Abstract
The central dogma of molecular biology defines the major route for the transfer of genetic information from genomic DNA to messenger RNA to three-dimensional proteins that affect structure and function. Like alternative splicing, the post-transcriptional conversion of adenosine to inosine (A-to-I) by RNA editing can dramatically expand the diversity of the transcriptome to generate multiple, functionally distinct protein isoforms from a single genomic locus. While RNA editing has been identified in virtually all tissues, such post-transcriptional modifications have been best characterized in RNAs encoding both ligand- and voltage-gated ion channels and neurotransmitter receptors. These RNA processing events have been shown to play an important role in the function of the encoded protein products and, in several cases, have been shown to be critical for the normal development and function of the nervous system.
1 Introduction
The conversion of adenosine to inosine (A-to-I) by RNA editing is increasingly identified as a post-transcriptional modification in which genomically-encoded sequences are altered through the site-specific deamination of specific adenosine residue(s) in precursor and mature mRNAs, tRNAs and primary miRNA transcripts (Blow et al. 2006; Gerber et al. 1998; Gott and Emeson 2000). An inosine within the open reading frame (ORF) of an mRNA is read as guanosine during translation, which can lead to specific change(s) in the amino acid coding potential of the mRNA to alter the functional properties of the encoded protein product. Such alterations in the primary nucleotide sequence of mRNA transcripts can affect not only coding potential, but also can alter the structure, stability, translation efficiency and splicing patterns of the modified transcripts, thereby affecting numerous aspects of RNA function in the cell (Gott and Emeson 2000). For specific tRNAs, such editing events are often observed in the wobble position of the anticodon loop (position 34), playing a crucial role in protein synthesis by allowing alternative pairing with U, C, or A in the third position of codons (Crick 1966). Editing also has been shown to play an important role in miRNA expression and function, as A-to-I conversion can modulate the processing of miRNA precursors by Drosha and Dicer, alter the target selectivity of mature miRNAs and decrease miRNA stability (Kawahara et al. 2007; Yang et al. 2006).
2 Identification of A-to-I Editing Targets
A-to-I editing is generally identified as an adenosine to guanosine (A-to-G) discrepancy during comparisons of genomic and cDNA sequences that result from the base-pairing of cytosine to inosine (like guanosine) during reverse transcriptase-mediated first-strand cDNA synthesis. At least eight mRNA transcripts with A-to-I modifications in the ORF were initially noted in mammals, based upon the serendipitous identification of such A-to-G disparities (Table 1). Most notably, subunits of the α-amino-3-hydroxy-5-methyl-isoxazole-4-propionate (AMPA) subtype of ionotropic glutamate receptor (GluR-2, GluR-3 and GluR-4), subunits of the kainate subtype of glutamate-gated ion channel (GluR-5 and GluR-6) and transcripts encoding the 2C-subtype of serotonin receptor (5HT2C) were shown to undergo A-to-I modifications that change the amino acid coding potential of the mature mRNAs, producing protein products with altered functional properties (Emeson and Singh 2001; Rueter and Emeson 1998). Recently, numerous laboratories have developed more directed approaches to identify mRNA targets of A-to-I editing, employing both biochemical and computer-based (in silico) strategies (Athanasiadis et al. 2004; Blow et al. 2004; Kikuno et al. 2002; Kim et al. 2004; Levanon et al. 2004; Ohlson et al. 2007). The earliest of these approaches took advantage of proposed sequence conservation surrounding editing sites in two evolutionarily distant species of fruit fly, Drosophila melanogaster and Drosophila pseudoobscura (Hoopengardner et al. 2003). By comparing sequences for 914 candidate genes that included ion channels, G-protein coupled receptors, proteins involved in fast synaptic neurotransmission and transcription factors, Hoopengardner and colleagues identified 41 genes containing regions within coding sequences that displayed unusually high sequence conservation compared with surrounding sequences. Further characterization of the mRNAs encoded by these genes allowed the identification of 16 additional edited RNA species in Drosophila, encoding primarily ligand- and voltage-gated ion channels and components of the synaptic release machinery (Hoopengardner et al. 2003). The identification of numerous editing targets in the Drosophila nervous system is consistent with the observation that the prominent phenotype observed in editing-deficient flies involves nervous system dysfunction and neurodegeneration (Hoopengardner et al. 2003; Palladino et al. 2000). Of the newly identified editing events in flies however, only transcripts from the kcna1 gene, encoding a voltage-gated potassium channel (Kv1.1), have been validated as a target in the mammalian transcriptome (Bhalla et al. 2004; Hoopengardner et al. 2003).
Table 1.
Functional consequence of A-to-I editing
|
More recently, additional in silico approaches have attempted to identify and validate A-to-G discrepancies between genomic and cDNA sequences on a genome/transcriptome-wide scale, predicting >12,000 editing sites in the human transcriptome of which >94% occur primarily in non-coding regions of RNA transcripts containing short interspersed elements (SINEs) of the Alu and L1 subclass (Athanasiadis et al. 2004; Blow et al. 2004; Kikuno et al. 2002; Kim et al. 2004; Levanon et al. 2004). Although the biological significance of editing in Alu sequences has not been fully examined, it has been proposed that the editing within this primate-specific class of SINE elements may modulate alternative splicing, chromatin structure and the retention of highly edited RNAs in the nucleus (Chen et al. 2008; Moller-Krull et al. 2008). Despite the success of in silico strategies in the identification and validation of novel editing sites, few codon-altering (recoding) A-to-I modifications were identified in mRNAs. Among these additional editing targets are transcripts encoding filamin A (FLNA), bladder cancer associated protein (BLCAP), cytoplasmic FMR1 interacting protein 2 (CYFIP2) and insulin-like growth factor binding protein 7 (IGFBP7) (Table 1). Despite their expression in discrete regions of the brain, the role(s) for these proteins in central nervous system function or the consequences of editing have not been determined.
3 Mammalian ADAR enzymes
A-to-I editing of precursor and mature mRNAs and primary miRNA transcripts is mediated by a family of double-stranded RNA-specific adenosine deaminases (ADARs) that catalyze the hydrolytic deamination of the C-6 position within the purine ring (Polson et al. 1991) and have been the topic of numerous reviews (Bass 2002; Hogg et al. 2011; Nishikura 2010). In mammals, three ADAR proteins (ADAR1, ADAR2 and ADAR3) have been purified and their corresponding genes have been identified (Chen et al. 2000; Hough and Bass 1994; Kim et al. 1994; Liu et al. 1997; O’Connell et al. 1995). ADAR1 and ADAR2 have been shown to be expressed in almost all cell types examined (Melcher et al. 1996b; Wagner et al. 1990) and are able to convert A-to-I in extended regions of duplex RNA within pre-mRNAs, mRNAs, primary miRNA transcripts and viral RNAs (Berg et al. 2001; Luciano et al. 2004; Schaub and Keller 2002; Yang et al. 2006). The expression of ADAR3 has been detected only in post-mitotic neurons in brain regions such as the amygdala and thalamus (Chen et al. 2000), yet ADAR3 has not demonstrated any catalytic activity using synthetic dsRNA or known ADAR substrates (Chen et al. 2000; Maas et al. 2003). ADAR1 and ADAR2 have overlapping yet distinct patterns of editing with some sites edited by only one enzyme and other sites edited equally well by both (Bass 2002; Hogg et al. 2011; Nishikura 2010).
The ADAR1 gene specifies two major protein isoforms, an interferon (IFN) inducible 150 kDa protein (p150) and a ubiquitous, constitutively expressed N-terminally truncated 110 kDa protein (p110), encoded by transcripts with alternative exon 1 structures that initiate from different promoters (George and Samuel 1999). The predicted protein sequence of ADAR1 indicates that it contains three copies of a dsRNA-binding motif (dsRBM), a motif shared among numerous dsRNA-binding proteins (Burd and Dreyfuss 1994; Fierro-Monti and Mathews 2000), a nuclear localization signal in the third dsRBM (Eckmann et al. 2001), and a region homologous to the catalytic domain of other known adenosine and cytidine deaminases. The amino-terminus of the p150 isoform also contains two Z-DNA binding domains, the first of which (Zα) overlaps with a leucine-rich nuclear export signal (Poulsen et al. 2001). The Z-DNA binding domains also have been proposed to tether ADAR1 to sites of transcription (Herbert and Rich 1996) or to mediate interactions between ADARs and other proteins (Poulsen et al. 2001). The locations of the nuclear localization and nuclear export sequences in ADAR1 are consistent with observations that the p150 isoform shuttles between the cytoplasm and the nucleus, whereas the p110 protein is localized predominantly to the nucleus (Eckmann et al. 2001; Fritz et al. 2009; Patterson and Samuel 1995; Strehblow et al. 2002). Alternative splicing within exon 7 of ADAR1 generates two distinct mRNA isoforms that differ by 26 amino acids that encode the linker region between the third double-stranded RNA binding motif and the catalytic domain to affect site-selective editing efficiency (Liu et al. 1999; Liu et al. 1997). Total ablation of ADAR1 expression in mice results in embryonic lethality at day 11.5, manifested by liver disintegration (Hartner et al. 2004) and widespread apoptosis (Wang et al. 2004), suggesting that ADAR1 may promote survival of numerous tissues by editing dsRNAs required for protection against programmed cell death. Similar embryonic lethality also results from selective loss of the p150 isoform, indicating a critical role for this ADAR1 isoform in embryonic development (Ward et al. 2011). Humans that are heterozygous for an ADAR1 loss-of-function allele demonstrate dyschromatosis symmetrica hereditaria, a recessive genetic disorder characterized by pea-sized hyperpigmented and hypopigmented macules on the hands and feet (Gao et al. 2005; Miyamura et al. 2003; Suzuki et al. 2005), whereas no such phenotype is observed in mice that are heterozygous for an ADAR1-null allele (Hartner et al. 2004; Wang et al. 2004).
ADAR2 is an 80 kilodalton protein with structural features similar to those observed for ADAR1 (Melcher et al. 1996b). ADAR2 contains a nuclear localization signal and two dsRNA binding motifs, sharing approximately 25% amino acid sequence similarity with the dsRBMs of ADAR1. ADAR2 also contains an adenosine deaminase domain sharing 70% amino acid similarity with ADAR1, as well as three zinc-chelating residues conserved in the deaminase domain of both enzymes. As with ADAR1, multiple cDNA isoforms of ADAR2 have been identified in rats, mice and humans including alternative splicing events in mRNA regions encoding the deaminase domain and near the amino terminus (Gerber et al. 1997; Lai et al. 1997; Rueter et al. 1999). Of particular interest is an alternative splicing event that introduces an additional 47 nucleotides near the 5′-end of the ADAR2 coding region, resulting in a frameshift that is predicted to produce a 9 kD protein (82 aa) lacking the dsRBMs and catalytic deaminase domain required for protein function (Rueter et al. 1999). This alternative splicing event is dependent upon the ability of ADAR2 to edit its own pre-mRNA, converting an intronic adenosine-adenosine (AA) to an adenosine-inosine (AI) dinucleotide that effectively mimics the highly conserved AG sequence normally found at 3′-splice junctions. These observations indicate that RNA editing may serve as a novel mechanism for the regulation of alternative splicing and provides an autoregulatory strategy by which ADAR2 can modulate its own level of expression (Feng et al. 2006; Rueter et al. 1999). Ablation of ADAR2 expression in mutant mice results in death between postnatal day 0 (P0) and P20, as mutant animals become progressively seizure-prone after P12 (Higuchi et al. 2000).
4 ADAR Substrates in the Central Nervous System
4.1 Glutamate-gated ion channels
4.1.1 AMPA Receptors
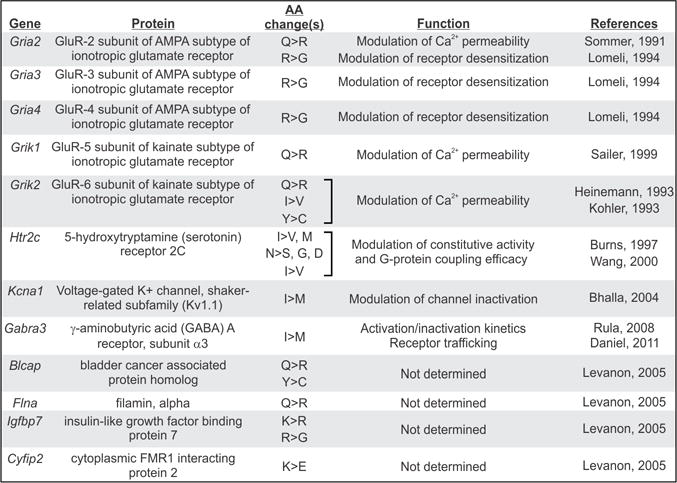
Ionotropic glutamate receptors (iGluRs) are involved in fast synaptic neurotransmission and in the establishment and maintenance of synaptic plasticity critical to learning and memory. Three subtypes of iGluRs, named according to selective agonists for each receptor subtype, include N-methyl-D-aspartate (NMDA) receptors, α-amino-3-hydroxy-5-methyl-isoxazole-4-propionate (AMPA) receptors and kainate receptors (Ozawa et al. 1998). Ionotropic glutamate receptors are tetrameric and their subunits share a similar core structure: three transmembrane segments (M1, M3 and M4), a pore loop (M2), a large extracellular N-terminal domain, and a highly-regulated, variably-sized C-terminal domain (Fig. 1). The N-terminus and a long hydrophilic region between the M3 and M4 transmembrane segments form a two-domain structure that is responsible for ligand-binding (Wollmuth and Sobolevsky 2004).
Figure 1. Summary of the RNA editing events in mouse transcripts encoding ionotropic glutamate receptor subunits.
