Abstract
The effects of obesity and a high-fat (HF) diet on whole body and tissue-specific metabolism of lactating dams and their offspring were examined in C57/B6 mice. Female mice were fed low-fat (LF) or HF diets before and throughout pregnancy and lactation. HF-fed mice were segregated into lean (HF-Ln) and obese (HF-Ob) groups before pregnancy by their weight gain response. Compared to LF-Ln dams, HF-Ln, and HF-Ob dams exhibited a greater positive energy balance (EB) and increased dietary fat retention in peripheral tissues (P < 0.05). HF-Ob dams had greater dietary fat retention in liver and adipose compared to HF-Ln dams (P < 0.05). De novo synthesized fat was decreased in tissues and milk from HF-fed dams compared to LF-Ln dams (P < 0.05). However, less dietary and de novo synthesized fat was found in the HF-Ob mammary glands compared to HF-Ln (P < 0.05). Obesity was associated with reduced milk triglycerides relative to lean controls (P < 0.05). Compared to HF diet alone obesity has additional adverse affects, impairing both lipid metabolism as well as milk fat production. Growth rates of LF-Ln litters were lower than HF-Ln and HF-Ob litters (P < 0.05). Total energy expenditure (TEE) of HF-Ob litters was reduced relative to HF-Ln litters, whereas their respiratory exchange ratios (RERs) were increased (P < 0.05). Collectively these data show that consumption of a HF diet significantly affects maternal and neonatal metabolism and that maternal obesity can independently alter these responses.
Introduction
Milk is a complex substance tailored by evolution to meet the diverse neonatal growth and nutrient requirements of individual mammalian species. Breast milk has been linked to numerous health benefits for human infants including lower incidence of infectious disease and reduced risk for obesity and childhood diabetes (1,2). The demands of milk production in fully lactating females act as a whole body metabolic challenge, requiring significant amounts of additional energy that is provided by both increased dietary intake and mobilization from maternal stores (3,4). The metabolic changes that occur during lactation involve an integrated response to the changing hormone levels, increased nutrient load, and energy demand for milk production.
Obesity has been described as a metabolically inflexible state; defined as impaired regulatory responses to metabolic challenges such as fasting, exercise, and overfeeding (5–7). This also appears to be true with lactation, as obese women have difficulty breast feeding (8,9). In rodents, some studies have shown that prenatal obesity has been associated with impaired mammary gland development, lactation, and pup growth (10–13), while others have shown increased pup growth (14,15). Regardless of these discrepancies in the literature, diet-induced obesity results in long-term consequences on the health and disease susceptibility of offspring (14,16). Invariably, however, the rodent models of obesity have been produced and maintained by feeding a diet high in fat. As such, the interpretation of these studies is complicated by the possibility that dietary differences may have contributed in part, or in total, to these outcomes. Therefore, animal models that include lean controls chronically exposed to the same high-fat (HF) diet are necessary to tease out the effects of a HF diet from those of obesity. Models that allow this specific comparison have been developed in mice, rats, and Japanese macaques (14,17–20). However, to date the effects of obesity on maternal metabolism during lactation, after controlling for HF diet, have not been reported.
To address the issue that exposure to chronic high-fat feeding may complicate the interpretation of the effects of obesity on lactation, we studied maternal and pup metabolism during lactation in the C57/B6 mice. This inbred line, while genetically homogeneous, exhibits a heterogeneous response to high-fat feeding, with some developing obesity and some remaining lean (19,21). Inbred species are presumed to be genetically homogeneous, however, they exhibit a range of responses that are presumed to be a function of epigenetic events caused by environmental, biological, and social influences imposed on the dams or the offspring themselves during their development. We hypothesized that obesity would inhibit the maternal response to the metabolic demands of lactation, beyond those incurred by HF, and that this impaired response would have adverse consequences on lactation, pup metabolism, and pup growth.
Methods and Procedures
Experimental paradigm of virgin and lactating mice
Female C57/B6J mice were purchased from Jackson Labs (Bar Harbor, Maine) at 7 weeks of age. After 1 week of acclimation, mice were placed on either a low fat (LF) (68% kcal carbohydrate, 12% kcal fat; #D08032201) or HF (34% kcal carbohydrate, 46% kcal fat; #D08032202) diet with both diets containing 10% kcal derived from sucrose and a standard dye (0.5%) to help keep the diets distinguishable. These diets were utilized for the duration of the study (Research Diets, New Brunswick NJ). Mice were housed in the UC Denver's Center for Comparative Medicine with free access to food and water for the study's duration (22–24 °C; 12:12 h light-dark cycle). The University of Colorado Denver Institutional Animal Care and Use Committee approved all procedures and housing conditions used in the study.
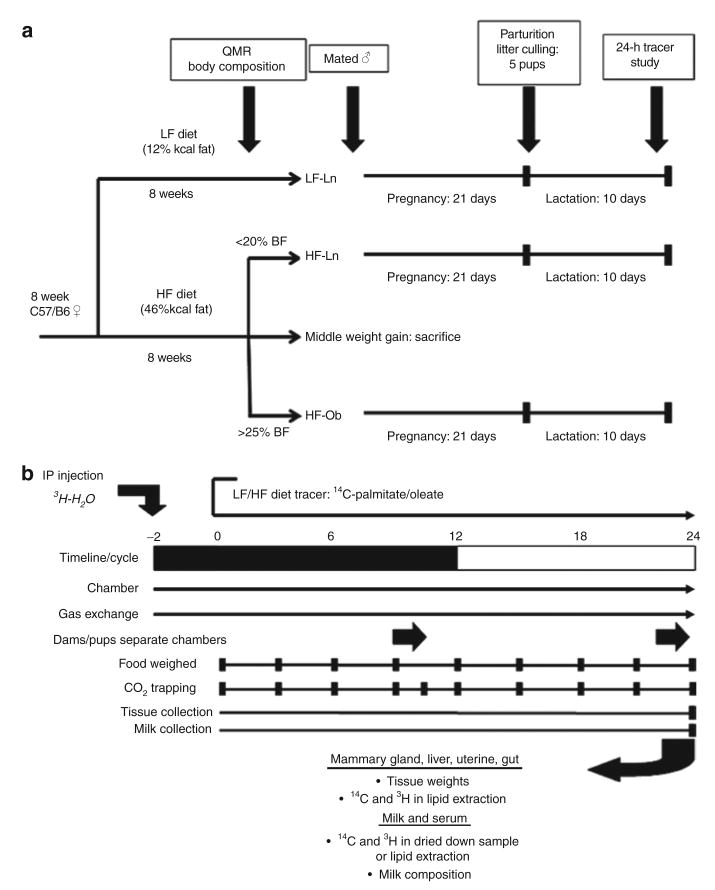
Animals fed LF diet were studied as LF lean controls. Based on body weight and body fat, the remaining mice fed the HF diet were classified as either HF lean (HF-Ln) controls or HF obese (HF-Ob). Briefly and illustrated in Figure 1a, mice were ranked by their rate of weight gain over 9–12 weeks in this obesogenic environment. Those in the top tertile of weight gain and had >25% body fat were classified as obese, and those in the lower tertile and had <20% body fat were considered lean. Mice in the middle tertile were excluded from further studies. Body composition was performed by quantitative magnetic resonance (QMR; EchoMRI-900 Whole Body Composition Analyzer; Echo Medical Systems, Houston, TX) in conscious mice before pregnancy and at the time of the study (lactation day 10 (L10)). Following 2 months on the study diets, mice in each group were placed into estrous and mated to C57/B6 males. All mice were housed 2 per cage and pregnant mice were transferred to individual housing toward the end of pregnancy (P18). After parturition, litters were normalized to five pups and weights were taken every day for 9–11 days.
Figure 1.
