Abstract
Purpose
To determine the most cost-effective treatment for patients with newly diagnosed neovascular macular degeneration: monthly or as-needed bevacizumab injections, or monthly or as-needed ranibizumab injections.
Design
Cost effectiveness analysis
Participants
Hypothetical cohort of 80-year-old patients with newly-diagnosed neovascular macular degeneration.
Methods
Using a mathematical model with a 20-year time horizon, we compared the incremental cost-effectiveness of treating a hypothetical cohort of 80-year-old patients with newly diagnosed neovascular macular degeneration using monthly bevacizumab, as-needed bevacizumab, monthly ranibizumab, or as-needed ranibizumab. Data came from the Comparison of Age-related macular degeneration Treatment Trial (CATT), the Medicare Fee Schedule, and the medical literature.
Main Outcome Measures
Costs, quality-adjusted life years (QALYs), and incremental costs per QALY gained.
Results
Compared with as-needed bevacizumab, the incremental cost-effectiveness ratio of monthly bevacizumab is $242 357/QALY. Monthly ranibizumab gains an additional 0.02 QALYs versus monthly bevacizumab at an incremental cost-effectiveness ratio of more than $10 million/QALY. As-needed ranibizumab was dominated by monthly bevacizumab, meaning it was more costly and less effective. In sensitivity analyses assuming a willingness to pay of $100 000/QALY, the annual risk of serious vascular events would have to be at least 2.5 times higher with bevacizumab than that observed in the CATT trial for as-needed ranibizumab to have an incremental cost-effectiveness ratio of <$100 000/QALY. In another sensitivity analysis, even if every patient receiving bevacizumab experienced declining vision by one category (e.g., from 20/25–20/40 to 20/50–20/80) after 2 years but every patient receiving ranibizumab retained their vision level, as-needed ranibizumab would have an incremental cost-effectiveness ratio of $97 340/QALY.
Conclusion
Even after considering the potential for differences in risks of serious adverse events and therapeutic effectiveness, bevacizumab confers considerably greater value than ranibizumab for the treatment of neovascular macular degeneration.
Age-related macular degeneration (AMD) is the leading cause of blindness among adults older than 65 years. With the aging of the United States (U.S.) population, by 2020 nearly 3 million persons are expected to experience AMD-related visual impairment.1–3 AMD causes blurring, distortion, and eventual loss of central vision and almost always affects health-related quality of life (HRQL).4,5
For many years, the conventional first-line treatment for extrafoveal neovascular AMD was focal argon laser photocoagulation (FALP). The Macular Photocoagulation Study demonstrated that patients with extrafoveal choroidal neovascularization who underwent FALP were 35% less likely than untreated patients to experience severe vision loss at 18 months, and 18% less likely at 5 years.6,7 Although FALP effectively stabilized best-corrected visual acuity (BCVA), the treatment improved vision in few patients and was contraindicated in those with subfoveal disease. Photodynamic therapy (PDT) with verteporfin, an alternative to FALP, became available in 2000. An advantage of PDT over FALP was the ability to safely treat not only patients with extrafoveal choroidal neovascularization but also those with occult and subfoveal disease. However, similar to FALP, PDT treatment with verteporfin stabilized the disease but improved BCVA in few patients.8