A schematic representation of the proposed topology for GluR subunits is presented, based upon the topology determined for GluR-1 (Hollman, 1994), indicating the relative positions of editing sites. The genomic, mRNA and amino acid sequences surrounding the edited regions are shown and modified nucleosides are presented in inverse lettering.
The first example of A-to-I editing in mammalian mRNAs was identified in transcripts encoding the GluR-2 subunit of the AMPA receptor in which a genomically-encoded glutamine codon (CAG) was altered to an arginine codon (CIG) (Melcher et al. 1995; Rueter et al. 1995; Sommer et al. 1991; Yang et al. 1995). The substitution of a positively-charged arginine residue for a neutrally-charged glutamine residue at the apex of the membrane reentrant pore loop (M2) changes the conductance properties of channels containing an edited GluR-2(R) subunit (Verdoorn et al. 1991). Heteromeric AMPA channels that contain the edited GluR-2(R) subunit are relatively impermeant to Ca2+ ions and show a linear current–voltage (I–V) relationship, whereas channels that lack or contain a non-edited GluR-2(Q) subunit show a double-rectifying I–V relationship and an increased Ca2+ conductance (Dingledine et al. 1992; Hollmann et al. 1991; Sommer et al. 1991; Verdoorn et al. 1991). Quantitative PCR analyses of adult rat, mouse and human brain RNA have demonstrated that virtually all GluR-2 transcripts encode this critical arginine residue within M2 while GluR-1, −3 and −4 transcripts encode only a glutamine at the analogous position (Higuchi et al. 2000; Sommer et al. 1991).
RNA editing is also responsible for an A-to-I modification in exon 13 of RNAs encoding the GluR-2, −3 and −4 AMPA receptor subunits to alter a genomically-encoded arginine (AGA) to a glycine (IGA) codon at position 764 (R/G site) to modulate the rate of recovery from receptor desensitization (Fig. 1). Because of faster recovery and a tendency for slower desensitization rates, heteromeric AMPA channels containing edited (glycine-containing) subunits show larger steady-state currents than the non-edited forms (Lomeli et al. 1994). Immediately following this edited codon, an alternative splicing event incorporates one of two mutually-exclusive exons referred to as ‘flip’ and ‘flop’ that encode a portion of the ligand-binding domain (Fig. 1) (Sommer et al. 1990). Combinations of editing and splicing generate a variety of channels with unique kinetic properties (Koike et al. 2000; Krampfl et al. 2002; Lomeli et al. 1994). Editing at the Q/R and R/G sites together, play a role in receptor trafficking, as editing of the Q/R site attenuates formation of GluR-2 homo-tetramers and leads to retention of the GluR-2(R) subunit in the endoplasmic reticulum (ER) (Greger et al. 2003; Greger et al. 2002; Greger et al. 2007). Indeed, non-edited GluR-2(Q) is released to form homomeric channels on the cell surface while edited subunits remain unassembled in the ER (Greger et al. 2003). Interestingly, several studies have shown that editing at the R/G site (Greger et al. 2006) and flip/flop alternative splicing (Coleman et al. 2006) also play roles in AMPA receptor trafficking.
To determine the biological significance of Q/R site editing, mutant mice were engineered to solely express the non-edited form of the GluR-2 transcript by Cre-mediated deletion of the editing complementary sequence (ECS), an intronic region required for formation of the RNA duplex that is essential for A-to-I conversion. A dominant-lethal phenotype was revealed as heterozygous mutant animals appeared healthy until postnatal day 14 (P14), when they begin to develop seizures that lead to death by P20. This phenotype resulted from dramatically increased AMPA receptor permeability to Ca2+, concomitant with neuronal degeneration (Brusa et al. 1995). In subsequent studies, mice homozygous for a null allele of ADAR2 were shown to die of seizures before P20, a phenotype nearly identical to that seen in mice deficient in GluR-2 editing (Q/R site). This early postnatal lethality was rescued by a targeted mutation in which the wild-type GluR-2 allele was modified to express transcripts with a genomically encoded arginine [GluR-2(R)], thereby circumventing the requirement for editing (Higuchi et al. 2000). Together, these studies highlight the physiological importance of GluR-2 editing (Q/R site) in normal brain function. However, it also should be noted that Ca2+-permeable AMPA receptors have been identified following mechanical or ischemic brain injury (Rump et al. 1996; Spaethling et al. 2008) or in specific brain regions such as cerebellar Bergmann glia (Burnashev et al. 1992; Iino et al. 2001), yet this Ca2+ permeability is thought to result largely from an absence of GluR-2 subunit incorporation into functional AMPA channels rather than the absence of GluR-2 editing.
The editing of GluR-2 mRNA has been implicated recently in the etiology of sporadic amyotrophic lateral sclerosis (ALS), a progressive neurodegenerative disorder involving primarily motor neurons of the cerebral cortex, brain stem and spinal cord, eventually leading to death from respiratory failure (Naganska and Matyja 2011). The editing of GluR-2 transcripts (Q/R site) in spinal motor neurons of ALS patients appears to be inefficient compared to control patients or unaffected neurons (Hideyama et al. 2010), suggesting that the inclusion of the GluR-2(Q) subunit into heteromeric AMPA channels results in a Ca2+-mediated excitotoxicity that contributes to cell death (Kawahara et al. 2004; Kawahara et al. 2003; Kawahara et al. 2006; Kwak and Kawahara 2005; Takuma et al. 1999). Support for this hypothesis was recently demonstrated in a mutant mouse line where ADAR2 expression was specifically ablated in ~50% of motor neurons using a conditional ADAR2-null allele in combination with Cre recombinase under the control of the vesicular acetylcholine transporter promoter. Motor neurons lacking ADAR2, expressing only non-edited GluR2(Q) subunits, were subject to a slow death, but could be rescued by expression of the Glur-2(R) allele (Hideyama et al. 2010), thus providing the first example of a human neurodegenerative disorder resulting from editing defects and further emphasizing the importance of GluR-2 editing in normal CNS function.
4.1.2 Kainate Receptors
Kainate (KA) receptors, like AMPA receptors, mediate fast excitatory neurotransmission and are widely expressed in a number of brain regions including the neocortex, the caudate/putamen, the CA3 region of the hippocampus, the reticular thalamus and the cerebellar granular layer (Seeburg 1993). There are five KA subunits encoded by distinct genes: GluR-5, GluR-6, GluR-7, KA-1 and KA-2. GluR-5 and GluR-6 form homomeric channels with a high affinity for kainate, but are not activated by AMPA (Bettler et al. 1990; Egebjerg et al. 1991). These two subunits are distinct from the other KA receptor subunits as their RNAs are modified by A-to-I editing (Sommer et al. 1991). Like GluR-2, RNAs for GluR-5 and GluR-6 have a Q/R editing site in a region encoding the hydrophobic pore domain (M2) which can alter the calcium permeability of heteromeric KA receptors containing a GluR-6 subunit (Egebjerg and Heinemann 1993). There are two additional editing sites in the M1 region of GluR-6 and editing leads to the substitution of a valine (ITT) for a genomically-specified isoleucine (ATT) codon (I/V site) and the substitution of a cysteine (TIC) for a tyrosine (TAC) codon (Y/C site). These editing events provide the possibility of eight different edited variants of GluR-6 subunits, all of which are expressed to a varying extent in the CNS, although fully edited GluR-6 transcripts represent the most abundantly expressed isoform in the adult nervous system (Kohler et al. 1993; Ruano et al. 1995).
Electrophysiological studies have revealed that channels with editing events in the M1 region of GluR-6 exhibit increased calcium permeability when the M2 pore encodes an arginine at the Q/R site, in direct contrast to the decreased calcium permeability shown for R-containing GluR-2 channels (Kohler et al. 1993). When the M1 region of GluR-6 is not edited, encoding an isoleucine and tyrosine at the I/V and Y/C sites, respectively, the presence of an arginine in M2 does little to alter calcium permeability (Kohler et al. 1993). Recombinant homomeric receptors composed of unedited kainate receptor subunits [GluR-5(Q) and GluR-6(Q)] demonstrate additional functional differences from those containing edited receptor isoforms [GluR-5(R) and GluR-6(R)], including a linear I–V relationship rather than double-rectifying properties, a single low conductance state rather than multiple conductance states and a highly significant increase in the permeability of Cl− ions (Chittajallu et al. 1999). The physiological relevance of these functional alterations has yet to be identified however, as studies of mutant mice capable of expressing only the edited GluR-5(R) isoform had no obvious developmental abnormalities or deficits in a number of behavioral paradigms (Sailer et al. 1999). While mutant mice solely expressing the non-edited GluR-6(Q) subunit appeared normal, they exhibited increased NMDA receptor-independent long-term potentiation in hippocampal slices and increased susceptibility to kainate-induced seizures, suggesting a role for GluR-6 (Q/R site) editing the modulation of synaptic plasticity and seizure vulnerability (Vissel et al. 2001).
4.2 The serotonin 2C receptor (5HT2C)
Serotonin (5-hydroxytryptamine; 5HT) is a monoaminergic neurotransmitter that modulates numerous sensory and motor processes as well as a wide variety of behaviors including sleep, appetite, pain perception, locomotion, thermoregulation, hallucinations, and sexual behavior (Werry et al. 2008). The multiple actions of 5HT are mediated by specific interaction with multiple receptor subtypes. Pharmacological, physiologic and molecular cloning studies have provided evidence for fifteen distinct 5HT receptor subtypes which have been subdivided into seven families (5HT1–5HT7) based on relative ligand binding affinities, genomic structure, amino acid sequence similarities and coupling to specific signal transduction pathways (Barnes and Sharp 1999; Bockaert et al. 2006; Hoyer et al. 1994; Hoyer et al. 2002). The 5HT2 family of receptors includes three receptor subtypes: 5HT2A, 5HT2B and 5HT2C, which belong to the G-protein-coupled receptor (GPCR) superfamily. The G-protein:5HT2C receptor interactions occur in highly conserved regions of the second and third intracellular loops to potentiate subsequent signal transduction pathways via Gαq/11, Gα12/13 and Gαi to modulate effector molecules such as phospholipases C, D and A2, as well as the extracellular signal-regulated kinases 1 and 2 (Berg et al. 1994; Berg et al. 1998; Werry et al. 2005; Werry et al. 2008). 5HT2C mRNA expression has been shown to be widely distributed in neocortical areas, hippocampus, nucleus accumbens, amygdala, choroid plexus, dorsal striatum and substantia nigra (Pasqualetti et al. 1999; Pompeiano et al. 1994), suggestive of physiologic roles in reward behavior, locomotion, energy balance, and also when dysregulated, in the development of certain disease states such as obesity, epilepsy, anxiety, sleep disorders and motor dysfunction (Giorgetti and Tecott 2004). Many of these anatomical predictions for 5HT2C function have been supported by analyses of 5HT2C-null mice that exhibit adult-onset obesity, seizures and decreased cocaine-mediated locomotor activity and reward behavior (Abdallah et al. 2009; Brennan et al. 1997; Giorgetti and Tecott 2004; Rocha et al. 2002; Tecott et al. 1995).
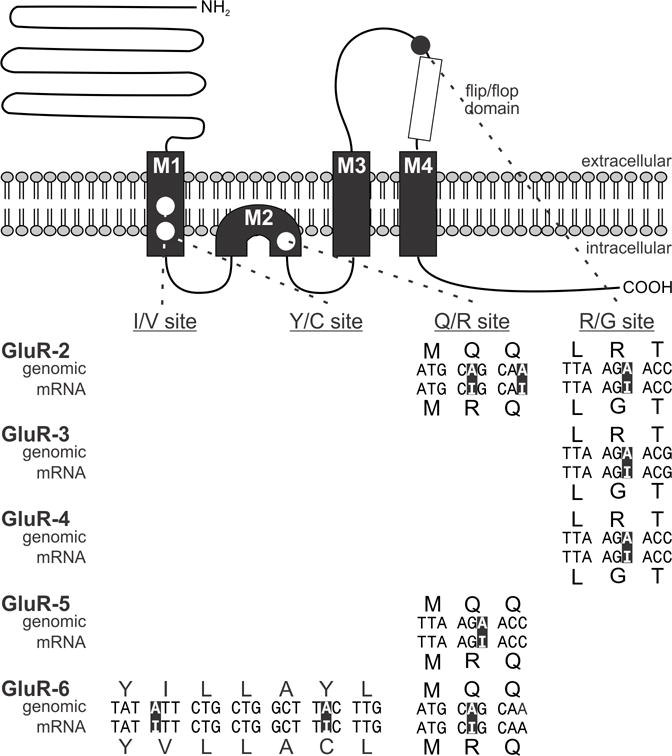
RNA transcripts encoding the 5HT2C receptor undergo up to five A-to-I editing events that predict alterations in the identity of three amino acids within the second intracellular loop of the receptor to generate as many as 24 receptor isoforms from 32 edited mRNA species (Fig. 2) (Burns et al. 1997; Wang et al. 2000). Sequence analysis of cDNAs isolated from dissected rat, mouse and human brains predicted the region-specific expression of 7 major 5HT2C isoforms encoded by eleven distinct mRNA (Abbas et al. 2010; Burns et al. 1997; Morabito et al. 2010; Niswender et al. 1999), suggesting that differentially edited 5HT2C receptors may serve distinct biological functions in those regions in which they are expressed. Sequencing studies have further revealed that edited mRNAs encoding isoforms with valine, serine and valine (VSV) or valine, asparagine and valine (VNV) at amino acids 157, 159 and 161 are the most highly expressed in a majority of dissected brain regions isolated from human and rat/mouse brains, respectively (Burns et al. 1997; Fitzgerald et al. 1999), whereas the major 5HT2C transcripts in the choroid plexus encode the less edited (INV) and non-edited (INI) receptor isoforms (Burns et al. 1997; Morabito et al. 2010). Functional comparisons in heterologous expression systems, between the non-edited (INI) and the fully-edited (VGV) 5HT2C isoforms revealed a 40-fold decrease in serotonergic potency to stimulate phosphoinositide hydrolysis for the VGV isoform due to reduced Gq/11-protein coupling efficiency and decreased coupling to other signaling pathways (Burns et al. 1997; Niswender et al. 1999; Price et al. 2001). In addition, cells expressing more highly edited 5HT2C receptors (e.g. VSV and VGV) demonstrate considerably reduced (or absent) constitutive activation in the absence of ligand compared to cells expressing the non-edited isoform (Niswender et al. 1999). This reduction in coupling efficiency and constitutive activity derives from a difference in the ability of edited 5HT2C isoforms to spontaneously isomerize to the active R* conformation, a form of the receptor that interacts efficiently with G-proteins in the absence of agonist (Burns et al. 1997; Niswender et al. 1999). As a consequence, the observed potency of agonists with increased affinity for the R* state is disproportionately reduced (Werry et al. 2008) and the ‘functional selectivity’ of receptor stimulus may be lost (Berg et al. 2001). More recent studies have indicated that alterations in 5HT2C editing are observed in suicide victims with a history of major depression (Berg et al. 2001; Gurevich et al. 2002b; Iwamoto and Kato 2003), and in response to antidepressant and antipsychotic treatment (Englander et al. 2005; Gurevich et al. 2002b), suggesting that editing of 5HT2C transcripts may be involved in psychiatric disorders and also may represent a homeostatic mechanism whereby 5HT2C receptor signaling is stabilized in the face of changing synaptic serotonergic input (Englander et al. 2005; Gurevich et al. 2002a).
