Abstract
Gestational diabetes mellitus is a major complication of human pregnancy. The oral clearance (CL) of glyburide, an oral antidiabetic drug, increases 2-fold in pregnant women during late gestation versus nonpregnant controls. In this study, we examined gestational age–dependent changes in maternal-fetal pharmacokinetics (PK) of glyburide and metabolites in a pregnant mouse model. Nonpregnant and pregnant FVB mice were given glyburide by retro-orbital injection. Maternal plasma was collected over 240 minutes on gestation days (gd) 0, 7.5, 10, 15, and 19; fetuses were collected on gd 15 and 19. Glyburide and metabolites were quantified using high-performance liquid chromatography–mass spectrometry, and PK analyses were performed using a pooled data bootstrap approach. Maternal CL of glyburide increased approximately 2-fold on gd 10, 15, and 19 compared with nonpregnant controls. Intrinsic CL of glyburide in maternal liver microsomes also increased as gestation progressed. Maternal metabolite/glyburide area under the curve ratios were generally unchanged or slightly decreased throughout gestation. Total fetal exposure to glyburide was <5% of maternal plasma exposure, and was doubled on gd 19 versus gd 15. Fetal metabolite concentrations were below the limit of assay detection. This is the first evidence of gestational age–dependent changes in glyburide PK. Increased maternal glyburide clearance during gestation is attributable to increased hepatic metabolism. Metabolite elimination may also increase during pregnancy. In the mouse model, fetal exposure to glyburide is gestational age–dependent and low compared with maternal plasma exposure. These results indicate that maternal glyburide therapeutic strategies may require adjustments in a gestational age–dependent manner if these same changes occur in humans.
Introduction
Gestational diabetes mellitus (GDM) complicates 5–14% of human pregnancies (Jovanovic and Pettitt, 2001; Paglia and Coustan, 2011). Like type 2 diabetes, the pathology of GDM is a combination of increased insulin resistance and decreased insulin sensitivity. If left untreated, GDM poses significant risks to the mother, fetus, and neonate. Such risks include maternal hypertension, preeclampsia, and cesarean delivery, as well as fetal/neonatal morbidities including macrosomia, hypoglycemia, and increased risk of metabolic syndrome, type 2 diabetes, and obesity for the offspring later in life (American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics, 2001; HAPO Study Cooperative Research Group, 2002). While insulin resistance occurs in normal pregnancy, women with GDM experience insulin resistance beyond their ability to compensate with increased insulin production, leading to hyperglycemia. Although insulin has been the standard of care for pharmacotherapeutic treatment of GDM, oral antidiabetic agents, such as glyburide, have gained increasing popularity because of their ease of administration, lower cost, and comparable efficacy to insulin (Langer et al., 2000).
Diagnosis of GDM generally takes place during the second trimester of pregnancy. It is well established that physiologic, biochemical, and hormonal changes during pregnancy can alter the pharmacokinetics (PK) of drugs throughout gestation (e.g., increased hepatic blood flow and glomerular filtration, and/or changes in the expression of drug-metabolizing enzymes and transporters) (Klieger et al., 2009). However, data are quite limited regarding the PK of glyburide during pregnancy, particularly in relation to gestational age. One study estimated a 2-fold increase in the oral clearance of glyburide in women with GDM in the third trimester of pregnancy compared with nonpregnant women with type 2 diabetes mellitus (Hebert et al., 2009). Formation clearance of 4-trans-hydroxycyclohexyl glyburide (M1), a pharmacologically active metabolite, was also increased more than 2-fold in the GDM group. Glyburide crosses the human placenta, but at a much slower rate compared with the placental transfer marker antipyrine (Nanovskaya et al., 2006), which was not expected given the low molecular weight and high lipophilicity of glyburide. This low rate of placental transfer could be explained by extremely high plasma protein binding of glyburide (Nanovskaya et al., 2006) and efflux transport at the apical membrane of the syncytiotrophoblasts in humans and mice. The ATP-binding cassette transporters breast cancer resistance protein (BCRP) and P-glycoprotein (P-gp) have been implicated as placental barriers to glyburide (Gedeon et al., 2006, 2008b; Zhou et al., 2008; Hemauer et al., 2010). Although multidrug resistance proteins (MRP1 and MRP3) also transport glyburide (Gedeon et al., 2006; Hemauer et al., 2010), human placenta perfusion studies suggest that MRPs may only play a minor role in the transport of glyburide across human placenta (Gedeon et al., 2008a). The time course and mechanism by which pregnancy changes glyburide PK throughout gestation remain largely unexplained. Therefore, the main objective of this study was to investigate gestational age–dependent changes in maternal-fetal disposition of glyburide and the mechanisms behind these changes.
Since hepatic CYP3A and CYP2C9 activities are significantly induced during human pregnancy (Hebert et al., 2008; Feghali and Mattison, 2011), cytochrome P450 induction could be one possible mechanism for the increased maternal glyburide clearance during pregnancy. Like in humans, glyburide clearance in pregnant mice was similarly doubled on gestation day (gd) 15 compared with nonpregnant controls (Zhou et al., 2010b), suggesting that the pregnant mouse may be an appropriate animal model to study glyburide PK during pregnancy. The mRNA levels of several hepatic Cyp3a isoforms and CYP3A activity in pregnant mice are significantly increased in a gestational age–dependent manner compared with nonpregnant controls (Zhang et al., 2008; Shuster et al., 2013). Therefore, we used pregnant mice to study gestational age–dependent effects on maternal-fetal disposition of glyburide.
In vitro microsomal incubation studies suggest that glyburide is extensively metabolized in the human liver by CYP3A4, CYP2C9, and CYP2C19 to six major metabolites: M1, 4-cis-hydroxycyclohexyl glyburide (M2a), 3-cis-hydroxycyclohexyl glyburide (M2b), 3-trans-hydroxycyclohexyl glyburide (M3), 2-trans-hydroxycyclohexyl glyburide (M4), and ethylene-hydroxylated glyburide (M5) (Ravindran et al., 2006; Zharikova et al., 2009). Because M1 and M2b are potentially pharmacologically active (Rydberg et al., 1994) and limited data exist regarding metabolite PK in pregnant and nonpregnant individuals, we also examined maternal-fetal disposition of metabolites.
In this study, we first determined the maternal-fetal PK of glyburide and its metabolites throughout gestation in pregnant mice. We then investigated whether the intrinsic clearance of glyburide in microsomes isolated from the livers of pregnant mice was increased in a gestational age–dependent manner. The data obtained in this study will facilitate mechanistic understanding of changes in maternal-fetal PK of glyburide and its metabolites throughout gestation, which is imperative for gestational age–dependent therapeutic strategies.
Materials and Methods
Glyburide and glipizide were purchased from Sigma-Aldrich (St. Louis, MO). Glyburide metabolites M1, M2a, M2b, and M3 were purchased from Toronto Research Chemicals (Toronto, ON, Canada). M4 and M5 are not commercially available, and thus were not included in our metabolite analyses. Internal standards glyburide-d11 and 4-trans-hydroxycyclohexyl glyburide-d3,13C were also purchased from Toronto Research Chemicals. We obtained [cyclohexyl-2,3-3H(N)]-glyburide ([3H]Gly) (50 Ci/mmol) from PerkinElmer Life Sciences (Waltham, MA). NADPH tetrasodium salt hydrate, potassium phosphate monobasic KH2PO4, and EDTA were purchased from Sigma-Aldrich. Optima-grade (or high-performance liquid chromatography–grade) methanol, dimethylsulfoxide, polyethylene glycol 400, ethanol, n-hexane, methylene chloride, formic acid, ammonium formate, and water were obtained from Thermo Fisher Scientific (Waltham, MA) or Acros Organics (Pittsburgh, PA). Phosphate-buffered saline (PBS) was purchased from Corning Cellgro (Manassas, VA).
Animal Studies.
Wild-type FVB mice, aged 7–10 weeks, were purchased from Taconic Farms (Germantown, NY) and cared for in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Research Council. The animal protocol for this study was approved by the Institutional Animal Care and Use Committee at the University of Washington (Seattle, WA). Briefly, mice were maintained under 12-hour light/dark cycles, and food was provided ad libitum. Female mice, aged 7–10 weeks, were mated with male mice of the same age overnight using a female to male ratio of 2:1. We defined gd 1 as the presence of a sperm plug after overnight housing. Nonpregnant mice were defined as gd 0. Progression of pregnancy was monitored by visual inspection and body weight increase. Body weight was recorded on the day of dosing.
