Abstract
Electrical coupling of photoreceptors through gap junctions suppresses voltage noise, routes rod signals into cone pathways, expands the dynamic range of rod photoreceptors in high scotopic and mesopic illumination, and improves detection of contrast and small stimuli. In essentially all vertebrates, connexin 35/36 (gene homologues Cx36 in mammals, Cx35 in other vertebrates) is the major gap junction protein observed in photoreceptors, mediating rod-cone, cone-cone, and possibly rod-rod communication. Photoreceptor coupling is dynamically controlled by the day/night cycle and light/dark adaptation, and is directly correlated with phosphorylation of Cx35/36 at two sites, serine110 and serine 276/293 (homologous sites in teleost fish and mammals respectively). Activity of protein kinase A (PKA) plays a key role during this process. Previous studies have shown that activation of dopamine D4 receptors on photoreceptors inhibits adenylyl cyclase, down-regulates cAMP and PKA activity, and leads to photoreceptor uncoupling, imposing the daytime/light condition. In this study we explored the role of adenosine, a nighttime signal with a high extracellular concentration at night and a low concentration in the day, in regulating photoreceptor coupling by examining photoreceptor Cx35 phosphorylation in zebrafish retina. Adenosine enhanced photoreceptor Cx35 phosphorylation in daytime, but with a complex dose-response curve. Selective pharmacological manipulations revealed that adenosine A2a receptors provide a potent positive drive to phosphorylate photoreceptor Cx35 under the influence of endogenous adenosine at night. A2a receptors can be activated in the daytime as well by micromolar exogenous adenosine. However, the higher affinity adenosine A1 receptors are also present and have an antagonistic though less potent effect. Thus the nighttime/darkness signal adenosine provides a net positive drive on Cx35 phosphorylation at night, working in opposition to dopamine to regulate photoreceptor coupling via a push-pull mechanism. However, the lower concentration of adenosine present in the daytime actually reinforces the dopamine signal through action on the A1 receptor.
Keywords: adenosine, A2a receptor, A1 receptor, Cx36, photoreceptor
Introduction
The sensor array that performs the first step of visual information processing, the photoreceptors, accomplishes the herculean task of detecting light over roughly 10 orders of magnitude of intensity and coding this into information that can be used by second and higher order neurons in the visual system. Many mechanisms contribute to the ability to perform this task. At the low end of this range, individual rods can reliably detect single photons. However, the high gain required for this capability introduces noise, only some of which is controlled by mechanisms intrinsic to the individual photoreceptor. An important mechanism to control noise in photoreceptors is electrical coupling to neighbors via gap junctions. Such coupling improves signal:noise by dampening non-correlated voltage noise (Lamb & Simon, 1976), which in turn facilitates detection of contrast boundaries (Lebedev et al., 1998). Rod-cone coupling extends rod dynamic range in mesopic vision to stimuli with light intensity beyond that which saturates the rod synapses by detouring the rod signal into the cone pathway (Nelson, 1977; Attwell et al., 1987; Hornstein et al., 2005; Trumpler et al., 2008). Conversely, uncoupling of rod-cone gap junctions in bright light suppresses entry of the rod signal generated through rod saturation into the cone pathway (Wang & Mangel, 1996).
The plasticity of photoreceptor gap junctions follows the day/night cycle as well as light/dark adaptation (Ribelayga et al., 2002; Ribelayga et al., 2008). Li et al. (Li et al., 2009; Li & O’Brien, 2012; Li et al., 2013) have established a strong correlation between photoreceptor coupling and gap junction phosphorylation, mediated through protein kinase A (PKA). In daytime when PKA activity is low, gap junction Cx35/36 is dephosphorylated and photoreceptors are weakly coupled, while at night when PKA is activated, Cx35/36 is strongly phosphorylated and photoreceptor coupling becomes robust. The neuromodulator dopamine, a light signal (Witkovsky, 2004), is released in daytime and under light exposure. Acting through its D4/D2-like receptors in photoreceptors, dopamine suppresses adenylyl cyclase activity, reduces cAMP level and PKA activity, and uncouples photoreceptors. It is not clear whether the nighttime low level of dopamine alone is sufficient to enhance photoreceptor coupling, or whether a co-regulator of adenylyl cyclase and PKA activity is involved. Adenosine is a likely candidate to co-regulate adenylyl cyclase as it is a neuromodulator that is produced in the retina at highest levels in the nighttime and in darkness (Ribelayga & Mangel, 2005), several adenosine receptor types have been found in the retina (Blazynski, 1990; Kvanta et al., 1997; Zhang et al., 2006), and adenosine modulates both rod and cone calcium currents (Stella et al., 2002; Stella et al., 2003; Stella et al., 2007). Furthermore, A2a receptors have been shown recently to play an important role in regulating photoreceptor coupling in the mouse retina (Li et al., 2013). The current study investigated the role of adenosine in controlling photoreceptor gap junction phosphorylation in zebrafish retina. The results suggest that adenosine plays a complex role in regulating photoreceptor coupling, opposing dopamine to enhance coupling when adenosine levels are high at night, but reinforcing dopamine to suppress coupling when levels are low during the day.