Figure 2. Summary of RNA recoding events in serotonin 2C receptor RNA and protein isoforms.
Schematic representation of the predicted topology and primary amino acid sequence for the mouse 5HT2C receptor is presented along with the positions of amino acid alterations within the second intracellular loop resulting from RNA editing events (colored circles). Nucleotide and predicted amino acid sequence alignments between 5HT2C genomic, mRNA and cDNA sequences are shown; the positions of the five editing sites (A–E) are indicated and nucleotide discrepancies and predicted alterations in amino acid sequence are shown with colors corresponding to each codon in which they reside.
Chronic administration of IFN-α for the treatment of hepatitis C, hairy cell leukemia, AIDS-related Kaposi’s sarcoma, chronic myelogenous leukemia, and melanoma have been shown to produce depressive symptoms that adversely affect disease outcome because of their negative impact on a patient’s quality of life, their interference with treatment adherence, and the development of serious complications, including suicide (Ademmer et al. 2001; Valentine et al. 1998; Zdilar et al. 2000). The mechanism by which chronic IFN-α treatment induces depression has yet to be established, although serotonin-mediated effects have been implicated (Cai et al. 2005; Lotrich et al. 2009; Menkes and MacDonald 2000). In vitro studies have demonstrated that IFN-α treatment of glioblastoma cell lines can alter the editing pattern for 5HT2C transcripts by increasing the expression of the IFN-inducible isoform of ADAR1 (p150) (Yang et al. 2004), providing a mechanism by which cytokines could induce depression by affecting the editing of numerous ADAR targets in the nervous system to alter subsequent neurotransmitter receptor function.
While the editing of 5HT2C transcripts has been shown to modulate multiple aspects of 5HT2C receptor signaling and expression in heterologous systems (Berg et al. 2001; Burns et al. 1997; Fitzgerald et al. 1999; Flomen et al. 2004; Marion et al. 2004; Niswender et al. 1999; Price et al. 2001; Wang et al. 2000), until recently, the physiologic importance for the existence of multiple 5HT2C isoforms had not been fully explored. Mutant mice solely expressing the fully-edited isoform of the 5HT2C receptor display several phenotypic characteristics of Prader-Willi Syndrome (PWS), a maternally-imprinted human disorder resulting from a loss of paternal gene expression on chromosome 15q11–13 (Goldstone 2004; Nicholls and Knepper 2001). Mutant mice display a failure to thrive, decreased somatic growth and neonatal muscular hypotonia, followed by post-weaning hyperphagia, in addition to a strain-specific neonatal lethality that is shared with other mouse models of PWS (Chamberlain et al. 2004). These observations are consistent with recent analyses indicating that 5HT2C RNA editing is increased in autopsy samples from PWS patients (Kishore and Stamm 2006) and a mouse model (PWS-ICdel) that also demonstrates alterations in 5HT2C-related behaviors (Doe et al. 2009). Previous studies have shown that a maternally imprinted small nucleolar RNA within the Prader-Willi critical region (snord115) can alter both the splicing and editing of 5HT2C transcripts (Kishore and Stamm 2006), providing a provoking and straightforward mechanism by which 5HT2C RNA processing patterns may be linked with the 15q11–13 locus.
4.3 The α3 subunit of the GABAA receptor (Gabra3)
γ-aminobutyric acid (GABA) is the main inhibitory neurotransmitter in the vertebrate central nervous system. The GABAA subtype of GABA receptor is a ligand-gated chloride channel composed of an assembly of five individual subunits, of which there are nineteen classes (α1–6, β1–3, γ1–3, δ, ɛ, ρ1–3, θ, and π) (Olsen and Sieghart 2008, 2009). Different combinations of these subunits generate pharmacologically and functionally distinct isoforms of the GABAA receptor, which typically contain two α subunits (D’Hulst et al. 2009). The GABAA receptor is a target for many classes of drugs including barbiturates, benzodiazepenes, ethanol, anti-convulsants and anesthetics (D’Hulst et al. 2009; Fisher 2009; Henschel et al. 2008; Jenkins et al. 2001; Korpi et al. 2007; Low et al. 2000; MacDonald et al. 1989; Meera et al. 2010; Mehta and Ticku 1999; Mohler and Okada 1977; Rudolph et al. 1999; Speth et al. 1980).
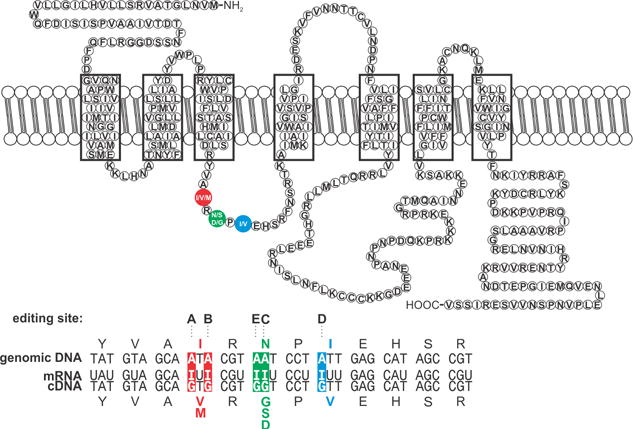
The α3 subunit of GABAA chloride channels is encoded by the Gabra3 gene, which is located on the X chromosome in both humans and mice (Bell et al. 1989; Derry and Barnard 1991). The mouse Gabra3 transcript was first identified as a potential substrate of A-to-I editing using microarray analyses of whole mouse brain RNA that co-immunoprecipitated with ADAR2. This enriched RNA population was further analyzed using a bioinformatics paradigm designed to identify extended regions of dsRNA (e.g. stem-loop structures) within the enriched transcripts (Ohlson et al. 2005; Ohlson et al. 2007). Using this method, an RNA editing event was found in a region encoding sequences immediately adjacent to the extracellular transmembrane 2/3 linker of the α3 subunit (Fig. 3), a region known to be important for channel gating (Ernst et al. 2005). Subsequent sequence analyses verified the presence of an editing site which causes the recoding of a genomically-encoded isoleucine (ATA) to methionine (ATI) codon in cDNA clones isolated from adult mouse brain (Enstero et al. 2010; Rula et al. 2008; Wahlstedt et al. 2009).
Figure 3. Conservation of RNA editing in mammalian Gabra3 transcripts.
The predicted topology for the α3-subunit of the GABAA receptor is shown with the position of the editing-dependent amino acid alteration (I/M site) immediately adjacent to the extracellular transmembrane 2/3 linker. Nucleotide and predicted amino acid sequence alignments between Gabra3 genomic and mRNA from several mammalian species are shown with the positions of the I/M editing site presented with inverse lettering.
To examine functional changes caused by editing, two groups have utilized heterologous expression systems, in which an edited (M) or a non-edited (I) version of the α3 subunit [α3(I) or α3(M)] was expressed along with two additional GABAA subunits to form a functional GABAA channel for electrophysiological analyses in human embryonic kidney (HEK) cells. These experiments revealed that GABAA heteromers containing a non-edited α3(I) subunit have faster activation and deactivation kinetics and slower desensitization than those containing the edited α3(M) isoform. In addition, channels with α3(I) subunits are more outwardly rectifying, assisting in the inhibition of action potentials (Nimmich et al. 2009; Rula et al. 2008). The EC50 of GABA for the non-edited is approximately 50% of that observed for channels containing the edited α3 subunit, although the effect of various allosteric modulators is not altered (Nimmich et al. 2009). While all six GABAA α subunits contain a homologous, conserved isoleucine in their third transmembrane domain, only the α3 subunit is edited (Nimmich et al. 2009; Rula et al. 2008). However, mutational analysis revealed that substitution of a methionine for an isoleucine in the analogous position in the α1 subunit resulted in similar functional alterations (Nimmich et al. 2009). In addition to the electrophysiological changes resulting from the editing of the Gabra3 RNA, more recent studies have revealed that GABAA receptor trafficking and localization are also affected by A-to-I conversion at the I/M site (Daniel et al. 2011). GABAA receptors with an α3(M) subunit were expressed to a lesser extent on the cell surface than heteromeric channels containing a non-edited α3(I) isoform, and α3(M) protein levels were significantly decreased. The substitution of a methionine for an isoleucine in the analogous position of the α1 subunit also modulated receptor cell-surface expression, indicating the importance of this amino acid residue for receptor trafficking (Daniel et al. 2011).
In the mature brain, activation of GABAA receptors produces a hyperpolarizing influx of chloride ions. While the α1 subunit is the predominant α subtype in the adult brain, the α2, α3 and α5 subtypes are much more highly expressed in the developing CNS (Laurie et al. 1992). In the developing nervous system however, the chloride gradient in cells is reversed from that of mature neurons, causing chloride ions to flow out from GABAA channels in response to GABA stimulation. This chloride efflux creates an excitatory response to GABA that can stimulate action potentials as well as relieve the voltage-dependent block of NMDA receptors (Leinekugel et al. 1999). These depolarizing currents occur during a period of robust synaptic development and are crucial for a number of developmental processes including proliferation and synaptogenesis (Ben-Ari 2007; Cancedda et al. 2007; Ge et al. 2006). Concomitant with the age-dependent switch from excitation to inhibition for heteromeric GABAA receptors is the changing expression of α3 subunit which begins to decline at P7 from the elevated levels observed during embryogenesis (Bosman et al. 2002; Daniel et al. 2011; Hutcheon et al. 2004; Laurie et al. 1992; Rula et al. 2008; Wahlstedt et al. 2009). The editing of Gabra3 transcripts has a reversed developmental pattern however, as editing is low during embryonic development and increases dramatically into adulthood (Ohlson et al. 2007; Rula et al. 2008; Wahlstedt et al. 2009). This developmental pattern of α3 subunit expression provides for the generation of high levels of embryonic GABAA receptors containing the non-edited a3(I) subunit that are ideally suited to respond to prolonged elevations of GABA, thereby producing robust, long-lived depolarization that may trigger the production of sodium and calcium-mediated action potentials (Rula et al. 2008). Editing of Gabra3 transcripts remains nearly constant after birth in humans (Nicholas et al. 2010), suggesting that the most important role for the non-edited α3(I) isoform is played out during embryogenesis and early development, while the α3(M) subunit, encoded by edited Gabra3 transcripts, is essential throughout the remainder of life for normal inhibitory function.
4.4 Voltage-gated potassium channel (Kv1.1)
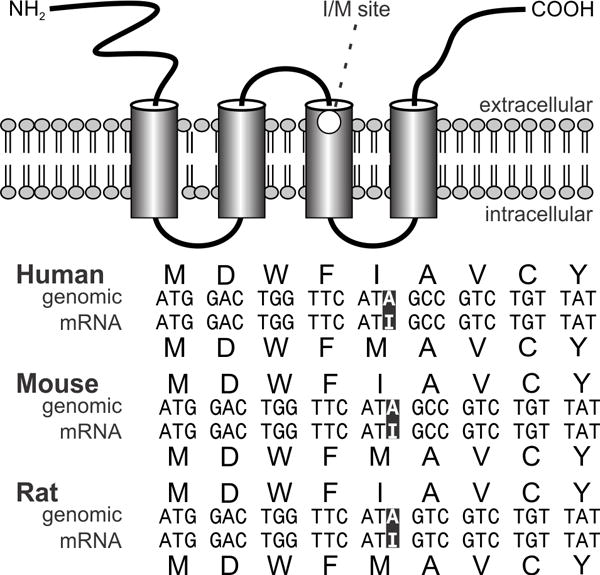
Voltage-gated K+ (Kv) channels are key regulators of neuronal membrane excitability, functioning to control resting membrane potentials (Pongs 1999), spontaneous firing rates (Enyedi and Czirjak 2010), the back propagation of action potentials into dendrites (Hoffman et al. 1997) and neurotransmitter release (Ishikawa et al. 2003). Kv channel protein subunits are encoded by at least 40 different genes in humans, and are grouped into twelve subfamilies (Gutman et al. 2005; Jan and Jan 1997). Neurons typically express multiple types of Kv channels with distinct time- and voltage-dependent properties and subcellular distributions that differentially contribute to the regulation of firing properties and signal integration. Kv channels are formed by the tetrameric assembly of integral membrane protein subunits (MacKinnon 1991) that each contain six transmembrane segments (S1–6) and intracellular amino- and carboxyl-termini (Fig. 4) (Baldwin et al. 1992; Gutman et al. 2005; MacKinnon 1991). Each functional channel consists of four α-subunits arranged around a central axis that generate an ion conduction pathway formed by the S6 segments, a K+ ion selectivity filter formed by the connecting loops between the S5 and S6 segments and a transmembrane voltage sensor (S4 segment) sensor responsible for voltage-dependent gating which contains basic amino acid residues at every third position (Doyle et al. 1998; Long et al. 2005).
