Abstract
Our recent studies revealed that blocking class I/II histone deacetylases (HDACs) inhibits renal interstitial fibroblast activation and proliferation and alleviates development of renal fibrosis. However, the effect of class III HDAC, particularly sirtuin 1 and 2 (SIRT1 and SIRT2), inhibition on renal fibrogenesis remains elusive. Here, we demonstrate that both SIRT1 and SIRT2 were expressed in cultured renal interstitial fibroblasts (NRK-49F). Exposure of NRK-49F to sirtinol, a selective inhibitor of SIRT1/2, or EX527 (6-chloro-2,3,4,9-tetrahydro-1H-carbazole-1-carboxamide), an inhibitor for SIRT1, resulted in reduced expression of fibroblast activation markers (α-smooth muscle actin, fibronectin, and collagen I) as well as proliferation markers (proliferating cell nuclear antigen, cyclin D1, cyclin E) in dose- and time-dependent manners. Treatment with a SIRT2 inhibitor, AGK2 (2-cyano-3-[5-(2,5-dichlorophenyl)-2-furanyl]-N-5-quinolinyl-2-propenamide), also dose- and time-dependently inhibited renal fibroblast activation and, to a lesser extent, cell proliferation. Furthermore, silencing of either SIRT1 or SIRT2 by small interfering RNA exhibited similar inhibitory effects. In a mouse model of obstructive nephropathy, administration of sirtinol attenuated deposition of collagen fibrils as well as reduced expression of α-smooth muscle actin, collagen I, and fibronectin in the injured kidney. SIRT1/2 inhibition–mediated antifibrotic effects are associated with dephosphorylation of epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor-β (PDGFRβ), and signal transducer and activator of transcription 3. Thus, SIRT1/2 activity may contribute to renal fibroblast activation and proliferation as well as renal fibrogenesis through activation of at least EGFR and PDGFRβ signaling. Blocking SIRT1/2 activation may have therapeutic potential for the treatment of chronic kidney disease.
Introduction
Chronic kidney disease (CKD) can progress to end-stage renal failure through aberrant activation and growth of renal interstitial fibroblasts. Differentiation of fibroblasts into myofibroblasts is characterized by expression of a high level of α-smooth muscle actin and fibronectin (Liu, 2004; Neilson, 2006; Wynn, 2008; Meran and Steadman, 2011). Accumulation of myofibroblasts results in production of excessive amounts of extracellular matrix proteins including collagen I and subsequent progression of renal fibrosis. The mechanism of myofibroblast activation under various pathologic conditions is not completely understood, but multiple cytokines/growth factor receptors, including epidermal growth factor receptor (EGFR) and platelet-derived growth factor receptor-β (PDGFRβ), have been shown to be involved in this process (Ludewig et al., 2000; Pang et al., 2009, 2010; Liu et al., 2011, 2012). Activation of these tyrosine kinase receptors leads to activation of multiple intracellular signaling pathways, including signal transducer and activator of transcription 3 (STAT3). STAT3 has also been implicated in the activation of renal interstitial fibroblasts and development of renal fibrosis (Pang et al., 2010).
Emerging evidence indicates that post-translational modification such as acetylation/deacetylation also plays an important role in the regulation of gene expression and functional proteins associated with cell proliferation and differentiation in a variety of cell types. This modification is regulated by histone acetyltransferases and histone deacetylases (HDACs). HDACs are a group of enzymes that catalyze the removal of acetyl groups from lysine residues in histone and nonhistone proteins. They are classified into four groups: class I (HDAC1, 2, 3, and 8), class II (HDAC4, 5, 6, 7, 9, and 10), class III (SIRT1–7, also known as sirtuins), and class IV (HDAC11) (Pang and Zhuang, 2010). Among them, class I and class II HDACs have been extensively studied for their role in the activation of fibroblasts and the pathogenesis of several chronic diseases, including fibrosis (Kook et al., 2003; Lee et al., 2007; Marchion and Munster, 2007; Liu et al., 2008). It was reported that inhibition of class I and II HDACs with trichostatin A (TSA) reduced platelet-derived growth factor–induced proliferation of NIH 3T3 fibroblasts (Catania et al., 2006) and prevented transforming growth factor-β–induced α-smooth muscle actin (α-SMA) expression and morphologic changes in cultured human skin fibroblasts (Rombouts et al., 2002). Treatment with TSA or MS-275 [pyridin-3-ylmethyl 4-((2-aminophenyl)carbamoyl)benzylcarbamate], a specific inhibitor of class I HDAC, also blocked activation and proliferation of renal interstitial fibroblasts and attenuated progression of renal interstitial fibrosis in an animal model of obstructive kidney injury (Pang et al., 2009; Liu et al., 2013). Furthermore, TSA administration can reverse atrial fibrosis and arrhythmia vulnerability in animal models (Liu et al., 2008). These studies indicate that class I/II HDACs play an essential role in mediating activation of fibroblasts and tissue fibrosis, and suggest that targeting class I/II HDACs would be a therapeutic approach for treatment of CKD.
Studies have also been conducted to elucidate the role of class III HDACs, in particular, SIRT1, in tissue fibrosis, but results are controversial. Wang et al. (2008) reported that most of the class III HDAC activities, including those of SIRT1, were increased in patients with primary myelofibrosis. However, resveratrol, a naturally occurring compound believed to be an activator of SIRT1, prevents fibrosis in a bleomycin-induced lung injury model (Akgedik et al., 2012). Similarly, activation of SIRT1 by resveratrol, or SRT1720 [N-(2-(3-(piperazin-1-ylmethyl)imidazo[2,1-b]thiazol-6-yl)phenyl)quinoxaline-2-carboxamide hydrochloride], also inhibits renal fibrosis (Li et al., 2010). In contrast, treatment with SRT1720 exacerbates lung fibrosis in mice (Imanishi et al., 2012). The mechanism behind these diverse effects of SIRT1 activators on tissue fibrosis is not clear, but may be associated with their nonspecific properties. Emerging evidence indicated that resveratrol and SRT1720 are not directly targeting SIRT1 and exhibit multiple off-target activities (Pacholec et al., 2010). In particular, resveratrol has also been reported to function as a pan-inhibitor of class I/II and IV HDACs (Venturelli et al., 2013). Given the multiple targets of these SIRT1 activators, it is necessary to further elucidate the functional role of SIRT1 in tissue fibrosis by using its more specific inhibitors.
Recently, several SIRT inhibitors have been developed, and their efficacy has been tested in treating tumors and other diseases. Sirtinol is a potent inhibitor of SIRT1/2 that can induce senescence-like growth arrest in human breast cancer MCF-7 and lung cancer H1299 cells (Ota et al., 2006) and enhanced chemosensitivity to camptothecin and cisplatin in PC3, DU145, and HeLa cells (Kojima et al., 2008; Jin et al., 2010; Peck et al., 2010). EX527 (6-chloro-2,3,4,9-tetrahydro-1H-carbazole-1-carboxamide) is a selective SIRT1 inhibitor (200- to 500-fold selective) which can induce a cell cycle arrest in MCF-7 cells (Peck et al., 2010). Moreover, a study with AGK2 (2-cyano-3-[5-(2,5-dichlorophenyl)-2-furanyl]-N-5-quinolinyl-2-propenamide), a selective inhibitor of SIRT2 (15-fold more selective to SIRT2 than SIRT1), reported that SIRT2 can protect neurons from death (He et al., 2012). As renal fibrogenesis is involved in fibroblast survival, proliferation, and differentiation, similar to the processes in tumorigenesis, this raises the possibility that SIRT1/2 inhibitors might be able to suppress activation and proliferation of renal fibroblasts as well as development of renal fibrosis.
To test this hypothesis, we investigated the effects of sirtinol, EX527, and AGK2 on renal interstitial fibroblast proliferation and activation in vitro, and confirmed the role of SIRT1 and SIRT2 in those processes using small interfering RNA. Further, we assessed the therapeutic effect of sirtinol on renal fibrosis in a murine model of renal interstitial fibrosis induced by unilateral ureteral obstruction.
Materials and Methods
Chemicals and Antibodies.
Antibodies to fibronectin, collagen I(A2), EGFR, glyceraldehyde-3-phosphate dehydrogenase, proliferating cell nuclear antigen (PCNA), SIRT1, and SIRT2 were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). α-SMA, α-tubulin, sirtinol, EX527, AGK2, and all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO). All other antibodies used in this study were purchased from Cell Signaling Technology (Danvers, MA). Small interfering RNA (siRNA) specific for rat SIRT1 was obtained from Santa Cruz Biotechnology, Inc., and siRNA specific for rat SIRT2 was obtained from Life Technologies (Grand Island, NY). SIRT1 and SIRT2 activity assay kits were obtained from Abcam (Cambridge, MA).
Cell Culture and Treatment.
Rat renal interstitial fibroblasts (NRK-49F) were cultured in Dulbecco’s modified Eagle's medium with F12 containing 5% fetal bovine serum, 0.5% penicillin and streptomycin in an atmosphere of 5% CO2, and 95% air at 37°C. To determine the effects of sirtinol, EX527, and AGK2 on fibroblast activation, these inhibitors were directly added to subconfluent NRK-49F cells and then incubated for the indicated time as described in the figure legends.
Transfection of siRNA into Cells.
siRNA oligonucleotides targeted specifically to rat SIRT1 or SIRT2 were used in this study. In a six-well plate, NRK-49F cells were seeded to 50–60% confluence in antibiotic-free medium and grown for 24 hours. Then cells in each well were transfected with siRNA (200 pmol) specific for SIRT1 or SIRT2 using BioT (Bioland Scientific, Paramount, CA) according to the manufacturer’s instructions. In parallel, scrambled siRNA (200 pmol) was used as control for off-target changes in NRK-49F. Twenty-four hours after transfection, the medium was changed and cells were incubated for an additional 24 hours before being harvested for analysis.
Animals and Experimental Design.