In recent years, new therapeutic options revolutionized the treatment of neovascular AMD. Antivascular endothelial growth factor (anti-VEGF) agents, including pegaptanib, ranibizumab (Lucentis, Genentech/Roche), and bevacizumab (Avastin, Genentech/Roche), are antibodies or antibody fragments that bind and block VEGF. The Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab In the Treatment of Neovascular AMD (MARINA) proved that intravitreal injections of ranibizumab, 0.3 or 0.5 mg, were more efficacious than sham treatment at preserving and improving vision.9 The Anti-VEGF antibody for the treatment of predominantly classic choroidal neovascularization in AMD (ANCHOR) trial showed that either dose was better than PDT with verteporfin.10 More recently, large randomized, controlled trials (RCTs), including the Comparison of Age-related macular degeneration Treatment Trial (CATT),11,12 directly compared the efficacy of ranibizumab and bevacizumab in patients with neovascular AMD. After two years’ follow-up, using similar dosing regimens, the CATT trial found bevacizumab to be noninferior in efficacy to ranibizumab. The study also compared monthly dosing with an as-needed regimen of these agents and found that participants who received monthly dosing of the agents regained slightly more vision.12 Although CATT is providing high-quality evidence of the comparative efficacy and safety of ranibizumab and bevacizumab for neovascular AMD and several studies in the literature demonstrate the cost-effectiveness of anti-VEGF agents relative to supportive care13–20 or PDT with verteporfin,15,19,21–23 little is known about the cost-effectiveness of bevacizumab relative to ranibizumab.24 To our knowledge, only two studies25,26 have directly compared the cost-effectiveness of these two agents for neovascular AMD. Because these studies predated the head-to-head RCTs, the researchers needed to make several assumptions about the safety and efficacy of bevacizumab and ranibizumab. The high-quality data now available from these trials, on the safety, efficacy, and differences in outcomes using different anti-VEGF dosing regimens, can be used to evaluate which anti-VEGF treatment confers the greatest societal value.
A rigorous cost-effectiveness analysis comparing bevacizumab and ranibizumab would be useful to providers and policymakers—and ultimately, patients—given the high prevalence of neovascular AMD, the risks for potentially serious side effects associated with use of these agents, the need in many patients for multiple injections, and differences in efficacy based on the dosing regimen used. And, perhaps most important, is the cost consideration: More than $1.6 billion is spent annually on ranibizumab therapy for retinal diseases,27 and ranibizumab was the single largest expenditure for Medicare Part B in 2010, accounting for nearly 10% of the entire drug budget.28
METHODS
Study Design
We developed a Markov model to capture the total costs and HRQL for patients with newly diagnosed neovascular AMD under four treatment options: monthly bevacizumab injections, as-needed bevacizumab injections, monthly ranibizumab injections, and as-needed ranibizumab injections. A societal perspective was taken to encompass all parties affected by the treatment choice: patients, providers, and payers. The model followed a hypothetical cohort of patients aged 80 years (the mean age for neovascular AMD onset) with neovascular AMD (as defined in CATT) over a 20-year time horizon. Markov modeling is a standard method used in health technology assessments and has been used in previous cost-effectiveness analyses for neovascular AMD.29 Table 1 (available at http://aaojournal.org) defines commonly used terminology in cost-effectiveness analyses.
Health States
A Markov model has patients transition through health states over time. We followed patients through health states based on BCVA levels (Figure 1, available at http://aaojournal.org). We assume that the BCVA level used captures vision in the better-seeing eye.
Progression Rates
Vision in each intervention group followed the observed BCVAs from CATT at years 1 and 2 (Table 2).11,12 Because to our knowledge no previous study has reported on the natural history of neovascular AMD treated with anti-VEGF agents beyond 2–3 years, we evaluated BCVA in the longer term using various scenarios. In our baseline model, we assumed that the distribution of BCVA from CATT was unchanged after year 2 for all treatment groups. In sensitivity analyses, we allowed the BCVA of patients in each treatment group to decline each year.
Table 2.