Figure 4. Schematic diagram of the structures of mammalian Kv α- and β-subunits comprising voltage-gated K+ channels of the Shaker-related subfamily.
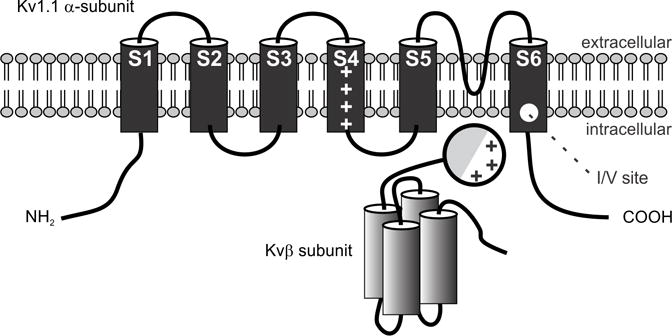
Shown is the proposed topology for α-subunits expressing non-inactivating K-channels and a possible topology for Kvβ1 which contains four α-helices and an amino terminal inactivating ball domain with lipophilic (shaded) and charged regions. This domain swings upon depolarization into the pore and causes rapid inactivation of the channel. The position of the transmembrane voltage sensor (S4), the K+ ion selectivity filter (S5–S6 linker) and the I/V editing site in the α-subunit are indicated (Adapted from Heinemann, 1994).
The first cloned potassium channel gene was encoded by the Shaker locus in Drosophila (Kamb et al. 1987; Papazian et al. 1987; Tempel et al. 1987). Subsequently, a Kv channel gene (Kcna1) exhibiting amino acid sequence similarity to Shaker was cloned in mice and humans (Curran et al. 1992; Tempel et al. 1988) and shown to encode the Kv1.1 α-subunit, a member of the Kv1 subfamily of voltage-gated potassium channels (Curran et al. 1992; Klocke et al. 1993). The Kv1 subfamily, also known as the Shaker-related family, consists of eight different genes (Kv1.1–1.8) (Gutman et al. 2005). Kv1.1 channels are delayed rectifiers that open upon cell depolarization and mediate an outward potassium current, thereby repolarizing the cell and attenuating the action potential (Baranauskas 2007). The Kv1.1-subtype of Kv1 channels is expressed in brain, skeletal muscle, heart, retina and pancreatic islets (Gutman et al. 2005). The Kv1.1 subunit forms both homomeric and heteromeric channels with other members of the Kv1 family, generating extensive functional diversity, as subunit composition greatly affects the kinetic and pharmacological properties (Al-Sabi et al. 2010; Coleman et al. 1999; Deal et al. 1994; Rasband et al. 2001; Schmidt et al. 1999; Shamotienko et al. 1997; Sokolov et al. 2007). In mammals, the tetramer of α-subunits is joined by four associated β-subunits, giving a 4α-4β stoichiometry (Parcej et al. 1992; Rhodes et al. 1997). An inactivating particle belonging to the β-subunits binds to the inner vestibule of the channel pore region shortly after channel activation to block current flow (Fig. 4), a process known as fast inactivation (Rettig et al. 1994).
In addition to forming a variety of channels types with other Kv1.x subunits, transcripts encoding the Kv1.1 subunit are diversified further by RNA editing. An isoleucine (ATT) to valine (ITT) codon change occurs as the result of an A-to-I editing event to recode a conserved isoleucine (Ile400) within the Kv family of voltage-gated potassium channels in a region encoding the sixth transmembrane domain (S6), which lines the inner vestibule of the ion-conducting pore (Fig. 4) (Bhalla et al. 2004; Hoopengardner et al. 2003). The extent of editing varies within different regions of the brain, with highest levels in medulla, spinal cord and thalamus (Hoopengardner et al. 2003). Kv1.1 channels containing edited Kv1.1(V) subunits display a 20-fold decrease in the inactivation rate at negative membrane potentials that may result from a reduced affinity for the binding of the inactivating particle of the β-subunits (Bhalla et al. 2004). In addition to reduced inactivation by the gating particle, editing of Kv1.1 RNAs also reduced the blockage of Kv1.1 channels by endogenous, highly unsaturated signaling lipids such as arachidonic acid, docosahexaenoic acid and anandamide by reducing the affinity of the pore residues for these blocking agents (Decher et al. 2010). Highly unsaturated fatty acids have been shown previously to convert non-inactivating Kv channels to rapidly inactivating channels (Honore et al. 1994; Oliver et al. 2004) through occlusion of the permeation pathway, similar to drugs that produce ‘open-channel block’. Open-channel block by drugs and lipids was strongly reduced in Kv1.1 channels whose amino acid sequence was altered by RNA editing in the pore cavity and in Kv1.x heteromeric channels containing edited Kv1.1 subunits (Decher et al. 2010). In the Drosophila Shaker (Kv1), Shab (Kv2) and squid sqKv2 channels, the position analogous to I400 in Kv1.1, is also edited to produce an isoleucine-to-valine substitution that reduces the sensitivity of these channels to highly unsaturated fatty acids (Bhalla et al. 2004; Decher et al. 2010; Patton et al. 1997; Ryan et al. 2008).
Previous studies have indicated that mutant animals with alterations in the fast inactivation rate of Kv1.1 have been shown to have behavioral and neurologic deficits associated hippocampal learning deficits (Giese et al. 1998) and episodic ataxia type-1 (Herson et al. 2003). In a rat model of chronic epilepsy, a 4-fold increase in Kv1.1 editing levels was observed in the entorhinal cortex of chronic epileptic animals and a reduced potency for the seizure-inducing Kv open channel blocker, 4-aminopyridine (4-AP), suggesting that increased editing of Kv1.1 transcripts contributes to the reduced ictogenic potential of 4-AP (Streit et al. 2011). These observations indicate that the combined effects of open-channel block of Kv1 channels by highly unsaturated lipids together with differential RNA editing can alter the pharmacology of Kv1.x channels and may contribute to the fine-tuning of neuronal signaling in different brain regions.
5 Conclusions
Initially identified as an RNA modification in the anticodon loop of tRNAs from animal, plant and eubacterial origin (Bjork 1995), the deamination of adenosine-to-inosine has become increasingly recognized as a critical RNA processing event to generate diversity in both the transcriptome and proteome (Blow et al. 2006; Gerber et al. 1998; Gott and Emeson 2000). Early studies found mRNA targets of editing based upon the chance identification of adenosine to guanosine (A-to-G) discrepancies between genomic and cDNA sequences that result from the similar base-pairing properties of inosine and guanosine during cDNA synthesis. These mRNA recoding events involved transcripts encoding proteins important for synaptic signaling including ionotropic glutamate receptor subunits (GluR-2, −3, −4, −5 and −6; Fig. 1) and the 2C-subtype of serotonin receptor (Fig. 2). This bias for editing events in the nervous system was thought to reflect either a complexity of neuronal function that requires extensive proteome diversity or simply the small number of neuroscience-focused laboratories that initially were examining this novel RNA processing event. Using primarily transcripts encoding the GluR-2 subunit of the AMPA receptor or synthetic duplex RNAs, numerous groups made significant early advances concerning the molecular mechanisms underlying A-to-I conversion including both the cis-active regulatory sequences/structures and the enzymes (ADARs) responsible for editing using heterologous expression and in vitro editing systems (Higuchi et al. 1993; Kim and Nishikura 1993; Lomeli et al. 1994; Maas et al. 1996; Melcher et al. 1996b; Melcher et al. 1995; O’Connell et al. 1995; Polson and Bass 1994; Rueter et al. 1995; Yang et al. 1995). While the identification of this limited repertoire of substrates proved to be invaluable, the serendipitous nature of such substrate identification raised numerous questions regarding the number of RNAs modified by A-to-I conversion and those tissues in which they were expressed.
While ADAR1 and ADAR2 have been shown to be expressed in almost all cell types examined (Melcher et al. 1996b; Wagner et al. 1990), the observation that ADAR expression levels are greatest in the brain (Melcher et al. 1996a; Melcher et al. 1996b), accompanied by the development of a candidate-based approach to identify mRNA targets of A-to-I editing in Drosophila (Hoopengardner et al. 2003), further supported the idea that editing was enriched in the nervous system. The majority of ADAR targets identified using this strategy were voltage- or ligand-gated ion channels or components of the synaptic vesicular release machinery and independently identified substrates for editing in Drosophila also encoded nervous system signaling components including the paralysis (para) voltage-gated Na+ channel (Hanrahan et al. 2000), the cacophony (cac) voltage-gated Ca2+ channel (Peixoto et al. 1997; Smith et al. 1998) and the glutamate-gated Cl− channel (DrosGluCl-α) (Semenov and Pak 1999). In addition, flies lacking Drosophila ADAR (dADAR) expression exhibited extreme behavioral deficits including temperature-sensitive paralysis, locomotor uncoordination, tremors which increased in severity with age and neurodegeneration (Palladino et al. 2000). Despite this apparent preponderance of editing events in the nervous system, few studies have focused upon editing in peripheral tissues. More recent studies using a transcriptome-wide analysis of neuronal and non-neuronal tissues (cerebellum, frontal lobe, corpus callosum, diencephalon, small intestine, kidney and adrenal gland) identified and validate numerous, novel editing events in multiple transcripts, yet the extent of editing for these RNAs was generally less for tissues outside the central nervous system (Li et al. 2009). The recent development of massively parallel, high-throughput sequencing strategies (Bentley et al. 2008; Mortazavi et al. 2008) should provide an effective strategy for the identification of A-to-G discrepancies between genomic and cDNA sequences from multiple tissues in a single organism. This experimental paradigm not only provides for a quantitative analysis of novel editing sites, but also reduces the possibility that any observed A-to-G disparity results from a single nucleotide polymorphism.
From alterations in coding potential to changes in structure, stability, translation efficiency and splicing, RNA editing can affect almost all aspects of cellular RNA function. In most cases, RNA editing of protein-coding genes has been shown to generate multiple protein isoforms and to diversify protein function. While the first ten years of inquiry into the mechanisms of A-to-I conversion provided dramatic advances in our understanding of the enzymatic activities and biochemical mechanisms underlying this RNA processing event, the second decade of study has largely focused upon further identification of ADAR substrates and a determination of how such editing-mediated changes in coding potential can affect protein function. The ultimate biological relevance of RNA editing resides with the specific substrates that are modified by this process. As we enter a third decade of investigation, many investigators will strive to further identify RNA targets of A-to-I conversion using state-of-the art sequencing technologies. Other laboratories will examine the functional consequences of identified recoding sites in novel ADAR targets and determine whether dysregulation of editing for specific transcripts is associated with an alteration in phenotype or associations with human disorders.
Acknowledgments
This work was supported by funding from the National Institutes of Health, the Vanderbilt Silvio O. Conte Center for Neuroscience Research and the Vanderbilt Kennedy Center.