The unilateral ureteral obstruction (UUO) model was established in male C57 black mice that weighed 20–25 g (The Jackson Laboratory, Bar Harbor, ME) as described in our previous studies (Pang et al., 2009, 2010). In brief, the abdominal cavity was exposed via a midline incision, and the left ureter was isolated and ligated. The contralateral kidney was used as a control to examine the effects of inhibition of SIRT1 and SIRT2 on renal fibrosis after UUO injury. Sirtinol, an inhibitor that can inhibit both SIRT1 (IC50 131 μM) and SIRT2 (IC50 38 μM) at 50 mg/kg (prepared in 50 μl of dimethylsulfoxide), was immediately administered i.p. after ureteral ligation and then given daily for 6 days. Selection of this dose of sirtinol was according to a previous report (Legutko et al., 2011). Control mice were injected with an equal volume of dimethylsulfoxide. The animals were sacrificed and the kidneys were collected at day 7 after UUO for protein analysis and histologic examination. All experimental procedures were performed according to the US Guidelines to the Care and Use of Laboratory Animals and approved by the Lifespan Animal Welfare Committee.
Masson Trichrome Staining.
For assessment of renal fibrosis, Masson trichrome staining was performed according to the protocol provided by the manufacturer (Sigma-Aldrich). The collagen tissue area (blue color) was quantitatively measured using ImageJ software developed at the National Institutes of Health. The positive staining area from each microscopic field (200×) was calculated and graphed.
Immunoblot Analysis.
After various treatments, cells were washed once with ice-cold phosphate-buffered saline and harvested in a cell lysis buffer. Proteins (25 μg) were separated by SDS-PAGE and transferred to nitrocellulose membranes. After incubation with 5% nonfat milk for 1 hour at room temperature, membranes were incubated with a primary antibody overnight at 4°C and then incubated with appropriate horseradish peroxidase–conjugated secondary antibody for 1 hour at room temperature. Bound antibodies were visualized by chemiluminescence detection.
SIRT1 and SIRT2 Activity Assay.
SIRT1 and SIRT2 activities from cells and kidney tissue were measured using fluorometric assay kits (Abcam) according to the manufacturer’s instructions. The Assay Kit (Fluorometric) measures the activity of SIRT by the basic principle of changing a reaction of SIRT1 or SIRT2 into the activity of the protease. The samples were mixed well and incubated for 10 minutes at room temperature, and the fluorescence intensity was measured every 30 seconds for a total of 60 minutes immediately after the addition of fluorosubstrate peptide (20 µM final concentration). SIRT1 activity was measured by excitation at 360 nm and emission at 460 nm, and SIRT2 activity was measured by excitation at 485 nm and emission at 535 nm. The enzymatic activity was expressed in relative fluorescence units.
Densitometry Analysis.
The semiquantitative analysis of different proteins was carried out using ImageJ software developed at the National Institutes of Health. The quantification is based on the intensity (density) of the band, which is calculated by the area and pixel value of the band. The quantification data are given as a ratio between target protein and loading control (house-keeping protein).
Statistical Analysis.
Data are presented as means ± S.D. and were subjected to one-way analysis of variance. Multiple means were compared using Tukey’s test, and differences between two groups were determined by Student’s t test. P < 0.01 was considered statistically significant.
Results
Sirtinol Inhibits Activation and Proliferation of Renal Interstitial Fibroblasts.
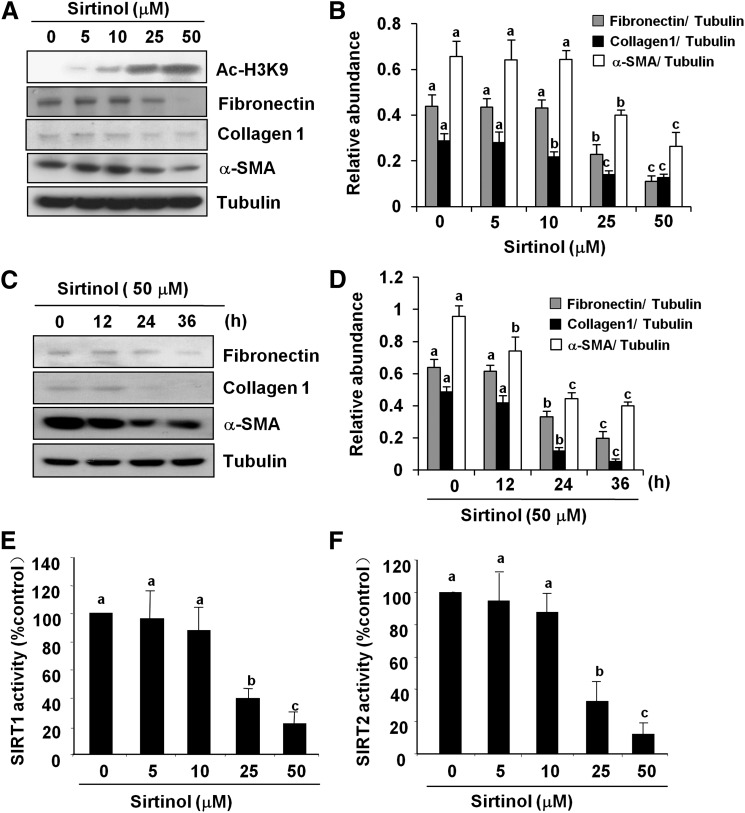
Activation of renal fibroblasts is the predominate mechanism for development and progression of renal fibrosis. To examine whether the two major members of class III HDACs, namely, SIRT1 and SIRT2, would be involved in renal fibroblast activation, rat renal interstitial fibroblast cells (NRK-49F) were exposed to various concentrations of sirtinol, a selective inhibitor for both SIRT1 and SIRT2, and then their activation and proliferation were examined. As shown in Fig. 1, sirtinol dose-dependently inhibited the expression of fibroblast activation markers: α-SMA, fibronectin, and collagen I, a major extracellular matrix (ECM) protein. Densitometry analysis of the immunoblot results demonstrated that sirtinol reduced expression of α-SMA, fibronectin, and collagen 1 by approximately 50, 50, and 70%, respectively, at a dose of 50 μM (Fig. 1, A and B). To ensure that this reduction is due to the inhibition of SIRT deacetylase activity, we also examined the effect of sirtinol on acetylation of histone H3 at lysine 9 (acetyl-H3K9) by Western blot analysis, and measured SIRT1 and SIRT2 activity using fluorometric assay kits with fluoro-substrate peptide as substrate. Consistent with the effect of sirtinol on renal fibroblast activation, sirtinol treatment also increased the level of acetyl-H3K9 (Fig. 1A) and decreased SIRT1 and SIRT2 activity in a dose-dependent manner (5–50 μM) (Fig. 1, E and F).
Fig. 1.
Sirtinol inhibits renal fibroblast activation. Normally cultured NRK-49F cells were treated with sirtinol (0–50 μM) for 36 hours (A and B) or treated with 50 μM sirtinol for the indicated time (C and D). Then, cell lysates were prepared and subjected to immunoblot analysis with antibodies for acetyl-H3K9 (Ac-H3K9), α-SMA, collagen I, fibronectin, or α-tubulin. Representative immunoblots from three or more experiments are shown (A and C). The levels of α-SMA, collagen I, and fibronectin were quantified by densitometry and normalized with α-tubulin (B and D). The activity of SIRT1 and SIRT2 in fibroblasts treated with sirtinol was measured by an enzymatic assay kit with fluorescence-labeled acetylated peptide as substrate. The value was expressed as the percentages of inhibition in each sample relative to controls (E and F). Values are the means ± S.D. of three independent experiments. Bars with different letters (a–c) are significantly different from one another (P < 0.01).
The time course study demonstrated that the expression level of α-SMA was significantly decreased within 12 hours after 50 μM sirtinol treatment and was further decreased more than 2-fold at 36 hours. Similarly, sirtinol largely suppressed expression of fibronectin and collagen I at 36 hours (Fig. 1, C and D).
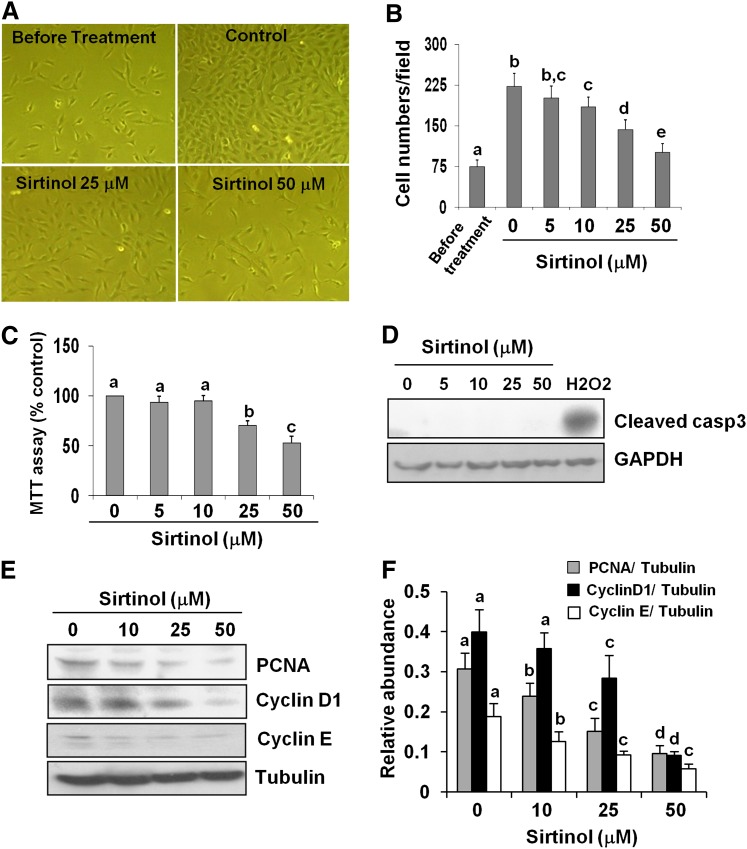
Next, we assessed the effect of sirtinol on the proliferation of cultured renal fibroblasts. Figure 2A shows that treatment of fibroblasts with sirtinol reduced the number of cells without altering cellular morphology. This effect of sirtinol occurred in a dose-dependent manner with the maximum inhibition at 50 μM, as indicated by cell counting and the MTT assay [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Fig. 2, B and C]. However, treatment with sirtinol up to 48 hours at doses from 5 to 50 μM did not cause cell death, as shown by the absence of cleaved caspase-3. As a positive control, cleaved caspase-3 was clearly detected in cells exposed to H2O2 (Fig. 2D). In addition, we observed a dose-dependent reduction in the expression of proliferation markers, PCNA, cyclin D1, and cyclin E at 36 hours after NRK-49F exposed to sirtinol (Fig. 2, E and F).