Vision outcomes and adverse events after year 1 and 2 from the Comparison of Age-Related Macular Degeneration Treatment Trial
| Monthly Bevacizumab | As Needed Bevacizumab | Monthly Ranibizumab | As Needed Ranibizumab | |||||
|---|---|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 1 | Year 2 | Year 1 | Year 2 | Year 1 | Year 2 | |
| Proportion of patients in each vision state | ||||||||
| 20/12–20/20 | 17.0% | 13.2% | 14.8% | 13.9% | 14.8% | 17.9% | 13.3% | 16.7% |
| 20/25–20/40 | 50.6% | 47.3% | 46.9% | 48.2% | 52.5% | 50.0% | 49.5% | 46.6% |
| 20/50–20/80 | 17.7% | 24.0% | 21.0% | 18.3% | 18.3% | 17.2% | 23.2% | 22.3% |
| 20/100–20/160 | 7.9% | 10.9% | 8.9% | 11.2% | 8.1% | 8.2% | 8.1% | 8.7% |
| ≤ 20/200 | 6.8% | 4.7% | 8.5% | 8.4% | 6.3% | 6.7% | 6.0% | 5.7% |
| Adverse event rates* | ||||||||
| Endophthalmitis | 1.4% | 0.68% | 0.00% | 0.68% | 1.00% | 0.33% | 0.00% | 0.33% |
| VTE | 1.40% | 0.85% | 0.33% | 0.85% | 0.00% | 0.17% | 0.67% | 0.17% |
| MI | 0.70% | 0.68% | 0.33% | 0.68% | 0.66% | 0.67% | 1.01% | 0.67% |
| CVA | 0.70% | 0.68% | 0.67% | 0.68% | 1.00% | 0.67% | 0.34% | 0.67% |
| Vascular Death | 0.70% | 1.19% | 1.67% | 1.19% | 0.66% | 1.34% | 0.67% | 1.34% |
VTE = venous thrombotic event; MI = myocardial infarction; CVA = cerebrovascular accident
Year 2 event rates were reported only for each drug, and not frequency of delivery, so the year 2 adverse event rates are the same for “monthly” and “as needed”.
Serious Systemic and Ocular Adverse Events
Using CATT data, we tracked rates of cerebrovascular accident (CVA), myocardial infarction (MI), and venous thrombotic events (VTEs) (Table 2).11,12 Once a patient experienced a CVA, MI, or VTE, the costs increased, HRQL declined, and the risk for death increased for the remainder of the patient’s life (Figure 1, available at http://aaojournal.org). We also tracked patients with blindness due to endophthalmitis.30,31 Age-adjusted mortality data from U.S. life tables were incorporated to capture rates for persons with neovascular AMD.32
Costs
Direct medical costs of managing neovascular AMD were based on office-based Centers for Medicare and Medicaid Services (CMS) allowables for 2011 in Michigan and included the costs of eye-care provider visits; ancillary testing (optical coherence tomography [OCT] and intravenous fluorescein angiography [IVFA]), to evaluate for and quantify the amount of neovascular AMD present; each intervention; treatment of side effects caused by the interventions; and costs associated with blindness when BCVA remained ≤20/200 (Table 3). For pharmaceutical products administered in the office, such as bevacizumab and ranibizumab, we included the drug cost, professional fee, and facility fee reimbursed by CMS in 2011. The cost of all drugs paid for outside the office setting was calculated by using Red Book data from 2012.40 All costs were adjusted for inflation to 2012 dollars. The number of office visits and injections for each therapeutic regimen came from CATT.11,12
Table 3.