References
- Abbas AI, Urban DJ, Jensen NH, Farrell MS, Kroeze WK, Mieczkowski P, Wang Z, Roth BL. Assessing serotonin receptor mRNA editing frequency by a novel ultra high-throughput sequencing method. Nucleic acids research. 2010;38:e118. doi: 10.1093/nar/gkq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah L, Bonasera SJ, Hopf FW, O’Dell L, Giorgetti M, Jongsma M, Carra S, Pierucci M, Di Giovanni G, Esposito E, Parsons LH, Bonci A, Tecott LH. Impact of serotonin 2C receptor null mutation on physiology and behavior associated with nigrostriatal dopamine pathway function. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:8156–65. doi: 10.1523/JNEUROSCI.3905-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ademmer K, Beutel M, Bretzel R, Jaeger C, Reimer C. Suicidal ideation with IFN-alpha and ribavirin in a patient with hepatitis C. Psychosomatics. 2001;42:365–7. doi: 10.1176/appi.psy.42.4.365. [DOI] [PubMed] [Google Scholar]
- Al-Sabi A, Shamotienko O, Dhochartaigh SN, Muniyappa N, Le Berre M, Shaban H, Wang J, Sack JT, Dolly JO. Arrangement of Kv1 alpha subunits dictates sensitivity to tetraethylammonium. The Journal of general physiology. 2010;136:273–82. doi: 10.1085/jgp.200910398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasiadis A, Rich A, Maas S. Widespread A-to-I RNA editing of Alu-containing mRNAs in the human transcriptome. PLoS biology. 2004;2:e391. doi: 10.1371/journal.pbio.0020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin TJ, Isacoff E, Li M, Lopez GA, Sheng M, Tsaur ML, Yan YN, Jan LY. Elucidation of biophysical and biological properties of voltage-gated potassium channels. Cold Spring Harbor symposia on quantitative biology. 1992;57:491–9. doi: 10.1101/sqb.1992.057.01.054. [DOI] [PubMed] [Google Scholar]
- Baranauskas G. Ionic channel function in action potential generation: current perspective. Molecular neurobiology. 2007;35:129–50. doi: 10.1007/s12035-007-8001-0. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Bass BL. RNA editing by adenosine deaminases that act on RNA. Annual review of biochemistry. 2002;71:817–46. doi: 10.1146/annurev.biochem.71.110601.135501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MV, Bloomfield J, McKinley M, Patterson MN, Darlison MG, Barnard EA, Davies KE. Physical linkage of a GABAA receptor subunit gene to the DXS374 locus in human Xq28. Am J Hum Genet. 1989;45:883–8. [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. GABA excites and sculpts immature neurons well before delivery: modulation by GABA of the development of ventricular progenitor cells. Epilepsy currents / American Epilepsy Society. 2007;7:167–9. doi: 10.1111/j.1535-7511.2007.00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley DR, Balasubramanian S, Swerdlow HP, Smith GP, Milton J, Brown CG, Hall KP, Evers DJ, Barnes CL, Bignell HR, Boutell JM, Bryant J, Carter RJ, Keira Cheetham R, Cox AJ, Ellis DJ, Flatbush MR, Gormley NA, Humphray SJ, Irving LJ, Karbelashvili MS, Kirk SM, Li H, Liu X, Maisinger KS, Murray LJ, Obradovic B, Ost T, Parkinson ML, Pratt MR, Rasolonjatovo IM, Reed MT, Rigatti R, Rodighiero C, Ross MT, Sabot A, Sankar SV, Scally A, Schroth GP, Smith ME, Smith VP, Spiridou A, Torrance PE, Tzonev SS, Vermaas EH, Walter K, Wu X, Zhang L, Alam MD, Anastasi C, Aniebo IC, Bailey DM, Bancarz IR, Banerjee S, Barbour SG, Baybayan PA, Benoit VA, Benson KF, Bevis C, Black PJ, Boodhun A, Brennan JS, Bridgham JA, Brown RC, Brown AA, Buermann DH, Bundu AA, Burrows JC, Carter NP, Castillo N, Chiara ECM, Chang S, Neil Cooley R, Crake NR, Dada OO, Diakoumakos KD, Dominguez-Fernandez B, Earnshaw DJ, Egbujor UC, Elmore DW, Etchin SS, Ewan MR, Fedurco M, Fraser LJ, Fuentes Fajardo KV, Scott Furey W, George D, Gietzen KJ, Goddard CP, Golda GS, Granieri PA, Green DE, Gustafson DL, Hansen NF, Harnish K, Haudenschild CD, Heyer NI, Hims MM, Ho JT, Horgan AM, et al. Accurate whole human genome sequencing using reversible terminator chemistry. Nature. 2008;456:53–9. doi: 10.1038/nature07517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KA, Clarke WP, Sailstad C, Saltzman A, Maayani S. Signal transduction differences between 5-hydroxytryptamine type 2A and type 2C receptor systems. Molecular pharmacology. 1994;46:477–84. [PubMed] [Google Scholar]
- Berg KA, Cropper JD, Niswender CM, Sanders-Bush E, Emeson RB, Clarke WP. RNA-editing of the 5-HT(2C) receptor alters agonist-receptor-effector coupling specificity. British journal of pharmacology. 2001;134:386–92. doi: 10.1038/sj.bjp.0704255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, Clarke WP. Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Molecular pharmacology. 1998;54:94–104. [PubMed] [Google Scholar]
- Bettler B, Boulter J, Hermans-Borgmeyer I, O’Shea-Greenfield A, Deneris ES, Moll C, Borgmeyer U, Hollmann M, Heinemann S. Cloning of a novel glutamate receptor subunit, GluR5: expression in the nervous system during development. Neuron. 1990;5:583–95. doi: 10.1016/0896-6273(90)90213-y. [DOI] [PubMed] [Google Scholar]
- Bhalla T, Rosenthal JJ, Holmgren M, Reenan R. Control of human potassium channel inactivation by editing of a small mRNA hairpin. Nature structural & molecular biology. 2004;11:950–6. doi: 10.1038/nsmb825. [DOI] [PubMed] [Google Scholar]
- Bjork GR. Genetic dissection of synthesis and function of modified nucleosides in bacterial transfer RNA. Progress in nucleic acid research and molecular biology. 1995;50:263–338. doi: 10.1016/s0079-6603(08)60817-x. [DOI] [PubMed] [Google Scholar]
- Blow M, Futreal PA, Wooster R, Stratton MR. A survey of RNA editing in human brain. Genome research. 2004;14:2379–87. doi: 10.1101/gr.2951204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blow MJ, Grocock RJ, van Dongen S, Enright AJ, Dicks E, Futreal PA, Wooster R, Stratton MR. RNA editing of human microRNAs. Genome biology. 2006;7:R27. doi: 10.1186/gb-2006-7-4-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockaert J, Claeysen S, Becamel C, Dumuis A, Marin P. Neuronal 5-HT metabotropic receptors: fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell and tissue research. 2006;326:553–72. doi: 10.1007/s00441-006-0286-1. [DOI] [PubMed] [Google Scholar]
- Bosman LW, Rosahl TW, Brussaard AB. Neonatal development of the rat visual cortex: synaptic function of GABAA receptor alpha subunits. J Physiol. 2002;545:169–81. doi: 10.1113/jphysiol.2002.026534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan TJ, Seeley WW, Kilgard M, Schreiner CE, Tecott LH. Sound-induced seizures in serotonin 5-HT2c receptor mutant mice. Nature genetics. 1997;16:387–90. doi: 10.1038/ng0897-387. [DOI] [PubMed] [Google Scholar]
- Brusa R, Zimmermann F, Koh DS, Feldmeyer D, Gass P, Seeburg PH, Sprengel R. Early-onset epilepsy and postnatal lethality associated with an editing-deficient GluR-B allele in mice. Science. 1995;270:1677–80. doi: 10.1126/science.270.5242.1677. [DOI] [PubMed] [Google Scholar]
- Burd CG, Dreyfuss G. Conserved structures and diversity of functions of RNA-binding proteins. Science. 1994;265:615–21. doi: 10.1126/science.8036511. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Khodorova A, Jonas P, Helm PJ, Wisden W, Monyer H, Seeburg PH, Sakmann B. Calcium-permeable AMPA-kainate receptors in fusiform cerebellar glial cells. Science. 1992;256:1566–70. doi: 10.1126/science.1317970. [DOI] [PubMed] [Google Scholar]
- Burns CM, Chu H, Rueter SM, Hutchinson LK, Canton H, Sanders-Bush E, Emeson RB. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature. 1997;387:303–8. doi: 10.1038/387303a0. [DOI] [PubMed] [Google Scholar]
- Cai W, Khaoustov VI, Xie Q, Pan T, Le W, Yoffe B. Interferon-alpha-induced modulation of glucocorticoid and serotonin receptors as a mechanism of depression. Journal of hepatology. 2005;42:880–7. doi: 10.1016/j.jhep.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Cancedda L, Fiumelli H, Chen K, Poo MM. Excitatory GABA action is essential for morphological maturation of cortical neurons in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:5224–35. doi: 10.1523/JNEUROSCI.5169-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SJ, Johnstone KA, DuBose AJ, Simon TA, Bartolomei MS, Resnick JL, Brannan CI. Evidence for genetic modifiers of postnatal lethality in PWS-IC deletion mice. Human molecular genetics. 2004;13:2971–7. doi: 10.1093/hmg/ddh314. [DOI] [PubMed] [Google Scholar]
- Chen CX, Cho DS, Wang Q, Lai F, Carter KC, Nishikura K. A third member of the RNA-specific adenosine deaminase gene family, ADAR3, contains both single- and double-stranded RNA binding domains. RNA. 2000;6:755–67. doi: 10.1017/s1355838200000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LL, DeCerbo JN, Carmichael GG. Alu element-mediated gene silencing. The EMBO journal. 2008;27:1694–705. doi: 10.1038/emboj.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittajallu R, Braithwaite SP, Clarke VR, Henley JM. Kainate receptors: subunits, synaptic localization and function. Trends in pharmacological sciences. 1999;20:26–35. doi: 10.1016/s0165-6147(98)01286-3. [DOI] [PubMed] [Google Scholar]
- Coleman SK, Moykkynen T, Cai C, von Ossowski L, Kuismanen E, Korpi ER, Keinanen K. Isoform-specific early trafficking of AMPA receptor flip and flop variants. J Neurosci. 2006;26:11220–9. doi: 10.1523/JNEUROSCI.2301-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman SK, Newcombe J, Pryke J, Dolly JO. Subunit composition of Kv1 channels in human CNS. Journal of neurochemistry. 1999;73:849–58. doi: 10.1046/j.1471-4159.1999.0730849.x. [DOI] [PubMed] [Google Scholar]
- Crick FH. Codon–anticodon pairing: the wobble hypothesis. Journal of molecular biology. 1966;19:548–55. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- Curran ME, Landes GM, Keating MT. Molecular cloning, characterization, and genomic localization of a human potassium channel gene. Genomics. 1992;12:729–37. doi: 10.1016/0888-7543(92)90302-9. [DOI] [PubMed] [Google Scholar]
- D’Hulst C, Atack JR, Kooy RF. The complexity of the GABAA receptor shapes unique pharmacological profiles. Drug Discov Today. 2009;14:866–75. doi: 10.1016/j.drudis.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Daniel C, Wahlstedt H, Ohlson J, Bjork P, Ohman M. Adenosine-to-inosine RNA editing affects trafficking of the gamma-aminobutyric acid type A (GABA(A)) receptor. J Biol Chem. 2011;286:2031–40. doi: 10.1074/jbc.M110.130096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal KK, Lovinger DM, Tamkun MM. The brain Kv1.1 potassium channel: in vitro and in vivo studies on subunit assembly and posttranslational processing. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14:1666–76. doi: 10.1523/JNEUROSCI.14-03-01666.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decher N, Streit AK, Rapedius M, Netter MF, Marzian S, Ehling P, Schlichthorl G, Craan T, Renigunta V, Kohler A, Dodel RC, Navarro-Polanco RA, Preisig-Muller R, Klebe G, Budde T, Baukrowitz T, Daut J. RNA editing modulates the binding of drugs and highly unsaturated fatty acids to the open pore of Kv potassium channels. The EMBO journal. 2010;29:2101–13. doi: 10.1038/emboj.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry JM, Barnard PJ. Mapping of the glycine receptor alpha 2-subunit gene and the GABAA alpha 3-subunit gene on the mouse X chromosome. Genomics. 1991;10:593–7. doi: 10.1016/0888-7543(91)90441-g. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Hume RI, Heinemann SF. Structural determinants of barium permeation and rectification in non-NMDA glutamate receptor channels. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1992;12:4080–7. doi: 10.1523/JNEUROSCI.12-10-04080.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe CM, Relkovic D, Garfield AS, Dalley JW, Theobald DE, Humby T, Wilkinson LS, Isles AR. Loss of the imprinted snoRNA mbii-52 leads to increased 5htr2c pre-RNA editing and altered 5HT2CR-mediated behaviour. Human molecular genetics. 2009;18:2140–8. doi: 10.1093/hmg/ddp137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Eckmann CR, Neunteufl A, Pfaffstetter L, Jantsch MF. The human but not the Xenopus RNA-editing enzyme ADAR1 has an atypical nuclear localization signal and displays the characteristics of a shuttling protein. Molecular biology of the cell. 2001;12:1911–24. doi: 10.1091/mbc.12.7.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egebjerg J, Bettler B, Hermans-Borgmeyer I, Heinemann S. Cloning of a cDNA for a glutamate receptor subunit activated by kainate but not AMPA. Nature. 1991;351:745–8. doi: 10.1038/351745a0. [DOI] [PubMed] [Google Scholar]
- Egebjerg J, Heinemann SF. Ca2+ permeability of unedited and edited versions of the kainate selective glutamate receptor GluR6. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:755–9. doi: 10.1073/pnas.90.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeson R, Singh M. Adenosine-to-inosine RNA editing: substrates and consequences. In: Bass B, editor. RNA Editing. Oxford University Press; Oxford: 2001. pp. 109–138. [Google Scholar]