Material and Methods
Animals
Wild type AB zebrafish were purchased from ZFIN (Eugene, OR), raised, bred, and maintained on a 14 hr light/ 10 hr dark cycle according to protocols approved by the institutional animal care and use committee at the University of Texas Health Science Center at Houston. Randomly selected adult fish of both sexes were transferred to a 12 hr light/ 12 hr dark cycle for more than 2 weeks before experiments. Nighttime studies were completed at least 2.5 hr prior to light onset in the darkness while daytime studies were initiated at least 2.5 hr after light onset under photopic light. Nighttime experiments were conducted with assistance of infrared night vision systems (B.E. Meyers Electro Optics, Redmond WA; Night Optics USA Inc., Huntington Beach, CA).
Eyecup preparation
Fish were anesthetized with 0.15% tricaine (Sigma-Aldrich, St. Louis, MO) and sacrificed by transection of the spinal cord adjacent to the head. The eyes were enucleated, the lens removed, and the remaining eyecups incubated in Ames’ medium with L-glutamine (USBiological, Swampscott, MA) at room temperature for 30 min. A series of drugs was applied in Ames’ medium: adenosine (Sigma-Aldrich); CGS-21680 (4-[2-[[6-Amino-9-(N-ethyl-β-D-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl]benzene propanoic acid hydrochloride; Tocris Bioscience, Minneapolis, MN); 5′-(N-Ethylcarboxamido) adenosine (NECA; Sigma-Aldrich); 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX; Tocris), 8-(3-Chlorostyryl)caffeine (CSC; Tocris), SCH 442416 (2-(2-Furanyl)-7-[3-(4-methoxyphenyl)propyl]-7H-pyrazolo [4,3-e][1,2,4] triazolo [1,5-c] pyrimidin-5-amine; Tocris), MRS 1706 (N-(4-Acetylphenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl)phenoxy]acetamide; Tocris), and 3,7-dimethyl-1-propargylxanthine (DMPX; Sigma-Aldrich). Drugs were dissolved in water (adenosine only) or dimethyl sulfoxide (DMSO) and diluted in Ames’ medium. The final concentration of DMSO in drug-treated and the corresponding control groups was no more than 0.1%. Following drug treatments, eyecups were fixed with 1.5% N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (Sigma-Aldrich) for 30 min at room temperature, then cryoprotected in 30% sucrose at 4°C overnight, and cryostat sectioned into 16μm thick slices.
Immunohistochemistry and imaging
Phosphorylation was estimated using a phospho-specific antibody against serine 276 (S276) of Cx35 (Kothmann et al., 2007). Retina tissue was co-labeled with a pan-specific antibody for all Cx35 as a reference. As described previously (Kothmann et al., 2007; Li et al., 2009), sections were soaked in phosphate buffered saline with 0.1% Triton X-100 (PBST) for 15 min, and then blocked with 10% donkey serum with PBST for 1 hr. Retina sections were incubated overnight at 4°C with a primary antibody mix containing 2.5 μg/ml monoclonal mouse anti-Cx35 (mCx35; EMD Millipore, Billerica, MA) and 1 μg/ml rabbit anti-phospho-S276 (P-S276). Sections were washed with PBS and incubated with Cy3-conjugated donkey anti-mouse IgG (0.9 μg/ml) and Alexa 488-conjugated donkey anti-rabbit IgG (1.25 μg/ml), for 2 hr at room temperature. Images were taken using a Zeiss LSM 510 Meta confocal microscope (Thornwood, NY) with identical settings of pinhole, laser power, contrast, and brightness parameters. Each confocal scan was in a 24 × 13 μm rectangle in the outer plexiform layer (OPL) at the region of the middle 1/3 between optic nerve and the retinal periphery. Three such scans at 0.3 μm intervals were stacked as one image. Five images represented one animal and 2–7 animals were used for each experimental condition.
Data analysis
12-bit images were analyzed using SimplePCI (Hamamatsu Photonics, Bridgewater, NJ). As described previously (Kothmann et al., 2009; Li et al., 2009; Li et al., 2013), the regions of interest (ROI) were automatically selected by applying a uniform threshold in the channel of mCx35 labeling that included most visible Cx35 plaques. The intensity of P-S276 and mCx35 was measured in each ROI, and the phosphorylation level was estimated by calculating the ratio of P-S276/mCx35. As found previously (Li et al., 2009; Li et al., 2013), phosphorylation levels of individual gap junctions were not normally distributed, but showed a skewed distribution with predominant weight close to zero (data not shown). In order to compare conditions, the median of P-S276/mCx35 from all ROI of 5 images was used to determine the phosphorylation level of OPL Cx35 in each animal.
Dose-response curves were generated using GraphPad Prism software (La Jolla, CA). Statistical analyses were performed in GraphPad Prism or SAS (Cary, NC). All comparisons were made using one-way or two-way analysis of variance (ANOVA). Bonferroni post-hoc multiple comparison tests were used to investigate differences between treatment groups. When appropriate, a log transformation was performed on the phosphorylation level ratio to meet the ANOVA assumption that the dependent variable is independently normally distributed with group mean and a constant variance. Orthogonal polynomial contrast was used to investigate multiphasic phenomena within the dose-response curves. This technique decomposes the dose-response effect into a linear combination of polynomial trends (contrasts). Each contrast is orthogonal to the others, in that it is independent of the others and therefore has 1 degree of freedom. To test the significance of each polynomial, we used an F-test that tests the mean square of the contrast divided by the mean square error from the ANOVA.