Glyburide was dissolved in 0.5% (v/v) dimethylsulfoxide, 10% (v/v) ethanol, 39.5% (v/v) saline, and 50% (v/v) polyethylene glycol 400 at a concentration of 0.5 mg/ml. Under anesthesia (2–5% isoflurane), pregnant (gd 7.5, 10, 15, and 19) or nonpregnant (gd 0) mice were administered 20 µg glyburide per mouse by retro-orbital injection (40 µl each injection). The 20-μg dose was selected to achieve maternal plasma concentrations in the 10–1000 ng/ml range, which is comparable with steady state plasma concentrations observed in pregnant women during the third trimester of pregnancy, as we demonstrated in previous studies with pregnant and nonpregnant mice receiving a glyburide dose of 1 mg/kg body weight (Zhou et al., 2008, 2010b; Hebert et al., 2009). The same dose of glyburide (20 µg) was used across gestational ages because the increase in body weight during pregnancy is mainly due to the presence of placenta and fetuses, which do not significantly contribute to the overall distribution volume and metabolic clearance of glyburide. In addition, glyburide is not administered based on body weight in clinical practice; therefore, maintaining the same dose across gestation better mimics clinical scenarios. Depth of anesthesia was evaluated using the front-toe pinch method. At various times (0.5, 5, 10, 20, 40, 60, 120, 180, and 240 minutes) after glyburide administration, animals (n = 3–5 per time point) were euthanized under anesthesia by cardiac puncture (i.e., a total of approximately 30 samples for each gestation day). Maternal blood was collected in heparinized microcentrifuge tubes (BD Biosciences, San Jose, CA) and centrifuged at 1000g for 10 minutes at 4°C. Plasma was collected and stored at −80°C until further analysis. Individual whole fetuses were collected from mice dosed on gd 15 and 19. Maternal mouse livers were also collected (n = 5 to 6 per gestation day) for microsomal preparation from mice that had not been dosed with glyburide. Tissues were immediately rinsed with PBS, snap-frozen in liquid nitrogen, and stored at −80°C until use.
Quantification of Glyburide and Metabolites in Maternal Plasma and Fetal Homogenates.
Glyburide and metabolite quantification in maternal plasma and fetal homogenates was performed using a previously validated high-performance liquid chromatography–mass spectrometry (HPLC-MS) method with some modifications (Naraharisetti et al., 2007). Most notable was the use of protein precipitation for the isolation of glyburide and metabolites from maternal plasma and fetal homogenates in place of liquid–liquid extraction. In brief, for every 100 µl maternal plasma, 450 µl methanol and 20 µl working internal standards (0.5 ng/µl glyburide-d11 and 0.15 ng/µl 4-trans-hydroxycyclohexyl glyburide-d3,13C) were added into a 1.5-ml microcentrifuge tube. Plasma samples were briefly vortexed and centrifuged at 20,800g for 10 minutes at 4°C. Supernatants were transferred to disposable clean glass tubes and evaporated using nitrogen gas. Samples were reconstituted in 75 µl initial mobile phase and 2 µl was injected per sample for HPLC-MS analysis. A calibration curve was prepared identically using human plasma as a matrix with a dynamic range of 10–4000 ng/ml for glyburide and 1.2–120 ng/ml for M1–M3.
Individual whole fetuses were homogenized in 1.5–2.5 ml PBS using an Omni Bead Ruptor Homogenizer (Omni International, Kennesaw, GA). For every 500 µl fetal homogenates, 50 µl 2 M HCl, 4 ml 60/40 (v/v) n-hexane/methylene chloride, and 20 µl working internal standard (0.5 ng/µl glyburide-d11) were added in a 13 × 100-mm borosilicate glass culture tube. Fetal samples were vortexed 30 seconds and centrifuged at 1970g for 10 minutes at 4°C. Supernatants were transferred to disposable clean glass tubes and evaporated using nitrogen gas. Each sample was reconstituted in 75 µl 1% formic acid in methanol and 2 µl was injected per sample for HPLC-MS analysis. A calibration curve for glyburide was prepared using 500 µl blank fetal homogenate (matched by gestational age) as the matrix, over a dynamic range of 0.05–2 ng glyburide. Calibration according to the analyte amount was chosen for fetal tissue analysis to accommodate the variations in fetal tissue specimen size and homogenate dilutions. No matrix effect was observed; that is, glyburide extraction recovery and instrument response did not vary over the range of fetal homogenate concentrations prepared on samples taken from gd 15 and 19 (0.06–0.6 g fetus/ml PBS).
The previously validated HPLC-MS method did not include M2a and M3 for lack of commercially available standards at the time (Naraharisetti et al., 2007). Those standards are now available; therefore, we modified the above method slightly to incorporate the separation and quantification of M1–M3 in maternal plasma. These metabolites were quantifiable in maternal plasma, but were not detectable in fetal tissue samples.
In brief, HPLC-MS was performed using an Agilent series 1100 high-performance liquid chromatographer interfaced with an Agilent G1956B single quadrupole mass spectrometer (Agilent Technologies, Palo Alto, CA). Separation of glyburide and all metabolites in maternal plasma and fetal homogenates was achieved using an Ace 3 C8 column (150 mm × 2.1 mm, 3 μm) with gradient elution. The mobile phases consisted of methanol containing 5 mM ammonium formate (B) and water containing 5 mM ammonium formate at pH 6.0 (A). The flow rate was set to 0.4 ml/min. The gradient was 42.5% methanol for the first 5 minutes, then increased linearly to 90% for 5.1 minutes, and finally decreased back to 42.5% for the remainder of the 11-minute run time. The mass spectrometer was run in atmospheric pressure ionization-electrospray positive ionization mode with a capillary voltage of 3500 V and a fragmentation voltage of 90 V for M1–M3 and trans-4-hydroxy glyburide-d3,13C or 115 V for glyburide and glyburide-d11. The drying gas temperature was 350°C, the nitrogen drying gas flow rate was 12 l/min, and the nebulizer pressure was 35 psi. Ions monitored were 494 m/z for glyburide, 505 m/z for glyburide-d11, 510 m/z for M1–M3, and 514 m/z trans-4-hydroxy glyburide d3,13C. Select ion chromatograms that are generated from sample extracts and demonstrate the separation of glyburide and metabolites are presented in Supplemental Fig. 1. The lower limits of quantification in maternal plasma were 10 ng/ml for glyburide and 1.2 ng/ml for M1–M3 (Supplemental Fig. 2). Blank human plasma spiked with glyburide and metabolites was used for quality control samples for maternal plasma and fetal homogenate analyses. Interday and intraday variability of glyburide and metabolites were less than 5 and 2%, respectively.
Plasma Protein Binding.
Mouse plasma protein binding was determined by ultrafiltration using Microcon-10 kDa Centrifugal Filters with Ultracel-10 membranes (EMD Millipore Corporation, Billerica, MA). [3H]Gly (1 ng) in methanol was aliquoted into 1.5-ml Eppendorf tubes and evaporated to dryness. Maternal plasma collected after glyburide administration on gd 0, 7.5, 10, 15, and 19 was added to each tube to a total volume of 220 µl. Samples were briefly vortexed and allowed to equilibrate for 30 minutes at 37°C. Two 100-µl aliquots from each sample were transferred to ultrafiltration cartridges, equilibrated for 30 minutes at 37°C, and centrifuged at 1000g for 10 minutes. Eight microliters of filtrate and unfiltered plasma were counted on a liquid scintillation counter. The fraction unbound (fu) of glyburide was calculated as the percentage of radioactivity of the filtrate to the radioactivity of the corresponding unfiltered plasma. Nonspecific binding of [3H]Gly was determined using PBS rather than plasma, and was 15.8% ± 4.8% (n = 5 determinations done in duplicate).
Glyburide Depletion Kinetics in Maternal Mouse Liver Microsomes.
Microsomes were prepared from individual maternal mouse livers (n = 5 to 6 per gestation day) as previously described (Thummel et al., 1993; Paine et al., 1997). Briefly, approximately 1 g mouse liver tissue was homogenized in 3 ml homogenization buffer (50 mM KPi buffer containing 0.25 M sucrose and 1 mM EDTA) using an Omni Bead Ruptor Homogenizer (Omni International). The homogenate was centrifuged at 15,000g for 30 minutes at 4°C, and the supernatant was centrifuged at 120,000g for 70 minutes at 4°C. Microsomal pellets were carefully resuspended in washing buffer (10 mM KPi, 0.1 mM KCl, and 1 mM EDTA, pH 7.4) and centrifuged again at 120,000g for 70 minutes at 4°C. Microsomal pellets were finally resuspended in 1 ml storage buffer (50 mM KPi, 0.25 M sucrose, and 10 mM EDTA, pH 7.4), and stored at −80°C until use. Microsomal protein concentrations were determined using the Pierce BCA Protein Assay Kit (Thermo Scientific, Rockford, IL) with bovine serum albumin as the standard.
