Abstract
Background
Dabrafenib (GSK2118436) is a potent ATP-competitive inhibitor of BRAF kinase and was highly selective for mutant BRAF in kinase panel screening, cell lines, and xenografts.
Methods
A Phase I trial of dabrafenib was conducted to evaluate safety and tolerability in patients with incurable solid tumours. Efficacy at the recommended Phase II dose (RP2D) was studied in patients with BRAF-mutant tumours, including those with non-V600E mutations, in three cohorts: (1) metastatic melanoma, (2) melanoma with untreated brain metastases, and (3) non-melanoma solid tumours.
Findings
184 patients enrolled, and 150 mg twice daily was chosen as the RP2D, based on safety, pharmacokinetic, and pharmacodynamic data. At the RP2D in patients with V600 BRAF-mutant melanoma, a response rate of 69% (a confirmed response rate of 50%) was observed overall and a 78% response rate (a confirmed response rate of 56%) in V600E BRAF-mutant melanoma. In V600 BRAF-mutant melanoma, responses were durable, with 17 patients (47%) on treatment for more than 6 months and a median progression-free survival (PFS) of 5·5 months. Responses were observed in patients with non-V600E BRAF mutations, including V600K and V600G. In the RP2D expansion of melanoma with untreated brain metastases, nine of ten patients (90%) showed reduction in brain lesion size and the median PFS was 4.2 months. Among BRAF-mutant non-melanoma solid tumours, antitumour activity was observed in gastrointestinal stromal tumour, papillary thyroid, non-small cell lung, ovarian, and colorectal cancer.
Interpretation
Dabrafenib is a highly active inhibitor of V600-mutant BRAF with a high response rate in V600E melanoma, and is the first drug of its class to demonstrate activity in melanoma brain metastases.
Funding
This study was funded and sponsored by GlaxoSmithKline
Keywords: BRAF-mutant, BRAF mutation, V600, V600E, V600K, V600G, K601E, V600_K601E, melanoma, metastatic melanoma, stage IV melanoma, brain metastases, dabrafenib, GSK2118436, papillary thyroid carcinoma, colorectal carcinoma, non-small cell lung carcinoma, gastrointestinal stromal tumour, ovarian cancer
Introduction
Activating oncogenic mutations of BRAF occur in many tumour types including cutaneous melanoma (50%), papillary thyroid (46%), borderline ovarian tumours (34%), biliary tract (11%), colorectal (10%), non-small cell lung cancer (NSCLC; 2%), and hairy cell leukaemia (100%).1,2 The most common mutation, substitution of valine with glutamic acid at amino acid position 600 (V600E), locks BRAF into its active conformation, with a ten-fold increase in activity over wild-type BRAF.1 In 7–21% of BRAF-mutant melanoma, substitution with lysine (V600K) results in similarly activated BRAF.2,5 Other less frequent activating mutations also occur.2,5 Mutant BRAF correlates with poorer prognosis in colorectal cancer,3 papillary thyroid cancer,4 and metastatic melanoma.5
Metastatic melanoma carries a poor prognosis with a median overall survival of 9–11 months.6 Patients with melanoma brain metastases fare worse, with a median survival of 4–5 months.7 Brain metastases are present in 20% of stage IV patients at diagnosis,8 40–45% of all stage IV patients,7,8 and contribute to death in 20–54% of stage IV patients.9 Systemic therapies have limited efficacy in melanoma brain metastases, with a response rate of no more than 10%.10–11. 12 Treatment of brain metastases includes surgery, or stereotactic or palliative whole-brain radiotherapy.10,13
BRAF-mutant melanoma displays features of oncogene addiction in vitro.14 The BRAF inhibitor vemurafenib (PLX4032/RG7204) showed clinical activity in patients with V600E-mutant BRAF metastatic melanoma, with a confirmed response rate of 48% and improved survival compared with dacarbazine.15 Patients with brain metastases were excluded from published clinical trials of vemurafenib.14,15
Dabrafenib (GSK2118436) is a potent ATP-competitive inhibitor of BRAF kinase and was highly selective for mutant BRAF in kinase panel screening, cell lines, and xenografts.16 A Phase I trial of dabrafenib was conducted in patients with incurable solid tumours, enriching with BRAF-mutant cancers, including a cohort with untreated, asymptomatic melanoma brain metastases.
Methods
Study Design
The primary objectives were to determine the safety, tolerability and recommended Phase II dose (RP2D) of dabrafenib; the secondary objectives included assessment of tumour response, and establishing the pharmacokinetic and pharmacodynamic profiles.
An accelerated dose titration design (Supplementary Methods) was used, starting at 12 mg daily (21-day cycle). Dose cohorts were expanded up to 20 patients to collect adequate data on safety, pharmacokinetics, or pharmacodynamics. Treatment continued until disease progression, intolerable toxicity, or withdrawal of consent. The maximum tolerated dose (MTD) was the highest dose at which no more than one of six patients experienced a dose-limiting toxicity (DLT) (Supplementary Methods).
An RP2D was chosen based on safety, pharmacokinetic, pharmacodynamic, and response data. Efficacy at the RP2D was studied in patients with BRAF-mutant tumours in three distinct cohorts: (1) metastatic melanoma, (2) asymptomatic untreated melanoma brain metastases, and (3) non-melanoma solid tumours.
Patients
Eligibility criteria included written informed consent, histologically confirmed diagnosis of a solid tumour for which there was no curative therapy, age ≥18 years, Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≤1, and adequate organ function (Supplementary Methods). Presence of a BRAF mutation was initially optional but later mandatory due to absence of activity in BRAF wild-type tumours.
Eligibility criteria for the expansion cohort of melanoma patients with brain metastases included brain metastases ≥3 mm, no symptoms attributable to brain metastases, and no prior surgical resection or stereotactic radiosurgery to target lesions, or whole-brain radiotherapy.
Study Assessments
Primary outcome measures
Toxicity was assessed with the Common Terminology Criteria for Adverse Events (CTCAE) v3.0. Safety evaluations were performed as described in Supplementary Methods. Dermatological examination was performed at baseline and as clinically indicated.
Secondary outcome measures
Tumour response was assessed by the Response Evaluation Criteria in Solid Tumours (RECIST) version 1.0.17 Baseline radiological assessment was performed within 35 days before initiating treatment. Initial restaging occurred at 9 weeks and 6 weeks in the escalation and expansion cohorts, respectively. Subsequent restaging occurred every 9 weeks.
Brain metastases were assessed with gadolinium-enhanced, T1-weighted brain magnetic resonance imaging (MRI). The sum of diameters from all brain lesions measuring ≥3 mm at baseline was calculated for each brain lesion assessment. Percentage change from baseline was calculated for each post-baseline assessment.