Results
Adenosine enhances photoreceptor gap junction phosphorylation in daytime, light-adapted retina
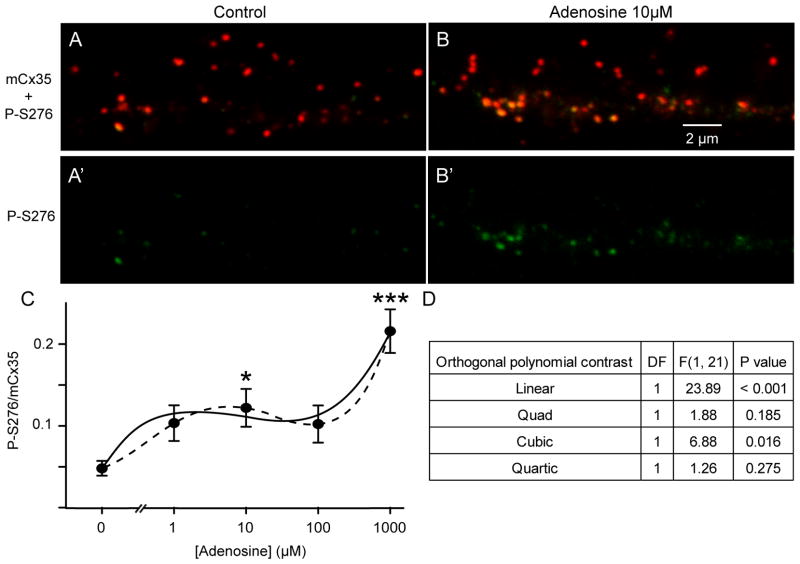
In the zebrafish retina, Cx35 gap junctions in the OPL are associated with cone-cone and rod-cone contacts, as well as some unidentified bipolar cells (Li et al., 2009). The phosphorylation state of OPL gap junctions is well correlated to tracer coupling through networks of coupled photoreceptors including both cones and rods (Li et al., 2009; Li & O’Brien, 2012; Li et al., 2013), and can be used as a proxy to qualitatively assess the degree of photoreceptor coupling. To investigate the role of adenosine signaling in the control of photoreceptor coupling, we examined the phosphorylation state of Cx35 in zebrafish OPL in light-adapted isolated eyecups from daytime, light-adapted fish. As previously reported (Li et al., 2009), Cx35 was poorly phosphorylated in the light-adapted preparation (figure 1A, A′), suggesting that photoreceptor coupling was low. Incubation of light-adapted eyecups for 20–25 min with 10 μM adenosine increased the phosphorylation level of Cx35 (figure 1B, B′), indicating that photoreceptor coupling was enhanced. A dose-response curve for adenosine (figure 1C) revealed a multiphasic relationship, suggesting that several different mechanisms responsive to adenosine influence Cx35 phosphorylation. Exogenously added adenosine significantly elevated Cx35 phosphorylation at 10 and 1000 μM, but not at 100 μM, compared to 0 added adenosine (one-way ANOVA with Bonferonni post-hoc multiple comparison). To determine whether the data were consistent with a multiphasic fit, we performed a one-way ANOVA followed by orthogonal polynomial contrasts. Since there were 5 levels of adenosine concentration, the most parsimonious model was identified among 4 orders of polynomial trends, 1st (linear), 2nd (quadratic), 3nd (cubic), and 4th (quartic) (figure 1D). The cubic polynomial fit (solid line in figure 1C) provided a significant improvement of fit over the quadratic fit (p=0.016), while the quartic polynomial (dashed line in figure 1C) did not significantly improve the fit over the cubic polynomial (p=0.275) (figure 1D). Thus, the best model is a triphasic dose response relationship. This relationship suggests that several different mechanisms responsive to adenosine influence Cx35 phosphorylation.
Figure 1.
Effects of adenosine on the phosphorylation level of OPL gap junctions in daytime, light-adapted retina. A) Confocal micrograph of immunostaining for total Cx35 (red) and phospho-S276 Cx35 (green) in the OPL of light-adapted daytime zebrafish retina in an eyecup preparation. Phosphorylated Cx35 (shown alone in A′) is very low. Scale bar in B applies to all images in A and B. B) 25 minute incubation with 10 μM adenosine increased Cx35 phosphorylation (phospho-S276 Cx35 shown alone in B′). C) Dose-response relationship of Cx35 phosphorylation level with adenosine concentration. Curves fit to the data are a third order polynomial model (solid line) and a fourth order polynomial model (dashed line). Data are means ± SE of median values for 4–7 animals. * P<0.05; ** P<0.01; *** P<0.001 compared to 0 added adenosine. D) Results table of orthogonal polynomial contrast following one-way ANOVA to assess the best fit of multiphasic models to the adenosine dose-response data. The orthogonal polynomial contrast tested the 4 possible orders of polynomial trends including linear, quadratic, cubic, and quartic terms; each contrast has 1 degree of freedom (DF) and they are orthogonal. The F value is obtained by taking the mean square for the contrast (DF=1) divided by the mean square for error (DF=21). The cubic polynomial fit (solid line in Fig1C) provided a significant improvement in fit over a quadratic model, while no significant improvement resulted from the quartic fit (dashed line in Fig 1C).