Glyburide depletion reaction mixtures contained 0.3 mg/ml microsomal protein and 0.5 µM glyburide (dissolved in <1% v/v methanol) in 200 µl 100 mM KH2PO4 buffer (1 mM EDTA, pH 7.4). The concentration of glyburide selected was below the Km values of CYP3A4 and CYP3A5 for glyburide depletion, which are both reported to be approximately 5 µM (Zhou et al., 2010a). After preincubation for 5 minutes, reactions were initiated by adding NADPH to a final concentration of 1 mM. Incubations without NADPH were used as negative controls. Reactions were stopped at 0, 3, 6, 10, 15, 20, and 30 minutes with 200 µl ice-cold methanol. After the addition of 20 µl glipizide (internal standard, 1 ng/µl), samples were briefly vortexed and centrifuged at 20,800g for 10 minutes at 4°C. The supernatant was transferred to a 96-well plate and 1 µl was injected for analysis by HPLC-MS. The glyburide concentration remaining at the various time points was analyzed graphically on a semilogarithmic plot. The first-order rate constant for glyburide depletion (kdep, min−1) was estimated by least-squares regression of the log-linear portion of the depletion curve. The intrinsic clearance (CLint) was calculated using the following equation (with incubation volume in milliliters and the amount of microsomal protein in milligrams):
Statistically significant differences of CLint between gd 0 and other gestation days were determined using the Kruskal–Wallis test followed by the Dunn’s multiple comparison test assuming a significance level of 0.05.
Quantification of Glyburide in Mouse Liver Microsomes.
Chromatographic separation of glyburide in extracts of mouse liver microsomal incubates was achieved using an Agilent Extend C18 column (50 mm × 2.1 mm, 5 µm) with gradient elution. The mobile phases consisted of methanol (B) and water containing 0.1% formic acid (A). The flow rate was set to 0.4 ml/min. The gradient was 30% methanol for the first 2 minutes, increased linearly to 75% for 3 minutes, held for 1 minute at 75% methanol, and finally decreased back to 30% for the remainder of the 9-minute run time. The mass spectrometer was run in atmospheric pressure ionization-electrospray positive ionization mode with a capillary voltage of 3500 V and a fragmentation voltage of 90 V for glyburide and glipizide. The drying gas temperature was 350°C, the nitrogen drying gas flow rate was 10 l/min, and the nebulizer pressure was 35 psi. Ions monitored were 494 m/z for glyburide and 446 m/z for glipizide.
Pharmacokinetic and Statistical Analysis of Glyburide and Metabolites in Pregnant Mice.
Maternal PK parameters were estimated for each gestation day using a pooled data bootstrap method as previously described (Mager and Göller, 1998) with some modifications. Briefly, the following steps were used to obtain PK parameter estimates. First, concentration time points from one gd group were sampled randomly with replacement 30 times using R programming software (R Statistical Computing, Vienna, Austria; R Core Team, 2014). Second, a two-compartment model was fit to the bootstrapped pseudo-concentration time profiles using the following equation and the following upper constraint: Ct = Ae−αt + Be−βt (A + B ≤ 12,500 ng⋅ml−1), where Ct is the plasma concentration at time t after dosing. The upper constraint for the sum of A and B was set to the highest possible blood concentration of glyburide in a 20-g female mouse given a 20 µg retro-orbital dose (i.e., 20 µg distributed in a blood volume of 1.6 ml or 8% of mouse total body weight). Third, A, B, α, β, and average body weight of mice in the given pseudo-profile were used to calculate area under concentration time curves from 0 to 240 minutes (AUC0–240 min), CL with and without body weight normalization (CL and CLbw), central volume of distribution with and without body weight normalization (Vc and Vc,bw), volume of distribution at steady state or β phase with and without body weight normalization (Vss and Vss,bw or Vβ and Vβ,bw), and mean body residence time. The average body weight of the mice used to generate the pseudo-profile was used to calculate body weight normalized estimates of clearance and volume of distribution. Fourth, steps 1–3 were repeated 10,000 times to create a distribution of PK parameter estimates. Fifth, based on the distribution of PK parameter estimates, 95% confidence intervals were obtained for all PK parameters. The above five steps were repeated for all gd groups. To determine whether PK parameters for pregnant mice (gd 7.5, 10, 15, and 19) were significantly different from those of nonpregnant mice (gd 0), we calculated two-sided P values using permutation tests with 10,000 replications (Westfall and Young, 1993). If the P value was less than 0.05, the difference was considered statistically significant.
The maternal metabolite area under the curve (AUC) for each gd group and fetal glyburide AUC for gd 15 and 19 were estimated using the same above-described bootstrapping method. Fetal glyburide concentrations were determined in individual fetuses and averaged by litter prior to bootstrap analysis. Due to the complexity of maternal metabolite kinetics and fetal glyburide kinetics, only noncompartmental analyses were feasible. We chose to estimate AUCs using the linear trapezoidal rule. To evaluate whether compartmental and noncompartmental approaches produce comparable results, the AUCs of glyburide in maternal plasma were estimated using noncompartmental analysis as well. This allowed calculations of the maternal metabolite to glyburide AUC ratios as well as the fetal/maternal glyburide AUC ratios. Statistical significance between maternal metabolite AUCs and metabolite to glyburide AUC ratios estimated on gd 7.5, 10, 15, or 19 versus gd 0 were determined as described in the above paragraph. Likewise, statistical comparisons of fetal glyburide AUCs or AUC ratios between gd 15 and 19 were conducted using the same method.
Differences in fetal glyburide concentrations were compared between gd 15 and 19 at each of nine time points (from 0 to 240 minutes) and were evaluated for statistical significance. Because glyburide was quantified in individual fetuses, fetal concentrations derived from the same litter were not considered statistically independent. For that reason, a generalized estimating equation approach was used to account for that dependence. Differences in fetal glyburide concentrations between gd 15 and 19 at each time point (between 0 and 240 minutes) were therefore determined using a generalized estimating equation with an independent correlation structure. The same approach was used to model differences between fetal/maternal glyburide concentration ratios on gd 15 and 19 at each time point. In both cases, the R package geepack (Yan and Fine, 2004; Halekoh et al., 2006) was used to perform the calculations, and calculated P values were adjusted for multiple testing using a Bonferroni correction.
Results
Maternal Glyburide Disposition Changed in a Gestational Age–Dependent Manner.
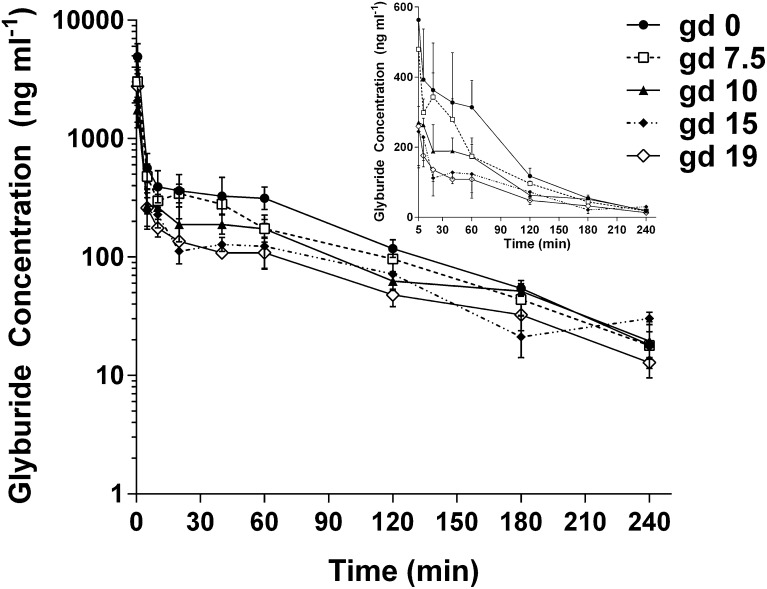
Wild-type FVB mice of various gestational ages (gd 0, 7.5, 10, 15, and 19) were administered 20 µg glyburide per mouse by retro-orbital injection. As shown in Fig. 1, within each gd group, maternal plasma glyburide concentrations decreased over time from 0 to 240 minutes in a biexponential fashion. As gestation advanced, glyburide concentrations showed a progressive decrease at nearly every time point; the largest differences between gestational ages occurred in the first 60 minutes after dosing. These changes are reflected by decreases in the coefficient of the slow exponential term (B) when the bootstrapped plasma concentration data were fit to a biexponential equation. However, distribution (α) and elimination (β) rate constants remained unchanged during pregnancy (Table 1). All of the derived two-compartmental model parameters for the maternal plasma glyburide PK are shown in Table 1. The AUC0–240 min of glyburide steadily decreased throughout gestation by as much as 50% on gd 15 and 19 compared with nonpregnant controls (20.7, 21.2, and 47.9 µg⋅min⋅ml−1 on gd 15, 19, and 0, respectively; P = 0.001). Accordingly, glyburide CL increased more than 2-fold on gd 15 and 19 compared with nonpregnant controls (0.97, 0.94, and 0.42 ml⋅min−1 on gd 15, 19, and 0, respectively; P < 0.05). CLbw estimates also demonstrated a gestational age–dependent increase (approximately 1.5-fold increase on gd 15 and 19 versus gd 0). Vc was unaffected by pregnancy; however, Vβ and Vss showed a significant 2-fold increase on gd 10 and nearly tripled on gd 15 and 19 compared with nonpregnant controls. The mean body residence time did not significantly change throughout gestation since the distribution and elimination rate constants did not vary across gestational ages. Maternal plasma protein binding (fu) of glyburide was not significantly affected by pregnancy throughout gestation.