Tumour genotyping for BRAF was performed with accredited assays (Supplementary Methods). Central genotyping by Response Genetics, Inc. (RGI; Los Angeles, CA, USA) was mandatory in the expansion cohorts.
Pharmacokinetic evaluation of dabrafenib and its metabolites was performed at multiple time points on Days 1, 8 and/or 15 of Cycle 1, and up to Cycle 4 in the expansion cohorts. Pharmacodynamic endpoints included baseline and Day 15 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) imaging, and paired pre-dose and on-treatment tumour biopsies in selected patients.
Statistical Analysis
For the response rate endpoint for melanoma, the 95% exact confidence intervals (CIs) were calculated. Median progression-free survival (PFS) and duration of response were estimated using the Kaplan–Meier method. Calculation of estimated 95% CI for median PFS was performed using SAS 9.1 with the Bookmeyer and Crowley method.18 PFS was defined as the interval between first dose and earlier date of disease progression or death due to any cause. If the patient received a new anticancer therapy prior to the date of progression or death, PFS was censored at the last adequate disease assessment prior to initiation of therapy. If the patient did not experience progression or death, PFS was censored at the date of last adequate disease assessment. Duration on treatment was defined as the time from first dose to the earlier date of last dose or data cut (25 March 2011). For patients with confirmed response, response duration was defined as the interval between first documented response and the earlier date of disease progression or death due to any cause. Censoring rules for response duration were the same as for PFS. Analysis of BRAF-mutant melanoma patients at the RP2D included those from both the escalation and expansion cohorts, and excluded patients who had received a prior BRAF or MEK inhibitor.
Role of the Funding Source
This study (NCT00880321) was funded, initiated, administrated, and sponsored by GlaxoSmithKline, which also provided data analysis services. All authors had access to all study data and are responsible for the decision to publish.
Results
Patients
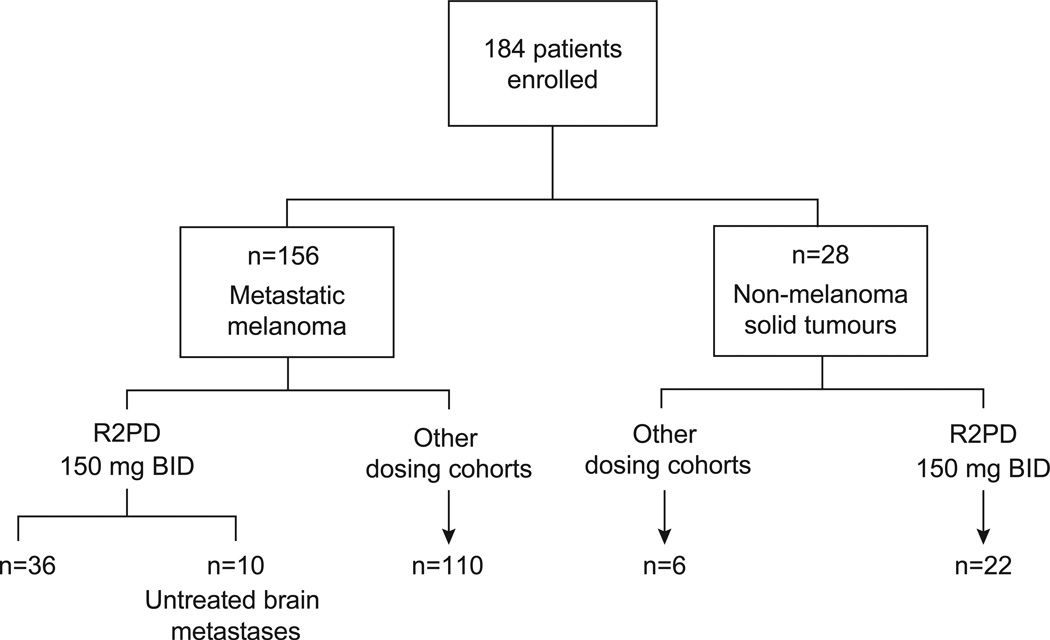
There were 184 patients enrolled, including 156 with melanoma, at eight study centres (Figure 1; Supplementary Results). Among the melanoma patients, three were BRAF wild-type, and 153 had the following BRAF mutations: V600E (n=130), V600K (n=18), V600G (n=1), K601E (n=2), V600_K601E (n=1), and unknown mutation type (n=1). Table 1A lists the baseline characteristics of the 36 V600 BRAF-mutant melanoma patients treated at the RP2D. Poor prognosis features were common, including 32 patients (89%) with stage M1c. Table 1A also includes ten V600 BRAF-mutant melanoma patients with untreated brain metastases treated at the RP2D. Twenty-eight patients with other solid-tumour malignancies included (Table 1B): 14 papillary thyroid, 11 colorectal, one ovarian, one NSCLC, and one gastrointestinal stromal tumour (GIST). Among the 28 non-melanoma patients, 26 had V600E BRAF mutations, one colorectal cancer patient had a BRAF mutation of unspecified type, and one colorectal cancer patient’s BRAF status was unknown.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram
Table 1.