- Englander MT, Dulawa SC, Bhansali P, Schmauss C. How stress and fluoxetine modulate serotonin 2C receptor pre-mRNA editing. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:648–51. doi: 10.1523/JNEUROSCI.3895-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enstero M, Akerborg O, Lundin D, Wang B, Furey TS, Ohman M, Lagergren J. A computational screen for site selective A-to-I editing detects novel sites in neuron specific Hu proteins. BMC bioinformatics. 2010;11:6. doi: 10.1186/1471-2105-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi P, Czirjak G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiological reviews. 2010;90:559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- Ernst M, Bruckner S, Boresch S, Sieghart W. Comparative models of GABAA receptor extracellular and transmembrane domains: important insights in pharmacology and function. Molecular pharmacology. 2005;68:1291–300. doi: 10.1124/mol.105.015982. [DOI] [PubMed] [Google Scholar]
- Feng Y, Sansam CL, Singh M, Emeson RB. Altered RNA editing in mice lacking ADAR2 autoregulation. Molecular and cellular biology. 2006;26:480–8. doi: 10.1128/MCB.26.2.480-488.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro-Monti I, Mathews MB. Proteins binding to duplexed RNA: one motif, multiple functions. Trends in biochemical sciences. 2000;25:241–6. doi: 10.1016/s0968-0004(00)01580-2. [DOI] [PubMed] [Google Scholar]
- Fisher JL. The anti-convulsant stiripentol acts directly on the GABA(A) receptor as a positive allosteric modulator. Neuropharmacology. 2009;56:190–7. doi: 10.1016/j.neuropharm.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald LW, Iyer G, Conklin DS, Krause CM, Marshall A, Patterson JP, Tran DP, Jonak GJ, Hartig PR. Messenger RNA editing of the human serotonin 5-HT2C receptor. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1999;21:82S–90S. doi: 10.1016/S0893-133X(99)00004-4. [DOI] [PubMed] [Google Scholar]
- Flomen R, Knight J, Sham P, Kerwin R, Makoff A. Evidence that RNA editing modulates splice site selection in the 5-HT2C receptor gene. Nucleic acids research. 2004;32:2113–22. doi: 10.1093/nar/gkh536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz J, Strehblow A, Taschner A, Schopoff S, Pasierbek P, Jantsch MF. RNA-regulated interaction of transportin-1 and exportin-5 with the double-stranded RNA-binding domain regulates nucleocytoplasmic shuttling of ADAR1. Molecular and cellular biology. 2009;29:1487–97. doi: 10.1128/MCB.01519-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Wang PG, Yang S, Hu XL, Zhang KY, Zhu YG, Ren YQ, Du WH, Zhang GL, Cui Y, Chen JJ, Yan KL, Xiao FL, Xu SJ, Huang W, Zhang XJ. Two frameshift mutations in the RNA-specific adenosine deaminase gene associated with dyschromatosis symmetrica hereditaria. Archives of dermatology. 2005;141:193–6. doi: 10.1001/archderm.141.2.193. [DOI] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–93. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George CX, Samuel CE. Human RNA-specific adenosine deaminase ADAR1 transcripts possess alternative exon 1 structures that initiate from different promoters, one constitutively active and the other interferon inducible. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:4621–6. doi: 10.1073/pnas.96.8.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber A, Grosjean H, Melcher T, Keller W. Tad1p, a yeast tRNA-specific adenosine deaminase, is related to the mammalian pre-mRNA editing enzymes ADAR1 and ADAR2. The EMBO journal. 1998;17:4780–9. doi: 10.1093/emboj/17.16.4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber A, O’Connell MA, Keller W. Two forms of human double-stranded RNA-specific editase 1 (hRED1) generated by the insertion of an Alu cassette. RNA. 1997;3:453–63. [PMC free article] [PubMed] [Google Scholar]
- Giese KP, Storm JF, Reuter D, Fedorov NB, Shao LR, Leicher T, Pongs O, Silva AJ. Reduced K+ channel inactivation, spike broadening, and after-hyperpolarization in Kvbeta1.1-deficient mice with impaired learning. Learning & memory. 1998;5:257–73. [PMC free article] [PubMed] [Google Scholar]
- Giorgetti M, Tecott LH. Contributions of 5-HT(2C) receptors to multiple actions of central serotonin systems. European journal of pharmacology. 2004;488:1–9. doi: 10.1016/j.ejphar.2004.01.036. [DOI] [PubMed] [Google Scholar]
- Goldstone AP. Prader-Willi syndrome: advances in genetics, pathophysiology and treatment. Trends in endocrinology and metabolism: TEM. 2004;15:12–20. doi: 10.1016/j.tem.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Gott JM, Emeson RB. Functions and mechanisms of RNA editing. Annual review of genetics. 2000;34:499–531. doi: 10.1146/annurev.genet.34.1.499. [DOI] [PubMed] [Google Scholar]
- Greger IH, Akamine P, Khatri L, Ziff EB. Developmentally regulated, combinatorial RNA processing modulates AMPA receptor biogenesis. Neuron. 2006;51:85–97. doi: 10.1016/j.neuron.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Greger IH, Khatri L, Kong X, Ziff EB. AMPA receptor tetramerization is mediated by Q/R editing. Neuron. 2003;40:763–74. doi: 10.1016/s0896-6273(03)00668-8. [DOI] [PubMed] [Google Scholar]
- Greger IH, Khatri L, Ziff EB. RNA editing at arg607 controls AMPA receptor exit from the endoplasmic reticulum. Neuron. 2002;34:759–72. doi: 10.1016/s0896-6273(02)00693-1. [DOI] [PubMed] [Google Scholar]
- Greger IH, Ziff EB, Penn AC. Molecular determinants of AMPA receptor subunit assembly. Trends in neurosciences. 2007;30:407–16. doi: 10.1016/j.tins.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Gurevich I, Englander MT, Adlersberg M, Siegal NB, Schmauss C. Modulation of serotonin 2C receptor editing by sustained changes in serotonergic neurotransmission. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002a;22:10529–32. doi: 10.1523/JNEUROSCI.22-24-10529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich I, Tamir H, Arango V, Dwork AJ, Mann JJ, Schmauss C. Altered editing of serotonin 2C receptor pre-mRNA in the prefrontal cortex of depressed suicide victims. Neuron. 2002b;34:349–56. doi: 10.1016/s0896-6273(02)00660-8. [DOI] [PubMed] [Google Scholar]
- Gutman GA, Chandy KG, Grissmer S, Lazdunski M, McKinnon D, Pardo LA, Robertson GA, Rudy B, Sanguinetti MC, Stuhmer W, Wang X. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacological reviews. 2005;57:473–508. doi: 10.1124/pr.57.4.10. [DOI] [PubMed] [Google Scholar]
- Hanrahan CJ, Palladino MJ, Ganetzky B, Reenan RA. RNA editing of the Drosophila para Na(+) channel transcript. Evolutionary conservation and developmental regulation. Genetics. 2000;155:1149–60. doi: 10.1093/genetics/155.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartner JC, Schmittwolf C, Kispert A, Muller AM, Higuchi M, Seeburg PH. Liver disintegration in the mouse embryo caused by deficiency in the RNA-editing enzyme ADAR1. The Journal of biological chemistry. 2004;279:4894–902. doi: 10.1074/jbc.M311347200. [DOI] [PubMed] [Google Scholar]
- Heinemann S, Rettig J, Scott V, Parcej DN, Lorra C, Dolly J, Pongs O. Journal of Physiology. Vol. 88. Paris: 1994. The inactivation behaviour of voltage-gated K-channels may be determined by association of alpha- and beta-subunits; pp. 173–80. [DOI] [PubMed] [Google Scholar]
- Henschel O, Gipson KE, Bordey A. GABAA receptors, anesthetics and anticonvulsants in brain development. CNS Neurol Disord Drug Targets. 2008;7:211–24. doi: 10.2174/187152708784083812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert A, Rich A. The biology of left-handed Z-DNA. The Journal of biological chemistry. 1996;271:11595–8. doi: 10.1074/jbc.271.20.11595. [DOI] [PubMed] [Google Scholar]
- Herson PS, Virk M, Rustay NR, Bond CT, Crabbe JC, Adelman JP, Maylie J. A mouse model of episodic ataxia type-1. Nature neuroscience. 2003;6:378–83. doi: 10.1038/nn1025. [DOI] [PubMed] [Google Scholar]
- Hideyama T, Yamashita T, Suzuki T, Tsuji S, Higuchi M, Seeburg PH, Takahashi R, Misawa H, Kwak S. Induced loss of ADAR2 engenders slow death of motor neurons from Q/R site-unedited GluR2. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:11917–25. doi: 10.1523/JNEUROSCI.2021-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M, Maas S, Single FN, Hartner J, Rozov A, Burnashev N, Feldmeyer D, Sprengel R, Seeburg PH. Point mutation in an AMPA receptor gene rescues lethality in mice deficient in the RNA-editing enzyme ADAR2. Nature. 2000;406:78–81. doi: 10.1038/35017558. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Single FN, Kohler M, Sommer B, Sprengel R, Seeburg PH. RNA editing of AMPA receptor subunit GluR-B: a base-paired intron-exon structure determines position and efficiency. Cell. 1993;75:1361–70. doi: 10.1016/0092-8674(93)90622-w. [DOI] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–75. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- Hogg M, Paro S, Keegan LP, O’Connell MA. RNA editing by mammalian ADARs. Advances in genetics. 2011;73:87–120. doi: 10.1016/B978-0-12-380860-8.00003-3. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Hartley M, Heinemann S. Ca2+ permeability of KA-AMPA–gated glutamate receptor channels depends on subunit composition. Science. 1991;252:851–3. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- Honore E, Barhanin J, Attali B, Lesage F, Lazdunski M. External blockade of the major cardiac delayed-rectifier K+ channel (Kv1.5) by polyunsaturated fatty acids. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:1937–41. doi: 10.1073/pnas.91.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopengardner B, Bhalla T, Staber C, Reenan R. Nervous system targets of RNA editing identified by comparative genomics. Science. 2003;301:832–6. doi: 10.1126/science.1086763. [DOI] [PubMed] [Google Scholar]
- Hough RF, Bass BL. Purification of the Xenopus laevis double-stranded RNA adenosine deaminase. The Journal of biological chemistry. 1994;269:9933–9. [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacological reviews. 1994;46:157–203. [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacology, biochemistry, and behavior. 2002;71:533–54. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Hutcheon B, Fritschy JM, Poulter MO. Organization of GABA receptor alpha-subunit clustering in the developing rat neocortex and hippocampus. Eur J Neurosci. 2004;19:2475–87. doi: 10.1111/j.0953-816X.2004.03349.x. [DOI] [PubMed] [Google Scholar]
- Iino M, Goto K, Kakegawa W, Okado H, Sudo M, Ishiuchi S, Miwa A, Takayasu Y, Saito I, Tsuzuki K, Ozawa S. Glia-synapse interaction through Ca2+-permeable AMPA receptors in Bergmann glia. Science. 2001;292:926–9. doi: 10.1126/science.1058827. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Nakamura Y, Saitoh N, Li WB, Iwasaki S, Takahashi T. Distinct roles of Kv1 and Kv3 potassium channels at the calyx of Held presynaptic terminal. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:10445–53. doi: 10.1523/JNEUROSCI.23-32-10445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto K, Kato T. RNA editing of serotonin 2C receptor in human postmortem brains of major mental disorders. Neuroscience letters. 2003;346:169–72. doi: 10.1016/s0304-3940(03)00608-6. [DOI] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Cloned potassium channels from eukaryotes and prokaryotes. Annual review of neuroscience. 1997;20:91–123. doi: 10.1146/annurev.neuro.20.1.91. [DOI] [PubMed] [Google Scholar]
- Jenkins A, Greenblatt EP, Faulkner HJ, Bertaccini E, Light A, Lin A, Andreasen A, Viner A, Trudell JR, Harrison NL. Evidence for a common binding cavity for three general anesthetics within the GABAA receptor. J Neurosci. 2001;21:RC136. doi: 10.1523/JNEUROSCI.21-06-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamb A, Iverson LE, Tanouye MA. Molecular characterization of Shaker, a Drosophila gene that encodes a potassium channel. Cell. 1987;50:405–13. doi: 10.1016/0092-8674(87)90494-6. [DOI] [PubMed] [Google Scholar]
- Kawahara Y, Ito K, Sun H, Aizawa H, Kanazawa I, Kwak S. Glutamate receptors: RNA editing and death of motor neurons. Nature. 2004;427:801. doi: 10.1038/427801a. [DOI] [PubMed] [Google Scholar]
- Kawahara Y, Kwak S, Sun H, Ito K, Hashida H, Aizawa H, Jeong SY, Kanazawa I. Human spinal motoneurons express low relative abundance of GluR2 mRNA: an implication for excitotoxicity in ALS. Journal of neurochemistry. 2003;85:680–9. doi: 10.1046/j.1471-4159.2003.01703.x. [DOI] [PubMed] [Google Scholar]
- Kawahara Y, Sun H, Ito K, Hideyama T, Aoki M, Sobue G, Tsuji S, Kwak S. Underediting of GluR2 mRNA, a neuronal death inducing molecular change in sporadic ALS, does not occur in motor neurons in ALS1 or SBMA. Neuroscience research. 2006;54:11–4. doi: 10.1016/j.neures.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Kawahara Y, Zinshteyn B, Sethupathy P, Iizasa H, Hatzigeorgiou AG, Nishikura K. Redirection of silencing targets by adenosine-to-inosine editing of miRNAs. Science. 2007;315:1137–40. doi: 10.1126/science.1138050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuno R, Nagase T, Waki M, Ohara O. HUGE: a database for human large proteins identified in the Kazusa cDNA sequencing project. Nucleic acids research. 2002;30:166–8. doi: 10.1093/nar/30.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DD, Kim TT, Walsh T, Kobayashi Y, Matise TC, Buyske S, Gabriel A. Widespread RNA editing of embedded alu elements in the human transcriptome. Genome research. 2004;14:1719–25. doi: 10.1101/gr.2855504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim U, Nishikura K. Double-stranded RNA adenosine deaminase as a potential mammalian RNA editing factor. Seminars in cell biology. 1993;4:285–93. doi: 10.1006/scel.1993.1034. [DOI] [PubMed] [Google Scholar]