Fig. 1.
Maternal plasma concentration time profiles of glyburide in wild-type FVB pregnant mice throughout gestation. Mice were administered 20 µg glyburide per mouse by retro-orbital injection. Data from gd 0 (●), gd 7.5 (□), gd 10 (▴), gd 15 (♦), and gd 19 (⋄) are shown as the mean ± S.D. (n = 3–5 mice per time point). The main figure shows a semilogarithmic plot and the insert in the upper right corner shows a rectilinear plot (including data from 5 to 240 minutes). Nonpregnant mice are referred to as gd 0.
TABLE 1.
Gestational age–dependent pharmacokinetics of glyburide in maternal plasma using a two-compartmental model
Data are reported as the mean (95% confidence interval) or the mean ± S.D. fu data are shown as mean ± S.D. from six maternal plasma samples for each gestation day, with duplicate determinations for each maternal plasma sample. Body weight data are the mean ± S.D. from approximately 30 mice per gestation day.
| Parameter | gd 0 |
gd 7.5 |
gd 10 |
gd 15 |
gd 19 |
||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Mean | P Value | Mean | P Value | Mean | P Value | Mean | P Value | |
| AUC0–240 min (µg⋅min⋅ml−1) | 47.9 (40.5–54.1) | 37.2 (30.6–42.1)a | 0.021a | 29.5 (23.1–35.0)a | 0.002a | 20.7 (16.1–26.3)a | 0.001a | 21.2 (16.3–25.5)a | 0.001a |
| CL (ml⋅min−1) | 0.42 (0.37–0.49) | 0.54 (0.48–0.65)a | 0.025a | 0.68 (0.57–0.87)a | 0.003a | 0.97 (0.76–1.24)a | 0.025a | 0.94 (0.79–1.23)a | 0.020a |
| CLbw (ml⋅min−1⋅g−1) | 0.018 (0.016–0.021) | 0.022 (0.020–0.027)a | 0.038a | 0.026 (0.022–0.033)a | 0.010a | 0.030 (0.024–0.039)a | 0.036a | 0.028 (0.023–0.036)a | 0.030a |
| Vc (ml) | 2.1 (1.7–5.2) | 4.0 (2.4–32.9) | 0.124 | 3.9 (1.7–56.3) | 0.205 | 5.2 (2.7–50.2) | 0.137 | 3.5 (1.8–8.7) | 0.398 |
| Vc,bw (ml⋅g−1) | 0.09 (0.07–0.22) | 0.17 (0.10–1.33) | 0.143 | 0.15 (0.07–2.24) | 0.240 | 0.161 (0.084–1.630) | 0.310 | 0.102 (0.052–0.254) | 0.837 |
| Vβ (ml) | 35.5 (29.1–48.3) | 43.7 (34.7–57.0) | 0.219 | 68.8 (51.7–103.3)a | 0.012a | 89.9 (67.2–152)a | 0.039a | 93.6 (65.0–170)a | 0.030a |
| Vβ, bw (ml⋅g−1) | 1.51 (1.24–2.06) | 1.82 (1.45–2.37) | 0.270 | 2.61 (1.95–3.93)a | 0.023a | 2.81 (2.10–4.84) | 0.089 | 2.73 (1.92–4.88) | 0.146 |
| Vss (ml) | 21.2 (18.2–27.1) | 29.2 (23.2–41.2) | 0.067 | 39.8 (28.3–61.7)a | 0.014a | 56.2 (38.4–84.6)a | 0.013a | 49.3 (33.3–71.5)a | 0.014a |
| Vss,bw (ml⋅g−1) | 0.90 (0.77–1.15) | 1.22 (0.97–1.71) | 0.090 | 1.51 (1.09–2.31)a | 0.033a | 1.75 (1.20–2.69) | 0.052 | 1.44 (0.98–2.13) | 0.135 |
| A (ng⋅ml−1) | 9020 (3432–11154) | 4602 (454–7999) | 0.150 | 4851 (250–11317) | 0.210 | 3680 (251–7316) | 0.155 | 5556 (1565–10891) | 0.280 |
| α (min−1) | 0.67 (0.38–0.83) | 0.60 (0.20–0.84) | 0.600 | 0.68 (0.19–0.98) | 0.939 | 0.84 (0.18–1.35) | 0.750 | 0.82 (0.37–1.28) | 0.657 |
| B (ng⋅ml−1) | 431 (325–543) | 382 (283–462) | 0.465 | 243 (169–299)a | 0.007a | 189 (127–238)a | 0.023a | 159 (93–216)a | 0.020a |
| β (min−1) | 0.012 (0.009–0.014) | 0.012 (0.01–0.014) | 0.712 | 0.010 (0.007–0.012) | 0.287 | 0.011 (0.006–0.015) | 0.756 | 0.010 (0.006–0.013) | 0.558 |
| MBRT (min−1) | 50.8 (44.9–59.3) | 54.3 (46.7–64.4) | 0.532 | 58.7 (48.3–72.9) | 0.288 | 58.1 (43.0–75.5) | 0.548 | 52.2 (40.8–63.7) | 0.870 |
| fu (%) | 2.19 ± 0.25 | 2.45 ± 0.67 | 0.394 | 2.27 ± 0.59 | 0.766 | 2.00 ± 0.11 | 0.260 | 2.27 ± 0.52 | 0.741 |
| Body weight (g) | 24 ± 2.2 | 24 ± 1.2 | 26 ± 1.7 | 32 ± 2.3 | 34 ± 4.7 | ||||
MBRT, mean body residence time.
PK parameters on gd 7.5, 10, 15, or 19 with statistically significant (P < 0.05) differences versus gd 0.
Maternal Metabolite Exposure Relative to Glyburide Exposure Was Unchanged during Pregnancy.
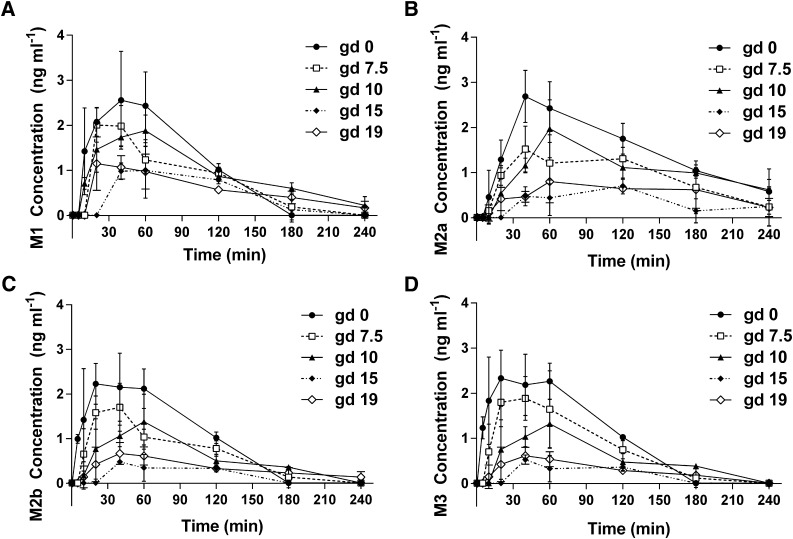
Primary metabolites of glyburide (M1, M2a, M2b, and M3) were also quantified in all maternal plasma samples collected. As shown in Fig. 2, within each gd group, maternal plasma concentrations of all four metabolites first increased to a maximum at times around 30–60 minutes and then decreased over time to 240 minutes. The concentrations of all four metabolites (0–4 ng⋅ml−1) were approximately 1000 times lower compared with those of glyburide (10–6000 ng⋅ml−1). Similar to glyburide, metabolite concentrations at most time points tended to decrease as gestation progressed; however, the differences in metabolite concentrations and AUC0–240 min estimates compared with nonpregnant controls were greatest on gd 15 and slightly reversed on gd 19 (rather than the sustained increase on gd 15 and 19 in the case of glyburide). Indeed, AUC0–240 min for M1 significantly decreased by 200% on gd 15 and 150% on gd 19 compared with gd 0 (129 and 141 ng⋅min⋅ml−1 compared with 251 ng⋅min⋅ml−1, respectively) (Table 2). AUC0–240 min for M2a, M2b, and M3 all decreased by 300% on gd 15 compared with gd 0 (Table 2). Although the metabolite to glyburide AUC ratios for M1 and M2a on gd 10 or for M2b and M3 on gd 15 were significantly increased or decreased, respectively, these ratios were <1% for all metabolites across all gestational ages. Maternal plasma AUCs of glyburide estimated using noncompartmental analysis shown in Table 2 were comparable to those calculated using a two-compartmental model shown in Table 1.