Fig. 2.
Sirtinol inhibits renal fibroblast proliferation. NRK-49F cells were cultured in medium with 5% fetal bovine serum and treated with sirtinol (0–50 μM) for 36 hours (A–E). Cells were randomly photographed in bright field (200×) (A) and cell proliferation was measured by counting cell number (B) or the MTT assay (C). To measure cell death, cultured NRK-49F cells were exposed to the same concentrations (0–50 μM) of sirtinol for 48 hours or treated with 1 mM H2O2 for 3 hours as positive control. Cell lysates were subjected to immunoblot analysis for cleaved caspase-3 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; D). Cell lysates were prepared and subjected to immunoblot analysis with antibodies for PCNA, cyclin D1, cyclin E, or α-tubulin (E). Representative immunoblots from three experiments are shown. The levels of PCNA, cyclin D1, and cyclin E were quantified by densitometry and normalized with α-tubulin (F). Values are the means ± S.D. of three independent experiments. Bars with different letters (a–e) are significantly different from one another (P < 0.01).
Collectively, our data indicate that class III HDACs, especially SIRT1 and/or SIRT2, are required for renal fibroblast activation and proliferation.
EX527 and AGK2 Inhibit Activation and Proliferation of Renal Interstitial Fibroblasts.
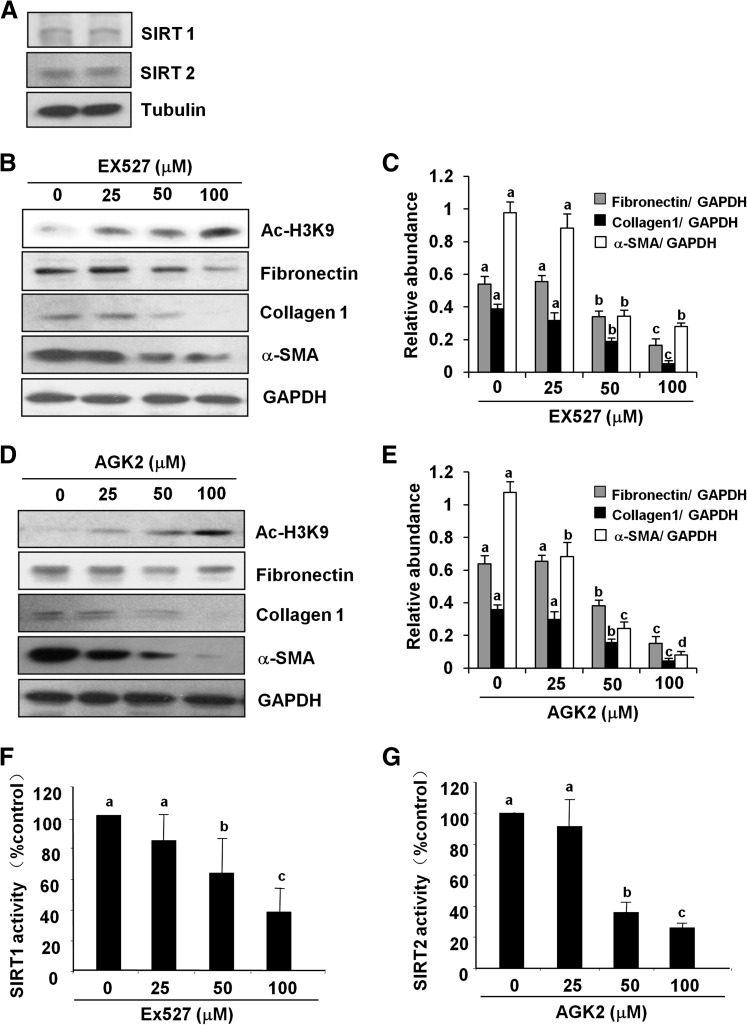
To further understand the role of SIRT1 and/or SIRT2 in regulating activation and proliferation of renal interstitial fibroblasts, we first examined expression of SIRT1 and SIRT2 in cultured NRK-49F cells. As shown in Fig. 3A, both SIRT1 and SIRT2 were expressed normally in cultured NRK-49F. Incubation of NRK-49F with different concentrations (25–100 μM) of EX527 (a selective inhibitor of SIRT1) or AGK2 (specific inhibitor of SIRT2) inhibited their deacetylase activity as shown by the dose-dependent increase in the expression of acetyl-H3K9 (Fig. 3, B and D) and decrease in the enzymatic activity of SIRT1 and SIRT2, respectively (Fig. 3, F and G). At 50 μM, EX527 was effective in reducing the expression level of α-SMA, collagen I, and fibronectin, and treatment with 100 μM of EX527 further decreased (more than 3-fold) expression of these molecules (Fig. 3, B and C). Similarly, exposure of cells to AGK2 dose-dependently reduced expression of α-SMA, collagen I, and fibronectin. AGK2 at a concentration of 25 μM significantly reduced the expression of α-SMA, but did not affect the levels of collagen I and fibronectin. A significant reduction in the level of all of these molecules was observed at a concentration of 50 μM, and a 3-fold reduction was seen in their expression when treated with 100 μM of AGK2 (Fig. 3, D and E).
Fig. 3.
SIRT1 and SIRT2 inhibitors suppress renal fibroblast activation. The cell lysates prepared from cultured NRK-49F cells were subjected to immunoblot analysis for the expression of SIRT1 and SIRT2 (A). NRK-49F cells were treated with EX527 (0–100 μM) and AGK2 (0–100 μM) for 36 hours. Then, cell lysates were prepared and subjected to immunoblot analysis with antibodies for acetyl-H3K9 (Ac-H3K9), α-SMA, collagen I, fibronectin, or glyceraldehyde-3-phosphate dehydrogenase (GAPDH; B and D). Representative immunoblots from three experiments are shown. The levels of α-SMA, collagen I, and fibronectin were quantified by densitometry and normalized with GAPDH (C and E). SIRT1 and SIRT2 activity was measured in NRK-49F cells treated with EX527 and AGK2 by an enzymatic assay kit with fluorescence-labeled acetylated peptide as substrate. The value was expressed as the percentage of inhibition in each sample relative to controls (F and G). Values are the means ± S.D. of three independent experiments. Bars with different letters (a–d) are significantly different from one another (P < 0.01).
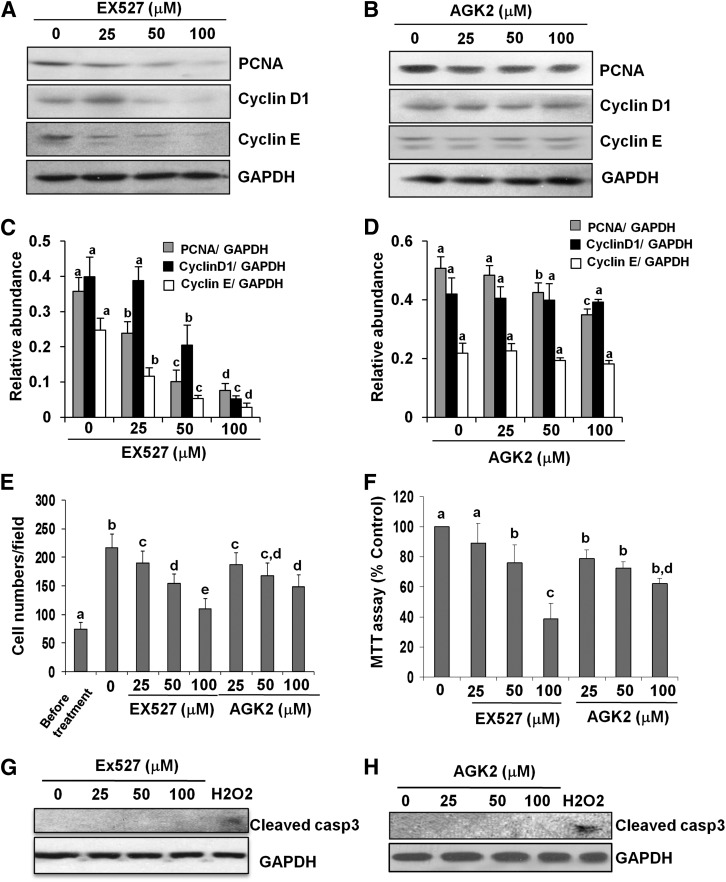
To evaluate the role of SIRT1 or SIRT2 in the proliferation of activated fibroblasts, NRK-49F cells were exposed to EX527 and AGK2 at 25–100 μM as indicated earlier. As shown in Fig. 4 (A and C), inhibition of SIRT1 with EX527 reduced expression of PCNA, cyclin D1, and cyclin E in a dose-dependent manner, with the maximum effect at a concentration of 100 μM. In contrast, blocking SIRT2 with AGK2 did not affect the expression level of cyclin D1 and cyclin E, although a slight reduction in the expression of PCNA was observed in cells exposed to 100 μM (Fig. 4, B and D). Further, MTT assay and counting of the number of cells confirmed a differential effect of EX527 and AGK2 on cell proliferation (Fig. 4, E and F). It is notable that treatment with EX527 or AGK2 at concentrations of 25–100 μM did not cause death of renal fibroblasts as indicated by absence of cleaved caspase-3 fragments in NRK-49F cells treated with either inhibitor, whereas exposure of the cells to 1 mM H2O2 resulted in caspase-3 cleavage (Fig. 4, G and H). This suggests that reduction in the number of NRK-49F by these two inhibitors was due to inhibition of their proliferation rather than induction of cell death. Collectively, SIRT1 inhibitor can significantly block expression of proteins involved in cell cycle progression, but SIRT2 inhibitor has less influence on the expression of those proteins. Although SIRT1 and SIRT2 both contribute to renal fibroblast activation, they play a distinct role in regulating cell proliferation.
Fig. 4.