Experimental design for feeding paradigm and dual-tracer study. The overall study design and feeding paradigm are shown in a. In addition, a timeline for mating, pregnancy, and lactation are included. The dual-tracer study is depicted in b where a metabolic monitoring chamber was used to assess the fuel utilization and lipid trafficking of exogenous fat in lactating mice.
Intake, expenditure, fuel utilization, and activity
Energy balance (EB) and fuel utilization were assessed by a metabolic monitoring system (Figure 1b) housed in the Center for Human Nutrition's Animal Satellite Facility. The multi-chamber indirect calorimeter unit (Columbus Instruments, Columbus, OH), modified for performing in vivo dietary tracer studies (22), was used. Up to eight mice could be continuously monitored, obtaining measurements of oxygen consumption (vO2) and CO2 production (vCO2) from each chamber at 10-min intervals. Metabolic rate was calculated with the Weir equation (23): Metabolic rate = 3.941 × vO2 + 1.106 × vCO2.
Extrapolation of metabolic rate over the 24-h period provided estimates of total energy expenditure (TEE) (kcal/day, by multiplying by 60 min/h and 24 h/day and dividing by 1 kcal/1,000 cal). Respiratory exchange ratio (RER) was calculated as the ratio of CO2 production to O2 consumption (vCO2/vO2). Each metabolic cage was equipped with an animal activity meter (Opto-Max; Columbus Instruments), which consists of a one-dimensional series of infrared beams that, when broken by the animals' movement, allows for the measurement of total activity.
Dual-tracer experimental design protocol
A 24 h dual-tracer experiment in combination with indirect calorimetry was performed to acquire a comprehensive assessment of EB, whole body fuel utilization, oxidation and tissue-specific trafficking of dietary fat, and the net retention of fuel via de novo lipogenesis (Figure 1b). Mice were placed in the metabolic monitoring system and maintained to acclimate them to the environment. The dual-tracer approach was based upon our previous studies of weight regain (22).
Two hours before the beginning of the 24 h tracer study, an intraperitoneal injection of 100 μCi 3H2O was given to the dams. In rodents, this has been shown to equilibrate with body water within 2 h and its concentration remains relatively steady over the following 24 h period (24). Incorporation of tritium into lipid pools was estimated in lipid extracted tissues as previously described (22).
1-[14C]Palmitate and 1-[14C]oleate tracer was blended into the LF or HF diet in a ratio that reflected the relative ratio of palmitate/oleate in the diets (1:3). The specific activities were 0.99 μCi/kcal for LF diet and 0.45 μCi/kcal for the HF diet. Intake was measured every 3 h, and the absolute amount of tracer consumed reflected the total dietary lipid ingested over the 24-h period.
Collection of co2, milk, and tissue
Oxidation and tissue-specific trafficking of the ingested fat was then monitored for 24 h. An exhaust line from each chamber was vented into a fume hood, where 0.25 mmol of CO2 could be collected from expired air. CO2 was collected into 3 ml of a 10:1:1 mixture of methanol, methylbenzethonium (Hyamine) hydroxide (Sigma-Aldrich, St Louis, MO), and 0.08% phenolphthalein. At each time point, (every 3 h) the time to saturation and flow rate was measured for each chamber. At 2 time points, once at the end of the dark cycle and once at the end of the light cycle, dams were placed in separate adjacent chamber for 1.5 h to quantify fuel utilization of the dams and litters separately. For litters, the steady state measurements of vO2 and vCO2 and the oxidation of dietary fat in the dark and light cycle (Figure 1) were extrapolated to 12 h values for each cycle. The dam alone measurements were obtained via simple subtraction of the litter alone measurements from the dam/litter unit measurements. Following the 24 h tracer protocol, lactating dams were anesthetized via IP injection of Avertin (32 mg/ml working solution: #T48402 Sigma-Aldrich) and milk letdown was induced with an injection of oxytocin (4USP units/mouse Vetone, #NDC13985-039-02). Lactating mice were milked using a vacuum apparatus and then sacrificed by cervical dislocation. Blood was collected and centrifuged to obtain serum samples. Tissues were collected, weighed and snap frozen in liquid nitrogen for determination of net retention of 14C and 3H in the lipid fractions of the #4 mammary gland, liver and gonadal (uterine) fat pad.
Serum, milk, and tissue tracer analysis
Aliquots of serum were dried down overnight and analyzed for 14C and 3H and expressed per ml. Total serum tracer was calculated as 14C or 3H activity/ml × 0.0385 (%body mass accounted for by serum) × body weight as previously described (25). Milk lipid was extracted as described below and 14C and 3H were measured via scintillation counting and expressed per ml and also 24 h total milk tracer. 14C and 3H content within liver, mammary gland, and uterine adipose was determined after extraction of lipid with chloroform-methanol as previously described (2:1, vol/vol (26)). Phases were separated with the addition of H2SO4 and centrifugation. The lower phase was collected and allowed to dry to completion under nitrogen gas. Tracer content in samples was measured with a Beckman LS6500 scintillation counter (Beckman Coulter, Brea, CA), employing a dual-window for 3H and 14C, with internal quench correction and after bleaching with hydrogen peroxide to control for color quench. For 14C content, disintegrations/min were converted to μCi, and then the specific activity of fat in the diet was used to convert this value to calories of dietary fat. For 3H content, disintegrations/min were converted to nCi. Tracer content was expressed as total tissue.
Milk volume and composition analysis
Milk water content was calculated as the difference in weights before and after an aliquot of milk was dried down overnight and then placed under a stream of nitrogen gas to ensure the complete evaporation of all water. Lipid was extracted from 25 μl of milk after overnight incubation at 4 °C in a 2 N Dole's extraction mixture (isopropanol: heptane: 1N H2SO4, 40:10:1) and centrifugation at 1,000g for 30 min at room temperature (27). The upper phase was removed, dried down under nitrogen gas, and the weight of the remaining lipid was taken. Protein and lactose were measured via colorimetric assays on milk samples diluted at 1:100. DC Protein Assay (#500-0116; Bio-Rad, Richmond, CA) for protein analysis and Abcam Lactose Assay (ab83384; Abcam, Cambridge, MA) for lactose analysis. All components were measured as mg/ml and expressed as a percentage of total weight.
Milk volume was estimated from the milk energy output estimated in Supplementary Table S1 online and the milk composition (Table 3).
Table 3. Milk composition on lactation day 10.
| LF-Ln | HF-Ln | HF-Ob | |
|---|---|---|---|
| Milk volume (ml) | 6.6 ± 0.5 | 10.7 ± 0.6* | 10.8 ± 0.7* |
| Water, % | 49.9 ± 6.4 | 54.2 ± 3.3 | 60.5 ± 6.4 |
| Lipid, % | 26.8 ± 6.1 | 21.9 ± 5.1 | 11.8 ± 3.5*,** |
| Protein, % | 19.5 ± 1.7 | 21.3 ± 2.4 | 24.2 ± 3.6 |
| Lactose, % | 3.8 ± 0.4 | 2.6 ± 0.4* | 3.4 ± 0.8 |
Data expressed as % weight.
HF-Ln, high-fat lean dams; HF-Ob, high-fat obese dams; LF-Ln, low-fat lean dams.
LF-Ln vs. HF-Ln or HF-Ob, P < 0.05;
HF-Ln vs. HF-Ob, P < 0.05.