Input Parameters for the Costs, Utilities, and Side Effects Included in the Markov Model of Neovascular Age-Related Macular Degeneration
| Parameter Value | Value in USD (range) | Reference |
|---|---|---|
| Costs (2012 USB) | ||
| Visits and diagnostic testing | ||
| Initial office visit | 242 | CPT 99204 |
| Subsequent office visits | 185 | CPT 99214 |
| Optical coherence tomography at each visit | 74 | CPT 92134 |
| Fluorescein angiography, at initial evaluation | 260 | CPT 99235 |
| Interventions | ||
| Intravitreal ranibizumab | 2389 | CPT 67028 |
| Intravitreal bevacizumab | 356 | CPT 67028 |
| Costs of Managing Sequelae | ||
| Short-term | ||
| Endophthalmitis* | 4140 | CPT 67015/67028 |
| Blindness | 2846 | Frick33 |
| VTE | 32141 | Mahan34 and MacDougall35 |
| MI | 18326 | Freeman36 |
| CVA | 13307 | Freeman36 |
| Long-term | ||
| MI | 3557 | Freeman36 |
| CVA | 62796 | Freeman36 |
| Utilities | ||
| Health States | Brown37 | |
| 20/12–20/20 | 0.92 | |
| 20/25–20/40 | 0.84 | |
| 20/50–20/80 | 0.76 | |
| 20/100–20/160 | 0.66 | |
| ≤ 20/200 | 0.61 | |
| Short-term side effects (QALYs lost)^ | ||
| Endophthalmitis | −0.1 | Aaberg38 |
| VTE | −0.004 | Bajaj39 |
| Long-term Side Effects (annual utility)† | ||
| Blindness from endophthalmitis | 0.37 | Brown37 |
| CVA | 0.39 | Freeman36 |
| MI | 0.84 | Freeman36 |
| Relative risk of mortality after MI or CVA | 2 | Assumption |
| After MI | ||
| All-other nonvascular mortality | Varies by age | CDC Wonder database32 |
VTE = venous thrombotic event; MI = myocardial infarction; CVA = cerebrovascular accident, QALY = quality-adjusted life years; USD = United states dollars; CDC = Centers for Disease Control and Prevention; CPT = Current Procedural Terminology
Reference: 2011 Medicare Payments for physician and facility fees; Red Book. All costs adjusted to 2012 prices
includes cost of topical antibiotics, corticosteroids and cycloplegics
Short term side effects affected patients only during the first year after receipt of the intervention
Long term side effects affected patients for the remainder of time they cycled through the model
Utilities
The main value of treating neovascular AMD comes from the HRQL gained by improving or maintaining BCVA. The measure used was quality-adjusted life-years (QALYs), to allow for comparisons with interventions for other diseases. A QALY incorporates quality and length of life, with one QALY representing 1 year in perfect health. HRQL, or “utility,” is quantified as a value ranging from 1.00 (perfect health) to 0.00 (death). This “utility” is multiplied by the number of years in a particular health state to get the QALYs. We incorporated utility scores for each BCVA level as captured by Brown and colleagues37; these scores range from 0.97 for 20/20 BCVA to 0.61 for <20/200 (Table 3). Because neovascular AMD affects the macula and often spares the peripheral retina, patients uncommonly experience BCVA <20/200 from neovascular AMD alone. Utility scores obtained from the literature for complications of the various interventions and utility scores for MI, CVA, VTE, and death are included in Table 3. These parameters were varied in sensitivity analyses.
All costs are in 2012 U.S. dollars. Costs and health utilities were discounted at 3% annually. Interventions with higher costs and worse health outcomes than other interventions are considered dominated. Undominated interventions a and b were compared to each other by using an incremental cost-effectiveness ratio (ICER) or net monetary benefit (NMB), defined as:
in which TC is the total cost; E, effectiveness measured in QALYs; WTP, willingness to pay for a QALY; a, the intervention of interest; and b, a lower-cost undominated alternative intervention.41 We used TreeAge Pro 2012 Health Care (TreeAge Software, Williamstown, MA) to calculate and compare costs and health effects of the interventions.
Sensitivity Analyses
We performed sensitivity analyses on the estimates of costs, utilities, and health state transitions to cover a range of assumptions. One-way sensitivity analyses were performed on all parameters to determine which ones had the largest impact on results. We also conducted several two-way sensitivity analyses. For some analyses, we used a WTP amount of $100 000/QALY to determine which intervention was best under different circumstances. Although there is some debate about what is an appropriate WTP amount, this value is in the range of those commonly used.42 Finally, we conducted a probabilistic sensitivity analysis using Monte Carlo simulation of all input assumptions simultaneously and created a cost-effectiveness acceptability curve to determine how robust the results were to changes in all parameters and how likely each therapy was to be the most cost-effective option.