Patient baseline demographics and clinical characteristics
| A: Patients with melanoma treated with dabrafenib at the recommended Phase II dose | |||
|---|---|---|---|
| Melanoma (n=36) (excludes patients with untreated brain metastases) |
Melanoma with untreated brain metastases (n=10) |
||
| Median age, years (range) | 54·5 (26–81) | 62·5 (23–82) | |
| Gender | Male | 21 (58) | 6 (60) |
| Female | 15 (42) | 4 (40) | |
| BRAF mutation | V600E | 27 (75) | 9 (90) |
| V600K | 8 (22) | 1 (10) | |
| V600G | 1 (3) | 0 | |
| ECOG PS | 0 | 22 (61) | 3 (30) |
| 1 | 13 (36) | 6 (60) | |
| 2 | 1 (3) | 1 (10) | |
| Stage M1c | 32 (89) | 10 (100) | |
| Elevated LDH | 17 (47) | 6 (60) | |
| History of treated stable brain metastases* | 1 (3) | 1 (10%)* | |
| Number of untreated brain metastases | 1 | N/A | 5 (50) |
| 2 | N/A | 2 (20) | |
| >3 | N/A | 3 (30) | |
| Median size of untreated brain metastases ≥ 3 mm (range) | 5 mm (3–15 mm) | ||
| Number of prior systemic regimens in the metastatic setting | 0 | 10 (28) | 4 (40) |
| 1 | 17 (47) | 3 (30) | |
| 2 | 6 (17) | 2 (20) | |
| ≥3 | 3 (9) | 1 (10) | |
| Country enrolled from | USA | 20 (56) | 5 (50) |
| Australia | 16 (44) | 5 (50) | |
| B: Non-melanoma patients treated with dabrafenib at any dose | ||||||
|---|---|---|---|---|---|---|
| PTC (n=14) | CRC (n=11) | NSCLC (n=1) |
GIST (n=1) |
Ovarian cancer (n=1) |
||
| Age, years (range) | 61·0 (44–83) |
57·0 (45–75) | 52·0 | 61·0 | 24·0 | |
| Gender | Male | 5 (36) | 7 (64) | 0 | 1 (100) | 0 |
| Female | 9 (64) | 4 (36) | 1 (100) | 0 | 1 (100) | |
| BRAF mutation | V600E | 14 (100) | 9* (82) | 1 (100) | 1 (100) | 1 (100) |
| Prior BRAF/MEK inhibitor therapy | 1 (7) | 1 (9) | 0 | 1 (100) | 0 | |
| Post-baseline disease assessment available as of data cut | 10 (71) | 11 (100) | 1 (100) | 1 (100) | 1 (100) | |
| Dose | 150 mg BID | 13 (93) | 7 (64) | 1 (100) | 1 (100) | 0 |
| 100 mg BID | 0 | 1 (9) | 0 | 0 | 1 (100) | |
| 100 mg TID | 1 (7) | 3 (27) | 0 | 0 | 0 | |
| ECOG PS | 0 | 3 (21) | 2 (18) | 1 (100) | 0 | 1 (100) |
| 1 | 11 (79) | 9 (82) | 0 | 0 | 0 | |
| Not reported | 0 | 0 | 0 | 1 (100) | 0 | |
| Elevated LDH | 0 | 3 (27) | 1 (100) | 0 | 0 | |
| Number of prior systemic regimens in the metastatic setting | 0 | 9 (64) | 4 (36) | 0 | 0 | 0 |
| 1 | 2 (14) | 4 (36) | 0 | 0 | 0 | |
| 2 | 2 (14) | 1 (9) | 0 | 1 (100) | 1 (100) | |
| ≥3 | 1 (7) | 2 (18) | 1 (100) | 0 | 0 | |
| Country enrolled from | USA | 13 (93) | 9 (82) | 1 (100) | 1 (100) | 0 |
| Australia | 1 (7) | 2 (18) | 0 | 0 | 1 (100) | |
One patient with a previously treated brain metastasis had a separate measurable untreated target brain metastasis.
ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase.
All data are n (%), unless stated otherwise.
One CRC patient had a BRAF mutation of unspecified type, and one CRC patient’s BRAF status was unknown.
BID, twice daily; CRC, colorectal cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; GIST, gastrointestinal stromal tumour; LDH, lactate dehydrogenase; NSCLC, non-small cell lung cancer; PTC, papillary thyroid cancer; TID, three times a day.
All data are n (%), unless stated otherwise.
Adverse Events
Among patients treated at all dose levels, the most common treatment-related adverse events (AEs) grade 2 or higher included cutaneous squamous cell carcinoma (SCC) or keratoacanthoma (11%), fatigue (8%), and pyrexia (6%) (Table 2). Dose reductions were required in 13 patients (7%), primarily for pyrexia (3%, n=5), fatigue (2%, n=3), and neutropenia (1%, n=2). No deaths or discontinuations of treatment resulted from AEs, and 140 patients (76%) experienced no drug-related toxicity higher than grade 2.
Table 2.
Treatment-related adverse events of grade 2 or higher observed in at least 5% of patients
| Toxicity grade |
≤75 mg BID (n=54) |
100 mg BID (n=10) |
100 mg TID (n=20) |
150 mg BID (n=70) |
200 mg BID (n=20) |
300 mg BID (n=10) |
Total (N=184) | |
|---|---|---|---|---|---|---|---|---|
| Cutaneous squamous cell carcinoma | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 4 (7) | 0 | 5 (25) | 5 (7) | 4 (20) | 2 (20) | 20 (11) | |
| Total | 4 (7) | 0 | 5 (25) | 5 (7) | 4 (20) | 2 (20) | 20 (11)* | |
| Fatigue | 2 | 4 (7) | 1 (10) | 0 | 3 (4) | 3 (15) | 1 (10) | 12 (7) |
| 3 | 0 | 1 (10) | 0 | 0 | 0 | 1 (10) | 2 (1) | |
| Total | 4 (7) | 2 (20) | 0 | 3 (4) | 3 (15) | 2 (20) | 14 (8) | |
| Pyrexia | 2 | 2 (4) | 2 (20) | 1 (5) | 3 (4) | 1 (5) | 1 (10) | 10 (5) |
| 3 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 1 (<1) | |
| Total | 2 (4) | 2 (20) | 1 (5) | 4 (6) | 1 (5) | 1 (10) | (6) |
Keratoacanthoma was observed in three patients, of whom two also developed cutaneous SCC. A total of 21 patients (11%) experienced SCC or keratoacanthoma.
All data are n (%).
Dose escalation reached 300 mg twice daily (BID) without identification of a MTD. DLTs were observed in three of 20 patients at 200 mg BID (grade 3 cutaneous SCC, grade 3 syncope, grade 2 pyrexia) and two of ten patients at 300 mg BID (grade 4 hyponatraemia, grade 3 cutaneous SCC). The RP2D of 150 mg BID was selected because a minimal increase in exposure was noted with 200 mg BID and no increase in the proportion of responders, near-maximal pharmacodynamic effect was observed in tumour biomarker and FDG-PET studies, and DLTs were present at 200 mg.
Cutaneous SCC was observed in 20 patients (11%) at doses ≥50 mg BID, with median onset at 67 days (range 9–217 days). All SCCs were low-grade, well-differentiated tumours, and no recurrence or metastasis was observed after local excision. Keratoacanthoma was observed in three patients, of whom two also developed cutaneous SCC. Neither cutaneous SCC nor keratoacanthoma caused discontinuation of treatment. Treatment-related non-malignant skin lesions were also observed, including hyperkeratosis (n=48, 26%) and actinic keratosis (n=18, 10%). Common hyperkeratotic lesions included verruca vulgaris, seborrhoeic hyperkeratoses, and plantar–palmar hyperkeratosis. Treatment-related actinic keratosis of grade 2 or above occurred in only one patient. Treatment-related hyperkeratotic lesions of grade 2 or above occurred in six patients (3%).
Thirty-seven patients (20%) experienced treatment-related pyrexia, which typically occurred without neutropenia or infection. The median time to first pyrexia was 31 days (range 1–532 days). Pyrexia was a serious AE in seven patients (4%).