Fig. 2.
Maternal plasma concentration time profiles of metabolites M1–M3 in wild-type FVB pregnant mice throughout gestation. (A) M1. (B) M2a. (C) M2b. (D) M3. Data from gd 0 (●), gd 7.5 (□), gd 10 (▴), gd 15 (♦), and gd 19 (⋄) are shown as the mean ± S.D. (n = 3–5 mice per time point). Nonpregnant mice are referred to as gd 0.
TABLE 2.
Gestational age–dependent AUCs of glyburide and metabolites in maternal plasma using noncompartmental analyses
Data are reported as the mean (95% confidence interval). The metabolite/glyburide AUC ratios were corrected for differences in the molarity between glyburide (494 g⋅mol−1) and metabolites (510 g⋅mol−1).
| Parameter | gd 0 |
gd 7.5 |
gd 10 |
gd 15 |
gd 19 |
||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Mean | P Value | Mean | P Value | Mean | P Value | Mean | P Value | |
| GLY AUC0–240 min (ng⋅min⋅ml−1) | 55,169 (46,859–65,378) | 39,729 (32,827–46,781)a | 0.013a | 29,308 (25,112–33,771)a | <0.001a | 25,302 (19,276–31,635)a | <0.001a | 24,216 (19,689–30,361)a | <0.001a |
| M1 AUC0–240 min (ng⋅min⋅ml−1) | 251 (204–294) | 187 (156–219) | 0.084 | 232 (205–258) | 0.596 | 129 (88–172)a | 0.006a | 141 (114–163)a | 0.011a |
| M2a AUC0–240 min (ng⋅min⋅ml−1) | 359 (305–401) | 221 (166–265)a | 0.001a | 256 (206–295)a | 0.007a | 101 (61–149)a | <0.001a | 131 (97–169)a | <0.001a |
| M2b AUC0–240 min (ng⋅min⋅ml−1) | 238 (208–274) | 159 (134–188)a | 0.009a | 141 (112–177)a | 0.005a | 47 (30–63)a | <0.001a | 83 (66–99)a | <0.001a |
| M3 AUC0–240 min (ng⋅min⋅ml−1) | 251 (223–285) | 188 (148–237)a | 0.044a | 137 (107–170)a | 0.002a | 50 (32–67)a | <0.001a | 70 (57–82)a | <0.001a |
| M1/GLY AUC ratio (%) | 0.44 (0.37–0.51) | 0.46 (0.37–0.55) | 0.793 | 0.77 (0.67–0.88)a | <0.001a | 0.49 (0.36–0.64) | 0.534 | 0.57 (0.43–0.69) | 0.091 |
| M2a/GLY AUC ratio (%) | 0.63 (0.53–0.71) | 0.54 (0.40–0.67) | 0.270 | 0.85 (0.70–0.98)a | 0.028a | 0.39 (0.25–0.55)a | 0.036a | 0.53 (0.38–0.70) | 0.298 |
| M2b/GLY AUC ratio (%) | 0.42 (0.35–0.48) | 0.39 (0.32–0.47) | 0.576 | 0.47 (0.37–0.57) | 0.485 | 0.18 (0.12–0.24)a | 0.003a | 0.33 (0.25–0.42) | 0.231 |
| M3/GLY AUC ratio (%) | 0.44 (0.37–0.51) | 0.46 (0.36–0.60) | 0.767 | 0.45 (0.36–0.55) | 0.867 | 0.19 (0.13–0.25)a | 0.003a | 0.28 (0.21–0.35)a | 0.041a |
GLY, glyburide.
Parameters on gd 7.5, 10, 15, or 19 with statistically significant (P < 0.05) differences versus gd 0.
Fetal Exposure to Glyburide Doubled from Mid to Late Gestation.
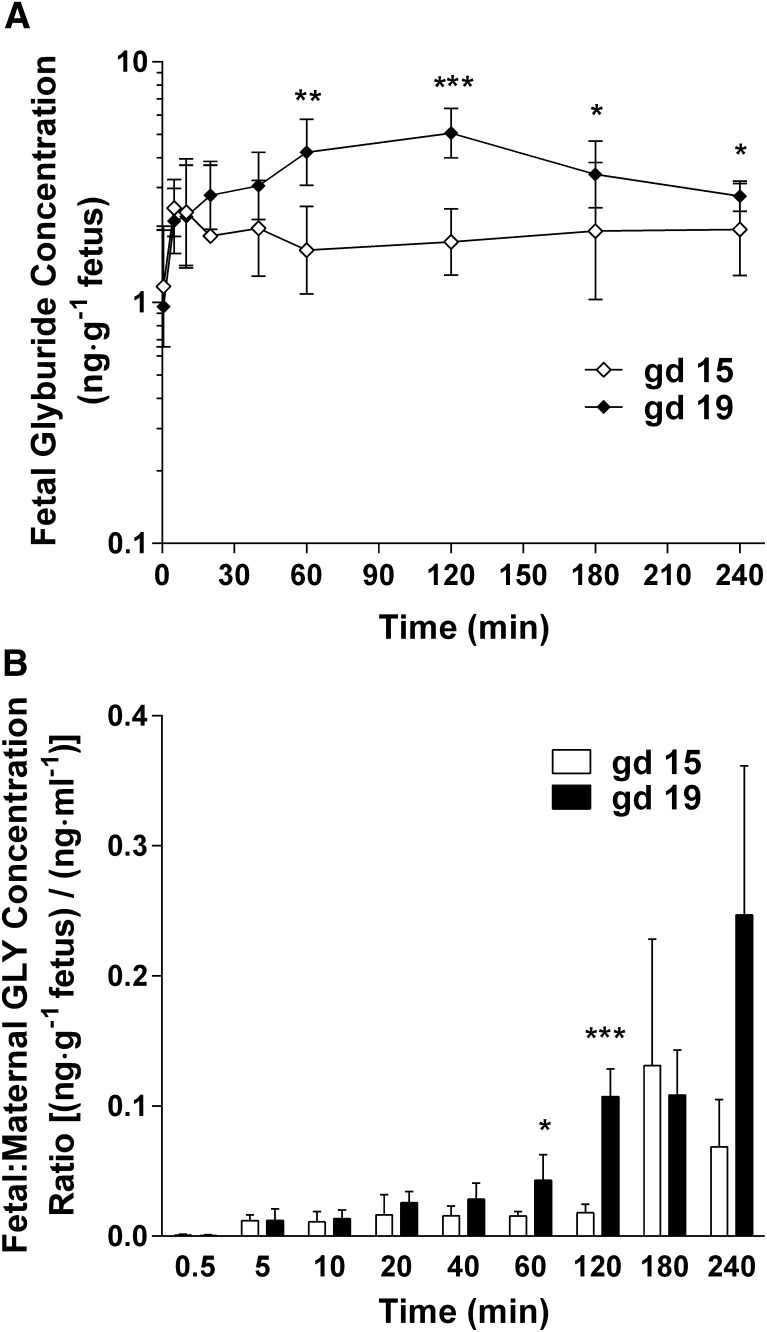
On gd 15 and 19, individual fetuses were collected simultaneously to determine fetal exposure to glyburide and metabolites. Concentrations of glyburide in fetal homogenates ranged from approximately 1 to 10 ng⋅g−1 fetus, and were lower overall on gd 15 compared with gd 19 (Fig. 3A). The fetal/maternal plasma concentration ratios of glyburide on gd 19 were also generally greater than those on gd 15 at several time points from 0.5 to 240 minutes (Fig. 3B). Consequently, the fetal AUC0–240 min of glyburide on gd 19 was significantly increased 2-fold compared with gd 15 (905 versus 462 ng⋅min⋅g−1, respectively; P < 0.001) (Table 3). The fetal/maternal plasma AUC0–240 min ratio of glyburide doubled from gd 15 to 19 as well (1.8% versus 3.7%, respectively; P = 0.007). Fetal concentrations of all four metabolites were below the detection limit for all time points on gd 15 and 19.