A = Milk lipid (g/ml) × 9 kcal/g lipid = Milk lipid kcal/ml
B = Milk protein (g/ml) × 4 kcal/g protein = Milk protein kcal/ml
C = Milk lactose (g/ml) × 4 kcal/g lactose = Milk lactose kcal/ml
A + B + C = Milk energy (kcal/ml)
Milk volume (ml/day) = (Milk energy output (kcal/day))/(Milk energy (kcal/ml))
Statistical analysis
Data are expressed as means ± s.e.m., and analyzed by ANOVA using SPSS (version 18.0), with the planned contrasts to examine the effect of a HF diet, with or without the presence of obesity, on lactation. In some cases, data were analyzed with one or more covariates (ANCOVA) to control for variation due to the specified variables. Relationships between parameters were examined with Pearson's correlation coefficient (linear) or Spearman's rho (nonlinear). Differences for planned contrasts and relationships were considered statistically significant when P < 0.05. Sample sizes varied as indicated in the tables and figures (n = 4–8), as some parameters were only measured in a subset of animals. Because the impact of the HF diet was expected to be substantial and confirmatory of published literature, fewer animals were employed for the LF-Ln group (n = 4). It should be recognized that comparisons with this group may yield some type 2 error in the analyses, owing to the small sample size. For the HF-Ln and HF-Ob groups, which revealed the novel effect of obesity with diet composition controlled, a larger samples size was employed (n = 8).
Results
Maternal body weight and morphometric characteristics
Body composition of LF-Ln, HF-Ln, or HF-Ob groups during mid-lactation (L9–L11) is depicted in Table 1. Body weights and compositions of LF-Ln and HF-Ln dams were not significantly different from each other. In contrast, HF-Ob dams had significantly greater body weights (P < 0.02) and fat mass values (P < 0.05) than either of the lean groups, indicating that the obese phenotype was maintained after parturition.
Table 1. Morphometric characteristics of dams: lactation day 10.
| LF-Ln | HF-Ln | HF-Ob | |
|---|---|---|---|
| Body composition | |||
| n | 4 | 4 | 4 |
| Body weight, g | 27.5 ± 0.16 | 27.8 ± 0.5 | 31.7 ± 1.7*,** |
| Fat-free mass (FFM), g | 24.3 ± 0.28 | 23.9 ± 0.37 | 25.3 ± 0.9 |
| Fat mass (FM), g | 3.1 ± 0.21 | 3.6 ± 0.26 | 6.5 ± 1.6*,** |
| % FM | 11.4 ± 0.77 | 13.2 ± 0.81 | 20.0 ± 3.8*,** |
| Tissue weights | |||
| n | 6 | 8 | 8 |
| GI tract, g | 4.0 ± 0.32 | 4.1 ± 0.28 | 4.3 ± 0.16 |
| Liver, g | 1.9 ± 0.11 | 1.8 ± 0.07 | 1.8 ± 0.09 |
| Mammary glanda, g | 0.34 ± 0.03 | 0.46 ± 0.03* | 0.46 ± 0.04* |
| Gonadal fat padb, g | 0.15 ± 0.02 | 0.12 ± 0.01 | 0.22 ± 0.02*,** |
GI, gastrointestinal tract; HF-Ln, high-fat lean dams; HF-Ob, high-fat obese dams; LF-Ln, low-fat lean dams.
A single #4 mammary gland.
A single gonadal (uterine) fat pad.
LF-Ln vs. HF-Ln or HF-Ob, P < 0.05;
HF-Ln vs. HF-Ob, P < 0.05.
Organ weights are shown in Table 1. The size of intestine and liver were not affected by a HF diet, nor were they affected by obesity despite the larger body mass of the HF-Ob dams. Whereas the mammary glands from HF-Ln and HF-Ob dams were significantly larger than LF-Ln dams (P < 0.02), no difference existed between HF-Ln and HF-Ob groups (P = 0.5) suggesting an effect of HF diet on mammary gland size. Consistent with having greater fat mass, HF-Ob dams had significantly larger uterine (gonadal) fat pads when compared with both LF-Ln and HF-Ln dams (P < 0.02).
Maternal energetics
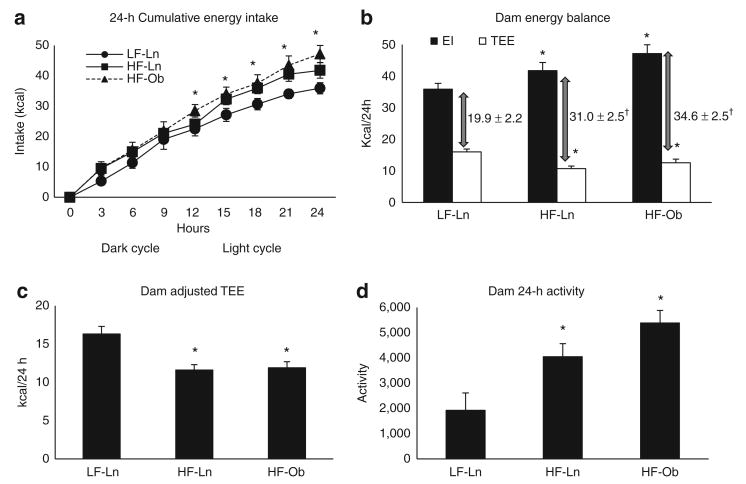
We hypothesized that the obese phenotype would inhibit the transition into negative energy imbalance during lactation. Cumulative energy intake (EI), EB, adjusted TEE, and activity of lactating dams is shown in Figure 2. Over the 24 h study, HF-fed dams consumed significantly more calories than did LF fed dams (P < 0.05) (Figure 2a,b). Interestingly, the cumulative EI of HF-fed dams began to diverge from LF-Ln dams during their light cycle, a time when feeding is typically minimal in rodents. HF diet induced a greater positive energy imbalance in both HF-Ln (P = 0.01) and HF-Ob (P = 0.02) dams compared to LF-Ln controls (Figure 2b). The greater positive energy imbalance was due to lower TEE in the HF-Ln (P < 0.001) and a greater EI (P = 0.04) relative to LF-Ln animals. HF-Ob dams had lower TEE (P = 0.01) as well as higher EI (P = 0.02) values than LF-Ln dams. In contrast, the TEE of HF-Ob dams was significantly greater than that of HF-Ln dams (P = 0.03). However, after controlling for the variation in basal metabolic mass (ANCOVA, lean mass as the covariate), the adjusted TEE was not different between HF-Ln and HF-Ob dams (Figure 2c). This suggested that the higher TEE in HF-Ob dams was the consequence of their greater metabolic mass. Additionally, because of the contribution of activity to TEE, we measured activity levels in these groups (Figure 2d). Surprisingly there was a significant increase in activity with HF feeding (P < 0.02), which tended to increase even further in the HF-Ob dams compared to HF-Ln mice (P = 0.06).
Figure 2.
Maternal energetics and activity. Cumulative energy intake (EI), energy balance, and activity were measured over the 24-h study and expressed as means ± s.e. Bars with a single asterisk are significantly different from low-fat lean dam (LF-Ln) controls and bars with a double asterisk are significantly different from high-fat lean dam (HF-Ln) controls (P < 0.05). (a) Cumulative energy intake was measured every 3 h over a 24-h period and expressed in kcal. (b) Energy balance showed as the difference between total energy expenditure (TEE) and total energy intake (EI) over 24-h and expressed as kcal/day (†LF-Ln vs. HF-Ln or HF-Ob, P < 0.05). (c) Adjusted total energy expenditure was examined by analysis of covariance, using lean mass as the covariate and expressed as kcal/24 h. (d) 24-h activity was measured using infrared laser beams. Each beam break was considered one activity count.