Pharmacokinetics
Pharmacokinetic analysis demonstrated that exposure was dose-proportional up to 300 mg BID on Day 1, but less than dose-proportional and lower after repeat dosing (Day 15). At the RP2D (150 mg BID), maximum plasma concentration (Cmax) was observed 2 hours post-dosing, and mean terminal half-life was 5·2 hours. The Day 15 geometric mean area under the plasma concentration-time curve over a 12-hour period and mean Cmax were 2619 ng*hr/ml (5·0 µM*hr) and 806 ng/ml (1·6 µM), respectively.
Pharmacodynamics
Paired tumour biopsies collected at baseline and after repeat dosing (Days 6–13) in eight patients at varying doses (70–200 mg BID) demonstrated a median inhibition of pERK of 83·9% (range 38·0–93·3%), calculated as a percentage change from baseline. Phospho-ERK decreased by 86·7% in the one patient who received 150 mg BID where this was evaluated. Similarly, FDG-PET performed at baseline and at Week 2 in 56 patients at varying doses (35 mg QD–300 mg BID) demonstrated decreases in mean maximum standardized uptake value (SUVmax) of tumour FDG uptake in 53 patients (95%) compared with baseline, with a median 60% decrease (percentage change from baseline range −100% to 19%).
Tumour Response
Partial or complete responses were observed at multiple doses (35–300 mg BID) and in multiple BRAF-mutant tumour types (Figure 1). Enrolment of BRAF wild-type melanoma was suspended when no responses were observed in three patients.
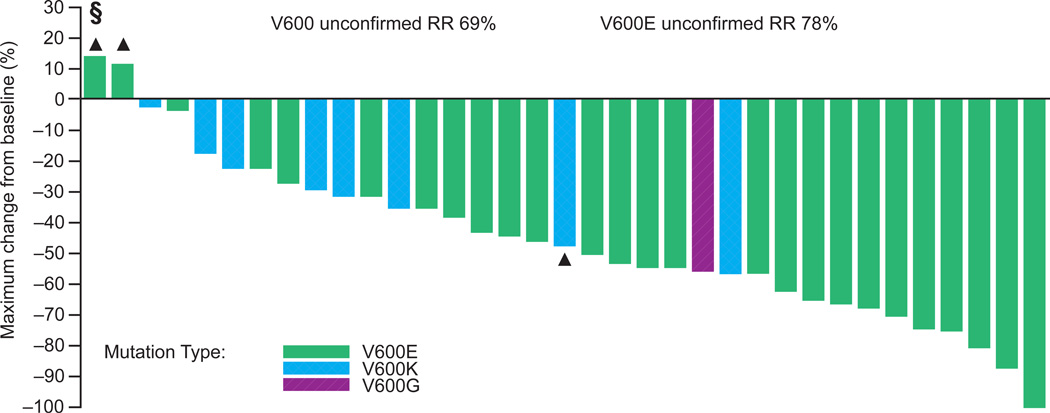
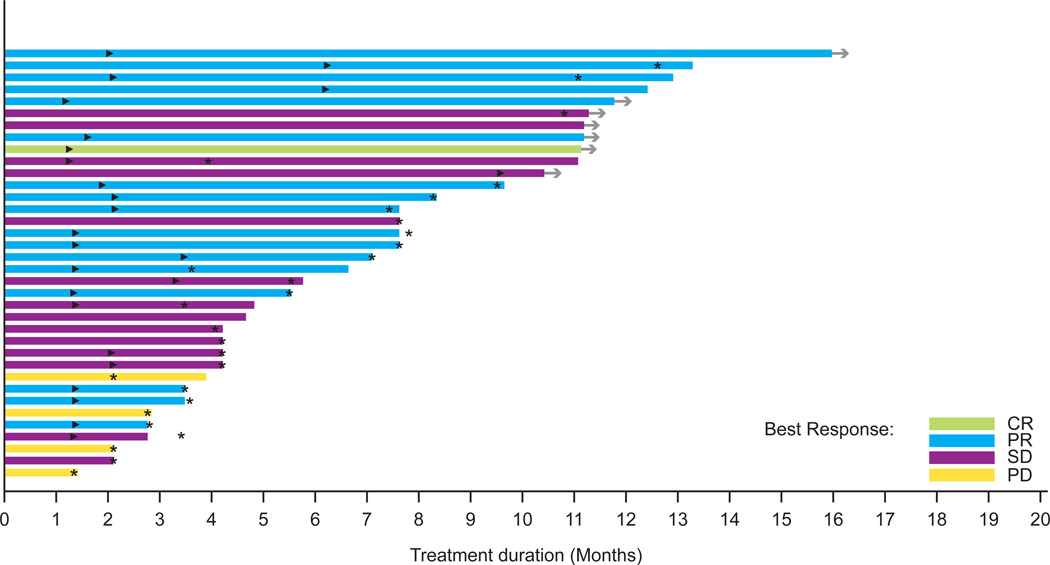
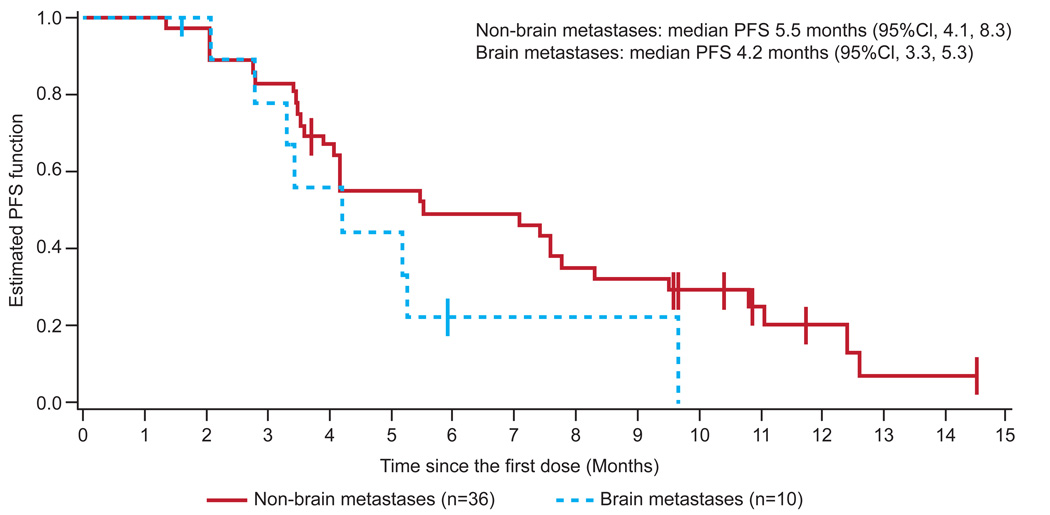
In patients with V600 BRAF-mutant melanoma treated at the RP2D (n=36), a response rate of 69% (95% CI 51·9–83·7) and confirmed response rate of 50% (95% CI 32·9–67·1) were observed overall. In V600E BRAF-mutant melanoma, a response rate of 78% (95% CI 57·7–91·4) and confirmed response rate of 56% (95% CI 35·3–74·5) were observed (Figure 2A, Figure S1). In V600 BRAF-mutant melanoma, antitumour activity was durable, with a median duration of response of 6·2 months (95% CI 4·2–7·7). Seventeen patients (47%) had not progressed at 6 months, and the median PFS was 5·5 months (95% CI 4·1–8·3) (Figures 3A and 3C). Median PFS among V600E patients was similar to V600K patients (5·5 vs. 5·6 months). A preliminary analysis of clinical factors associated with durability of response revealed a longer median PFS (7·8 months, 95% CI 5·5–12·4) with normal lactate dehydrogenase (LDH) compared with high LDH (4·2 months, 95% CI 3·4–7·6) and ECOG PS of 0 (7·4 months, 95% CI 4·1–11·1) compared with ECOG PS of 1 or 2 (4·2 months, 95% CI 3·4–7·8), among patients with V600 BRAF-mutant melanoma treated at the RP2D.