Fig. 3.
Fetal glyburide concentration time profiles and fetal/maternal plasma concentration ratios in mid-late gestation. Data from gd 15 (⋄) and gd 19 (♦) are shown as the mean ± S.D. (n = 20–40 fetuses per time point). (A) Semilogarithmic plot of fetal glyburide concentration over time. (B) Fetal/maternal plasma glyburide concentration ratios. Statistically significant differences in fetal concentrations and fetal/maternal plasma concentration ratios between gd 15 and 19 were determined using a generalized estimating equation approach with an independent correlation structure. P values were adjusted for multiple testing using a Bonferroni correction assuming a significance level of 0.05. *P < 0.05; **P < 0.01; ***P < 0.001. GLY, glyburide.
TABLE 3.
Gestational age–dependent maternal-fetal AUCs of glyburide using noncompartmental analyses
Data are reported as the mean (95% confidence interval).
| Parameter | gd 15 |
gd 19 |
|
|---|---|---|---|
| Mean | Mean | P Value | |
| Maternal AUC0–240 min (ng⋅min⋅ml−1) | 26,129 (20,129–32,478) | 24,216 (19,576–30,273) | 0.657 |
| Fetal AUC0–240 min (ng⋅min⋅g−1) | 462 (349–529) | 905 (726–1013)a | <0.001a |
| Fetal/maternal AUC ratio (%) | 1.8 (1.4–2.2) | 3.7 (2.8–4.6)a | 0.007a |
Statistically significant (P < 0.05) differences between gd 19 and 15.
Gestational Age–Dependent Changes in Maternal Glyburide Metabolism.
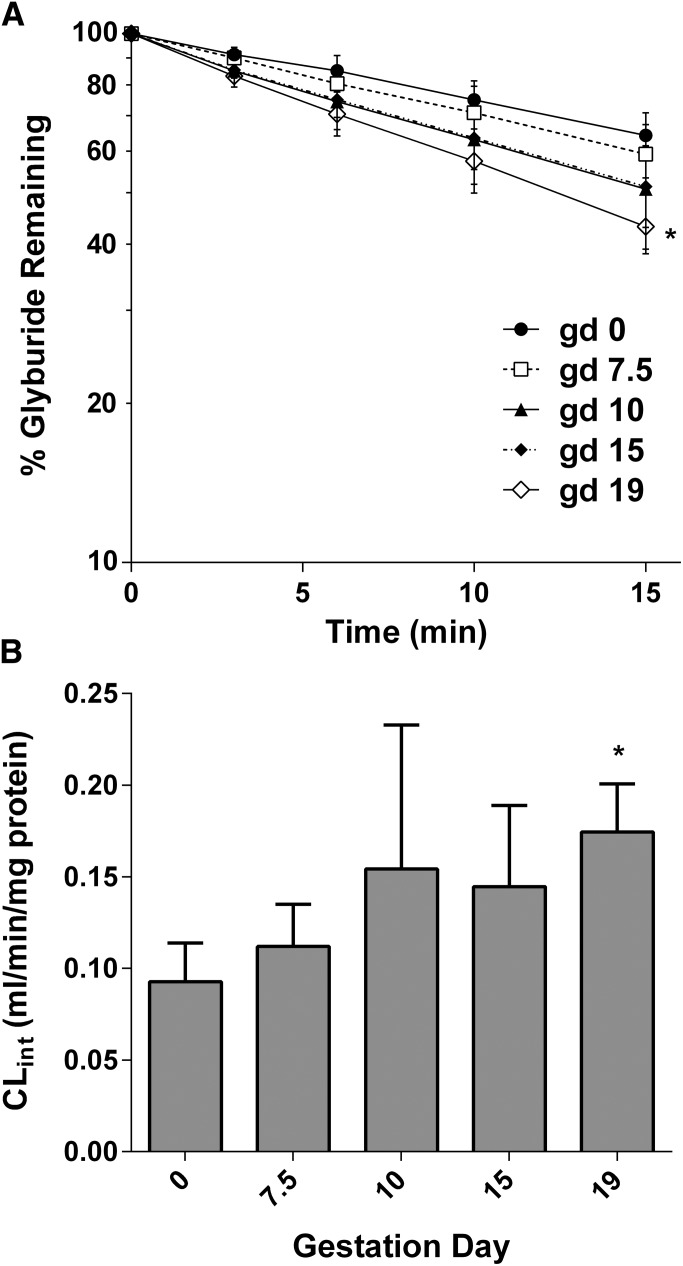
We hypothesized that increased glyburide CL in pregnant mice versus nonpregnant controls is the result of increased cytochrome P450–mediated metabolism of glyburide in the liver. To test this hypothesis, the glyburide depletion rate was measured in microsomes prepared from maternal livers collected on gd 0, 7.5, 10, 15, and 19. The mean time courses of glyburide depletion during incubation with liver microsomes for the five gestational age groups are shown in Fig. 4A. There was indeed a gestational age–dependent increase in glyburide CLint in the liver, which doubled by gd 19 versus gd 0 (0.17 versus 0.09 ml⋅min−1⋅mg protein−1, respectively) (Fig. 4B).
Fig. 4.
Gestational age–dependent depletion kinetics of glyburide in mouse liver microsomes. (A) Semilogarithmic plot of glyburide depletion over time in mouse liver microsomes. Data from gd 0 (●), gd 7.5 (□), gd 10 (▴), gd 15 (♦), and gd 19 (⋄) are shown as the mean ± S.D. (n = 4–6 livers per gestation day). (B) Gestational age–dependent changes in glyburide CLint. Data are the mean ± S.D. (n = 4–6 livers per gestation day). Statistically significant differences between gd 0 and gd 7.5, 10, 15 or 19, as indicated by an asterisk, were determined by the Kruskal–Wallis test followed by the Dunn’s multiple comparison test assuming a significance level of 0.05. Nonpregnant mice are referred to as gd 0.
Discussion
Previous clinical studies showed that the oral clearance of glyburide increased 2-fold during the third trimester in pregnant women with GDM compared with nonpregnant controls (Hebert et al., 2009). Diagnosis and treatment of GDM most often occur in the second trimester (Metzger et al., 2007). In addition, women who are at risk for developing GDM or have a previous history of GDM may be screened and treated earlier in pregnancy (Metzger et al., 2007). Therefore, it is important to understand glyburide PK changes throughout gestation, as well as how such changes affect glycemic control. In this study, we characterized maternal-fetal glyburide disposition throughout gestation in a pregnant mouse model, since it is not feasible to obtain data at this level of detail in pregnant women.
We found that the maternal plasma PK of glyburide changes in a gestational age–dependent manner, with the largest alterations occurring in mid-late gestation on gd 15 and 19 (Fig. 1; Table 1). In particular, maternal glyburide CL, Vβ, and Vss steadily increased over gestation and were approximately doubled by mid-late gestation. Glyburide has a low hepatic extraction ratio (approximately 0.1) with no significant renal CL. Therefore, the increase in maternal systemic CL could be accounted for by changes in fu in plasma and CLint of glyburide in the liver. The fu did not increase throughout gestation (Table 1), which was somewhat unexpected considering that plasma albumin is known to decrease during pregnancy (Anderson, 2005). However, the fu of glyburide also did not change in pregnant women with GDM compared with nonpregnant controls (Hebert et al., 2009). The reasons why the fu was unaffected by pregnancy are not clear. It is possible that glyburide can bind to other lipoproteins in the plasma and/or there are biochemical changes in maternal plasma that have offsetting modulation on plasma protein binding of glyburide. Since the fu of glyburide in maternal plasma did not significantly change throughout gestation, the increase in maternal glyburide CL and CLbw is most likely caused by an increase in hepatic CLint. Indeed, the glyburide depletion rate in mouse liver microsomes steadily increased as gestation progressed (Fig. 4). Using ketoconazole as a CYP3A inhibitor, we previously showed that glyburide was primarily metabolized by CYP3A in mouse liver (Zhou et al., 2010b). This is consistent with the finding that hepatic CYP3A activity is significantly induced by pregnancy in both humans and mice (Hebert et al., 2008; Zhang et al., 2008). This study further confirmed that hepatic CYP3A activity in pregnant mice is induced in a gestational age–dependent manner. It is not known which mouse CYP3A isoforms are responsible for increased glyburide metabolism during pregnancy as we and others have shown that mRNA levels of Cyp3a16, Cyp3a41, and Cyp3a44 are induced, whereas Cyp3a11, Cyp3a13, and Cyp3a25 genes are downregulated in a gestational age–dependent manner (Zhang et al., 2008; Shuster et al., 2013). Increases in Vss and Vβ most likely reflect increases in total body water and fat content during pregnancy, and further suggest that distribution of glyburide into maternal tissues is increased during pregnancy.