Although EB is normally a function of intake and expenditure (EB = EI–TEE), the caloric output due to milk production must be considered in lactating animals. Due to the small amounts of milk collected we were unable to determine the exact caloric value of the milk. However, when considering the fact that the only source of neonatal nutrition is milk, we can estimate the caloric value of milk by adding up litters' TEE and the calories contributing to litter growth. This calculation is shown in Supplementary Table S1 online. After estimating milk calories and recalculating EB to include those calories secreted into the milk, we estimate that HF-Ob dams were in a positive energy imbalance (11.4 ± 1.6 kcal/day) whereas HF-Ln and LF-Ln dams were near EB (0.7 ± 2.5 and −1.1 ± 3.7 kcal/day, respectively). Compared to either LF-Ln or HF-Ln, HF-Ob had a significantly greater EB (P < 0.05). Consistent with these measurements of EB, over the 24-h study HF-Ob dams gained on average 1.25 g whereas the HF-Ln only gained on average 0.6 g and the LF-Ln gained on average 0.2 g. These data support the hypothesis that obesity interferes with the negative energy imbalance induced by lactation.
Maternal whole body fuel utilization and dietary fat oxidation
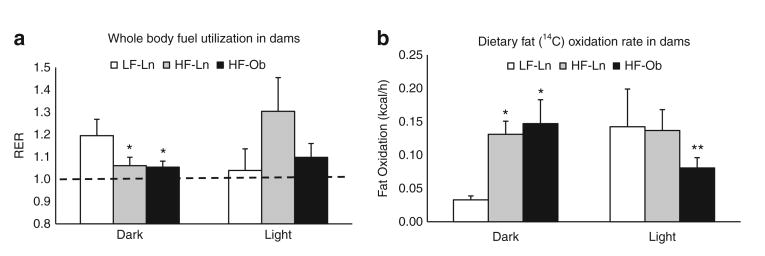
Rodents feed on a diurnal schedule, consuming the majority of their daily intake during the dark cycle, which can have an effect on their diurnal utilization of fuels (28) such that the dark cycle will be most reflective of dietary composition. RER is shown for the 12 h dark and light cycles in Figure 3a. Overall, all groups maintained an RER above 1.0 throughout the 24 h study, suggesting that they are favoring the oxidation of carbohydrate and are in a lipogenic state. As a reflection of the composition of their respective diets, the HF-Ln and HF-Ob dams have a significantly lower RER than the LF-Ln dams (P = 0.01) during the dark cycle. RER values of the LF-Ln and HF-Ln groups changed in different directions between the dark and light cycles, with RER decreasing for the LF-Ln group and increasing for the HF-Ln group (Figure 3a). In contrast, RER of HF-Ob dams did not change between dark and light cycles.
Figure 3.
Maternal whole body fuel utilization and dietary fat oxidation. The oxidation of dietary fat and whole body fuel utilization measured during dark and light cycles are expressed as means ± s.e. Bars with a single asterisk are significantly different from low-fat lean dam (LF-Ln) controls and bars with a double asterisk are significantly different from high-fat lean dam (HF-Ln) controls (P < 0.05). (a) Respiratory exchange ratio (RER; CO2/O2) derived from indirect calorimetry measurements. (b) Oxidation of dietary fat was assessed by measuring 14C-CO2 in expired air over 4.5 min at each time point.
Mean dietary fat oxidation rates for dark and light cycles over the 24 h study are shown in Figure 3b. Consistent with differences in fat composition of the HF and LF diets, dietary fat oxidation rates of HF-Ln and HF-Ob dams during the dark cycle were significantly greater than that of the LF-Ln group (P < 0.006). Fat oxidation rates in the dark cycle were not significantly different between HF-Ln and HF-Ob groups. However, light cycle fat oxidation rates for the LF-Ln and HF-Ln groups were comparable and tended to be higher than that of the HF-Ob group (P < 0.07). In the HF-Ob, we suspect the diurnal fluctuation that traffics dietary fat to the liver during the dark cycle, followed by its release in the light cycle, is impaired, consistent with what we have observed in nonlactating obese animals (22). More dietary fat is retained in their liver and peripheral adipose tissue and they fail to induce lipogenesis to support milk production (Table 2). Their whole body metabolism over the day (RER) remains relatively constant and the dietary fat is stored away and not remobilized later in the day, so dietary fat oxidation declines.
Table 2. 24-h net retention of dietary fat and de novo-derived lipid in lactating dams.
| LF-Ln | HF-Ln | HF-Ob | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Dietary fat (cal) | DNL (nCi) | Dietary fat (cal) | DNL (nCi) | Dietary fat (cal) | DNL (nCi) | |
| n | 5 | 5 | 5 | 5 | 5 | 5 |
| Total tissue | ||||||
| Liver | 84.9 ± 14.9 | 240.0 ± 24.3 | 144.5 ± 16.4* | 88.7 ± 24.3* | 242.0 ± 41.8*,** | 78.5 ± 16.9* |
| Mammary glanda | 44.2 ± 4.4 | 57.6 ± 2.0 | 153.7 ± 25.4* | 47.6 ± 8.1 | 114.6 ± 10.3* | 32.0 ± 7.2* |
| Gonadal fat padb | 20.9 ± 4.0 | 31.3 ± 6.3 | 30.3 ± 3.7* | 6.5 ± 0.5* | 43.1 ± 6.4*,** | 9.4 ± 2.3* |
| Total serum (at 24 h) | 0.7 ± 0.06 | 5.4 ± 1.9 | 0.4 ± 0.03* | 22.9 ± 7.5* | 0.4 ± 0.05* | 7.4 ± 1.3** |
| Total milk (over 24 h) | 1,687.0 ± 463 | 4,491 ± 1,326 | 10,872 ± 875* | 4,667 ± 681 | 7,390 ± 1,560*,** | 2,938 ± 652** |
Net retention of dietary fat in tissue lipid traced with 14C-fatty acid in the diet and expressed as calories. Net retention of de novo-derived lipid (DNL) measured as 3H-lipid incorporation from 3H-H2O and expressed as nCi.
HF-Ln, high-fat lean dams; HF-Ob, high-fat obese dams; LF-Ln, low-fat lean dams.
A single #4 mammary gland.
A single gonadal (uterine) fat pad.
LF-Ln vs. HF-Ln or HF-Ob, P < 0.05;
HF-Ln vs. HF-Ob, P < 0.05.
Tissue-specific retention of dietary fat
Dietary fat tracer was used to determine differences in fuel trafficking during lactation between lean and obese dams and was estimated from the net retention of ingested 14C-labeled dietary fat. Net retention of dietary fat energy (cal) per total weight (Table 1) of liver, mammary gland, and uterine (gonadal) adipose over a 24 h period is shown in Table 2. Retention of dietary fat in the liver was significantly greater in the HF-Ln and HF-Ob groups (P < 0.005) compared to the LF-Ln group. In addition, HF-Ob dams had significantly greater dietary fat retention in their liver (P = 0.03) than HF-Ln dams suggesting an additional effect of obesity on dietary fat trafficking to the liver. Dietary fat retention in mammary glands of HF-Ln and HF-Ob dams was significantly greater than that observed in LF-Ln mice (P < 0.001). Unlike the effect of obesity on hepatic dietary fat retention, we found that HF-Ob dams tended to have less dietary fat in their mammary glands than HF-Ln mice (P = 0.06). HF diet and obesity also increased the amount of dietary fat retention in the uterine fat pad compared to LF-Ln dams (P < 0.05). Obesity lead to an additional increase in dietary fat retention within the uterine fat, as HF-Ob dams had significantly greater retention of 14C in their total uterine fat pad (P < 0.05) than HF-Ln dams.