A2a receptors contribute to enhancement of Cx35 phosphorylation
Adenosine activates a family of 4 known adenosine receptor subtypes with differing G-protein coupling. A1 and A3 receptors couple through Gi/o, while A2a and A2b receptors couple through Gs, and A2b can couple as well through Gq (Fredholm et al., 2000). As photoreceptor Cx35 is phosphorylated in a PKA-dependent manner (Li et al., 2009), the enhancement of phosphorylation by adenosine is consistent with a Gs effect on adenylyl cyclase, as would be provided by A2a or A2b receptors. To examine the adenosine receptor subtype contribution to the adenosine dose-response curve, we first examined the effects of the highly A2a selective agonist CGS 21680 on OPL gap junction phosphorylation in the light-adapted retina. Figure 2A shows that CGS significantly increased Cx35 phosphorylation level at 10 μM and above, orders of magnitude higher than the nanomolar Kd’s reported for human A2a receptors but lower than the high micromolar Kd’s reported for A2b receptors (Klotz, 2000). This is reminiscent of the very high EC50 for elongation of sunfish cone inner/outer segments (Rey & Burnside, 1999) and suggests that CGS is a poor agonist for zebrafish adenosine receptors or that multiple receptor subtypes with opposing actions contribute to the response.
Figure 2.
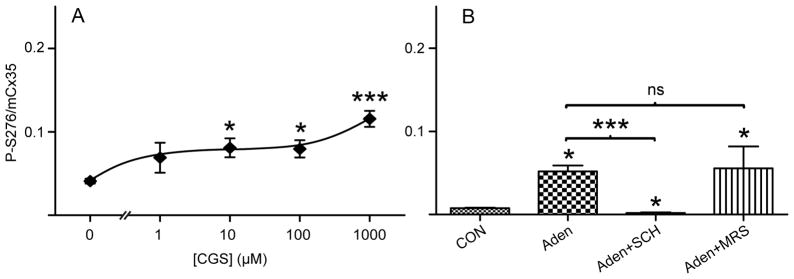
Contributions of A2 receptors to regulation of Cx35 phosphorylation. A) Dose-response relationship of Cx35 phosphorylation level for incubation of adenosine A2a receptor agonist CGS 21680. Data are means ± SE of median values for 2–7 animals. B) Comparison of Cx35 phosphorylation level for incubation of non-drug control, 10 μM adenosine, 10 μM adenosine plus 100 nM SCH 442416 (A2a receptor antagonist), and 10 μM adenosine plus 5 nM MRS 1706 (A2b receptor antagonist). Data are means ± SE of median values for 3–5 animals.
We next examined the ability of more selective A2 receptor antagonists to block the stimulatory effect of exogenous adenosine. 10 μM adenosine significantly increased Cx35 phosphorylation in the daytime, light-adapted eyecup (figure 2B). This effect was completely blocked by co-application of the A2a selective antagonist SCH 442416 (100 nM; figure 2B). The selective A2b antagonist MRS 1706 (5 nM; figure 2B) did not block the enhancement of Cx35 phosphorylation by 10 μM adenosine, suggesting that the A2a receptor provided the major influence at this concentration of adenosine.
A1 receptors are active in daytime and suppress Cx35 phosphorylation
Since the effective concentrations of adenosine and CGS can activate several types of adenosine receptors, we attempted to discriminate among them. We employed the partially selective adenosine receptor agonist NECA, which stimulates A1, A2a and A3 receptors with reported affinities in the low tens of nanomolar, but stimulates A2b receptors with low micromolar affinity (Klotz, 2000). There was little effect on OPL Cx35 phosphorylation at concentrations up to 100 nM (figure 3A, solid curve), but enhancement of phosphorylation at 1 μM and reduction again at 10 μM. The enhancement of phosphorylation at 1 μM is suggestive of the activity of an A2b receptor, but the lack of effect at nanomolar concentrations is not consistent with the A2a enhancement of phosphorylation seen in the previous experiments.
Figure 3.
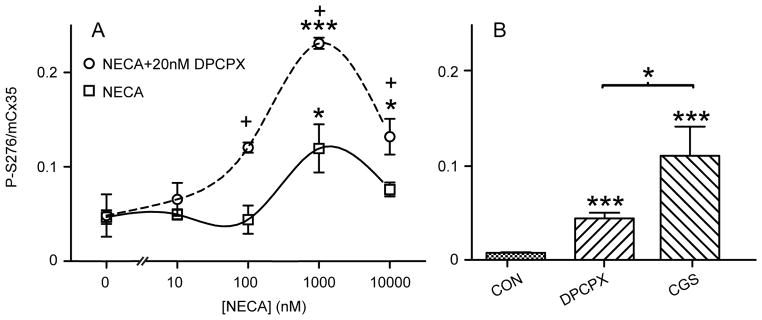
Contribution of A1 receptor to regulation of Cx35 phosphorylation. A) Dose-response relationship of Cx35 phosphorylation level for incubation of adenosine receptor agonist NECA alone (solid curve) and in the presence of 20 nM A1 receptor antagonist DPCPX (dashed curve). Asterisks mark significance compared to no drug while plus signs mark significance comparing NECA with NECA+DPCPX in two-way ANOVA followed by Bonferroni post-hoc tests. Data are means ± SE of median values for 2–7 animals. B) Comparison of Cx35 phosphorylation levels for incubation of no drug control, 20 nM DPCPX, and 10 μM CGS 21680. Data are means ± SE of median values for 3–5 animals.