Although the pharmacodynamics and therapeutic window of glyburide have not been well characterized, it is possible that 1.5- to 2-fold increases in glyburide CL (and CLbw) and corresponding decreases in AUC warrant consideration when determining appropriate therapeutic management of glyburide in pregnant women. If our animal data indeed reflect gestational age–dependent changes in maternal glyburide CL and AUC in humans, dosing adjustments may be required as early as the first or second trimester. This would be particularly important for pregnant women treated with glyburide starting in the first or second trimester.
Understanding maternal disposition of primary metabolites of glyburide is also important because some metabolites (e.g., M1 and M2b) are pharmacologically active (Balant et al., 1979; Rydberg et al., 1994). We expected that increased glyburide CL across gestation would lead to either no change or increase in the formation of M1–M3, if they are all derived from pathways that are upregulated; instead, maternal plasma AUCs of each of the measured metabolites decreased progressively, reaching a nadir by gd 15 and a slight reversal by gd 19 (Fig. 2; Table 2).
The AUC of a metabolite represents the balance of its formation and elimination rates. A decrease in metabolite AUC can possibly be explained by an increase in elimination clearance of the metabolite. Accelerated metabolite elimination could be due to increased secondary oxidative metabolism, increased phase II conjugation, and/or increased renal clearance of the metabolites. In humans, M1 undergoes glucuronidation mediated by uridine 5′-diphospho-glucuronosyltransferases (UGTs) and M2b is excreted unchanged in the urine (Naraharisetti et al., 2007). UGT1A1 and UGT1A4 expression is indeed induced during human pregnancy (Feghali and Mattison, 2011); however, mRNA levels of UGTs in pregnant mice are relatively unchanged (Shuster et al., 2013). Mechanisms of renal clearance (i.e., active tubular secretion and/or reabsorption) remain unknown for all glyburide metabolites in humans and mice. Further investigation is required to elucidate the mechanisms of the putative increase in metabolite elimination.
Another possible explanation for a decrease in metabolite AUC as gestation progresses is that the increase in glyburide CL is due to an increase in normally minor metabolic pathway(s) not represented by the measured metabolites; that is, upregulation of competing pathway(s) results in a decrease in formation of the measured metabolites. M1, M2a, M2b, M3, M4, and M5 are the primary metabolites of glyburide produced by the human liver, and glyburide is metabolized primarily to M5 by CYP19 in human placenta (Zharikova et al., 2009). It therefore is possible that decreases in concentrations of M1–M3 could be due to increased formation of M4 and/or M5 in the maternal liver and placenta. However, without commercially available standards for M4 and M5, we were unable to quantify gestational age–dependent changes in maternal concentrations of M4 and M5.
Table 2 also presents the metabolite/glyburide AUC ratio, which is governed by the ratio of a given metabolite’s formation clearance to its elimination clearance. It is a quantitative index reflecting either the joint or opposing effects of simultaneous changes in formation and elimination clearances of a primary metabolite. Mean AUC ratios for M1 and M2a were elevated on gd 10 and declined to near the gd 0 values by gd 15 and 19. This suggests that the increase in formation clearance of these two metabolites outpaced the increase in their elimination clearances by gd 10, Moreover, the changes in formation and elimination clearances became comparable as gestation progressed beyond gd 10. For M2b and M3, their AUC ratios declined across all the gestation days studied, suggesting that there was a greater increase in elimination clearance compared with formation clearance.
Fetal exposure to glyburide was <5% of maternal exposure, but was doubled on gd 19 versus gd 15 (Fig. 3; Table 3). This change is consistent with the finding that protein expression of BCRP in mouse placenta on gd 15 is 2 to 3 times greater than that on gd 19 (Wang et al., 2006). Glyburide is a substrate of mouse and human BCRP, which limit the transport of glyburide across the placenta barrier (Gedeon et al., 2006; Zhou et al., 2008; Hemauer et al., 2010). As BCRP protein expression decreases from gd 15 to gd 19, glyburide penetration across the placenta to the fetus increases. P-gp could also contribute to the gestational age–dependent changes in fetal exposure to glyburide because both human and mouse P-gp expression in the placenta decreases as gestation progresses (Mathias et al., 2005; Aleksunes et al., 2008; Zhang et al., 2008). M1–M3 were not detectable in fetal homogenates, suggesting that fetal exposure to these primary metabolites is negligible due to their low concentrations in maternal circulations. Since glyburide is highly bound to plasma proteins with a low volume of distribution, the actual fetal plasma concentrations may be higher than what we observed in fetal homogenates. Indeed, one clinical study showed that the mean ratio of the umbilical cord glyburide concentration at delivery to maternal plasma glyburide concentration was approximately 0.7 (Hebert et al., 2009), indicating that a substantial amount of glyburide can cross the placenta to the fetus. Therefore, although total fetal exposure in human pregnancy is not known, there could be times after drug administration that fetal concentrations are nearly as high as maternal plasma concentrations. Although the current dosage of glyburide is safe for use during pregnancy, dosage increases based on gestational age could raise concerns for fetal safety. In addition, since BCRP expression in human placenta decreases from approximately 28 weeks of gestation toward term (Meyer zu Schwabedissen et al., 2006), increased fetal exposure to glyburide in late pregnancy would be expected and may pose a safety concern that conflicts with the consideration to increase glyburide doses during pregnancy for improved maternal efficacy.
In summary, we have demonstrated gestational age–dependent maternal-fetal glyburide PK in pregnant mice. Results of this study suggest the possible need for increased glyburide dosages even in early pregnancy should the same PK changes occur in humans, and that the pregnant mouse is an appropriate animal model to study glyburide disposition during pregnancy.
Supplementary Material
Acknowledgments
The authors thank Dr. Laura Shireman for advice regarding the implementation of the generalized estimating equation approach and overall contribution to the statistical rigor of this work.
Abbreviations
- [3H]Gly
[cyclohexyl-2,3-3H(N)]-glyburide
- AUC
area under concentration time curve
- AUC0–240 min
area under concentration time curves from 0 to 240 minutes
- BCRP
breast cancer resistance protein
- CL
clearance
- CLbw
clearance with body weight normalization
- CLint
intrinsic clearance
- fu
fraction unbound
- gd
gestation day
- GDM
gestational diabetes mellitus
- HPLC-MS
high-performance liquid chromatography–mass spectrometry
- kdep
first-order rate constant for glyburide depletion
- M1
4-trans-hydroxycyclohexyl glyburide
- M2a
4-cis-hydroxycyclohexyl glyburide
- M2b
3-cis-hydroxycyclohexyl glyburide
- M3
3-trans-hydroxycyclohexyl glyburide
- M4
2-trans-hydroxycyclohexyl glyburide
- M5
ethylene-hydroxylated glyburide
- MRP
multidrug-resistance protein
- PBS
phosphate-buffered saline
- P-gp
P-glycoprotein
- PK
pharmacokinetics
- UGT
uridine 5′-diphospho-glucuronosyltransferase
- Vβ
volume of distribution at β phase
- Vβ,bw
volume of distribution at β phase with body weight normalization
- Vc
central volume of distribution
- Vc,bw
central volume of distribution with body weight normalization
- Vss
volume of distribution at steady state
- Vss,bw
volume of distribution at steady state with body weight normalization
Authorship Contributions
Participated in research design: Shuster, Shen, Hebert, Thummel, Mao.
Conducted experiments: Shuster, Risler.
Contributed new reagents or analytic tools: Risler, Liang.
Performed data analysis: Shuster, Risler, Liang, Rice.
Wrote or contributed to the writing of the manuscript: Shuster, Liang, Rice, Shen, Hebert, Thummel, Mao.