Net retention of lipid derived from de novo synthesis
To test the hypothesis that obesity impairs de novo lipogenesis during lactation, de novo synthesized lipid was estimated from net retention of 3H in lipid fractions (22) from liver, mam-mary gland, and adipose. Data are expressed as nCi per total tissue, Table 2. HF feeding led to significantly lower amounts de novo synthesized lipid retained in the liver (P < 0.001) and adipose (P < 0.001). De novo synthesized lipid retention in liver and adipose tissues of HF-Ln and HF-Ob groups were not different. De novo synthesized lipid began to decrease in the HF-Ln mammary gland compared to the LF-Ln and reached significantly lower levels in the HF-Ob mammary glands. Additionally, HF-Ob dams tended to have less de novo synthesized lipid in their mammary glands compared to HF-Ln dams (P = 0.06), suggesting that obesity related factors are acting specifically in the mammary gland to regulate de novo lipogenesis. Conversely, there may also be a defect in the uptake of de novo synthesized lipid from the liver in the HF-Ob mammary glands resulting in decreased retention of de novo synthesized fat.
Milk and serum analysis
Milk samples were taken at the end of the 24-h tracer study and analyzed for water, lipid, protein, and lactose content and presented in Table 3. There were no significant differences in water or protein content between groups. Compared to the LF-Ln milk, HF-Ln dams produced a lower percentage of lactose in their milk, although not a large decrease it was significant (P = 0.04). HF-Ob dams produced a significant decrease in the percentage of milk lipid, compared to LF-Ln (56% decrease, P = 0.02) and HF-Ln (46% decrease, P = 0.05) but had a similar amount of milk lactose compared to both lean groups.
Milk energy output was estimated from the energy deposited for litter growth and litter TEE (milk energy output = litter growth + litter TEE) and expressed as kcal/day in Supplementary Table S1 online. On L10, HF-Ln dams had the greatest (P < 0.05) milk energy output (30.4 ± 1.8 kcal/day) compared to both LF-Ln (22.2 ± 2.4 kcal/day) and HF-Ob dams (23.1 ± 2.0). Using the estimated milk energy output and the milk composition, we calculated the milk volume produced over the 24 h period (Table 3). Both HF-Ln and HF-Ob dams produced significantly more milk on L10 compared to LF-Ln dams (P < 0.001, P = 0.003, respectively). These data suggest that HF feeding promoted greater milk production at either the level of mammary gland function or pup feeding behavior.
Milk samples were also analyzed for dietary fat and de novo-derived lipid in order to determine differences in the sources of milk lipids. Milk tracer is presented in Table 2 as total 24 h levels and in Table 4 as per ml of milk. The amount of diet-derived fat in milk was significantly different between groups (P < 0.001) and it followed the same relationship observed in the mammary gland where LF-Ln had the least amount of dietary fat, HF-Ln had the greatest, and HF-Ob fell in the middle. This relationship held true when the dietary fat in milk was expressed over the total 24 h experiment (Table 2) or as per ml of milk (Table 4). De novo-derived lipids in milk from HF-Ln and HF-Ob dams were lower than in LF-Ln milk lipid but only reached significance in the HF-Ob dams (P = 0.02) when expressed as per ml of milk (Table 4). Additionally, HF-Ob dams had less de novo-derived lipid/ml of milk and total over the 24 h study compared to the HF-Ln dams (P = 0.02, P = 0.04, respectively).
Table 4. Dietary fat and de novo lipid content in serum and milk.
| LF-Ln | HF-Ln | HF-Ob | |
|---|---|---|---|
| n | 4 | 6 | 6 |
| Serum contenta | |||
| Dietary fat (cal/ml) | 0.67 ± 0.2 | 0.35 ± 0.03* | 0.35 ± 0.04* |
| DNL (nCi/ml) | 5.0 ± 2.5 | 9.1 ± 3.3 | 6.8 ± 1.4 |
| Milk contentb | |||
| Dietary fat (cal/ml) | 246 ± 57 | 1,046 ± 104* | 683 ± 120*,** |
| DNL (nCi/ml) | 658 ± 160 | 443 ± 65 | 278 ± 54*,** |
Dietary fat content traced with 14C-fatty acids in the diet and expressed as cal/ml. De novo-derived lipid (DNL) measured as 3H content and expressed as nCi/ml.
HF-Ln, high-fat lean dams; HF-Ob, high-fat obese dams; LF-Ln, low-fat lean dams.
Tracer contents were determined in dehydrated samples.
Tracer contents were determined in lipid extracted samples.
LF-Ln vs. HF-Ln or HF-Ob, P < 0.05;
HF-Ln vs. HF-Ob, P < 0.05.
Serum levels of dietary and de novo synthesized lipid were also measured and presented as total 24 h levels (Table 2) and as per ml (Table 4). Dietary fat contents were lower in the serum from both HF-Ln and HF-Ob dams compared to LF-Ln when expressed as total serum dietary fat (P < 0.001) or as per ml (P < 0.01). Total 24-h serum de novo synthesized lipid was the greatest in HF-Ln dams (P = 0.04) and not different between LF-Ln and HF-Ob (Table 2). There were no significant differences found in the amount of circulating de novo synthesized lipid when expressed as per ml of serum between groups, although HF-Ln dams showed a slight increase compared to both LF-Ln and HF-Ob dams similar to what was observed in the total serum calculation (Table 4). Total serum triglycerides were not different between the groups (35 ± 4 mg/dl)− they were all very high (as would be expected with lactation). These serum tracer values therefore represent enrichment of circulating triglycerides with either dietary or de novo derived lipid. The LF-Ln was enriched with dietary fat (likely from very-low-density lipoprotein release), the HF-Ln was enriched with de novo fat (also likely from the liver), and the HF-Ob is enriched with neither. Combined with the tissue tracer data (Table 2), these findings suggest that excess dietary fat was retained in peripheral tissue in the HF-Ob dams.
Litter growth, metabolism, and fuel utilization
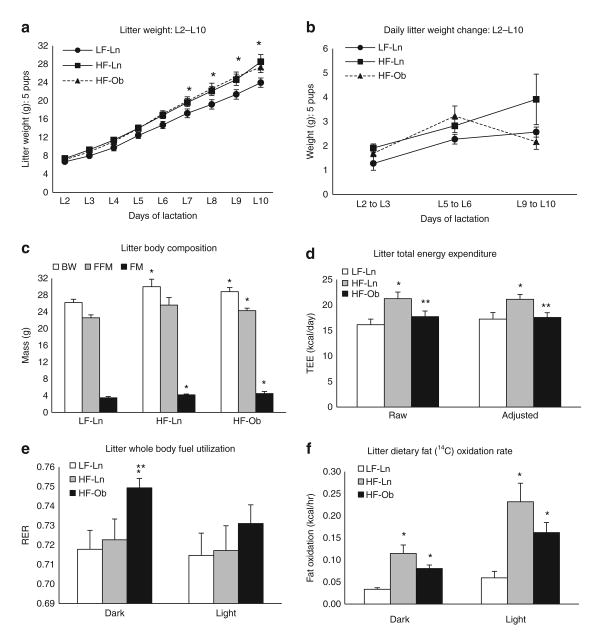
Litters were normalized to five pups on L2 and weighed each subsequent day until mid-lactation. The growth curve for these litters is depicted in Figure 4a. All litters weighed the same for the first 5 days of lactation, after which pups nursing HF-Ln and HF-Ob dams began to gain more weight than those nursing LF-Ln dams. This difference in weight gain remained through day 10 of lactation. Between L7 and L10, litters nursing on HF-Ln or HF-Ob dams weighed significantly more than those nursing LF-Ln dams (P < 0.05). There was no difference in the weights of litters nursing HF-Ln or HF-Ob dams (Figure 4a). Figure 4b depicts the daily weight change of litters from LF-Ln, HF-Ln, and HF-Ob dams between days L2 to L3, L5 to L6, and L9 to L10. Both LF and HF lean litters showed progressively increasing daily weight gains whereas HF-Ob litter weight gain became lower from L9 to L10. Decreased weight gain from L9 to L10 in HF-Ob litters may reflect the changes in milk composition observed in this group (Table 3). To determine whether pups from HF-fed dams gained more weight in fat-free mass or in fat mass, litters' body composition was measured just before sacrifice (Figure 4c). Elevated body mass in litters from HF-fed dams was a result of an increase in both fat free mass (HF-Ln P = 0.09, HF-Ob P = 0.05) and fat mass (HF-Ln P = 0.01, HF-Ob P = 0.04), suggesting that there was not a preferential deposition of one or the other.
