1989 Jan 27;243(4890):500–507. doi: 10.1126/science.2643164. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A., Loomis C. R., Merrill A. H., Jr, Bell R. M. Sphingosine inhibition of protein kinase C activity and of phorbol dibutyrate binding in vitro and in human platelets. J Biol Chem. 1986 Sep 25;261(27):12604–12609. [PubMed] [Google Scholar]
- Hannun Y. A. The sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem. 1994 Feb 4;269(5):3125–3128. [PubMed] [Google Scholar]
- Honda N., Hishida A., Ikuma K., Yonemura K. Acquired resistance to acute renal failure. Kidney Int. 1987 Jun;31(6):1233–1238. doi: 10.1038/ki.1987.136. [DOI] [PubMed] [Google Scholar]
- Humes H. D., Nguyen V. D., Cieslinski D. A., Messana J. M. The role of free fatty acids in hypoxia-induced injury to renal proximal tubule cells. Am J Physiol. 1989 Apr;256(4 Pt 2):F688–F696. doi: 10.1152/ajprenal.1989.256.4.F688. [DOI] [PubMed] [Google Scholar]
- Igarashi Y., Hakomori S., Toyokuni T., Dean B., Fujita S., Sugimoto M., Ogawa T., el-Ghendy K., Racker E. Effect of chemically well-defined sphingosine and its N-methyl derivatives on protein kinase C and src kinase activities. Biochemistry. 1989 Aug 22;28(17):6796–6800. doi: 10.1021/bi00443a002. [DOI] [PubMed] [Google Scholar]
- Iwata M., Herrington J., Zager R. A. Protein synthesis inhibition induces cytoresistance in cultured human proximal tubular (HK-2) cells. Am J Physiol. 1995 Jun;268(6 Pt 2):F1154–F1163. doi: 10.1152/ajprenal.1995.268.6.F1154. [DOI] [PubMed] [Google Scholar]
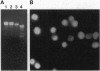
- Iwata M., Myerson D., Torok-Storb B., Zager R. A. An evaluation of renal tubular DNA laddering in response to oxygen deprivation and oxidant injury. J Am Soc Nephrol. 1994 Dec;5(6):1307–1313. doi: 10.1681/ASN.V561307. [DOI] [PubMed] [Google Scholar]
- Jaffrézou J. P., Herbert J. M., Levade T., Gau M. N., Chatelain P., Laurent G. Reversal of multidrug resistance by calcium channel blocker SR33557 without photoaffinity labeling of P-glycoprotein. J Biol Chem. 1991 Oct 15;266(29):19858–19864. [PubMed] [Google Scholar]
- Jarvis W. D., Kolesnick R. N., Fornari F. A., Traylor R. S., Gewirtz D. A., Grant S. Induction of apoptotic DNA damage and cell death by activation of the sphingomyelin pathway. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):73–77. doi: 10.1073/pnas.91.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. Y., Linardic C., Obeid L., Hannun Y. Identification of sphingomyelin turnover as an effector mechanism for the action of tumor necrosis factor alpha and gamma-interferon. Specific role in cell differentiation. J Biol Chem. 1991 Jan 5;266(1):484–489. [PubMed] [Google Scholar]
- Kubota M., Kitahara S., Shimasaki H., Ueta N. Accumulation of ceramide in ischemic human brain of an acute case of cerebral occlusion. Jpn J Exp Med. 1989 Apr;59(2):59–64. [PubMed] [Google Scholar]
- Linardic C. M., Jayadev S., Hannun Y. A. Brefeldin A promotes hydrolysis of sphingomyelin. J Biol Chem. 1992 Jul 25;267(21):14909–14911. [PubMed] [Google Scholar]
- Mathias S., Dressler K. A., Kolesnick R. N. Characterization of a ceramide-activated protein kinase: stimulation by tumor necrosis factor alpha. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10009–10013. doi: 10.1073/pnas.88.22.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthys E., Patel Y., Kreisberg J., Stewart J. H., Venkatachalam M. Lipid alterations induced by renal ischemia: pathogenic factor in membrane damage. Kidney Int. 1984 Aug;26(2):153–161. doi: 10.1038/ki.1984.149. [DOI] [PubMed] [Google Scholar]