Effects of SIRT1 and SIRT2 inhibitors on renal fibroblast proliferation. NRK-49F cells were cultured in medium with 5% fetal bovine serum and treated with EX527 (0–100 μM) and AGK2 (0–100 μM) for 36 hours (A–F). Then, cell lysates were prepared and subjected to immunoblot analysis with antibodies for PCNA, cyclin D1, cyclin E, or glyceraldehyde-3-phosphate dehydrogenase (GAPDH; A and B). Representative immunoblots from three experiments are shown. The levels of PCNA, cyclin D1, and cyclin E were quantified by densitometry and normalized with GAPDH (C and D). NRK-49F cells were treated with the indicated concentration of EX527 and AGK2 for 36 hours, cells were randomly photographed in bright field (200×), and cell proliferation was measured by cell counting (E) or the MTT assay (F). To measure cell death, cultured NRK-49F cells were exposed to the same concentrations (0–100 μM) of EX527 or AGK2 for 48 hours or treated with 1 mM H2O2 for 3 hours as positive control. Cell lysates were subjected to immunoblot analysis for cleaved caspase-3 and GAPDH (G and H). Values are the means ± S.D. of three independent experiments. Bars with different letters (a–e) are significantly different from one another (P < 0.01).
Knockdown of SIRT1 and SIRT2 Reduces Activation and Proliferation of Renal Fibroblasts.
To confirm the role of SIRT1 and SIRT2 in renal fibroblast activation and proliferation, NRK-49F cells were transfected with specific siRNA for SIRT1 and SIRT2. As shown in Fig. 5 (A and C), the knockdown efficiency of SIRT1 and SIRT2 was more than 70% when compared with control siRNA-transfected cells, and silencing of SIRT1 or SIRT2 did not affect expression of each other. The knockdown of both SIRT1 and SIRT2 significantly increased the level of acetyl-H3K9 and reduced the expression of α-SMA, collagen I, and fibronectin by 3- to 4-fold compared with cells transfected with control siRNA. Both SIRT1 and SIRT2 have a similar inhibitory effect on the expression of these fibroblast activation markers (Fig. 5, B and D). In contrast, the level of PCNA was decreased more than 90% in cells treated with SIRT1 siRNA, whereas PCNA was only decreased about 40% in SIRT2 siRNA–transfected cells (Fig. 5, B and E). These results are consistent with the inhibitory effect of SIRT1 and SIRT2 inhibitors on renal interstitial fibroblasts, and further suggest that both SIRT1 and SIRT2 are involved in the regulation of fibroblast activation, but SIRT1 contributes more to regulation of renal fibroblast cell proliferation.
Fig. 5.
Knockdown of SIRT1 and SIRT2 inhibits renal fibroblast activation. NRK-49F cells were transfected with siRNA targeting SIRT1 and SIRT2 or scrambled siRNA and then incubated in normal culture medium with 5% fetal bovine serum. At 48 hours after transfection, cell lysates were prepared for immunoblot analysis with antibodies against SIRT1, SIRT2, acetyl-H3K9 (Ac-H3K9), α-SMA, collagen I, fibronectin, PCNA, or glyceraldehyde-3-phosphate dehydrogenase (GAPDH; A and B). The levels of SIRT1, SIRT2, α-SMA, collagen I, fibronectin, and PCNA were quantified by densitometry and normalized with GAPDH (C–E). Values are the means ± S.D. of three independent experiments. Bars with different letters (a–c) are significantly different from one another (P < 0.01). Con, control.
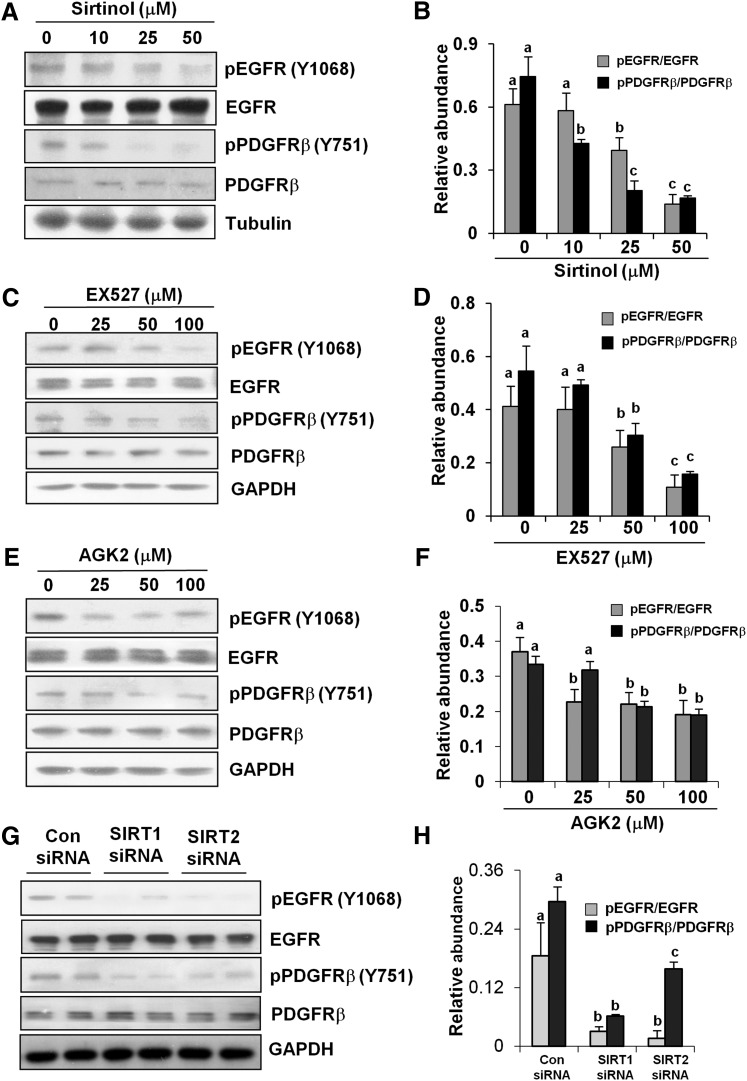
Inhibition of SIRT1 and SIRT2 Blocks the Phosphorylation of EGFR and PDGFRβ.
EGFR and PDGFRβ are two major cell surface receptors involved in renal fibroblast activation and proliferation (Ludewig et al., 2000; Terzi et al., 2000; Bonner, 2004). To demonstrate whether SIRT1/2 inhibition suppresses EGFR and PDGFRβ activation, we examined the effect of SIRT1 and SIRT2 inhibitors on phosphorylation (activation) of these two receptors. As shown in Fig. 6, A and B, and Supplemental Fig. 1, inhibition of both SIRT1 and SIRT2 with sirtinol significantly reduced the phosphorylation level of EGFR at Tyr1068 and Tyr845 as well as PDGFRβ at Tyr751 and Tyr579 in a dose-dependent fashion, with the maximum effect observed when cells were treated with 50 μM. In addition, cells exposed to EX527 also showed a significant decrease in the expression levels of phospho-EGFR at Tyr1068 and phospho-PDGFRβ at Tyr751 with a more than 3-fold reduction at 100 μM (Fig. 6, C and D). In contrast, the lower dose of AGK2 significantly reduced the level of phospho-EGFR at Tyr1068 and phospho-PDGFRβ at Tyr751, and this effect remained the same in cells treated with higher concentrations of AGK2 (Fig. 6, E and F). Of note, none of these inhibitors affected expression of total EGFR and PDGFR. These data indicate that blocking SIRT1 and SIRT2 can inhibit EGFR and PDGFRβ phosphorylation without affecting their expression.
Fig. 6.
Effects of SIRT1 and -2 inhibitors and siRNA on EGFR and PDGFRβ phosphorylation. Cultured NRK-49F cells were treated with sirtinol (0–50 μM), EX527 (0–100 μM), or AGK2 (0–100 μM) for 36 hours (A–F) or cells were transfected with siRNA targeting SIRT1 and SIRT2 or scrambled siRNA and then cultured for 48 hours (G and H). Cell lysates were prepared and subjected to immunoblot analysis with antibodies for phospho-EGFR (pEGFR; Tyr1068), phospho-PDGFRβ (pPDGFRβ; Tyr751), EGFR, PDGFRβ, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), or α-tubulin (A, C, E, and G). Representative immunoblots from three experiments are shown. The phosphorylated and total levels of EGFR and PDGFRβ were quantified by densitometry and phosphorylated protein levels were normalized to total protein levels (B, D, F, and H). Values are the means ± S.D. of three independent experiments. Bars with different letters (a–c) are significantly different from one another (P < 0.01). Con, control.
To confirm the effect of SIRT1 or SIRT2 inhibitors on EGFR and PDGFRβ phosphorylation, we also examined the effect of SIRT1 and SIRT2 knockdown on EGFR and PDGFRβ phosphorylation. In NRK-49F cells transfected with SIRT1 and SIRT2 siRNA, the phosphorylation level of EGFR was decreased by more than 80%, and both siRNAs had similar effects. In contrast, SIRT1 siRNA was superior to SIRT2 siRNA in suppressing expression of phospho-PDGFRβ. The expression level of total PDGFRβ was not affected by SIRT1 or SIRT2 siRNA (Fig. 6, G and H). These results together with data from SIRT1- and SIRT2-specific inhibitors suggest that both SIRT1 and SIRT2 are involved in regulating activation of EGFR and PDGFRβ, with SIRT1 playing a greater role in control of PDGFRβ activation.