Figure 2.
Antitumour response in patients treated with dabrafenib
A: V600 BRAF-mutant melanoma patients treated at the recommended Phase II dose (n=36)
§ One V600E patient developed disease progression prior to the first restaging. Tumour size change was not available and is not included in the figure.
▲ Patients with PD as best response.
RR, response rate.
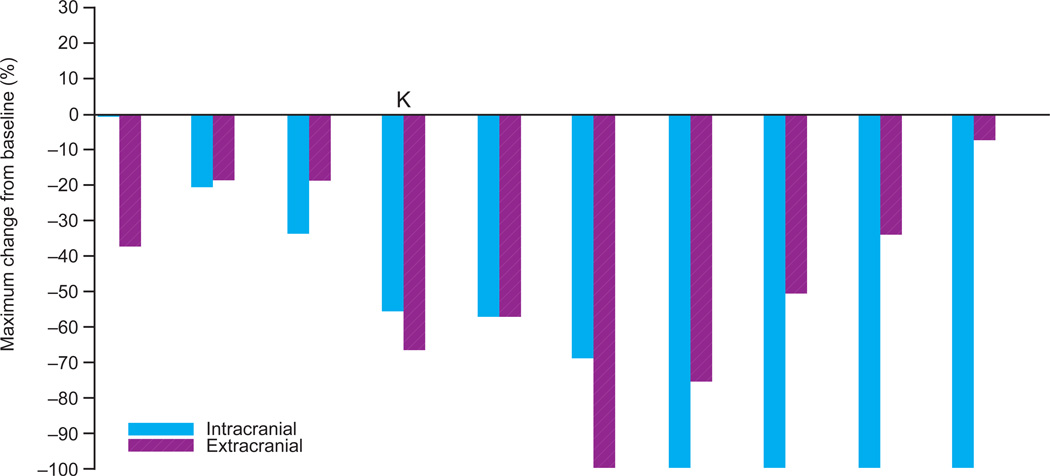
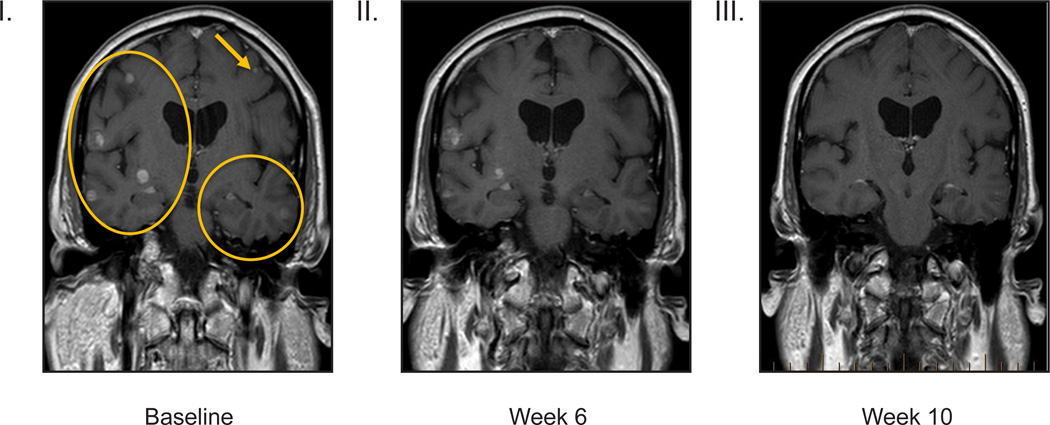
B: V600 BRAF-mutant melanoma patients with untreated brain metastases (n=10) and representative images from gadolinium-enhanced magnetic resonance imaging treated at the recommended Phase II dose
K V600K patient.
Panels I–III, V600E brain at baseline (I) Week 6 (II) and Week 10 (III). Best response by RECIST was 71% decrease, and the best intracranial decrease was 68%.
Figure 3.
V600 BRAF-mutant melanoma patients treated at the recommended Phase II dose
A: Duration on treatment for patients with melanoma (n=36)†
►Time of the first PR or CR
* Time of disease progression
→ Patients remaining in study
† The first restaging occurred at 9 weeks and 6 weeks in the escalation and expansion cohorts, respectively. Subsequent restaging occurred every 9 weeks.
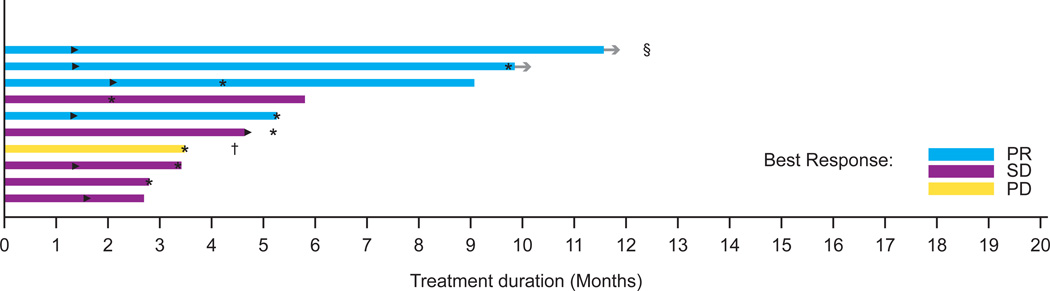
B: Duration on treatment in melanoma patients with untreated brain metastases (n=10)
►Time of the first PR or CR
* Time of disease progression
→ Patients remaining in study
† The patient’s best response was SD; however this was determined after the data cut used in this manuscript
§ The patient depicted as the top bar in the figure received gamma knife radiation at 5·9 months but did not meet criteria for progression by RECIST.