Figure 4.
Analysis of litter energy expenditure and fuel utilization. All values are expressed as a mean ± s.e. Bars with a single asterisk are significantly different from low-fat lean dam (LF-Ln) controls and bars with a double asterisk are significantly different from high-fat lean dam (HF-Ln) controls (P < 0.05). (a) Growth curve of litters normalized to five pups over the first 10 days of lactation. (b) Daily litter weight change of litters normalized to five pups between days L2 to L3, L5 to L6, and L9 to L10. (c) Litter body composition on the study day depicted as total body weight (BW), fat-free mass (FFM), and fat mass (FM). (d) Total energy expenditure (Raw) expressed over the 24 h as kcal/day; total energy expenditure (adjusted) was examined by analysis of covariance, using lean mass as the covariate. (e) Respiratory exchange ratio (RER; CO2/O2) derived from indirect calorimetry measurements is expressed during the dark and light cycle. (f) Oxidation of dietary fat was assessed by measuring 14C-CO2 in expired air over 4.5 min at each time point during the light and dark cycle.
To define the effect of maternal diet and obesity on litter metabolism we measured TEE, RER, and dietary fat oxidation in litters from LF-Ln, HF-Ln, and HF-Ob dams. Litter-specific TEE was measured after removing litters from their respective dams during the dark and light cycles and averaged over the 24 h study (Figure 4d). The HF-Ln litters had the highest TEE, which was significantly greater than the TEE of litters nursing from LF-Ln and HF-Ob dams (P = 0.006 and P = 0.02 respectively). After controlling for basal metabolic mass (ANCOVA, lean mass as covariate) these differences in TEE remained significant. Whole body fuel utilization was also calculated during the dark and light cycles for all litters (Figure 4e). Litters nursing from HF-Ob dams had significantly greater RER than those nursing from LF-Ln (P = 0.002) or HF-Ln (P = 0.02) dams during the dark cycle. However, during the light cycle, the RER of these litters were identical. The oxidation rates of maternally derived 14C-labeled lipid in litters from each group for the dark and light cycles is shown in Figure 4f. It is important to note that 14C-labeled lipid was solely derived from maternal consumption and passed through milk, as there was no observation of dietary dye in the pups' stomachs. The rates of litter oxidation of 14C-labeled lipid during both dark and light cycles was greater for litters nursing form HF-Ln and HF-Ob dams compared to those nursing from LF-Ln dams (P < 0.004). Litters nursing from HF-Ob mice tended to oxidize less dietary fat than HF-Ln, however these values did not reach significance (P = 0.08). The pattern of dietary fat oxidation in these litters closely mimics the differences in milk dietary fat composition observed between groups suggesting that dietary fat oxidation in the litters is driven by milk lipid composition.
Discussion
Lactation is a calorically demanding physiological state that presents significant challenges to maternal energy homeostasis. In humans and many animal models, these challenges are met by increasing energy consumption, decreasing energy expenditure, trafficking nutrients to the mammary gland, and enhancing de novo lipid synthesis (4,29). The novel observations of this study are that there are distinct consequences to this adaptive response with respect to consumption of a HF diet, in the presence or absence of obesity. A HF diet increases the trafficking of dietary lipid to adipose, liver, and mammary gland while subsequently decreasing de novo lipogenesis in these tissues and lowering TEE. These changes, however, do not appear to affect maternal metabolism in a way that compromises milk production. On the other hand, the addition of obesity blunts the diurnal fluctuation in metabolism, suppresses the induction of carbohydrate utilization, and alters the trafficking of dietary and de novo-derived calories to the mammary gland. Together the metabolic changes which occur in obese dams, compromises milk fat production and leads to detrimental consequences on EB and fuel utilization of their suckling pups.
HF diet during pregnancy and lactation has severe consequences on neonatal body composition (30), feeding behavior (15,31), and susceptibility to metabolic disease (32). However, few have actually studied the effects of HF diet on maternal metabolism during lactation. Shortly after parturition the metabolic demands of milk production induce a negative energy imbalance in lactating dams (33,34). In humans, acute challenges with a HF diet increases TEE and milk energy output, resulting in an even greater negative energy imbalance and more dietary fat in the milk (35). This effect is somewhat akin to the acute response to HF feeding in obesity-resistant female rats, which increase both TEE and fat oxidation to dissipate the excess energy (17). Although chronic HF feeding in the present study did increase the amount of dietary fat trafficked to the milk, the dams exhibited a decreased TEE at L10. Chronic verses acute consumption of a HF diet may be the cause for this difference in TEE. Additionally, we suspect that the observation of lower TEE in HF fed dams is a result of decreased de novo lipogenesis, as the energetic cost to make milk lipid is much greater than the cost of utilizing lipid from the diet. Importantly, activity increased with HF feeding therefore decreased TEE was not a result of lower activity in these groups. Consequently, during lactation TEE may be driven by energy utilized for milk production rather than EI or activity, suggesting that HF diet decreases the energy needed to produce milk.
Lower energy requirements for milk production in HF fed dams may be a consequence of trafficking excess dietary fat to the liver and mammary gland, lowering the carbohydrate uptake and decreasing the need for de novo synthesized milk lipid. Our data is consistent with this concept and with previous studies reporting reduced de novo lipogenesis in liver and mammary gland after HF feeding during lactation (36,37). Additionally, greater amounts of dietary fat in the milk of HF lean mice and lower amounts found in their serum, suggests that they are utilizing more dietary fat for milk lipid synthesis relative to de novo-derived fats, which has been reported in previous studies (36). Even so, de novo lipogenesis was still occurring in the HF-Ln dams (RER > 1) and this process was making a significant contribution, albeit to a lesser extent than the LF-Ln, to milk lipid synthesis. The lack of an effect of HF feeding on milk triglycerides in this study and in previous reports (38) suggests that the HF-Ln dams have simply adapted milk production to the available nutrients. Without the need to make as much milk fat via de novo lipogenesis, their energy requirements are lower than that of the LF-Ln. Unlike the HF-Ln dams, the HF-Ob dams fail to adapt milk synthesis by utilizing excess dietary fat, and the production of milk fat is compromised.
This failure to adapt to the challenges of lactation may be a consequence of a more general inability to adapt to other types of metabolic stress, like fasting, exercise, and overfeeding (7,22). This generalized impairment in metabolic regulation, referred to as metabolic inflexibility, would explain the sustained, low RER across the diurnal cycle of the obese dams. In contrast to obese animals, lean and weight reduced animals traffic dietary fat to the liver during their dark cycle, and remobilize it in the form of very-low-density lipoprotein later in their light cycle (22). A coincidental increase in dietary fat oxidation occurs during the light cycle as a result. The LF-Ln and HF-Ln exhibit this same diurnal fluctuation during lactation, whereas the HF-Ob do not. Their impaired metabolic regulation also blunts the diversion of ingested nutrients to the mammary gland for milk production. Studies have shown that obesity correlates with poor lactation performance in women (8,9) and impaired mammary gland function in rodents (10,11). However, due to different feeding paradigms and study designs, the effects of HF diet have not been well separated from the effects of obesity on lactation. The novel aspects of the present study are that we were able to show this effect of obesity, above and beyond the consequences of a diet high in fat, on maternal metabolism.