Inclusion of 20 nM of the selective A1 receptor inhibitor DPCPX resulted in an elevation of the dose-response relationship with a similar shape (figure 3A, dashed curve), which was significant at 100 nM through 10 μM NECA. This suggests that A1 receptor activation depressed Cx35 phosphorylation while other adenosine receptors enhanced it. The enhancement at nanomolar NECA is consistent with an A2a activity that was completely masked by A1 receptor activation. The reduction in Cx35 phosphorylation at the highest NECA concentration may reflect desensitization of an A2-type receptor (Mundell & Kelly, 1998), or perhaps recruitment of an inhibitory receptor such as the A3 or an indirect effect such as enhancement of dopamine release.
The substantial enhancement of the NECA dose-response curve by A1 receptor antagonist DPCPX revealed the activity of the A1 receptor in regulating photoreceptor gap junction phosphorylation, but it was not clear whether this receptor was activated only by the exogenous agonist. Figure 3B shows that 20 nM DPCPX significantly increased Cx35 phosphorylation in the daytime, light-adapted eyecup preparation even in the absence of exogenous agonists, indicating that the A1 receptor was activated by endogenous adenosine and was suppressing phosphorylation. The phosphorylation enhancement by A1 receptor inhibition was smaller than that produced by activation of both A2a and A1 receptors together with 10 μM CGS (figure 3B). The suppression induced by the A1 receptor nonetheless adds significantly to the inhibitory drive from activation of dopamine D4 receptors to keep the gap junction phosphorylation and photoreceptor coupling at a minimal level in the daytime light condition.
A2a receptors contribute to Cx35 phosphorylation in nighttime retina
In the nighttime state, the extracellular adenosine concentration is higher than in the daytime due to a darkness-induced increase in extracellular adenosine production from ATP and a circadian rhythm-driven increase in intracellular adenosine suppressing uptake of extracellular adenosine (Ribelayga & Mangel, 2005). To investigate further the role of adenosine receptors in regulating photoreceptor coupling, we examined Cx35 phosphorylation in dark-adapted eyecups from nighttime, dark-adapted zebrafish. As previously reported (Li et al., 2009), OPL Cx35 was relatively well phosphorylated in the nighttime, dark-adapted condition (figure 4A, A′). Application of 1 μM of the A2a receptor-selective antagonist 8-Chlorostyryl caffeine (CSC) strongly reduced Cx35 phosphorylation (figure 4B, B′). A dose-response relationship for the effect of CSC on Cx35 phosphorylation is shown in figure 4C. The monophasic curve shows an IC50 of 9.8 nM, consistent with reported values for the effects of CSC on the A2a receptor (Jacobson et al., 1993). A dose-response curve with the low affinity and less selective antagonist 3,7-dimethyl-1-propargylxanthine (DMPX) showed a biphasic relationship (figure 4D). The half maximal inhibition of Cx35 phosphorylation at approximately 1 μM is consistent with the low micromolar IC50’s reported for either A2a or A2b receptors, while enhancement at tens of micromolar is consistent with an effect on the A1 receptor, which has reported IC50’s from 10 to 45 μM (Muller & Jacobson, 2011). These data suggest that A2a receptor activation has a major role in enhancing Cx35 phosphorylation and photoreceptor coupling at night and that an inhibition by A1 receptors is superimposed upon the A2a effect.
Figure 4.

Influence of adenosine receptors on the phosphorylation level of OPL gap junctions in nighttime, dark-adapted retina. A) Confocal micrograph of immunostaining for total Cx35 (red) and phospho-S276 Cx35 (green) in the OPL of nighttime, dark-adapted zebrafish retina in an eyecup preparation. Phosphorylated Cx35 (shown alone in A′) is much higher than in daytime. B) 25 minute incubation with 1 μM A2a receptor antagonist CSC reduced Cx35 phosphorylation (phospho-S276 Cx35 shown alone in B′). Scale bar in B′ applies to all images in A and B. C) Dose-response relationship of Cx35 phosphorylation level for incubation of CSC. D) Dose-response relationship of Cx35 phosphorylation level for incubation of less selective adenosine receptor antagonist DMPX. Median values for 2 animals are shown.
Discussion
Scotopic vision at night is characterized by extensive rod input into cones and the post-receptoral neurons in the cone pathway. This input travels through gap junctions between rods and cones and is efficiently eliminated by light adaptation, dopamine and processes driven by a circadian clock (Witkovsky et al., 1988; Mangel et al., 1994; Wang & Mangel, 1996; Ribelayga et al., 2008). Recent work has shown that the changes in photoreceptor coupling are caused predominantly by changes in the phosphorylation state of photoreceptor gap junctions made of Cx36 (Cx35 in non-mammalian vertebrates) (Li et al., 2009; Li et al., 2013), although circadian-driven increases in Cx36 transcription and translation likely also contribute to the nocturnal increase in coupling (Katti et al., 2013). This suggests that regulation of the activities of protein kinases and phosphatases that control Cx36 phosphorylation is critical for short-term plasticity of photoreceptor coupling.