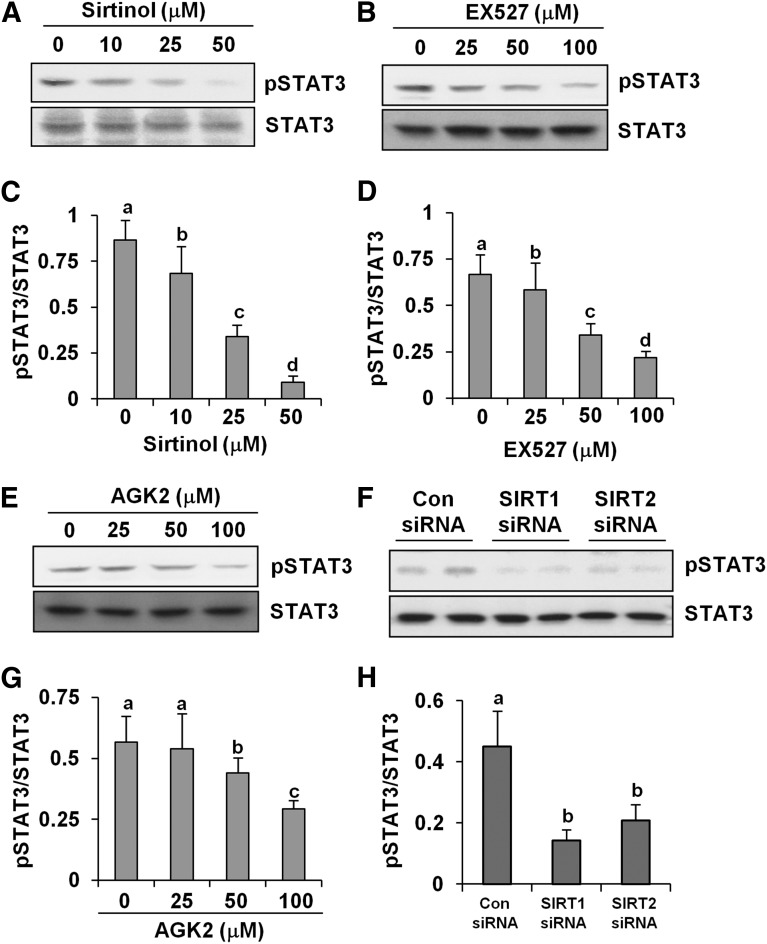
SIRT Inhibitors Block Phosphorylation of STAT3 in Cultured Renal Interstitial Fibroblasts.
STAT3 is one of the central effector molecules that mediate fibrogenic signaling (Pang et al., 2009, 2010). An acetylation/deacetylation cascade of STAT3 has been recently identified, which is essential for its enhanced transcriptional competence. As such, we examined the phosphorylation status of STAT3 at Tyr705 in NRK-49F cells treated with sirtinol, EX527, and AGK2. The phosphorylation level of STAT3 was remarkably reduced in a dose-dependent manner in NRK-49F cells exposed to all three inhibitors (Fig. 7, A–E and G). The highest concentrations of sirtinol (50 μM), EX527 (100 μM), and AGK2 (100 μM) reduced the level of phospho-STAT3 by approximately 90, 75, and 50%, respectively (Fig. 7, C, D, and G). In addition, these inhibitors did not alter the expression level of total STAT3. Consistent with these observations, knockdown of either SIRT1 or SIRT2 also reduced STAT3 phosphorylation without altering expression of total STAT3 (Fig. 7, F and H). Together, these data suggest that SIRT1 and SIRT2 contribute to regulation of STAT3 activation in renal fibroblasts.
Fig. 7.
SIRT inhibitors inhibit STAT3 phosphorylation in renal fibroblasts. NRK-49F cells were cultured in medium with 5% fetal bovine serum (FBS) and then treated with sirtinol (0–50 μM), EX527 (0–100 μM), and AGK2 (0–100 μM) for 36 hours (A–E and G). NRK-49F cells were transfected with siRNA targeting SIRT1 and SIRT2 or scrambled siRNA and incubated in normal culture medium with 5% FBS, and cells were harvested 48 hours after transfection for immunoblot analysis (F and H). After treatment, cell lysates were prepared and subjected to immunoblot analysis with antibodies for phospho-STAT3 (pSTAT3; Tyr705) or STAT3 (A, B, E, and F). Representative immunoblots from three experiments are shown. The phosphorylated and total levels of STAT3 were quantified by densitometry, and phosphorylated protein levels were normalized to total protein levels (C, D, G, and H). Values are the means ± S.D. of three independent experiments. Bars with different letters (a–d) are significantly different from one another (P < 0.01). Con, control.
SIRT Inhibitors Reduce Extracellular Signal-Regulated Kinase 1/2 and Protein Kinase B Phosphorylation in Cultured Renal Interstitial Fibroblasts.
To demonstrate whether SIRT1/2 activation is also involved in the activation of other signaling pathways, we examined the effect of SIRT1/2 inhibition on extracellular signal-regulated kinase 1/2 (ERK1/2) and protein kinase B (AKT) phosphorylation. As shown in Supplemental Fig. 2, treatment with sirtinol, EX527, or AGK2 also dose-dependently reduced phosphorylation of ERK1/2 and AKT. In addition, silencing of SIRT1 or SIRT2 resulted in similar inhibitory effects on these two kinases. Expression levels of total ERK1/2 or AKT were not affected by all of those treatments (Supplemental Fig. 2). Therefore, SIRT1 and SIRT2 activation is also critically involved in the regulation of ERK and AKT signaling pathways.
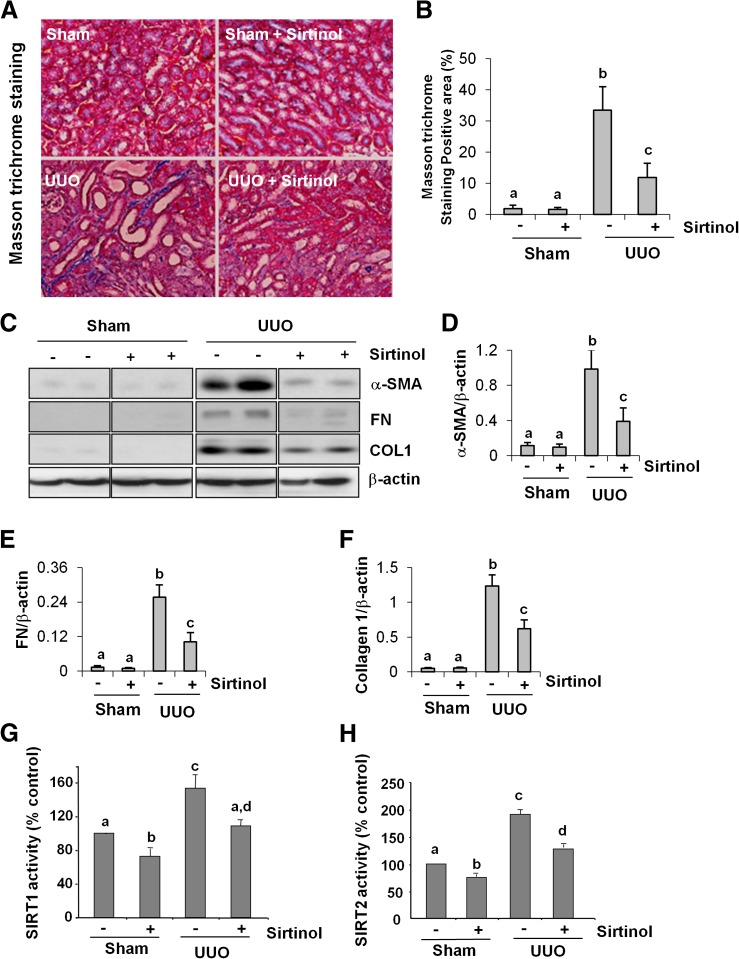
Sirtinol Attenuates Renal Fibroblast Activation and Deposition of ECM in a Mouse Model of Renal Fibrosis.
The major feature of renal fibrosis is activation of renal interstitial fibroblasts and excessive deposition of ECM components, such as fibronectin and collagen I, in the kidney. To assess the role of SIRT in the development of renal fibrosis, we further examined the effect of sirtinol on renal fibrogenesis in a murine model of renal fibrosis induced by UUO. As shown in Fig. 8, A and B, collagen fibrils are extensively deposited as a consequence of myofibroblast activation after UUO injury as evidenced by an increase in positive areas of ECM within the interstitial space under Masson trichrome staining. Administration of sirtinol dramatically reduced collagen deposition. Semiquantitative analysis of Masson trichrome–positive areas revealed that about a 30-fold increase of ECM component deposition in the obstructive kidney compared with control kidneys and sirtinol treatment reduced ECM deposition by more than 70% (Fig. 8B).
Fig. 8.
Administration of sirtinol attenuates development of renal fibrosis and deposition of ECM in obstructed kidneys. (A) Photomicrographs illustrating Masson trichrome staining of kidney tissue after treatment with or without sirtinol. (B) The Masson trichrome–positive tubulointerstitial area (blue in A) relative to the whole area from 10 random cortical fields (200×) (means ± S.D.) was analyzed. Data are represented as the mean ± S.D. (n = 6). Means with different superscript letters are significantly different from one another (P < 0.01). (C) Kidney tissue lysates were subjected to immunoblot analysis with antibodies against α-SMA, collagen I (COL1), fibronectin (FN), or β-actin. The levels of α-SMA, collagen I, and fibronectin were quantified by densitometry and normalized with β-actin (D–F). SIRT1 and SIRT2 activity was measured by an enzymatic assay with fluorescence-labeled acetylated peptide as substrate. The value was expressed as the percentage of inhibition in each sample relative to controls (G and H). Values are the means ± S.D. (n = 6). Bars with different letters (a–d) are significantly different from one another (P < 0.01).
Immunoblot analysis of whole-kidney tissue lysate indicated that there was a dramatic increase in the expression of α-SMA, collagen I, and fibronectin in the obstructed kidney after 7 days. Administration of sirtinol remarkably decreased the level of α-SMA (∼60%), fibronectin (∼60%), and collagen 1 (∼50%) (Fig. 8, C–F). An increase in the level of SIRT1 and SIRT2 activities was also detected in the kidney after UUO injury. Administration of sirtinol significantly reduced their activation (Fig. 8, G and H). Collectively, these data suggest that SIRT1 and SIRT2 inhibition attenuates the accumulation of myofibroblasts and deposition of ECM in the kidney after UUO injury.