- Kim U, Wang Y, Sanford T, Zeng Y, Nishikura K. Molecular cloning of cDNA for double-stranded RNA adenosine deaminase, a candidate enzyme for nuclear RNA editing. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:11457–61. doi: 10.1073/pnas.91.24.11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore S, Stamm S. Regulation of alternative splicing by snoRNAs. Cold Spring Harbor symposia on quantitative biology. 2006;71:329–34. doi: 10.1101/sqb.2006.71.024. [DOI] [PubMed] [Google Scholar]
- Klocke R, Roberds SL, Tamkun MM, Gronemeier M, Augustin A, Albrecht B, Pongs O, Jockusch H. Chromosomal mapping in the mouse of eight K(+)-channel genes representing the four Shaker-like subfamilies Shaker, Shab, Shaw, and Shal. Genomics. 1993;18:568–74. doi: 10.1016/s0888-7543(05)80358-1. [DOI] [PubMed] [Google Scholar]
- Kohler M, Burnashev N, Sakmann B, Seeburg PH. Determinants of Ca2+ permeability in both TM1 and TM2 of high affinity kainate receptor channels: diversity by RNA editing. Neuron. 1993;10:491–500. doi: 10.1016/0896-6273(93)90336-p. [DOI] [PubMed] [Google Scholar]
- Koike M, Tsukada S, Tsuzuki K, Kijima H, Ozawa S. Regulation of kinetic properties of GluR2 AMPA receptor channels by alternative splicing. J Neurosci. 2000;20:2166–74. doi: 10.1523/JNEUROSCI.20-06-02166.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi ER, Debus F, Linden AM, Malecot C, Leppa E, Vekovischeva O, Rabe H, Bohme I, Aller MI, Wisden W, Luddens H. Does ethanol act preferentially via selected brain GABAA receptor subtypes? the current evidence is ambiguous. Alcohol. 2007;41:163–76. doi: 10.1016/j.alcohol.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Krampfl K, Schlesinger F, Zorner A, Kappler M, Dengler R, Bufler J. Control of kinetic properties of GluR2 flop AMPA-type channels: impact of R/G nuclear editing. Eur J Neurosci. 2002;15:51–62. doi: 10.1046/j.0953-816x.2001.01841.x. [DOI] [PubMed] [Google Scholar]
- Kwak S, Kawahara Y. Deficient RNA editing of GluR2 and neuronal death in amyotropic lateral sclerosis. Journal of molecular medicine. 2005;83:110–20. doi: 10.1007/s00109-004-0599-z. [DOI] [PubMed] [Google Scholar]
- Lai F, Chen CX, Carter KC, Nishikura K. Editing of glutamate receptor B subunit ion channel RNAs by four alternatively spliced DRADA2 double-stranded RNA adenosine deaminases. Molecular and cellular biology. 1997;17:2413–24. doi: 10.1128/mcb.17.5.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12:4151–72. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinekugel X, Khalilov I, McLean H, Caillard O, Gaiarsa JL, Ben-Ari Y, Khazipov R. GABA is the principal fast-acting excitatory transmitter in the neonatal brain. Adv Neurol. 1999;79:189–201. [PubMed] [Google Scholar]
- Levanon EY, Eisenberg E, Yelin R, Nemzer S, Hallegger M, Shemesh R, Fligelman ZY, Shoshan A, Pollock SR, Sztybel D, Olshansky M, Rechavi G, Jantsch MF. Systematic identification of abundant A-to-I editing sites in the human transcriptome. Nature biotechnology. 2004;22:1001–5. doi: 10.1038/nbt996. [DOI] [PubMed] [Google Scholar]
- Li JB, Levanon EY, Yoon JK, Aach J, Xie B, Leproust E, Zhang K, Gao Y, Church GM. Genome-wide identification of human RNA editing sites by parallel DNA capturing and sequencing. Science. 2009;324:1210–3. doi: 10.1126/science.1170995. [DOI] [PubMed] [Google Scholar]
- Liu Y, Emeson RB, Samuel CE. Serotonin-2C receptor pre-mRNA editing in rat brain and in vitro by splice site variants of the interferon-inducible double-stranded RNA-specific adenosine deaminase ADAR1. The Journal of biological chemistry. 1999;274:18351–8. doi: 10.1074/jbc.274.26.18351. [DOI] [PubMed] [Google Scholar]
- Liu Y, George CX, Patterson JB, Samuel CE. Functionally distinct double-stranded RNA-binding domains associated with alternative splice site variants of the interferon-inducible double-stranded RNA-specific adenosine deaminase. The Journal of biological chemistry. 1997;272:4419–28. doi: 10.1074/jbc.272.7.4419. [DOI] [PubMed] [Google Scholar]
- Lomeli H, Mosbacher J, Melcher T, Hoger T, Geiger JR, Kuner T, Monyer H, Higuchi M, Bach A, Seeburg PH. Control of kinetic properties of AMPA receptor channels by nuclear RNA editing. Science. 1994;266:1709–13. doi: 10.1126/science.7992055. [DOI] [PubMed] [Google Scholar]
- Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- Lotrich FE, Ferrell RE, Rabinovitz M, Pollock BG. Risk for depression during interferon-alpha treatment is affected by the serotonin transporter polymorphism. Biological psychiatry. 2009;65:344–8. doi: 10.1016/j.biopsych.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, Fritschy JM, Rulicke T, Bluethmann H, Mohler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–4. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- Luciano DJ, Mirsky H, Vendetti NJ, Maas S. RNA editing of a miRNA precursor. RNA. 2004;10:1174–7. doi: 10.1261/rna.7350304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas S, Melcher T, Herb A, Seeburg PH, Keller W, Krause S, Higuchi M, O’Connell MA. Structural requirements for RNA editing in glutamate receptor pre-mRNAs by recombinant double-stranded RNA adenosine deaminase. The Journal of biological chemistry. 1996;271:12221–6. doi: 10.1074/jbc.271.21.12221. [DOI] [PubMed] [Google Scholar]
- Maas S, Rich A, Nishikura K. A-to-I RNA editing: recent news and residual mysteries. The Journal of biological chemistry. 2003;278:1391–4. doi: 10.1074/jbc.R200025200. [DOI] [PubMed] [Google Scholar]
- MacDonald RL, Rogers CJ, Twyman RE. Barbiturate regulation of kinetic properties of the GABAA receptor channel of mouse spinal neurones in culture. J Physiol. 1989;417:483–500. doi: 10.1113/jphysiol.1989.sp017814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 1991;350:232–5. doi: 10.1038/350232a0. [DOI] [PubMed] [Google Scholar]
- Marion S, Weiner DM, Caron MG. RNA editing induces variation in desensitization and trafficking of 5-hydroxytryptamine 2c receptor isoforms. The Journal of biological chemistry. 2004;279:2945–54. doi: 10.1074/jbc.M308742200. [DOI] [PubMed] [Google Scholar]
- Meera P, Olsen RW, Otis TS, Wallner M. Alcohol- and alcohol antagonist-sensitive human GABAA receptors: tracking delta subunit incorporation into functional receptors. Mol Pharmacol. 2010;78:918–24. doi: 10.1124/mol.109.062687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AK, Ticku MK. An update on GABAA receptors. Brain Res Brain Res Rev. 1999;29:196–217. doi: 10.1016/s0165-0173(98)00052-6. [DOI] [PubMed] [Google Scholar]
- Melcher T, Maas S, Herb A, Sprengel R, Higuchi M, Seeburg PH. RED2, a brain-specific member of the RNA-specific adenosine deaminase family. The Journal of biological chemistry. 1996a;271:31795–8. doi: 10.1074/jbc.271.50.31795. [DOI] [PubMed] [Google Scholar]
- Melcher T, Maas S, Herb A, Sprengel R, Seeburg PH, Higuchi M. A mammalian RNA editing enzyme. Nature. 1996b;379:460–4. doi: 10.1038/379460a0. [DOI] [PubMed] [Google Scholar]
- Melcher T, Maas S, Higuchi M, Keller W, Seeburg PH. Editing of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR-B pre-mRNA in vitro reveals site-selective adenosine to inosine conversion. The Journal of biological chemistry. 1995;270:8566–70. doi: 10.1074/jbc.270.15.8566. [DOI] [PubMed] [Google Scholar]
- Menkes DB, MacDonald JA. Interferons, serotonin and neurotoxicity. Psychological medicine. 2000;30:259–68. doi: 10.1017/s0033291799001774. [DOI] [PubMed] [Google Scholar]
- Miyamura Y, Suzuki T, Kono M, Inagaki K, Ito S, Suzuki N, Tomita Y. Mutations of the RNA-specific adenosine deaminase gene (DSRAD) are involved in dyschromatosis symmetrica hereditaria. American journal of human genetics. 2003;73:693–9. doi: 10.1086/378209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler H, Okada T. Benzodiazepine receptor: demonstration in the central nervous system. Science. 1977;198:849–51. doi: 10.1126/science.918669. [DOI] [PubMed] [Google Scholar]
- Moller-Krull M, Zemann A, Roos C, Brosius J, Schmitz J. Beyond DNA: RNA editing and steps toward Alu exonization in primates. Journal of molecular biology. 2008;382:601–9. doi: 10.1016/j.jmb.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Morabito MV, Abbas AI, Hood JL, Kesterson RA, Jacobs MM, Kump DS, Hachey DL, Roth BL, Emeson RB. Mice with altered serotonin 2C receptor RNA editing display characteristics of Prader-Willi syndrome. Neurobiology of disease. 2010;39:169–80. doi: 10.1016/j.nbd.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nature methods. 2008;5:621–8. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- Naganska E, Matyja E. Amyotrophic lateral sclerosis – looking for pathogenesis and effective therapy. Folia Neuropathol. 2011;49:1–13. [PubMed] [Google Scholar]
- Nicholas A, de Magalhaes JP, Kraytsberg Y, Richfield EK, Levanon EY, Khrapko K. Age-related gene-specific changes of A-to-I mRNA editing in the human brain. Mech Ageing Dev. 2010;131:445–7. doi: 10.1016/j.mad.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls RD, Knepper JL. Genome organization, function, and imprinting in Prader-Willi and Angelman syndromes. Annual review of genomics and human genetics. 2001;2:153–75. doi: 10.1146/annurev.genom.2.1.153. [DOI] [PubMed] [Google Scholar]
- Nimmich ML, Heidelberg LS, Fisher JL. RNA editing of the GABA(A) receptor alpha3 subunit alters the functional properties of recombinant receptors. Neurosci Res. 2009;63:288–93. doi: 10.1016/j.neures.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikura K. Functions and regulation of RNA editing by ADAR deaminases. Annual review of biochemistry. 2010;79:321–49. doi: 10.1146/annurev-biochem-060208-105251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Copeland SC, Herrick-Davis K, Emeson RB, Sanders-Bush E. RNA editing of the human serotonin 5-hydroxytryptamine 2C receptor silences constitutive activity. The Journal of biological chemistry. 1999;274:9472–8. doi: 10.1074/jbc.274.14.9472. [DOI] [PubMed] [Google Scholar]
- O’Connell MA, Krause S, Higuchi M, Hsuan JJ, Totty NF, Jenny A, Keller W. Cloning of cDNAs encoding mammalian double-stranded RNA-specific adenosine deaminase. Molecular and cellular biology. 1995;15:1389–97. doi: 10.1128/mcb.15.3.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlson J, Enstero M, Sjoberg BM, Ohman M. A method to find tissue-specific novel sites of selective adenosine deamination. Nucleic acids research. 2005;33:e167. doi: 10.1093/nar/gni169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlson J, Pedersen JS, Haussler D, Ohman M. Editing modifies the GABA(A) receptor subunit alpha3. RNA. 2007;13:698–703. doi: 10.1261/rna.349107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D, Lien CC, Soom M, Baukrowitz T, Jonas P, Fakler B. Functional conversion between A-type and delayed rectifier K+ channels by membrane lipids. Science. 2004;304:265–70. doi: 10.1126/science.1094113. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60:243–60. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–8. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa S, Kamiya H, Tsuzuki K. Glutamate receptors in the mammalian central nervous system. Progress in neurobiology. 1998;54:581–618. doi: 10.1016/s0301-0082(97)00085-3. [DOI] [PubMed] [Google Scholar]
- Palladino MJ, Keegan LP, O’Connell MA, Reenan RA. dADAR, a Drosophila double-stranded RNA-specific adenosine deaminase is highly developmentally regulated and is itself a target for RNA editing. RNA. 2000;6:1004–18. doi: 10.1017/s1355838200000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papazian DM, Schwarz TL, Tempel BL, Jan YN, Jan LY. Cloning of genomic and complementary DNA from Shaker, a putative potassium channel gene from Drosophila. Science. 1987;237:749–53. doi: 10.1126/science.2441470. [DOI] [PubMed] [Google Scholar]
- Parcej DN, Scott VE, Dolly JO. Oligomeric properties of alpha-dendrotoxin-sensitive potassium ion channels purified from bovine brain. Biochemistry. 1992;31:11084–8. doi: 10.1021/bi00160a018. [DOI] [PubMed] [Google Scholar]
- Pasqualetti M, Ori M, Castagna M, Marazziti D, Cassano GB, Nardi I. Distribution and cellular localization of the serotonin type 2C receptor messenger RNA in human brain. Neuroscience. 1999;92:601–11. doi: 10.1016/s0306-4522(99)00011-1. [DOI] [PubMed] [Google Scholar]
- Patterson JB, Samuel CE. Expression and regulation by interferon of a double-stranded-RNA-specific adenosine deaminase from human cells: evidence for two forms of the deaminase. Molecular and cellular biology. 1995;15:5376–88. doi: 10.1128/mcb.15.10.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton DE, Silva T, Bezanilla F. RNA editing generates a diverse array of transcripts encoding squid Kv2 K+ channels with altered functional properties. Neuron. 1997;19:711–22. doi: 10.1016/s0896-6273(00)80383-9. [DOI] [PubMed] [Google Scholar]