In photoreceptors, regulation of adenylyl cyclase activity, which in turn regulates PKA activity, has a dominant role in control of electrical coupling (Li & O’Brien, 2012). The light-adaptive signal is provided by dopamine, which activates a receptor with D2 pharmacology (Dearry & Burnside, 1986; Cohen & Blazynski, 1990; Krizaj et al., 1998) that has been shown to be a D4 receptor in mouse retina (Cohen et al., 1992; Nir et al., 2002; Jackson et al., 2009). In this study, we examined in the zebrafish retina whether adenosine provides a nocturnal signal to oppose the light-adaptive signal provided by dopamine. The results show that an adenosine A2a receptor activated by endogenous adenosine maintains photoreceptor Cx35 in a phosphorylated state at night in darkness, consistent with activation of photoreceptor adenylyl cyclase. Exogenously applied adenosine and adenosine receptor agonists enhance Cx35 phosphorylation in light-adapted daytime conditions as well. This effect is mediated predominantly by the A2a receptor as well. An A2b receptor could contribute to this effect at very high agonist concentrations, although there is no clear evidence for its presence in our results.
We have previously found that the phosphorylation state of OPL Cx35 and tracer coupling among zebrafish photoreceptors can be directly regulated by cAMP analogues that activate or inhibit PKA (Li et al., 2009). Our current results suggest that the pool of cAMP that controls photoreceptor coupling is indeed sensitive to both adenosine and dopamine receptors. In mouse photoreceptors, we have recently observed similar opposing actions of A2a and D4 receptors (Li et al., 2013). We further found that A2a and D4 receptor and adenylyl cyclase 1 expression is intricately linked. Both D4 receptor and AC1 transcripts are regulated cyclically along with A2a receptor transcript, and expression of both is suppressed in an A2a knockout animal. These results suggest that A2a and D4 receptors are interdependent, and they likely regulate the same pool of adenylyl cyclase.
The theme that adenosine A2a receptors and dopamine D2/D4 receptors regulate the same physiological processes in opposing fashion in photoreceptors has become established. A2a receptors drive elongation of sunfish cone myoids (Rey & Burnside, 1999), opposing the action of dopamine (Dearry & Burnside, 1986; Hillman et al., 1995). In similar fashion, A2-type adenosine receptors suppress calcium current through L-type calcium channels in salamander rods (Stella et al., 2002), while D2-like dopamine receptors enhance the current (Stella & Thoreson, 2000).
In addition to an action of the A2a receptor, we also observed an action of A1 receptors on zebrafish OPL gap junction phosphorylation in the daytime, light-adapted retina (figure 1E). A1 receptors, which couple through a Gi/o similar to dopamine D4 receptors, have an opposite effect on adenylyl cyclase as do A2a receptors (Fredholm et al., 2000). A1 receptors have affinity for adenosine around 100 nM, which is 2–3 fold higher than is exhibited by A2a receptors (Fredholm et al., 2011). Extracellular adenosine is always present in the retina, but is lower during the day and light-adapted conditions than at night (Ribelayga & Mangel, 2005). In the daytime, the low level of extracellular adenosine appears to preferentially activate A1 receptors, reinforcing the action of dopamine D4 receptors to suppress adenylyl cyclase activity. The level of A2a transcript is lower during the day than at night in mouse retina (Li et al., 2013), so activity of the A2a receptor is consequently lower in the day as well. It is not clear whether A1 receptor expression is also regulated through the day, as is A2a expression. Expression of all of the adenosine receptors is subject to regulation by a number of physiological variables (Fredholm et al., 2011), and such regulation would not be unexpected in the retina.
It should also be noted that adenosine receptors may not be expressed uniformly among photoreceptors. Stella et al. (2007) commented that while the calcium current of rods and all cone types in the salamander retina were suppressed by adenosine, an earlier study showed that the calcium current of large single cones was modulated in the opposite manner to rods and other cone types by cAMP analogs that inhibit or activate PKA (Stella & Thoreson, 2000). This suggests that an A1 receptor may mediate the dominant adenosine effect in large single cones while an A2-type receptor mediates the dominant effect in the other photoreceptor types (Stella et al., 2007). In the mouse retina, the in situ hybridization signal for A2a receptor mRNA was substantially more abundant in cones than in rods (Li et al., 2013), suggesting that receptor abundance may differ in the photoreceptor types. The gap junctions that we imaged in the zebrafish retina contain large populations of cone-cone and rod-cone synapses (Li et al., 2009). We did not detect populations of gap junctions that behaved differently with respect to pharmacological agents in the current study (data not shown), but it is possible that there are differences in signaling controlling Cx35 phosphorylation on the rod and cone sides of rod-cone gap junctions or among the different cone types. It should also be considered that the suppressive effect of A1 receptors on Cx35 phosphorylation we observed could be indirect, perhaps resulting from stimulation of dopamine release. Further research will be needed to clarify that mechanism.
Optimization of retinal circuits to perform under vastly different light regimes requires a rich variety of mechanisms, and the extracellular neuromodulators dopamine and adenosine provide signals to coordinate many of these. The action of a nocturnal adenosine signal on A2a receptors maintains high photoreceptor coupling in the dark-adapted state. Our observations suggest that light adaptation will engage the combined actions of dopamine and an A1 receptor to suppress coupling. This convergence of extracellular cues on a single signaling mechanism provides tight regulatory control for photoreceptor coupling, and may provide insight into other synaptic processes in the retina that are subject to dopamine signaling.