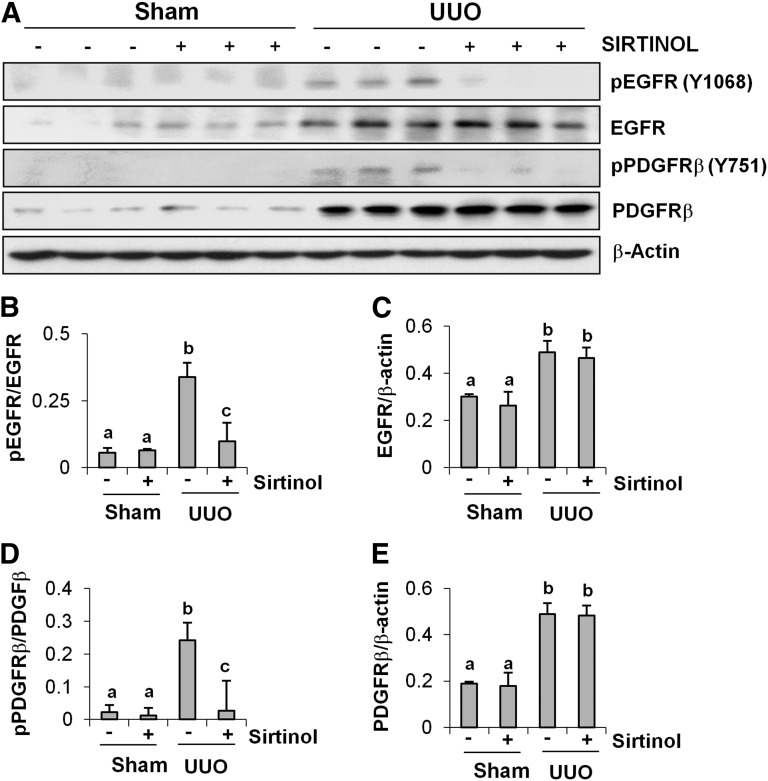
Sirtinol Inhibits Phosphorylation of EGFR and PDGFRβ in the Obstructive Kidney.
To determine the effect of sirtinol on EGFR and PDGFRβ activation in the obstructed kidney, we also examined phosphorylation levels of EGFR and PDGFRβ by immunoblot analysis. EGFR and PDGFRβ phosphorylation was induced in the kidney after UUO injury, but administration of sirtinol blocked their phosphorylation without affecting their expression, although UUO injury also increased the expression of total EGFR and PDGFRβ (Fig. 9, A, B, and D). In addition, EGFR and PDGFRβ expression was significantly increased in the obstructed kidney, and sirtinol did not affect their expression (Fig. 9, A, C, and E). These data are consistent with our observations in cultured renal fibroblasts, and suggest the importance of SIRT1/2 in regulating activation of these two tyrosine kinase receptors.
Fig. 9.
Effect of sirtinol on phosphorylation of EGFR and PDGFRβ in obstructed kidneys. Kidney tissue lysates were prepared and subjected to immunoblot analysis with antibodies for phospho-EGFR (pEGFR; Tyr1068), phospho-PDGFRβ (pPDGFRβ; Tyr751), EGFR, PDGFRβ, or β-actin (A). The phosphorylated and total levels of EGFR and PDGFRβ and β-actin were quantified by densitometry, and phosphorylated protein levels were normalized to total protein levels (B and D). The levels of EGFR and PDGFRβ were normalized with β-actin (C and E). Values are the means ± S.D. (n = 6). Bars with different letters (a–c) are significantly different from one another (P < 0.01).
Sirtinol Inhibits Phosphorylation and Expression of STAT3 in the Obstructive Kidney.
We also examined the effect of sirtinol on the phosphorylation status of STAT3 in the kidney of mice with UUO injury. UUO injury induced an increase in the expression of phospho-STAT3 (Tyr705) and total STAT3 in the kidney, whereas treatment with sirtinol decreased the phosphorylation level and expression of STAT3 (Fig. 10, A–C). Sirtinol treatment also reduced the basal level of phospho-STAT3 and total STAT3 in sham-operated kidney. Notably, the ratio of phospho-STAT3 to total STAT3 was still decreased in the injured kidney treated with sirtinol, compared with injured kidneys without sirtinol treatment (Fig. 10B). Together, our data suggest that SIRT1 and SIRT2 positively regulate STAT3 not only by promoting its phosphorylation, but also by modulating its expression and/or stability.
Fig. 10.
Effect of sirtinol on STAT3 phosphorylation in obstructed kidneys. Kidney tissue lysates were prepared and subjected to immunoblot analysis with antibodies for phospho-STAT3 (pSTAT3; Tyr705), STAT3, or β-actin (A). The phosphorylated and total levels of STAT3 and β-actin were quantified by densitometry, phosphorylated STAT3 level was normalized to total STAT3 (B), and STAT3 level was normalized with β-actin (C). Values are the means ± S.D. (n = 6). Bars with different letters (a–d) are significantly different from one another (P < 0.01).
Discussion
SIRTs have been reported to be involved in renal protection, antiaging, and neuron survival (He et al., 2010; Villalba and Alcain, 2012; Fan et al., 2013). However, the role of SIRTs, particularly individual isoforms, in renal interstitial fibroblast activation and renal fibrosis development is not well defined. In this study, we examined the effect of SIRT1/2 inhibition on renal fibroblast activation and proliferation and renal fibrogenesis. Our results showed that blocking SIRT1 and SIRT2 with a pan-inhibitor (sirtinol), isoform-selective inhibitors (EX527, AGK2), or siRNA inhibited activation and proliferation of cultured renal interstitial fibroblasts. Further, administration of sirtinol prevented development of renal fibrosis in a murine model of renal fibrosis induced by UUO injury. These results indicate that SIRT1/2 mediate activation and proliferation of renal interstitial fibroblasts and development of renal fibrogenesis, and suggest that SIRT1/2 inhibitors might be useful for the treatment of CKD.
Clearly, our results are in contrast to previous observations that activation of SIRT1 with resveratrol or SRT1720, which are believed to be SIRT1 activators, attenuated renal fibrosis (He et al., 2010; Li et al., 2010). The underlying mechanisms behind those opposing effects are not clear. A possible explanation is that resveratrol and SRT1720 exert their antifibrotic effect by acting on off-targets. In this context, studies have shown that both resveratrol and SRT1720 do not directly interact with SIRT1 and do not activate SIRT1 with native p53 peptide substrate in vitro (Beher et al., 2009; Huber et al., 2010; Pacholec et al., 2010). The broad selectivity assessment against over 100 targets also indicates that SRT1720 and resveratrol are highly promiscuous and would not serve as useful pharmacological tools for studying SIRT1 pathways (Beher et al., 2009; Huber et al., 2010; Pacholec et al., 2010). More astonishingly, resveratrol was reported to function as a pan-HDAC inhibitor of all 11 human HDACs of class I, II, and IV in tumor cells (Venturelli et al., 2013). Since class I and II HDACs have been shown to be involved in renal fibroblast activation and renal fibrogenesis (Pang et al., 2009; Liu et al., 2013), the resveratrol-mediated antifibrotic activity may be attached to the modulation of these two classes of HDACs.
Development of renal fibrosis is involved in the activation of multiple signaling pathways, including EGFR and PDGFRβ signaling. It has been documented that the phosphorylation level of PDGFRβ and EGFR is increased during the progression of interstitial fibrosis, and blocking each of them reduced the activation of renal fibroblasts in vitro and attenuated development of renal fibrosis in animal models (Ludewig et al., 2000; Pang et al., 2009; Liu et al., 2011). As such, the antifibrotic effect of SIRT1/2 inhibitors may occur through interfering with these profibrotic growth factor receptors. In the current study, we observed increased phosphorylation of PDGFRβ and EGFR in both cultured renal fibroblasts and obstructed kidney, and inhibition of either SIRT1 or SIRT2 resulted in their dephosphorylation, corresponding to the inhibitory effect of SIRT1/2 inhibitor, sirtinol, on activation/proliferation of renal fibroblasts and development of renal fibrosis. Along with this observation, we also demonstrated that blocking class I HDAC activity with MS-275 resulted in decreased EGFR phosphorylation in vitro and in vivo (Pang et al., 2009, 2011; Liu et al., 2013). However, in contrast to the ineffectiveness of SIRT inhibition on the expression of total EGFR, treatment with MS-275 was able to reduce expression levels of total EGFR. Currently, the molecular insight for class I and class III HDAC-elicited different regulations on growth factor receptors is not clear. Recent reports reveal that SIRT1 can suppress the activity/expression of a dephosphorylating machinery, protein tyrosine phosphatase 1B, which regulates various signaling including EGFR, PDGFRβ, and STAT3 (Sun et al., 2007; Gagarina et al., 2010). On this background, it is possible that inhibition of SIRT(s) by chemical inhibitor may increase the activity of protein tyrosine phosphatase 1B, and subsequently reduce phosphorylation of signaling molecules associated with fibrogenesis. However, how SIRT inhibition reduces EGFR and PDGFR phosphorylation needs further investigation.
SIRT1/2 may also regulate renal fibrosis through targeting intracellular signaling components downstream of cellular membrane receptors. Our earlier studies have shown that inhibition of class I/II HDACs with TSA induced STAT3 acetylation, which is accompanied by dephosphorylation of STAT3 at Tyr705. In this study, we found that blocking SIRT1/2 also inhibits STAT3 phosphorylation in both cultured renal fibroblasts and obstructed kidney. Additionally, we observed that SIRT1/2 inhibitors not only inhibited UUO-induced STAT3 phosphorylation in the kidney, but also reduced its phosphorylation in the sham-operated kidney. This suggests that the HDAC activity is required for regulating activation of STAT3 under both physiologic and pathologic conditions. Currently, it is unclear how SIRT1/2 is coupled to cellular machinery leading to STAT3 phosphorylation and expression. Given that inactivation of SIRT1/2 or other HDACs can induce or enhance protein acetylation, and that STAT3 acetylation was also observed in renal fibroblasts exposed to the class I/II inhibitor, it is possible that acetylation of STAT3 may counteract its phosphorylation. In this context, Kramer et al. (2009) reported that STAT1 acetylation can induce its binding to T-cell protein tyrosine phosphatase, which catalyzes STAT1 dephosphorylation (Kramer et al., 2009). Whether this functional acetyl-phospho switch, regulated by an acetylation/deacetylation balance, also modulates STAT3 phosphorylation requires further investigation. On the other hand, STAT3 acetylation may induce its dephosphorylation through upregulation of its negative regulators, the suppressor of cytokine signaling (SOCS) family members. In this regard, Xiong et al. (2012) reported that inhibition of HDACs with TSA leads to the hyperacetylation of histones associated with the SOCS1 and SOCS3 promoters and subsequently upregulates SOCS1 and SOCS3 expression. As SOCS1 and SOCS3 are the inhibitors of STAT3, their upregulation would suppress STAT3 phosphorylation (Xiong et al., 2012).