C: Kaplan–Meier progression-free survival curve
In the RP2D expansion of BRAF-mutant melanoma with asymptomatic untreated brain metastases, 26 baseline brain metastases were measured, with a size range of 3–15 mm. Nine of the ten patients (90%) achieved a decrease in brain lesions, and four of ten patients achieved complete resolution of all brain lesions (Figure 2B). One patient with many diffuse brain metastases (Figure and images 2B) had a 68% decrease in the sum of diameters of brain metastases. Decreasing size of extracranial metastases was observed in all nine patients with responding brain lesions. The median PFS was 4·2 months (95% CI 3·3–5·3, Figures 3B and 3C), and the most durable responder met the criteria for progressive disease at 15 months and remains on study at 19 months. All patients were alive at 5 months, and two survived beyond 12 months (based on data after data cut). Among the eight patients who progressed, the site of progression was in the brain alone in three patients, in extracranial sites alone in three patients, and in both brain and extracranial sites in two patients.
Responses were observed in melanoma patients with non-V600E BRAF mutations (n=22, Figures S2 and S3). Among the 18 melanoma patients with V600K who were treated at all doses, the response rate was 39% (95% CI 17·3–64·3) and the confirmed response rate was 22% (95% CI 6·4–47·6). The median PFS for the eight patients treated at 150 mg BID was 5·6 months (95% CI 3·9–40·8). Among patients treated at all dose levels, nine patients (50%) received treatment for at least 6 months. Among the three patients with non-V600 or complex BRAF mutations, PFS was 1·5 (K601E), 1·8 (V600_K601E), and 4·2 months (K601E), and no responses were observed.
Responses were observed in non-melanoma patients who were treated at all doses (Figures S4 and S5). Among 14 BRAF-mutant papillary thyroid cancer patients, four were not evaluable because the first restaging scan was not available at the data cut, one patient had prior MEK inhibitor therapy and had progressive disease as best response, and three of the nine remaining patients (33%) achieved partial response (PR) (two confirmed). The one patient with BRAF-mutant NSCLC achieved PR (83% decrease, unconfirmed). The one patient with BRAF-mutant GIST, who received prior MEK inhibitor therapy, achieved stable disease (SD; 17% decrease). The one patient with BRAF-mutant ovarian cancer achieved SD (28% decrease). Nine of the 11 colorectal cancer patients had BRAF-mutant evaluable disease; one had a confirmed PR (72% decrease), and seven had SD, including prolonged SD of more than 7 months in one patient.
In Figure 2A (and Figures S2, and S4), a total of five patients with progressive disease are not depicted in the waterfall plot because: these patients progressed early before the first restaging scans (two patients, Figures 2A and S4), tumour size change was not available (one patient, Figure S2), BRAF status was not known (one patient, Figure S4), or there was a BRAF mutation of unknown type (one patient, Figure S4).
Discussion
This Phase I study is the first to demonstrate activity of a targeted therapy in previously untreated melanoma brain metastases. Nine of ten patients with previously untreated asymptomatic melanoma brain metastases had contraction of brain metastases, including four with complete resolution and a 68% decrease in a patient with bulky brain metastases. The intracranial disease reduction was accompanied by extracranial reduction in every case. Patients with melanoma brain metastases typically survive less than 5 months; yet in this study, all 10 patients were alive at 5 months, and two patients had durable antitumor activity with survival beyond 12 months. One patient remains on treatment at 19 months.
Dabrafenib was synthesized specifically to prevent penetration of the blood–brain barrier because of potential neurotoxic effects on abundant wild-type BRAF in normal brain. Preclinical studies suggest minimal penetration of the intact blood–brain barrier by the parent drug after single-dose administration, as measured by brain concentrations, pERK inhibition, and PET imaging (unpublished data).
Disruption of the blood–brain barrier by brain macrometastases may be the mechanism that allows drugs to penetrate brain tumours.19 Relapse and/or progression in brain metastases after previous response is likely to be mediated by the same mechanisms observed in extracranial sites,20–23 although site-specific mechanisms such as “re-healing” of the blood–brain barrier also deserve exploration.
The responses in brain metastases highlight the inadequacy of the RECIST guidelines to assess response in small brain lesions.17,24 We applied a revised response criteria using gadolinium-enhanced MRI and a minimum size criterion of 3 mm,12 which enabled inclusion of most patients with brain metastases.
This study deliberately enriched for non-V600E BRAF-mutant metastatic melanoma and is the first study to report PFS in this group. Activity was demonstrated in V600K and V600G BRAF-mutant melanoma. While the response rate was lower in V600K than V600E BRAF-mutant melanoma, PFS was equivalent at RP2D. Larger studies that include non-V600E mutant melanoma will give more accurate estimates of antitumour activity, and future trials should not exclude these patients. In contrast, absence of response among patients with the K601E mutations parallels cell line data demonstrating less inhibition with vemurafenib.25
Dabrafenib also has activity against non-melanoma V600E BRAF-mutant cancers, including papillary thyroid, colorectal, ovarian, NSCLC, and GIST. Further studies should be performed with enrichment for BRAF-mutant subsets in these tumour types.
Toxicity from dabrafenib was mild, with only 24% of patients from all cohorts experiencing AEs higher than grade 2. Only three toxicities of grade 2 or higher were observed in more than 5% of patients, including cutaneous SCC or keratoacanthoma (11%), fatigue (8%), and pyrexia (6%). Arthralgia of grade 2 or higher occurred in less than 5% of patients. Photosensitivity, commonly reported with vemurafenib,15 was not observed. The photosensitivity induced by vemurafenib appears to be a property of the chemical structure of the drug, which is independent of BRAF inhibition. Pyrexia of at least grade 2 occurred in 6% of patients, usually as an isolated episode early in the course of treatment, and was managed using conservative measures, with drug continuation in nearly all cases. The spectrum of keratotic cutaneous lesions, including verruca, plantar–palmar hyperkeratoses, actinic keratoses, seborrhoeic keratoses, and SCC, was similar to that reported with vemurafenib. Non-cutaneous SCC was not observed. A universal clinico-histopathological classification of BRAF inhibitor-induced keratotic lesions is urgently needed to ensure consistent nomenclature and accurate comparisons between BRAF inhibitors, and to determine optimal treatment. These lesions may be caused by paradoxical activation of the MAP kinase pathway in BRAF wild-type cells26–28 (e.g. keratinocytes) carrying alterations in oncogenes upstream of BRAF (e.g. RAS).