Acknowledgments
We thank Dr. Christophe Ribelayga for critically reviewing this manuscript. This research was supported by the American Health Assistance Foundation (now BrightFocus Foundation) Macular Degeneration Research program, by NIH grant EY12857 and core grant EY10608, and by an unrestricted grant to the Department of Ophthalmology & Visual Science from Research to Prevent Blindness. Additional support was provided by the Vale-Asche Foundation through the Frederic B. Asche endowment.
References
- Attwell D, Borges S, Wu SM, Wilson M. Signal clipping by the rod output synapse. Nature. 1987;328:522–524. doi: 10.1038/328522a0. [DOI] [PubMed] [Google Scholar]
- Blazynski C. Discrete distributions of adenosine receptors in mammalian retina. J Neurochem. 1990;54:648–655. doi: 10.1111/j.1471-4159.1990.tb01920.x. [DOI] [PubMed] [Google Scholar]
- Cohen AI, Blazynski C. Dopamine and its agonists reduce a light-sensitive pool of cyclic AMP in mouse photoreceptors. Vis Neurosci. 1990;4:43–52. doi: 10.1017/s0952523800002753. [DOI] [PubMed] [Google Scholar]
- Cohen AI, Todd RD, Harmon S, O’Malley KL. Photoreceptors of mouse retinas possess D4 receptors coupled to adenylate cyclase. Proc Natl Acad Sci U S A. 1992;89:12093–12097. doi: 10.1073/pnas.89.24.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearry A, Burnside B. Dopaminergic regulation of cone retinomotor movement in isolated teleost retinas: I. Induction of cone contraction is mediated by D2 receptors. J Neurochem. 1986;46:1006–1021. doi: 10.1111/j.1471-4159.1986.tb00612.x. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Arslan G, Halldner L, Kull B, Schulte G, Wasserman W. Structure and function of adenosine receptors and their genes. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:364–374. doi: 10.1007/s002100000313. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, PIA, Jacobson KA, Linden J, Muller CE International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors--an update. Pharmacol Rev. 2011;63:1–34. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman DW, Lin D, Burnside B. Evidence for D4 receptor regulation of retinomotor movement in isolated teleost cone inner-outer segments. J Neurochem. 1995;64:1326–1335. doi: 10.1046/j.1471-4159.1995.64031326.x. [DOI] [PubMed] [Google Scholar]
- Hornstein EP, Verweij J, Li PH, Schnapf JL. Gap-junctional coupling and absolute sensitivity of photoreceptors in macaque retina. J Neurosci. 2005;25:11201–11209. doi: 10.1523/JNEUROSCI.3416-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CR, Chaurasia SS, Zhou H, Haque R, Storm DR, Iuvone PM. Essential roles of dopamine D4 receptors and the type 1 adenylyl cyclase in photic control of cyclic AMP in photoreceptor cells. J Neurochem. 2009;109:148–157. doi: 10.1111/j.1471-4159.2009.05920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Nikodijevic O, Padgett WL, Gallo-Rodriguez C, Maillard M, Daly JW. 8-(3-Chlorostyryl)caffeine (CSC) is a selective A2-adenosine antagonist in vitro and in vivo. FEBS Lett. 1993;323:141–144. doi: 10.1016/0014-5793(93)81466-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katti C, Butler R, Sekaran S. Diurnal and circadian regulation of connexin 36 transcript and protein in the mammalian retina. Invest Ophthalmol Vis Sci. 2013;54:821–829. doi: 10.1167/iovs.12-10375. [DOI] [PubMed] [Google Scholar]
- Klotz KN. Adenosine receptors and their ligands. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:382–391. doi: 10.1007/s002100000315. [DOI] [PubMed] [Google Scholar]
- Kothmann WW, Li X, Burr GS, O’Brien J. Connexin 35/36 is phosphorylated at regulatory sites in the retina. Vis Neurosci. 2007;24:363–375. doi: 10.1017/S095252380707037X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothmann WW, Massey SC, O’Brien J. Dopamine-stimulated dephosphorylation of connexin 36 mediates AII amacrine cell uncoupling. J Neurosci. 2009;29:14903–14911. doi: 10.1523/JNEUROSCI.3436-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaj D, Gabriel R, Owen WG, Witkovsky P. Dopamine D2 receptor-mediated modulation of rod-cone coupling in the Xenopus retina. J Comp Neurol. 1998;398:529–538. [PMC free article] [PubMed] [Google Scholar]
- Kvanta A, Seregard S, Sejersen S, Kull B, Fredholm BB. Localization of adenosine receptor messenger RNAs in the rat eye. Exp Eye Res. 1997;65:595–602. doi: 10.1006/exer.1996.0352. [DOI] [PubMed] [Google Scholar]
- Lamb TD, Simon EJ. The relation between intercellular coupling and electrical noise in turtle photoreceptors. J Physiol. 1976;263:257–286. doi: 10.1113/jphysiol.1976.sp011631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebedev DS, Byzov AL, Govardovskii VI. Photoreceptor coupling and boundary detection. Vision Res. 1998;38:3161–3169. doi: 10.1016/s0042-6989(98)00017-0. [DOI] [PubMed] [Google Scholar]