Furthermore, the reduction of STAT3 phosphorylation in response to SIRT1/2 inhibition may also be the consequence of STAT3 degradation. However, STAT3 degradation only occurs in the kidney treated with sirtinol, but not in cultured renal interstitial fibroblasts. Comparison of the ratio of phospho-STAT3/STAT3 in the kidney with or without SIRT1/2 inhibitors still shows a decrease in STAT3 phosphorylation. This finding suggests that STAT3 degradation may only in part contribute to STAT3 dephosphorylation. The mechanism by which STAT3 is degraded in the kidney after SIRT1/2 inhibition is poorly understood, but may be associated with its acetylation. In this context, it has been reported that the acetylation of a specific lysine creates a binding site for the recruitment of an E3-containing complex, which then ubiquitinates the target protein, leading to its degradation (Jeong et al., 2002; Lee et al., 2004). On this basis, it is possible that sirtinol-induced inhibition of deacetylation leads to hyperacetylation of STAT3, which in turn reduces the stability of STAT3. This hypothesis deserves to be examined in the future.
Our data showed that blocking SIRT1 and SIRT2 also suppresses ERK1/2 and AKT phosphorylation, suggesting that SIRT activation is involved in the activation of multiple intracellular signaling pathways. AKT is activated through a process that requires binding of AKT to phosphatidylinositol 3,4,5-trisphosphate [PIP3], which promotes phosphorylation of AKT by the upstream kinase phosphoinositide-dependent protein kinase 1 (PDK1). A recent study indicated that deacetylation by SIRT1 enhances binding of AKT and PDK1 to PIP3 and promotes their activation, whereas SIRT1 inhibitor–mediated acetylation of AKT and PDK1 reduces binding of AKT and PDK1 to PIP3, resulting in prevention of AKT phosphorylation (Sundaresan et al., 2011). Similarly, a recent study showed that SIRT2 directly interacts with AKT and is required for optimal activation of AKT (Ramakrishnan et al., 2014). Together with these reports, our study reveals that SIRT1 and 2 can regulate AKT signaling. But in the case of ERK1/2 signaling, it currently remains unclear how SIRT1 and SIRT2 regulate activation of ERK1/2 in renal fibroblasts. Since activation of ERK1/2 mostly occurs at the downstream of growth factor receptors, it is possible that dephosphorylation of ERK1/2 is secondary to inactivation of EGFR and PDGFRβ by SIRT1/2 inhibition. However, we cannot exclude the possibility that SIRT1/2 inhibition–mediated acetylation would also directly regulate ERK1/2 activation. Recent studies in different types of fibroblasts support this notion. A study in human diploid fibroblasts found that SIRT1 promoted cell proliferation and antagonized cellular senescence by partly activating ERK signaling (Huang et al., 2008). SIRT2 was also reported to promote myoblast cell (C2C12) proliferation by activation of the ERK1/2 pathway (Wu et al., 2014).
Increasing evidence has demonstrated that epigenetic modifications play a crucial role in the pathogenesis of tissue fibrosis. As such, the inhibition of HDAC enzymes may become an important therapeutic approach for chronic fibrotic kidney diseases. Clinical trials with HDAC inhibitors have shown promising results in cancer therapy. To date, two class I/II HDAC inhibitors are approved by the Food and Drug Administration for clinical use to treat tumors (Kelly et al., 2002; Marks and Xu, 2009). As the process of renal fibrogenesis is similar to tumorigenesis, it is possible that HDAC inhibitors including SIRT inhibitors may be an effective therapeutic treatment. Currently, more potent and highly specific inhibitors for SIRT1 and SIRT2 are being developed and have been tested for their efficacy in different disorders in animal models. Thus, it will be interesting to further assess the value of whether more specific inhibition of SIRT1 and -2 might be useful as a therapeutic intervention in renal diseases associated with fibroblast activation, especially CKD.
In contrast to the role of SIRTs in mediating renal fibrogenesis, activation of SIRTs, in particular SIRT1, has been reported to protect against acute kidney injury induced by ischemia/reperfusion (Fan et al., 2013). The protective effect of SIRT1 is associated with maintaining peroxisome function, promoting mitochondrial biogenesis (Funk and Schnellmann, 2013) and deacetylation of p53 (Kim et al., 2011). Thus, it appears that SIRTs play a role in both renal protection and fibrogenesis. As severe acute kidney injury would often cause renal fibrosis due to incomplete repair, it is suggested that activation of SIRT1 at the early stage and inhibition of SIRT1 at the late stage would promote renal structural and functional recovery and reduce fibrosis.
In summary, our study is the first to demonstrate that blockade of SIRT1 and SIRT2 with their highly selective inhibitors can inhibit the activation of renal interstitial fibroblasts in vitro, and that sirtinol treatment attenuates UUO-induced fibrosis in an animal model. These antifibrotic actions of SIRT1/2 inhibitors are associated with inhibition of EGFR and PDGFR as well as multiple intracellular signaling pathways. Additional studies are needed to address the mechanism by which SIRT1/2 inhibition leads to suppression of growth factor receptors and signaling associated with activation and proliferation of renal fibroblasts and renal fibrogenesis.
Supplementary Material
Abbreviations
- acetyl-H3K9
acetylation of histone H3 at lysine 9
- AGK2
2-cyano-3-[5-(2,5-dichlorophenyl)-2-furanyl]-N-5-quinolinyl-2-propenamide
- AKT
protein kinase B
- CKD
chronic kidney disease
- ECM
extracellular matrix
- EGFR
epidermal growth factor receptor
- ERK
extracellular signal-regulated kinase
- EX527
6-chloro-2,3,4,9-tetrahydro-1H-carbazole-1-carboxamide
- HDAC
histone deacetylase
- MS-275
pyridin-3-ylmethyl 4-((2-aminophenyl)carbamoyl)benzylcarbamate
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PCNA
proliferating cell nuclear antigen
- PDGFRβ
platelet-derived growth factor receptor-β
- PDK1
phosphoinositide-dependent protein kinase 1
- PIP3
phosphatidylinositol 3,4,5-trisphosphate
- siRNA
small interfering RNA
- SIRT
sirtuin
- α-SMA
α-smooth muscle actin
- SOCS
suppressor of cytokine signaling
- SRT1720
N-(2-(3-(piperazin-1-ylmethyl)imidazo[2,1-b]thiazol-6-yl)phenyl)quinoxaline-2-carboxamide hydrochloride
- STAT3
signal transducer and activator of transcription 3
- TSA
trichostatin A
- UUO
unilateral ureteral obstruction
Authorship Contributions
Participated in research design: Zhuang, Ponnusamy, Zhou, Zhao, Gong.
Conducted experiments: Ponnusamy, Zhou, Tang, Yan, Tolbert.
Contributed new reagents or analytic tools: Ponnusamy, Zhou.
Performed data analysis: Ponnusamy, Zhou.
Wrote or contributed to the writing of the manuscript: Zhuang, Ponnusamy.
Footnotes
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK085065]; Key Discipline Construction Project of Pudong Health Bureau of Shanghai (PWZxk2014-6); and the National Nature Science Foundation of China [81270778].