Together lower milk energy output and reduced TEE in obese dams resulted in a positive energy imbalance during lactation, which may be a driving force for impaired lipid trafficking to the mammary gland. Elevated trafficking of dietary fat to adipose and liver, as seen in nonlactating obese subjects (39), can disrupt the normal regulation of lipogenesis and lipolysis that is required in peripheral tissues for nutrient diversion to the mammary gland (29,38,40). Consistent with some reports (41), we found that obesity reduced milk fat production by L10 while not changing the protein or lactose content of the milk. In contrast, some studies have reported that HF-induced obesity leads to higher milk fat and lower protein in combination with lower milk production and retarded pup growth (10,12,42). When milk production was calculated from the estimated milk energy output and milk composition we found that HF-fed dams actually produced more milk over the 24 h. For the HF-Ln dams, milk composition was not different; therefore increased milk production resulted in elevated pup growth. Milk energy output was reduced in HF-Ob dams and as suggested in the report from Aoki et al. (41), the pups from HF-Ob dams may sense the decrease in energy content and suckle more to make up for it. Studies showing higher energy density of milk employed a diet that likely contained higher simple sugars, saturated fats, and trans-fats, whereas the present study employed a HF diet comprised of complex carbohydrates, low amounts of sucrose and soybean oil. The former paradigm may be more representative of the human obesity phenotype compared to the latter, however the differences in findings indicate a need for further examination of each dietary component and their effect on milk composition. Soybean oil-based diet may, in fact, show potential benefit for lactating women. Overall, these data support our hypothesis that the obese phenotype impairs maternal metabolism during lactation, resulting in significant alterations in milk composition that potentially compromise neonatal nutrition. The long-term effects of compromised nutrition in early development can have profound effects on neonatal growth and metabolism, predisposing the offspring of obese individuals to adult disease (14,15,30).
The effects of HF feeding during lactation on neonatal growth have been inconsistent (10,11,14,15,37). Some studies have reported impaired neonatal growth linked to lactation defects (10,11), whereas others have reported increased neonatal growth correlating with an obese phenotype (14,15). Our data agrees with evidence that implicate HF maternal diets induce increased neonatal growth. Increased milk energy output and milk production from lean dams fed a HF diet may explain the excess weight gain in their pups. Elevated energy expenditure and fat oxidation in these offspring support this concept. However, decreased milk fat and milk energy output from obese dams suggest that obese offspring have become more metabolically efficient allowing them to grow to the same extent with fewer calories. This “thrifty phenotype” is consistent with numerous reports showing that under nutrition in both perinatal and postnatal development can lead to metabolic efficiency and subsequent “catch-up” growth (43,44). Additionally, they may have adapted to the decreased milk energy content by increasing their time suckling and the total volume of milk consumed. Our observations cannot determine if the thrifty phenotype is solely the consequence of postnatal lactation defects, in utero epigenetic changes, or both. However, these studies provide the justification for cross-fostering studies that would be able to distinguish the impact of postnatal and in utero effects. Additionally, the inherited genetic makeup of the pups from obese dams may play a large role in the metabolic differences observed in this study. Obese offspring had an elevated RER and lower 14C fat oxidation rates, consistent with the presence of fewer fat calories in the milk from obese dams. However, these data may also reflect metabolic adaptations associated with storage of fat rather than its utilization for energy, as has been reported to occur in neonates exposed to under nutrition (45).
In summary, there is increasing evidence that diet and obesity can influence neonatal metabolism through in utero and postnatal mechanisms (14,15,20,31,46). In our study, obesity was induced by high-fat feeding before pregnancy, thus it is possible that both in utero and postnatal influences contributed to differences in the metabolic properties of neonates born to lean and obese dams. Nevertheless our data documenting differences in milk composition of lean and obese dams support the concept that maternal obesity, above and beyond the consequences of a high-fat diet, can influence neonatal metabolism through alterations in the nutrient content of milk.
Supplementary Material
Acknowledgments
This research was supported by National Institutes of Health grants R21HD050863 and POIHD38129 (j.L.M.) and DK038088 (P.S.M.) and a Colorado Clinical Translational Sciences Institute Predoctoral T32 Training Grant L1RR025778 (j.L.W., j.L.M., and P.S.M.). We appreciate the use of core services provided by the Colorado Nutrition and Obesity Research Center (NORC, DK48520).
J.L.M. and P.S.M. are co-senior investigators on this study.
Footnotes
Supplementary Material: Supplementary material is linked to the online version of the paper at http://www.nature.com/oby
Disclosure: The authors declared no conflict of interest.
References
- 1.Ip S, Chung M, Raman G, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep) 2007:1–186. [PMC free article] [PubMed] [Google Scholar]
- 2.Butte NF. Impact of infant feeding practices on childhood obesity. J Nutr. 2009;139:412S–416S. doi: 10.3945/jn.108.097014. [DOI] [PubMed] [Google Scholar]
- 3.Rasmussen KM. The influence of maternal nutrition on lactation. Annu Rev Nutr. 1992;12:103–117. doi: 10.1146/annurev.nu.12.070192.000535. [DOI] [PubMed] [Google Scholar]
- 4.McNamara JP. Lipid metabolism in adipose tissue during lactation: a model of a metabolic control system. J Nutr. 1994;124:1383S–1391S. doi: 10.1093/jn/124.suppl_8.1383S. [DOI] [PubMed] [Google Scholar]
- 5.Corpeleijn E, Saris WH, Blaak EE. Metabolic flexibility in the development of insulin resistance and type 2 diabetes: effects of lifestyle. Obes Rev. 2009;10:178–193. doi: 10.1111/j.1467-789X.2008.00544.x. [DOI] [PubMed] [Google Scholar]
- 6.Phielix E, Mensink M. Type 2 diabetes mellitus and skeletal muscle metabolic function. Physiol Behav. 2008;94:252–258. doi: 10.1016/j.physbeh.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 7.Storlien L, Oakes ND, Kelley DE. Metabolic flexibility. Proc Nutr Soc. 2004;63:363–368. doi: 10.1079/PNS2004349. [DOI] [PubMed] [Google Scholar]
- 8.Butte NF, Garza C, Stuff JE, Smith EO, Nichols BL. Effect of maternal diet and body composition on lactational performance. Am J Clin Nutr. 1984;39:296–306. doi: 10.1093/ajcn/39.2.296. [DOI] [PubMed] [Google Scholar]
- 9.Rasmussen KM. Association of maternal obesity before conception with poor lactation performance. Annu Rev Nutr. 2007;27:103–121. doi: 10.1146/annurev.nutr.27.061406.093738. [DOI] [PubMed] [Google Scholar]
- 10.Flint DJ, Travers MT, Barber MC, Binart N, Kelly PA. Diet-induced obesity impairs mammary development and lactogenesis in murine mammary gland. Am J Physiol Endocrinol Metab. 2005;288:E1179–E1187. doi: 10.1152/ajpendo.00433.2004. [DOI] [PubMed] [Google Scholar]
- 11.Rolls BJ, Rowe EA, Fahrbach SE, Agius L, Williamson DH. Obesity and high energy diets reduce survival and growth rates of rat pups. Proc Nutr Soc. 1980;39:51A. [PubMed] [Google Scholar]