- Li H, Chuang AZ, O’Brien J. Photoreceptor coupling is controlled by connexin 35 phosphorylation in zebrafish retina. J Neurosci. 2009;29:15178–15186. doi: 10.1523/JNEUROSCI.3517-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, O’Brien J. Regulation of gap junctional coupling in photoreceptors. In: Akutagawa E, Ozaki K, editors. Photoreceptors: Physiology, Types and Abnormalities. Nova Science; Hauppauge, NY: 2012. [Google Scholar]
- Li H, Zhang Z, Blackburn MR, Wang SW, Ribelayga CP, O’Brien J. Adenosine and Dopamine Receptors Coregulate Photoreceptor Coupling via Gap Junction Phosphorylation in Mouse Retina. J Neurosci. 2013;33:3135–3150. doi: 10.1523/JNEUROSCI.2807-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangel SC, Baldridge WH, Weiler R, Dowling JE. Threshold and chromatic sensitivity changes in fish cone horizontal cells following prolonged darkness. Brain Res. 1994;659:55–61. doi: 10.1016/0006-8993(94)90862-1. [DOI] [PubMed] [Google Scholar]
- Muller CE, Jacobson KA. Xanthines as Adenosine Receptor Antagonists. In: Fredholm BB, editor. Methylxanthines, Handbook of Experimental Pharmacology. 200. Vol. 200. Springer-Verlag; Berlin Heidelberg: 2011. pp. 151–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundell SJ, Kelly E. Evidence for co-expression and desensitization of A2a and A2b adenosine receptors in NG108-15 cells. Biochem Pharmacol. 1998;55:595–603. doi: 10.1016/s0006-2952(97)00466-8. [DOI] [PubMed] [Google Scholar]
- Nelson R. Cat cones have rod input: a comparison of the response properties of cones and horizontal cell bodies in the retina of the cat. J Comp Neurol. 1977;172:109–135. doi: 10.1002/cne.901720106. [DOI] [PubMed] [Google Scholar]
- Nir I, Harrison JM, Haque R, Low MJ, Grandy DK, Rubinstein M, Iuvone PM. Dysfunctional light-evoked regulation of cAMP in photoreceptors and abnormal retinal adaptation in mice lacking dopamine D4 receptors. J Neurosci. 2002;22:2063–2073. doi: 10.1523/JNEUROSCI.22-06-02063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey HL, Burnside B. Adenosine stimulates cone photoreceptor myoid elongation via an adenosine A2-like receptor. J Neurochem. 1999;72:2345–2355. doi: 10.1046/j.1471-4159.1999.0722345.x. [DOI] [PubMed] [Google Scholar]
- Ribelayga C, Cao Y, Mangel SC. The circadian clock in the retina controls rod-cone coupling. Neuron. 2008;59:790–801. doi: 10.1016/j.neuron.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribelayga C, Mangel SC. A circadian clock and light/dark adaptation differentially regulate adenosine in the mammalian retina. J Neurosci. 2005;25:215–222. doi: 10.1523/JNEUROSCI.3138-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribelayga C, Wang Y, Mangel SC. Dopamine mediates circadian clock regulation of rod and cone input to fish retinal horizontal cells. J Physiol. 2002;544:801–816. doi: 10.1113/jphysiol.2002.023671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella SL, Jr, Bryson EJ, Cadetti L, Thoreson WB. Endogenous adenosine reduces glutamatergic output from rods through activation of A2-like adenosine receptors. J Neurophysiol. 2003;90:165–174. doi: 10.1152/jn.00671.2002. [DOI] [PubMed] [Google Scholar]
- Stella SL, Jr, Bryson EJ, Thoreson WB. A2 adenosine receptors inhibit calcium influx through L-type calcium channels in rod photoreceptors of the salamander retina. J Neurophysiol. 2002;87:351–360. doi: 10.1152/jn.00010.2001. [DOI] [PubMed] [Google Scholar]
- Stella SL, Jr, Hu WD, Vila A, Brecha NC. Adenosine inhibits voltage-dependent Ca2+ influx in cone photoreceptor terminals of the tiger salamander retina. J Neurosci Res. 2007;85:1126–1137. doi: 10.1002/jnr.21210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella SL, Jr, Thoreson WB. Differential modulation of rod and cone calcium currents in tiger salamander retina by D2 dopamine receptors and cAMP. Eur J Neurosci. 2000;12:3537–3548. doi: 10.1046/j.1460-9568.2000.00235.x. [DOI] [PubMed] [Google Scholar]
- Trumpler J, Dedek K, Schubert T, de Sevilla Muller LP, Seeliger M, Humphries P, Biel M, Weiler R. Rod and cone contributions to horizontal cell light responses in the mouse retina. J Neurosci. 2008;28:6818–6825. doi: 10.1523/JNEUROSCI.1564-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Mangel SC. A circadian clock regulates rod and cone input to fish retinal cone horizontal cells. Proc Natl Acad Sci U S A. 1996;93:4655–4660. doi: 10.1073/pnas.93.10.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P. Dopamine and retinal function. Doc Ophthalmol. 2004;108:17–40. doi: 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Stone S, Besharse JC. Dopamine modifies the balance of rod and cone inputs to horizontal cells of the Xenopus retina. Brain Res. 1988;449:332–336. doi: 10.1016/0006-8993(88)91048-7. [DOI] [PubMed] [Google Scholar]
- Zhang M, Budak MT, Lu W, Khurana TS, Zhang X, Laties AM, Mitchell CH. Identification of the A3 adenosine receptor in rat retinal ganglion cells. Mol Vis. 2006;12:937–948. [PubMed] [Google Scholar]