Nearly all BRAF-mutant metastatic melanomas shrink after treatment with dabrafenib, but most patients developed acquired resistance, with a median PFS of 5·5 months at the RP2D. Patients with normal LDH or ECOG PS of 0 had a longer PFS than patients with high LDH or ECOG PS of 1 or 2, respectively, suggesting that burden of disease may influence response durability. Multiple possible mechanisms of acquired resistance have now been described.20–23,29 The frequency with which the downstream MAP kinase pathway remains active in tumours resistant to BRAF inhibition has led to an ongoing Phase I study of dabrafenibGSK2118436 in combination with the MEK inhibitor trametinib (GSK1120212), enriched for patients with BRAF mutant metastatic melanoma (NCT01072175).
This study is limited by sample size, as expected in a first-in-human Phase I study. Further trials are underway to confirm antitumour activity of single-agent dabrafenib, including a Phase II trial for patients with asymptomatic brain metastases (NCT01266967) and a Phase III randomized controlled trial of dabrafenib versus dacarbazine (NCT01227889).
In conclusion, dabrafenib is a highly active inhibitor of V600-mutant BRAF, with a high response rate in V600E melanoma, and is the first drug of its class to demonstrate efficacy in melanoma brain metastases. Clinical trials in melanoma have traditionally excluded patients with brain metastases based on preclinical predictions regarding drug distribution into the intact central nervous system. We hope that the advent of effective drug therapy for brain metastases of V600 BRAF-mutant melanoma will result in new trial designs that permit the inclusion of such patients in the future.
Research in context
Systematic Review
We searched Medline from 2002 for full papers reporting results from any clinical trial of potent BRAF inhibitors in patients with mutant BRAF cancers. We identified two relevant studies of vemurafenib, Phase I and Phase III, enriched for patients with BRAF-mutant metastatic melanoma.15,30 Patients with known untreated or progressing brain metastases were specifically excluded from these studies, and neither study reported activity in brain metastases. The Phase I study reported high unconfirmed responses (81%) in V600E BRAF-mutant metastatic melanoma patients.30 The Phase III study, comparing first-line vemurafenib with dacarbazine chemotherapy in patients with V600E mutant metastatic melanoma, showed confirmed responses in 48% and a 63% reduction in risk of death for vemurafenib-treated patients.15 Ten of 336 patients who received vemurafenib were retrospectively identified to carry the V600K mutation (present in 20% of BRAF-mutant melanomas),5 and had a response rate of 40%; however, survival in this subgroup was not reported. Within the Phase I study of vemurafenib, three patients with metastatic papillary thyroid cancer were included; one had a partial response and two had stable disease as best response. No other published clinical trials in BRAF-mutant non-melanoma solid tumours or non-V600E metastatic mutant melanoma were identified.
Interpretation
This study establishes activity of the BRAF inhibitor dabrafenib in V600E melanoma and other cancers, but extends the investigation of its activity into three new areas; the large group of patients with melanoma brain metastases, those with non-V600E BRAF-mutant melanoma, and those with non-melanoma, non-papillary thyroid tumours with mutant BRAF. The high response rate in melanoma brain metastases and the near-equivalent PFS for V600K BRAF-mutant melanoma compared with V600E justify the inclusion of such patients in further trials of potent BRAF inhibitors.
As neither vemurafenib nor dabrafenib demonstrate penetration of the blood-brain barrier, this study provides strong precedent for the inclusion of patients with brain metastases in future drug trials in melanoma, irrespective of predicted central nervous system exposure.
Supplementary Material
Acknowledgements
We thank the patients and their families for their participation.
We also thank Vicky Wegener, Natalie Byrne, Peter Carr, Arthur Clements, Matteo Carlino, Alexander Menzies, Pablo Fernandez-Penas, Rachael Anforth, Jennifer O'Leary, Julie Howle, Deirdre d'Souza, Catriona McNeil, Sayed Ali, Lydia Visintin, John Thompson, Michael Hughes, Peter Barry, Jonathon Stretch, Robyn Saw, Andrew Spillane, Kerwin Shannon, Michael Quinn, Raghwa Sharma, Richard Scolyer, Cliff Meldrum, Sandie Grierson, Candace Carter, Valerie Jakrot, Emma Zhang, Melvin Chin, Robyn Ward, Franziska Loehrer, Haby Henary, Judith Christmas, Catrina Coverdale, Colleen Fitzgerald, Rebecca Tunstall, John Sullivan, Francine McCarty, Christine Smith, Susannah Chang, Jolly Mazumdar, Monica Barbee, Vicki Goodman, Jeff Legos, Bill Bell, and Mary Richardson.
GVL supported by Fellowships from the Cancer Institute NSW (2009, 2011) and the Royal Australasian College of Physicians (2010).
Editorial support in the form of collating comments, fact-checking and graphic services was provided by Alison Scott at MediTech Media and was funded by GSK.
Footnotes
Author Contributions
Gerald Falchook and Georgina Long developed the manuscript, with support from Richard Kefford; all had full access to the study data. Bo Ma carried out statistical analyses of the data. All authors reviewed and commented on the manuscript throughout development and approved the final version.
Conflicts of Interest
Gerald Falchook received support for congress attendance from GlaxoSmithKline. The Department of Investigational Cancer Therapeutics, MD Anderson Cancer Center, Houston, Texas received research funds from GlaxoSmithKline on behalf of Gerald Falchook and Razelle Kurzrock. Georgina Long received honoraria from GlaxoSmithKline and Roche, support for congress attendance from GlaxoSmithKline, and payment for lectures from Merck, Sharp & Dohme and Pfizer. The Melanoma Institute Australia received educational grants from Roche. Michael Brown received consultancy fees from GlaxoSmithKline. Omid Hamid received payment for lectures from Genentech and BMS, and The Angeles Clinic received grants for clinical trials from Arqule, BMS, Genentech, and Roche. Drug Development Unit, Sarah Cannon Research Institute, Nashville received consulting fees and support for congress attendance on behalf of Jeffrey Infante, who has no additional personal conflicts of interest. Michael Millward received consultancy fees and the Cancer Council Trials and Sir Charles Gairdner Hospital and University of Western Australia received research funding from GlaxoSmithKline. Samuel Blackman, Martin Curtis, Bo Ma and Danielle Ouellet are employees of GlaxoSmithKline and hold stock options. Peter Lebowitz was an employee of GlaxoSmithKline at the time of the study and holds stock options. Richard Kefford received consultancy fees and support for travel from GlaxoSmithKline, and payment for lectures and board membership from Merck, Sharp & Dohme, Pfizer and Roche. The Melanoma Institute Australia, and University of Sydney, and Westmead Institute for Cancer Research received fees for board membership from Merck, Sharp & Dohme and Roche, and support for congress attendance and research grants from Roche. Tobias Arkenau, Kevin Kim and Anna Pavlick have no conflicts of interest.