- 12.Rolls BA, Gurr MI, van Duijvenvoorde PM, Rolls BJ, Rowe EA. Lactation in lean and obese rats: effect of cafeteria feeding and of dietary obesity on milk composition. Physiol Behav. 1986;38:185–190. doi: 10.1016/0031-9384(86)90153-8. [DOI] [PubMed] [Google Scholar]
- 13.Rasmussen KM. Effects of under- and overnutrition on lactation in laboratory rats. J Nutr. 1998;128:390S–393S. doi: 10.1093/jn/128.2.390S. [DOI] [PubMed] [Google Scholar]
- 14.Gorski JN, Dunn-Meynell AA, Hartman TG, Levin BE. Postnatal environment overrides genetic and prenatal factors influencing offspring obesity and insulin resistance. Am J Physiol Regul Integr Comp Physiol. 2006;291:R768–R778. doi: 10.1152/ajpregu.00138.2006. [DOI] [PubMed] [Google Scholar]
- 15.Levin BE. Interaction of perinatal and pre-pubertal factors with genetic predisposition in the development of neural pathways involved in the regulation of energy homeostasis. Brain Res. 2010;1350:10–17. doi: 10.1016/j.brainres.2009.12.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu G, Bazer FW, Cudd TA, Meininger CJ, Spencer TE. Maternal nutrition and fetal development. J Nutr. 2004;134:2169–2172. doi: 10.1093/jn/134.9.2169. [DOI] [PubMed] [Google Scholar]
- 17.Jackman MR, MacLean PS, Bessesen DH. Energy expenditure in obesity-prone and obesity-resistant rats before and after the introduction of a high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1097–R1105. doi: 10.1152/ajpregu.00549.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCurdy CE, Bishop JM, Williams SM, et al. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest. 2009;119:323–335. doi: 10.1172/JCI32661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burcelin R, Crivelli V, Dacosta A, Roy-Tirelli A, Thorens B. Heterogeneous metabolic adaptation of C57BL/6J mice to high-fat diet. Am J Physiol Endocrinol Metab. 2002;282:E834–E842. doi: 10.1152/ajpendo.00332.2001. [DOI] [PubMed] [Google Scholar]
- 20.Reifsnyder PC, Churchill G, Leiter EH. Maternal environment and genotype interact to establish diabesity in mice. Genome Res. 2000;10:1568–1578. doi: 10.1101/gr.147000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins S, Martin TL, Surwit RS, Robidoux J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiol Behav. 2004;81:243–248. doi: 10.1016/j.physbeh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Jackman MR, Steig A, Higgins JA, et al. Weight regain after sustained weight reduction is accompanied by suppressed oxidation of dietary fat and adipocyte hyperplasia. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1117–R1129. doi: 10.1152/ajpregu.00808.2007. [DOI] [PubMed] [Google Scholar]
- 23.WEIR JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol (Lond) 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Commerford SR, Pagliassotti MJ, Melby CL, Wei Y, Hill JO. Inherent capacity for lipogenesis or dietary fat retention is not increased in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1680–R1687. doi: 10.1152/ajpregu.2001.280.6.R1680. [DOI] [PubMed] [Google Scholar]
- 25.Caster WO, Poncelet J, Simon AB, Armstrong WD. Tissue weights of the rat I Normal values determined by dissection and chemical methods. Proc Soc Exp Biol Med. 1956;91:122–126. doi: 10.3181/00379727-91-22186. [DOI] [PubMed] [Google Scholar]
- 26.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 27.Dole VP, Meinertz H. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem. 1960;235:2595–2599. [PubMed] [Google Scholar]
- 28.Munday MR, Williamson DH. Diurnal variations in food intake and in lipogenesis in mammary gland and liver of lactating rats. Biochem J. 1983;214:183–187. doi: 10.1042/bj2140183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bauman DE, Currie WB. Partitioning of nutrients during pregnancy and lactation: a review of mechanisms involving homeostasis and homeorhesis. J Dairy Sci. 1980;63:1514–1529. doi: 10.3168/jds.s0022-0302(80)83111-0. [DOI] [PubMed] [Google Scholar]
- 30.Sewell MF, Huston-Presley L, Super DM, Catalano P. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol. 2006;195:1100–1103. doi: 10.1016/j.ajog.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 31.Chen H, Simar D, Lambert K, Mercier J, Morris MJ. Maternal and postnatal overnutrition differentially impact appetite regulators and fuel metabolism. Endocrinology. 2008;149:5348–5356. doi: 10.1210/en.2008-0582. [DOI] [PubMed] [Google Scholar]
- 32.Gluckman PD, Hanson MA, Beedle AS, Raubenheimer D. Fetal and neonatal pathways to obesity. Front Horm Res. 2008;36:61–72. doi: 10.1159/000115337. [DOI] [PubMed] [Google Scholar]
- 33.Denis RG, Williams G, Vernon RG. Regulation of serum leptin and its role in the hyperphagia of lactation in the rat. J Endocrinol. 2003;176:193–203. doi: 10.1677/joe.0.1760193. [DOI] [PubMed] [Google Scholar]
- 34.Malabu UH, Kilpatrick A, Ware M, Vernon RG, Williams G. Increased neuropeptide Y concentrations in specific hypothalamic regions of lactating rats: possible relationship to hyperphagia and adaptive changes in energy balance. Peptides. 1994;15:83–87. doi: 10.1016/0196-9781(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 35.Mohammad MA, Sunehag AL, Haymond MW. Effect of dietary macronutrient composition under moderate hypocaloric intake on maternal adaptation during lactation. Am J Clin Nutr. 2009;89:1821–1827. doi: 10.3945/ajcn.2008.26877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Del Prado M, Villalpando S, Gordillo J, Hernández-Montes H. A high dietary lipid intake during pregnancy and lactation enhances mammary gland lipid uptake and lipoprotein lipase activity in rats. J Nutr. 1999;129:1574–1578. doi: 10.1093/jn/129.8.1574. [DOI] [PubMed] [Google Scholar]
- 37.Grigor MR, Warren SM. Dietary regulation of mammary lipogenesis in lactating rats. Biochem J. 1980;188:61–65. doi: 10.1042/bj1880061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neville MC, Picciano MF. Regulation of milk lipid secretion and composition. Annu Rev Nutr. 1997;17:159–183. doi: 10.1146/annurev.nutr.17.1.159. [DOI] [PubMed] [Google Scholar]
- 39.Jackman MR, Kramer RE, MacLean PS, Bessesen DH. Trafficking of dietary fat in obesity-prone and obesity-resistant rats. Am J Physiol Endocrinol Metab. 2006;291:E1083–E1091. doi: 10.1152/ajpendo.00159.2006. [DOI] [PubMed] [Google Scholar]
- 40.Vernon RG. Lipid metabolism during lactation: a review of adipose tissue-liver interactions and the development of fatty liver. J Dairy Res. 2005;72:460–469. doi: 10.1017/S0022029905001299. [DOI] [PubMed] [Google Scholar]
- 41.Aoki N, Yamaguchi Y, Ohira S, Matsuda T. High fat feeding of lactating mice causing a drastic reduction in fat and energy content in milk without affecting the apparent growth of their pups and the production of major milk fat globule membrane components MFG-E8 and butyrophilin. Biosci Biotechnol Biochem. 1999;63:1749–1755. doi: 10.1271/bbb.63.1749. [DOI] [PubMed] [Google Scholar]
- 42.Shaw MA, Rasmussen KM, Myers TR. Consumption of a high fat diet impairs reproductive performance in Sprague-Dawley rats. J Nutr. 1997;127:64–69. doi: 10.1093/jn/127.1.64. [DOI] [PubMed] [Google Scholar]
- 43.Dulloo AG, Jacquet J, Seydoux J, Montani JP. The thrifty ‘catch-up fat’ phenotype: its impact on insulin sensitivity during growth trajectories to obesity and metabolic syndrome. Int J Obes (Lond) 2006;30(Suppl 4):S23–S35. doi: 10.1038/sj.ijo.0803516. [DOI] [PubMed] [Google Scholar]
- 44.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 45.Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 46.Desai M, Babu J, Ross MG. Programmed metabolic syndrome: prenatal undernutrition and postweaning overnutrition. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2306–R2314. doi: 10.1152/ajpregu.00783.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.