- Nguyen V. D., Cieslinski D. A., Humes H. D. Importance of adenosine triphosphate in phospholipase A2-induced rabbit renal proximal tubule cell injury. J Clin Invest. 1988 Sep;82(3):1098–1105. doi: 10.1172/JCI113666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeid L. M., Linardic C. M., Karolak L. A., Hannun Y. A. Programmed cell death induced by ceramide. Science. 1993 Mar 19;259(5102):1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- Ohta H., Sweeney E. A., Masamune A., Yatomi Y., Hakomori S., Igarashi Y. Induction of apoptosis by sphingosine in human leukemic HL-60 cells: a possible endogenous modulator of apoptotic DNA fragmentation occurring during phorbol ester-induced differentiation. Cancer Res. 1995 Feb 1;55(3):691–697. [PubMed] [Google Scholar]
- Ohta H., Yatomi Y., Sweeney E. A., Hakomori S., Igarashi Y. A possible role of sphingosine in induction of apoptosis by tumor necrosis factor-alpha in human neutrophils. FEBS Lett. 1994 Dec 5;355(3):267–270. doi: 10.1016/0014-5793(94)01218-0. [DOI] [PubMed] [Google Scholar]
- Okazaki T., Bell R. M., Hannun Y. A. Sphingomyelin turnover induced by vitamin D3 in HL-60 cells. Role in cell differentiation. J Biol Chem. 1989 Nov 15;264(32):19076–19080. [PubMed] [Google Scholar]
- Portilla D., Mandel L. J., Bar-Sagi D., Millington D. S. Anoxia induces phospholipase A2 activation in rabbit renal proximal tubules. Am J Physiol. 1992 Mar;262(3 Pt 2):F354–F360. doi: 10.1152/ajprenal.1992.262.3.F354. [DOI] [PubMed] [Google Scholar]
- Raines M. A., Kolesnick R. N., Golde D. W. Sphingomyelinase and ceramide activate mitogen-activated protein kinase in myeloid HL-60 cells. J Biol Chem. 1993 Jul 15;268(20):14572–14575. [PubMed] [Google Scholar]
- Rosenwald A. G., Pagano R. E. Inhibition of glycoprotein traffic through the secretory pathway by ceramide. J Biol Chem. 1993 Mar 5;268(7):4577–4579. [PubMed] [Google Scholar]
- Ryan M. J., Johnson G., Kirk J., Fuerstenberg S. M., Zager R. A., Torok-Storb B. HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 1994 Jan;45(1):48–57. doi: 10.1038/ki.1994.6. [DOI] [PubMed] [Google Scholar]
- Sadahira Y., Zheng M., Ruan F., Hakomori S., Igarashi Y. Sphingosine-1-phosphate inhibits extracellular matrix protein-induced haptotactic motility but not adhesion of B16 mouse melanoma cells. FEBS Lett. 1994 Feb 28;340(1-2):99–103. doi: 10.1016/0014-5793(94)80180-0. [DOI] [PubMed] [Google Scholar]
- Van Veldhoven P. P., Matthews T. J., Bolognesi D. P., Bell R. M. Changes in bioactive lipids, alkylacylglycerol and ceramide, occur in HIV-infected cells. Biochem Biophys Res Commun. 1992 Aug 31;187(1):209–216. doi: 10.1016/s0006-291x(05)81480-9. [DOI] [PubMed] [Google Scholar]
- Weinberg J. M. The cell biology of ischemic renal injury. Kidney Int. 1991 Mar;39(3):476–500. doi: 10.1038/ki.1991.58. [DOI] [PubMed] [Google Scholar]
- Weinberg J. M., Venkatachalam M. A., Roeser N. F., Davis J. A., Varani J., Johnson K. J. Amino acid protection of cultured kidney tubule cells against calcium ionophore-induced lethal cell injury. Lab Invest. 1991 Dec;65(6):671–678. [PubMed] [Google Scholar]
- Zager R. A. Acute renal failure in the setting of bone marrow transplantation. Kidney Int. 1994 Nov;46(5):1443–1458. doi: 10.1038/ki.1994.417. [DOI] [PubMed] [Google Scholar]
- Zager R. A., Baltes L. A., Sharma H. M., Jurkowitz M. S. Responses of the ischemic acute renal failure kidney to additional ischemic events. Kidney Int. 1984 Nov;26(5):689–700. doi: 10.1038/ki.1984.204. [DOI] [PubMed] [Google Scholar]
- Zager R. A. Heme protein-induced tubular cytoresistance: expression at the plasma membrane level. Kidney Int. 1995 May;47(5):1336–1345. doi: 10.1038/ki.1995.189. [DOI] [PubMed] [Google Scholar]
- Zager R. A. Obstruction of proximal tubules initiates cytoresistance against hypoxic damage. Kidney Int. 1995 Feb;47(2):628–637. doi: 10.1038/ki.1995.80. [DOI] [PubMed] [Google Scholar]