- Peixoto AA, Smith LA, Hall JC. Genomic organization and evolution of alternative exons in a Drosophila calcium channel gene. Genetics. 1997;145:1003–13. doi: 10.1093/genetics/145.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polson AG, Bass BL. Preferential selection of adenosines for modification by double-stranded RNA adenosine deaminase. The EMBO journal. 1994;13:5701–11. doi: 10.1002/j.1460-2075.1994.tb06908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polson AG, Crain PF, Pomerantz SC, McCloskey JA, Bass BL. The mechanism of adenosine to inosine conversion by the double-stranded RNA unwinding/modifying activity: a high-performance liquid chromatography-mass spectrometry analysis. Biochemistry. 1991;30:11507–14. doi: 10.1021/bi00113a004. [DOI] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain research. Molecular brain research. 1994;23:163–78. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Pongs O. Voltage-gated potassium channels: from hyperexcitability to excitement. FEBS letters. 1999;452:31–5. doi: 10.1016/s0014-5793(99)00535-9. [DOI] [PubMed] [Google Scholar]
- Poulsen H, Nilsson J, Damgaard CK, Egebjerg J, Kjems J. CRM1 mediates the export of ADAR1 through a nuclear export signal within the Z-DNA binding domain. Molecular and cellular biology. 2001;21:7862–71. doi: 10.1128/MCB.21.22.7862-7871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RD, Weiner DM, Chang MS, Sanders-Bush E. RNA editing of the human serotonin 5-HT2C receptor alters receptor-mediated activation of G13 protein. The Journal of biological chemistry. 2001;276:44663–8. doi: 10.1074/jbc.M106745200. [DOI] [PubMed] [Google Scholar]
- Rasband MN, Park EW, Vanderah TW, Lai J, Porreca F, Trimmer JS. Distinct potassium channels on pain-sensing neurons. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:13373–8. doi: 10.1073/pnas.231376298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig J, Heinemann SH, Wunder F, Lorra C, Parcej DN, Dolly JO, Pongs O. Inactivation properties of voltage-gated K+ channels altered by presence of beta-subunit. Nature. 1994;369:289–94. doi: 10.1038/369289a0. [DOI] [PubMed] [Google Scholar]
- Rhodes KJ, Strassle BW, Monaghan MM, Bekele-Arcuri Z, Matos MF, Trimmer JS. Association and colocalization of the Kvbeta1 and Kvbeta2 beta-subunits with Kv1 alpha-subunits in mammalian brain K+ channel complexes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:8246–58. doi: 10.1523/JNEUROSCI.17-21-08246.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha BA, Goulding EH, O’Dell LE, Mead AN, Coufal NG, Parsons LH, Tecott LH. Enhanced locomotor, reinforcing, and neurochemical effects of cocaine in serotonin 5-hydroxytryptamine 2C receptor mutant mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:10039–45. doi: 10.1523/JNEUROSCI.22-22-10039.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruano D, Lambolez B, Rossier J, Paternain AV, Lerma J. Kainate receptor subunits expressed in single cultured hippocampal neurons: molecular and functional variants by RNA editing. Neuron. 1995;14:1009–17. doi: 10.1016/0896-6273(95)90339-9. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Mohler H. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Rueter S, Emeson R. Adenosine-to-Inosine conversion in mRNA. In: Grosjean H, Benne R, editors. Modification and Editing of RNA. ASM Press; Washington D.C.: 1998. pp. 343–361. [Google Scholar]
- Rueter SM, Burns CM, Coode SA, Mookherjee P, Emeson RB. Glutamate receptor RNA editing in vitro by enzymatic conversion of adenosine to inosine. Science. 1995;267:1491–4. doi: 10.1126/science.7878468. [DOI] [PubMed] [Google Scholar]
- Rueter SM, Dawson TR, Emeson RB. Regulation of alternative splicing by RNA editing. Nature. 1999;399:75–80. doi: 10.1038/19992. [DOI] [PubMed] [Google Scholar]
- Rula EY, Lagrange AH, Jacobs MM, Hu N, Macdonald RL, Emeson RB. Developmental modulation of GABA(A) receptor function by RNA editing. J Neurosci. 2008;28:6196–201. doi: 10.1523/JNEUROSCI.0443-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rump A, Sommer C, Gass P, Bele S, Meissner D, Kiessling M. Editing of GluR2 RNA in the gerbil hippocampus after global cerebral ischemia. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1996;16:1362–5. doi: 10.1097/00004647-199611000-00034. [DOI] [PubMed] [Google Scholar]
- Ryan MY, Maloney R, Reenan R, Horn R. Characterization of five RNA editing sites in Shab potassium channels. Channels. 2008;2:202–9. doi: 10.4161/chan.2.3.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer A, Swanson GT, Perez-Otano I, O’Leary L, Malkmus SA, Dyck RH, Dickinson-Anson H, Schiffer HH, Maron C, Yaksh TL, Gage FH, O’Gorman S, Heinemann SF. Generation and analysis of GluR5(Q636R) kainate receptor mutant mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:8757–64. doi: 10.1523/JNEUROSCI.19-20-08757.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub M, Keller W. RNA editing by adenosine deaminases generates RNA and protein diversity. Biochimie. 2002;84:791–803. doi: 10.1016/s0300-9084(02)01446-3. [DOI] [PubMed] [Google Scholar]
- Schmidt K, Eulitz D, Veh RW, Kettenmann H, Kirchhoff F. Heterogeneous expression of voltage-gated potassium channels of the shaker family (Kv1) in oligodendrocyte progenitors. Brain research. 1999;843:145–60. doi: 10.1016/s0006-8993(99)01938-1. [DOI] [PubMed] [Google Scholar]
- Seeburg PH. The TINS/TiPS Lecture. The molecular biology of mammalian glutamate receptor channels. Trends in neurosciences. 1993;16:359–65. doi: 10.1016/0166-2236(93)90093-2. [DOI] [PubMed] [Google Scholar]
- Semenov EP, Pak WL. Diversification of Drosophila chloride channel gene by multiple posttranscriptional mRNA modifications. Journal of neurochemistry. 1999;72:66–72. doi: 10.1046/j.1471-4159.1999.0720066.x. [DOI] [PubMed] [Google Scholar]
- Shamotienko OG, Parcej DN, Dolly JO. Subunit combinations defined for K+ channel Kv1 subtypes in synaptic membranes from bovine brain. Biochemistry. 1997;36:8195–201. doi: 10.1021/bi970237g. [DOI] [PubMed] [Google Scholar]
- Smith LA, Peixoto AA, Hall JC. RNA editing in the Drosophila DMCA1A calcium-channel alpha 1 subunit transcript. Journal of neurogenetics. 1998;12:227–40. doi: 10.3109/01677069809108560. [DOI] [PubMed] [Google Scholar]
- Sokolov MV, Shamotienko O, Dhochartaigh SN, Sack JT, Dolly JO. Concatemers of brain Kv1 channel alpha subunits that give similar K+ currents yield pharmacologically distinguishable heteromers. Neuropharmacology. 2007;53:272–82. doi: 10.1016/j.neuropharm.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Sommer B, Keinanen K, Verdoorn TA, Wisden W, Burnashev N, Herb A, Kohler M, Takagi T, Sakmann B, Seeburg PH. Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science. 1990;249:1580–5. doi: 10.1126/science.1699275. [DOI] [PubMed] [Google Scholar]
- Sommer B, Kohler M, Sprengel R, Seeburg PH. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991;67:11–9. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- Spaethling JM, Klein DM, Singh P, Meaney DF. Calcium-permeable AMPA receptors appear in cortical neurons after traumatic mechanical injury and contribute to neuronal fate. Journal of neurotrauma. 2008;25:1207–16. doi: 10.1089/neu.2008.0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speth RC, Johnson RW, Regan J, Reisine T, Kobayashi RM, Bresolin N, Roeske WR, Yamamura HI. The benzodiazepine receptor of mammalian brain. Fed Proc. 1980;39:3032–8. [PubMed] [Google Scholar]
- Strehblow A, Hallegger M, Jantsch MF. Nucleocytoplasmic distribution of human RNA-editing enzyme ADAR1 is modulated by double-stranded RNA-binding domains, a leucine-rich export signal, and a putative dimerization domain. Molecular biology of the cell. 2002;13:3822–35. doi: 10.1091/mbc.E02-03-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit AK, Derst C, Wegner S, Heinemann U, Zahn RK, Decher N. RNA editing of Kv1.1 channels may account for reduced ictogenic potential of 4-aminopyridine in chronic epileptic rats. Epilepsia. 2011;52:645–8. doi: 10.1111/j.1528-1167.2011.02986.x. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Suzuki T, Inagaki K, Ito S, Kono M, Fukai K, Takama H, Sato K, Ishikawa O, Abe M, Shimizu H, Kawai M, Horikawa T, Yoshida K, Matsumoto K, Terui T, Tsujioka K, Tomita Y. Mutation analysis of the ADAR1 gene in dyschromatosis symmetrica hereditaria and genetic differentiation from both dyschromatosis universalis hereditaria and acropigmentatio reticularis. The Journal of investigative dermatology. 2005;124:1186–92. doi: 10.1111/j.0022-202X.2005.23732.x. [DOI] [PubMed] [Google Scholar]
- Takuma H, Kwak S, Yoshizawa T, Kanazawa I. Reduction of GluR2 RNA editing, a molecular change that increases calcium influx through AMPA receptors, selective in the spinal ventral gray of patients with amyotrophic lateral sclerosis. Annals of neurology. 1999;46:806–15. doi: 10.1002/1531-8249(199912)46:6<806::aid-ana2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374:542–6. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- Tempel BL, Jan YN, Jan LY. Cloning of a probable potassium channel gene from mouse brain. Nature. 1988;332:837–9. doi: 10.1038/332837a0. [DOI] [PubMed] [Google Scholar]
- Tempel BL, Papazian DM, Schwarz TL, Jan YN, Jan LY. Sequence of a probable potassium channel component encoded at Shaker locus of Drosophila. Science. 1987;237:770–5. doi: 10.1126/science.2441471. [DOI] [PubMed] [Google Scholar]
- Valentine AD, Meyers CA, Kling MA, Richelson E, Hauser P. Mood and cognitive side effects of interferon-alpha therapy. Seminars in oncology. 1998;25:39–47. [PubMed] [Google Scholar]
- Verdoorn TA, Burnashev N, Monyer H, Seeburg PH, Sakmann B. Structural determinants of ion flow through recombinant glutamate receptor channels. Science. 1991;252:1715–8. doi: 10.1126/science.1710829. [DOI] [PubMed] [Google Scholar]
- Vissel B, Royle GA, Christie BR, Schiffer HH, Ghetti A, Tritto T, Perez-Otano I, Radcliffe RA, Seamans J, Sejnowski T, Wehner JM, Collins AC, O’Gorman S, Heinemann SF. The role of RNA editing of kainate receptors in synaptic plasticity and seizures. Neuron. 2001;29:217–27. doi: 10.1016/s0896-6273(01)00192-1. [DOI] [PubMed] [Google Scholar]
- Wagner RW, Yoo C, Wrabetz L, Kamholz J, Buchhalter J, Hassan NF, Khalili K, Kim SU, Perussia B, McMorris FA, et al. Double-stranded RNA unwinding and modifying activity is detected ubiquitously in primary tissues and cell lines. Molecular and cellular biology. 1990;10:5586–90. doi: 10.1128/mcb.10.10.5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstedt H, Daniel C, Enstero M, Ohman M. Large-scale mRNA sequencing determines global regulation of RNA editing during brain development. Genome Res. 2009;19:978–86. doi: 10.1101/gr.089409.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Miyakoda M, Yang W, Khillan J, Stachura DL, Weiss MJ, Nishikura K. Stress-induced apoptosis associated with null mutation of ADAR1 RNA editing deaminase gene. The Journal of biological chemistry. 2004;279:4952–61. doi: 10.1074/jbc.M310162200. [DOI] [PubMed] [Google Scholar]
- Wang Q, O’Brien PJ, Chen CX, Cho DS, Murray JM, Nishikura K. Altered G protein-coupling functions of RNA editing isoform and splicing variant serotonin2C receptors. Journal of neurochemistry. 2000;74:1290–300. doi: 10.1046/j.1471-4159.2000.741290.x. [DOI] [PubMed] [Google Scholar]
- Ward SV, George CX, Welch MJ, Liou LY, Hahm B, Lewicki H, de la Torre JC, Samuel CE, Oldstone MB. RNA editing enzyme adenosine deaminase is a restriction factor for controlling measles virus replication that also is required for embryogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:331–6. doi: 10.1073/pnas.1017241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werry TD, Gregory KJ, Sexton PM, Christopoulos A. Characterization of serotonin 5-HT2C receptor signaling to extracellular signal-regulated kinases 1 and 2. Journal of neurochemistry. 2005;93:1603–15. doi: 10.1111/j.1471-4159.2005.03161.x. [DOI] [PubMed] [Google Scholar]
- Werry TD, Loiacono R, Sexton PM, Christopoulos A. RNA editing of the serotonin 5HT2C receptor and its effects on cell signalling, pharmacology and brain function. Pharmacology & therapeutics. 2008;119:7–23. doi: 10.1016/j.pharmthera.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Wollmuth LP, Sobolevsky AI. Structure and gating of the glutamate receptor ion channel. Trends Neurosci. 2004;27:321–8. doi: 10.1016/j.tins.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Yang JH, Sklar P, Axel R, Maniatis T. Editing of glutamate receptor subunit B pre-mRNA in vitro by site-specific deamination of adenosine. Nature. 1995;374:77–81. doi: 10.1038/374077a0. [DOI] [PubMed] [Google Scholar]
- Yang W, Chendrimada TP, Wang Q, Higuchi M, Seeburg PH, Shiekhattar R, Nishikura K. Modulation of microRNA processing and expression through RNA editing by ADAR deaminases. Nature structural & molecular biology. 2006;13:13–21. doi: 10.1038/nsmb1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Wang Q, Kanes SJ, Murray JM, Nishikura K. Altered RNA editing of serotonin 5-HT2C receptor induced by interferon: implications for depression associated with cytokine therapy. Brain research. Molecular brain research. 2004;124:70–8. doi: 10.1016/j.molbrainres.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Zdilar D, Franco-Bronson K, Buchler N, Locala JA, Younossi ZM. Hepatitis C, interferon alfa, and depression. Hepatology. 2000;31:1207–11. doi: 10.1053/jhep.2000.7880. [DOI] [PubMed] [Google Scholar]