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Akgedik R, Akgedik S, Karamanlı H, Uysal S, Bozkurt B, Ozol D, Armutcu F, Yıldırım Z. (2012) Effect of resveratrol on treatment of bleomycin-induced pulmonary fibrosis in rats. Inflammation 35:1732–1741 [DOI] [PubMed] [Google Scholar]
- Beher D, Wu J, Cumine S, Kim KW, Lu SC, Atangan L, Wang M. (2009) Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem Biol Drug Des 74:619–624 [DOI] [PubMed] [Google Scholar]
- Bonner JC. (2004) Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev 15:255–273 [DOI] [PubMed] [Google Scholar]
- Catania A, Iavarone C, Carlomagno SM, Chiariello M. (2006) Selective transcription and cellular proliferation induced by PDGF require histone deacetylase activity. Biochem Biophys Res Commun 343:544–554 [DOI] [PubMed] [Google Scholar]
- Fan H, Yang HC, You L, Wang YY, He WJ, Hao CM. (2013) The histone deacetylase, SIRT1, contributes to the resistance of young mice to ischemia/reperfusion-induced acute kidney injury. Kidney Int 83:404–413 [DOI] [PubMed] [Google Scholar]
- Funk JA, Schnellmann RG. (2013) Accelerated recovery of renal mitochondrial and tubule homeostasis with SIRT1/PGC-1α activation following ischemia-reperfusion injury. Toxicol Appl Pharmacol 273:345–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagarina V, Gabay O, Dvir-Ginzberg M, Lee EJ, Brady JK, Quon MJ, Hall DJ. (2010) SirT1 enhances survival of human osteoarthritic chondrocytes by repressing protein tyrosine phosphatase 1B and activating the insulin-like growth factor receptor pathway. Arthritis Rheum 62:1383–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Nie H, Hong Y, Sheng C, Xia W, Ying W. (2012) SIRT2 activity is required for the survival of C6 glioma cells. Biochem Biophys Res Commun 417:468–472 [DOI] [PubMed] [Google Scholar]
- He W, Wang Y, Zhang MZ, You L, Davis LS, Fan H, Yang HC, Fogo AB, Zent R, Harris RC, et al. (2010) Sirt1 activation protects the mouse renal medulla from oxidative injury. J Clin Invest 120:1056–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Gan Q, Han L, Li J, Zhang H, Sun Y, Zhang Z, Tong T. (2008) SIRT1 overexpression antagonizes cellular senescence with activated ERK/S6k1 signaling in human diploid fibroblasts. PLoS One 3:e1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber JL, McBurney MW, Distefano PS, McDonagh T. (2010) SIRT1-independent mechanisms of the putative sirtuin enzyme activators SRT1720 and SRT2183. Future Med Chem 2:1751–1759 [DOI] [PubMed] [Google Scholar]
- Imanishi S, Hayashi R, Ichikawa T, Suzuki K, Sasahara M, Kondo T, Ogawa H, Tobe K. (2012) SRT1720, a SIRT1 activator, aggravates bleomycin-induced lung injury in mice. Food Nutr Sci 3:157–163 [Google Scholar]
- Jeong JW, Bae MK, Ahn MY, Kim SH, Sohn TK, Bae MH, Yoo MA, Song EJ, Lee KJ, Kim KW. (2002) Regulation and destabilization of HIF-1alpha by ARD1-mediated acetylation. Cell 111:709–720 [DOI] [PubMed] [Google Scholar]
- Jin KL, Park JY, Noh EJ, Hoe KL, Lee JH, Kim JH, Nam JH. (2010) The effect of combined treatment with cisplatin and histone deacetylase inhibitors on HeLa cells. J Gynecol Oncol 21:262–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly WK, O’Connor OA, Marks PA. (2002) Histone deacetylase inhibitors: from target to clinical trials. Expert Opin Investig Drugs 11:1695–1713 [DOI] [PubMed] [Google Scholar]
- Kim DH, Jung YJ, Lee JE, Lee AS, Kang KP, Lee S, Park SK, Han MK, Lee SY, Ramkumar KM, et al. (2011) SIRT1 activation by resveratrol ameliorates cisplatin-induced renal injury through deacetylation of p53. Am J Physiol Renal Physiol 301:F427–F435 [DOI] [PubMed] [Google Scholar]
- Kojima K, Ohhashi R, Fujita Y, Hamada N, Akao Y, Nozawa Y, Deguchi T, Ito M. (2008) A role for SIRT1 in cell growth and chemoresistance in prostate cancer PC3 and DU145 cells. Biochem Biophys Res Commun 373:423–428 [DOI] [PubMed] [Google Scholar]
- Kook H, Lepore JJ, Gitler AD, Lu MM, Wing-Man Yung W, Mackay J, Zhou R, Ferrari V, Gruber P, Epstein JA. (2003) Cardiac hypertrophy and histone deacetylase-dependent transcriptional repression mediated by the atypical homeodomain protein Hop. J Clin Invest 112:863–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer OH, Knauer SK, Greiner G, Jandt E, Reichardt S, Gührs KH, Stauber RH, Böhmer FD, Heinzel T. (2009) A phosphorylation-acetylation switch regulates STAT1 signaling. Genes Dev 23:223–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW. (2004) Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions. Exp Mol Med 36:1–12 [DOI] [PubMed] [Google Scholar]
- Lee TM, Lin MS, Chang NC. (2007) Inhibition of histone deacetylase on ventricular remodeling in infarcted rats. Am J Physiol Heart Circ Physiol 293:H968–H977 [DOI] [PubMed] [Google Scholar]
- Legutko A, Marichal T, Fiévez L, Bedoret D, Mayer A, de Vries H, Klotz L, Drion PV, Heirman C, Cataldo D, et al. (2011) Sirtuin 1 promotes Th2 responses and airway allergy by repressing peroxisome proliferator-activated receptor-γ activity in dendritic cells. J Immunol 187:4517–4529 [DOI] [PubMed] [Google Scholar]
- Li J, Qu X, Ricardo SD, Bertram JF, Nikolic-Paterson DJ. (2010) Resveratrol inhibits renal fibrosis in the obstructed kidney: potential role in deacetylation of Smad3. Am J Pathol 177:1065–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. (2004) Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol 15:1–12 [DOI] [PubMed] [Google Scholar]
- Liu N, Guo JK, Pang M, Tolbert E, Ponnusamy M, Gong R, Bayliss G, Dworkin LD, Yan H, Zhuang S. (2012) Genetic or pharmacologic blockade of EGFR inhibits renal fibrosis. J Am Soc Nephrol 23:854–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, He S, Ma L, Ponnusamy M, Tang J, Tolbert E, Bayliss G, Zhao TC, Yan H, Zhuang S. (2013) Blocking the class I histone deacetylase ameliorates renal fibrosis and inhibits renal fibroblast activation via modulating TGF-beta and EGFR signaling. PLoS ONE 8:e54001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Levin MD, Petrenko NB, Lu MM, Wang T, Yuan LJ, Stout AL, Epstein JA, Patel VV. (2008) Histone-deacetylase inhibition reverses atrial arrhythmia inducibility and fibrosis in cardiac hypertrophy independent of angiotensin. J Mol Cell Cardiol 45:715–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Tolbert E, Pang M, Ponnusamy M, Yan H, Zhuang S. (2011) Suramin inhibits renal fibrosis in chronic kidney disease. J Am Soc Nephrol 22:1064–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig D, Kosmehl H, Sommer M, Böhmer FD, Stein G. (2000) PDGF receptor kinase blocker AG1295 attenuates interstitial fibrosis in rat kidney after unilateral obstruction. Cell Tissue Res 299:97–103 [DOI] [PubMed] [Google Scholar]
- Marchion D, Münster P. (2007) Development of histone deacetylase inhibitors for cancer treatment. Expert Rev Anticancer Ther 7:583–598 [DOI] [PubMed] [Google Scholar]
- Marks PA, Xu WS. (2009) Histone deacetylase inhibitors: Potential in cancer therapy. J Cell Biochem 107:600–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meran S, Steadman R. (2011) Fibroblasts and myofibroblasts in renal fibrosis. Int J Exp Pathol 92:158–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson EG. (2006) Mechanisms of disease: Fibroblasts—a new look at an old problem. Nat Clin Pract Nephrol 2:101–108 [DOI] [PubMed] [Google Scholar]
- Ota H, Tokunaga E, Chang K, Hikasa M, Iijima K, Eto M, Kozaki K, Akishita M, Ouchi Y, Kaneki M. (2006) Sirt1 inhibitor, Sirtinol, induces senescence-like growth arrest with attenuated Ras-MAPK signaling in human cancer cells. Oncogene 25:176–185 [DOI] [PubMed] [Google Scholar]
- Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, et al. (2010) SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem 285:8340–8351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang M, Kothapally J, Mao H, Tolbert E, Ponnusamy M, Chin YE, Zhuang S. (2009) Inhibition of histone deacetylase activity attenuates renal fibroblast activation and interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol 297:F996–F1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang M, Ma L, Gong R, Tolbert E, Mao H, Ponnusamy M, Chin YE, Yan H, Dworkin LD, Zhuang S. (2010) A novel STAT3 inhibitor, S3I-201, attenuates renal interstitial fibroblast activation and interstitial fibrosis in obstructive nephropathy. Kidney Int 78:257–268 [DOI] [PubMed] [Google Scholar]
- Pang M, Ma L, Liu N, Ponnusamy M, Zhao TC, Yan H, Zhuang S. (2011) Histone deacetylase 1/2 mediates proliferation of renal interstitial fibroblasts and expression of cell cycle proteins. J Cell Biochem 112:2138–2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang M, Zhuang S. (2010) Histone deacetylase: a potential therapeutic target for fibrotic disorders. J Pharmacol Exp Ther 335:266–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck B, Chen CY, Ho KK, Di Fruscia P, Myatt SS, Coombes RC, Fuchter MJ, Hsiao CD, Lam EW. (2010) SIRT inhibitors induce cell death and p53 acetylation through targeting both SIRT1 and SIRT2. Mol Cancer Ther 9:844–855 [DOI] [PubMed] [Google Scholar]
- Ramakrishnan G, Davaakhuu G, Kaplun L, Chung WC, Rana A, Atfi A, Miele L, Tzivion G. (2014) Sirt2 deacetylase is a novel AKT binding partner critical for AKT activation by insulin. J Biol Chem 289:6054–6066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts K, Niki T, Greenwel P, Vandermonde A, Wielant A, Hellemans K, De Bleser P, Yoshida M, Schuppan D, Rojkind M, et al. (2002) Trichostatin A, a histone deacetylase inhibitor, suppresses collagen synthesis and prevents TGF-beta(1)-induced fibrogenesis in skin fibroblasts. Exp Cell Res 278:184–197 [DOI] [PubMed] [Google Scholar]
- Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q. (2007) SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab 6:307–319 [DOI] [PubMed] [Google Scholar]
- Sundaresan NR, Pillai VB, Wolfgeher D, Samant S, Vasudevan P, Parekh V, Raghuraman H, Cunningham JM, Gupta M, Gupta MP. (2011) The deacetylase SIRT1 promotes membrane localization and activation of Akt and PDK1 during tumorigenesis and cardiac hypertrophy. Sci Signal 4:ra46. [DOI] [PubMed] [Google Scholar]
- Terzi F, Burtin M, Hekmati M, Federici P, Grimber G, Briand P, Friedlander G. (2000) Targeted expression of a dominant-negative EGF-R in the kidney reduces tubulo-interstitial lesions after renal injury. J Clin Invest 106:225–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturelli S, Berger A, Böcker A, Busch C, Weiland T, Noor S, Leischner C, Schleicher S, Mayer M, Weiss TS, et al. (2013) Resveratrol as a pan-HDAC inhibitor alters the acetylation status of jistone proteins in human-derived hepatoblastoma cells. PLoS ONE 8:e73097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba JM, Alcaín FJ. (2012) Sirtuin activators and inhibitors. Biofactors 38:349–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JC, Chen C, Dumlao T, Naik S, Chang T, Xiao YY, Sominsky I, Burton J. (2008) Enhanced histone deacetylase enzyme activity in primary myelofibrosis. Leuk Lymphoma 49:2321–2327 [DOI] [PubMed] [Google Scholar]
- Wu G, Song C, Lu H, Jia L, Yang G, Shi X, Sun S. (2014) Sirt2 induces C2C12 myoblasts proliferation by activation of the ERK1/2 pathway. Acta Biochim Biophys Sin (Shanghai) 46:342–345 [DOI] [PubMed] [Google Scholar]
- Wynn TA. (2008) Cellular and molecular mechanisms of fibrosis. J Pathol 214:199–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H, Du W, Zhang YJ, Hong J, Su WY, Tang JT, Wang YC, Lu R, Fang JY. (2012) Trichostatin A, a histone deacetylase inhibitor, suppresses JAK2/STAT3 signaling via inducing the promoter-associated histone acetylation of SOCS1 and SOCS3 in human colorectal cancer cells. Mol Carcinog 51:174–184 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.