Footnotes
This work was supported in part by the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development [Grant U10-HD047892]; the National Institutes of Health National Center for Advancing Translational Sciences [Grant TL1-RR025016]; and the National Institutes of Health National Institute of General Medical Sciences [Grant T32-GM007750]. D.L.S. is the recipient of the American Foundation for Pharmaceutical Education Predoctoral Fellowship in Pharmaceutical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development, or the National Institutes of Health National Center for Advancing Translational Sciences.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Aleksunes LM, Cui Y, Klaassen CD. (2008) Prominent expression of xenobiotic efflux transporters in mouse extraembryonic fetal membranes compared with placenta. Drug Metab Dispos 36:1960–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics (2001) Clinical management guidelines for obstetrician-gynecologists. Number 30, September 2001 (replaces Technical Bulletin Number 200, December 1994). Gestational diabetes. Obstet Gynecol 98:525–538 [PubMed] [Google Scholar]
- Anderson GD. (2005) Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet 44:989–1008 [DOI] [PubMed] [Google Scholar]
- Balant L, Fabre J, Loutan L, Samimi H. (1979) Does 4-trans-hydroxy-glibenclamide show hypoglycemic activity? Arzneimittelforschung 29:162–163 [PubMed] [Google Scholar]
- Feghali MN, Mattison DR. (2011) Clinical therapeutics in pregnancy. J Biomed Biotechnol 2011:783528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedeon C, Anger G, Lubetsky A, Miller MP, Koren G. (2008a) Investigating the potential role of multi-drug resistance protein (MRP) transporters in fetal to maternal glyburide efflux in the human placenta. J Obstet Gynaecol 28:485–489 [DOI] [PubMed] [Google Scholar]
- Gedeon C, Anger G, Piquette-Miller M, Koren G. (2008b) Breast cancer resistance protein: mediating the trans-placental transfer of glyburide across the human placenta. Placenta 29:39–43 [DOI] [PubMed] [Google Scholar]
- Gedeon C, Behravan J, Koren G, Piquette-Miller M. (2006) Transport of glyburide by placental ABC transporters: implications in fetal drug exposure. Placenta 27:1096–1102 [DOI] [PubMed] [Google Scholar]
- Halekoh U, Hojsgaard S, Yan J. (2006) The R Package geepack for generalized estimating equations. J Stat Softw 15:1–11 [Google Scholar]
- HAPO Study Cooperative Research Group (2002) The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study. Int J Gynaecol Obstet 78:69–77 [DOI] [PubMed] [Google Scholar]
- Hebert MF, Easterling TR, Kirby B, Carr DB, Buchanan ML, Rutherford T, Thummel KE, Fishbein DP, Unadkat JD. (2008) Effects of pregnancy on CYP3A and P-glycoprotein activities as measured by disposition of midazolam and digoxin: a University of Washington specialized center of research study. Clin Pharmacol Ther 84:248–253 [DOI] [PubMed] [Google Scholar]
- Hebert MF, Ma X, Naraharisetti SB, Krudys KM, Umans JG, Hankins GD, Caritis SN, Miodovnik M, Mattison DR, Unadkat JD, et al. Obstetric-Fetal Pharmacology Research Unit Network (2009) Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther 85:607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemauer SJ, Patrikeeva SL, Nanovskaya TN, Hankins GD, Ahmed MS. (2010) Role of human placental apical membrane transporters in the efflux of glyburide, rosiglitazone, and metformin. Am J Obstet Gynecol 202:383.e1–e383.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic L, Pettitt DJ. (2001) Gestational diabetes mellitus. JAMA 286:2516–2518 [DOI] [PubMed] [Google Scholar]
- Klieger C, Pollex E, Kazmin A, Koren G. (2009) Hypoglycemics: pharmacokinetic considerations during pregnancy. Ther Drug Monit 31:533–541 [DOI] [PubMed] [Google Scholar]
- Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O. (2000) A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med 343:1134–1138 [DOI] [PubMed] [Google Scholar]
- Mager H, Göller G. (1998) Resampling methods in sparse sampling situations in preclinical pharmacokinetic studies. J Pharm Sci 87:372–378 [DOI] [PubMed] [Google Scholar]
- Mathias AA, Hitti J, Unadkat JD. (2005) P-glycoprotein and breast cancer resistance protein expression in human placentae of various gestational ages. Am J Physiol Regul Integr Comp Physiol 289:R963–R969 [DOI] [PubMed] [Google Scholar]
- Metzger BE, Buchanan TA, Coustan DR, de Leiva A, Dunger DB, Hadden DR, Hod M, Kitzmiller JL, Kjos SL, Oats JN, et al. (2007) Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 30 (Suppl 2):S251–S260 [DOI] [PubMed] [Google Scholar]
- Meyer zu Schwabedissen HE, Grube M, Dreisbach A, Jedlitschky G, Meissner K, Linnemann K, Fusch C, Ritter CA, Völker U, Kroemer HK. (2006) Epidermal growth factor-mediated activation of the map kinase cascade results in altered expression and function of ABCG2 (BCRP). Drug Metab Dispos 34:524–533 [DOI] [PubMed] [Google Scholar]
- Nanovskaya TN, Nekhayeva I, Hankins GD, Ahmed MS. (2006) Effect of human serum albumin on transplacental transfer of glyburide. Biochem Pharmacol 72:632–639 [DOI] [PubMed] [Google Scholar]
- Naraharisetti SB, Kirby BJ, Hebert MF, Easterling TR, Unadkat JD. (2007) Validation of a sensitive LC-MS assay for quantification of glyburide and its metabolite 4-transhydroxy glyburide in plasma and urine: an OPRU Network study. J Chromatogr B Analyt Technol Biomed Life Sci 860:34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paglia MJ, Coustan DR. (2011) Gestational diabetes: evolving diagnostic criteria. Curr Opin Obstet Gynecol 23:72–75 [DOI] [PubMed] [Google Scholar]
- Paine MF, Khalighi M, Fisher JM, Shen DD, Kunze KL, Marsh CL, Perkins JD, Thummel KE. (1997) Characterization of interintestinal and intraintestinal variations in human CYP3A-dependent metabolism. J Pharmacol Exp Ther 283:1552–1562 [PubMed] [Google Scholar]
- R Core Team (2014) R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- Ravindran S, Zharikova OL, Hill RA, Nanovskaya TN, Hankins GD, Ahmed MS. (2006) Identification of glyburide metabolites formed by hepatic and placental microsomes of humans and baboons. Biochem Pharmacol 72:1730–1737 [DOI] [PubMed] [Google Scholar]
- Rydberg T, Jönsson A, Røder M, Melander A. (1994) Hypoglycemic activity of glyburide (glibenclamide) metabolites in humans. Diabetes Care 17:1026–1030 [DOI] [PubMed] [Google Scholar]
- Shuster DL, Bammler TK, Beyer RP, Macdonald JW, Tsai JM, Farin FM, Hebert MF, Thummel KE, Mao Q. (2013) Gestational age-dependent changes in gene expression of metabolic enzymes and transporters in pregnant mice. Drug Metab Dispos 41:332–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel KE, Kharasch ED, Podoll T, Kunze K. (1993) Human liver microsomal enflurane defluorination catalyzed by cytochrome P-450 2E1. Drug Metab Dispos 21:350–357 [PubMed] [Google Scholar]
- Wang H, Wu X, Hudkins K, Mikheev A, Zhang H, Gupta A, Unadkat JD, Mao Q. (2006) Expression of the breast cancer resistance protein (Bcrp1/Abcg2) in tissues from pregnant mice: effects of pregnancy and correlations with nuclear receptors. Am J Physiol Endocrinol Metab 291:E1295–E1304 [DOI] [PubMed] [Google Scholar]
- Westfall PH, Young SS. (1993) Resampling-Based Multiple Testing: Examples and Methods for P-Value Adjustment, Wiley, New York [Google Scholar]
- Yan J, Fine J. (2004) Estimating equations for association structures. Stat Med 23:859–874, discussion 875–877, 879–880 [DOI] [PubMed] [Google Scholar]
- Zhang H, Wu X, Wang H, Mikheev AM, Mao Q, Unadkat JD. (2008) Effect of pregnancy on cytochrome P450 3a and P-glycoprotein expression and activity in the mouse: mechanisms, tissue specificity, and time course. Mol Pharmacol 74:714–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zharikova OL, Fokina VM, Nanovskaya TN, Hill RA, Mattison DR, Hankins GD, Ahmed MS. (2009) Identification of the major human hepatic and placental enzymes responsible for the biotransformation of glyburide. Biochem Pharmacol 78:1483–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Naraharisetti SB, Liu L, Wang H, Lin YS, Isoherranen N, Unadkat JD, Hebert MF, Mao Q. (2010a) Contributions of human cytochrome P450 enzymes to glyburide metabolism. Biopharm Drug Dispos 31:228–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Naraharisetti SB, Wang H, Unadkat JD, Hebert MF, Mao Q. (2008) The breast cancer resistance protein (Bcrp1/Abcg2) limits fetal distribution of glyburide in the pregnant mouse: an Obstetric-Fetal Pharmacology Research Unit Network and University of Washington Specialized Center of Research Study. Mol Pharmacol 73:949–959 [DOI] [PubMed] [Google Scholar]
- Zhou L, Zhang Y, Hebert MF, Unadkat JD, Mao Q. (2010b) Increased glyburide clearance in the pregnant mouse model. Drug Metab Dispos 38:1403–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.