References
- 1.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 2.Catalogue of Somatic Mutations in Cancer (COSMIC) website Wellcome Trust Sanger Institute. [Accessed August 2011];2011 at http://www.sanger.ac.uk/genetics/CGP/cosmic/ [Google Scholar]
- 3.Roth AD, Tejpar S, Delorenzi M, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28:466–474. doi: 10.1200/JCO.2009.23.3452. [DOI] [PubMed] [Google Scholar]
- 4.Elisei R, Ugolini C, Viola D, et al. BRAFV600E Mutation and Outcome of Patients with Papillary Thyroid Carcinoma: A 15-Year Median Follow-Up Study. J Clin Endocrinol Metab. 2008;93:3943–3949. doi: 10.1210/jc.2008-0607. [DOI] [PubMed] [Google Scholar]
- 5.Long GV, Menzies AM, Nagrial AM, et al. Prognostic and Clinicopathologic Associations of Oncogenic BRAF in Metastatic Melanoma. J Clin Oncol. 2011;29:1239–1246. doi: 10.1200/JCO.2010.32.4327. [DOI] [PubMed] [Google Scholar]
- 6.Balch CM, Gershenwald JE, Soong S-j, et al. Final Version of 2009 AJCC Melanoma Staging and Classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies MA, Liu P, McIntyre S, et al. Prognostic Factors for Survival in Melanoma Patients With Brain Metastases. Cancer. 2011;117:1687–1696. doi: 10.1002/cncr.25634. [DOI] [PubMed] [Google Scholar]
- 8.Carlino MS, Atkins MB, Warneke CL, et al. Pigment Cell Melanoma Res. Vol. 2010. Blackwell Publishing Ltd; 2010. Differences between Australia (OZ) and the United States (US) in the patterns, prognosis, and treatment of melanoma CNS metastases: analysis from the PHAMOUS (prognostic heterogeneity in patients with advanced melanoma between OZ and the US) study; p. 946. [Google Scholar]
- 9.Skibber J, Soong S-j, Austin L, Balch C, Sawaya R. Cranial irradiation after surgical excision of brain metastases in melanoma patients. Ann Surg Oncol. 1996;3:118–123. doi: 10.1007/BF02305789. [DOI] [PubMed] [Google Scholar]
- 10.Mornex F, Thomas L, Mohr P, et al. A prospective randomized multicentre phase III trial of fotemustine plus whole brain irradiation versus fotemustine alone in cerebral metastases of malignant melanoma. Melanoma Res. 2003;13:97–103. doi: 10.1097/00008390-200302000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Agarwala SS, Kirkwood J, Gore M, et al. Temozolomide for the Treatment of Brain Metastases Associated With Metastatic Melanoma: A Phase II Study. J Clin Oncol. 2004;22:2101–2107. doi: 10.1200/JCO.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 12.Lawrence DP, Hamid O, McDermott DF, et al. J Clin Oncol; 2010 May 20, 2010; Chicago, USA. 2010. Phase II trial of ipilimumab monotherapy in melanoma patients with brain metastases; p. 8523. [Google Scholar]
- 13.Aoyama H, Shirato H, Tago M, et al. Stereotactic Radiosurgery Plus Whole-Brain Radiation Therapy vs Stereotactic Radiosurgery Alone for Treatment of Brain Metastases: A Randomized Controlled Trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 14.Karasarides M, Chiloeches A, Hayward R, et al. B-RAF is a therapeutic target in melanoma. Oncogene. 2004;23:6292–6298. doi: 10.1038/sj.onc.1207785. [DOI] [PubMed] [Google Scholar]
- 15.Chapman PB, Hauschild A, Robert C, et al. Improved Survival with Vemurafenib in Melanoma with BRAF V600E Mutation. N Engl J Med. 2011 doi: 10.1056/NEJMoa1103782. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laquerre S, Arnone M, Moss K, et al. A Selective Raf Kinase Inhibitor Induces Cell Death and Tumor Regression of Human Cancer Cell Lines Encoding B-RafV600E Mutation. Mol Cancer Ther. 2009;8(12 Suppl):B88. [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, et al. New Guidelines to Evaluate the Response to Treatment in Solid Tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.Brookmeyer R, Crowley J. A Confidence Interval for the Median Survival Time. Biometrics. 1982;38:29–41. [Google Scholar]
- 19.Gerstner ER, Fine RL. Increased Permeability of the Blood-Brain Barrier to Chemotherapy in Metastatic Brain Tumors: Establishing a Treatment Paradigm. J Clin Oncol. 2007;25:2306–2312. doi: 10.1200/JCO.2006.10.0677. [DOI] [PubMed] [Google Scholar]
- 20.Johannessen CM, Boehm JS, Kim SY, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468:968–972. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nazarian R, Shi H, Wang Q, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villanueva J, Vultur A, Lee JT, et al. Acquired Resistance to BRAF Inhibitors Mediated by a RAF Kinase Switch in Melanoma Can Be Overcome by Cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18:683–695. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagle N, Emery C, Berger MF, et al. Dissecting Therapeutic Resistance to RAF Inhibition in Melanoma by Tumor Genomic Profiling. J Clin Oncol. 2011 doi: 10.1200/JCO.2010.33.2312. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Boggaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 25.Yang H, Higgins B, Kolinsky K, et al. RG7204 (PLX4032), a Selective BRAF(V600E) Inhibitor, Displays Potent Antitumor Activity in Preclinical Melanoma Models. Cancer Res. 2010;70:5518–5527. doi: 10.1158/0008-5472.CAN-10-0646. [DOI] [PubMed] [Google Scholar]
- 26.Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-Dead BRAF and Oncogenic RAS Cooperate to Drive Tumor Progression through CRAF. Cell. 2010;140:209–221. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431–435. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 28.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poulikakos PI, Persaud Y, Janakiraman M, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E) Nature. 2011;480:387–390. doi: 10.1038/nature10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simon P, Freidlin B, Rubinstein L, et al. Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst. 1997;89:1138–1147. doi: 10.1093/jnci/89.15.1138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.