Since all information used in this analysis came from the medical literature and publically available sources (e.g., CMS fee schedule), institutional review board approval was unnecessary.
RESULTS
Base Model
Over 20 years, the expected costs for a single patient with newly diagnosed neovascular AMD receiving monthly bevacizumab, as-needed bevacizumab, monthly ranibizumab, and as-needed ranibizumab were $79 771, $65 267, $257 496, and $163 694, and the QALYs for a patient receiving these treatments were 6.66, 6.60, 6.68, and 6.64, respectively. The ICER of monthly bevacizumab over as-needed bevacizumab was $242 357/QALY. The ICER of monthly ranibizumab over as-needed bevacizumab was $10 708 377/QALY. As-needed ranibizumab was dominated by monthly bevacizumab because monthly bevacizumab had lower costs and higher QALYs (Figure 2, available at http://aaojournal.org, and Table 4).
Table 4.
Cost and Health Results of the Different Therapies for Neovascular Macular Degeneration
| Base model | |||
|---|---|---|---|
| Therapy | Cost (USD) | QALYs | ICER |
| As needed bevacizumab | 65267 | 6.60 | Lowest cost* |
| Monthly bevacizumab | 79771 | 6.66 | 242357 |
| As needed ranibizumab | 163694 | 6.64 | Dominated† |
| Monthly ranibizumab | 257496 | 6.68 | 10708377 |
| Base model (excluding serious systemic adverse events) | |||
| Therapy | Cost (USD) | QALYs | ICER |
| As needed bevacizumab | 54339 | 7.30 | Lowest cost* |
| Monthly bevacizumab | 68705 | 7.32 | 678250 |
| As needed ranibizumab | 169008 | 7.35 | Dominated‡ |
| Monthly ranibizumab | 276510 | 7.40 | 2569040 |
| Base model (excluding costs of visits and OCTs for those getting monthly injections) | |||
| Therapy | Cost (USD) | QALYs | ICER |
| As needed bevacizumab | 65267 | 6.60 | Dominated† |
| Monthly bevacizumab | 55261 | 6.66 | Lowest cost* |
| As needed ranibizumab | 163694 | 6.64 | Dominated† |
| Monthly ranibizumab | 233108 | 6.68 | 10715692 |
Intervention had the lowest costs so other interventions are measured against it. The lowest cost intervention will not have an ICER
dominated by monthly bevacizumab which had higher QALYs at a lower cost
dominated by “extended dominance” by monthly bevacizumab and monthly ranibizumab. If an ICER of as-needed ranibizumab were created relative to monthly bevacizumab, it would be 3760854, which is higher than the ICER of monthly ranibizumab relative to monthly bevacizumab, implying that there is greater value gained per dollar spent with monthly ranibizumab than with as-needed ranibizumab.
USD = United States dollars; QALY = Quality-adjusted life years; ICER = Incremental cost-effectiveness ratio; OCT = optical coherence tomography
Base Model Without Visits or OCT Costs for Monthly Treatment Recipients
Since some clinicians do not charge for a clinic visit or OCT scan when an injection will be performed—as would be the case for monthly ranibizumab or bevacizumab recipients—we reran our models excluding costs of all subsequent visits and OCT tests after those incurred at the initial examination. With this adjustment, the expected costs for a single patient with newly diagnosed neovascular AMD receiving monthly bevacizumab, as-needed bevacizumab, monthly ranibizumab, and as-needed ranibizumab were $55 261, $65 267, $233 108, and $163 694, and the QALYs were 6.66, 6.60, 6.68, and 6.64, respectively. The ICER of monthly ranibizumab over monthly bevacizumab was $10 715 692/QALY. Because monthly bevacizumab involved fewer office visits and OCTs, it was less expensive than as-needed bevacizumab (Table 4). If monthly bevacizumab entailed fewer than 5 office visits and OCTs annually, the total costs were lower than those for as-needed bevacizumab. If monthly bevacizumab had 5.0–7.8 office visits and OCTs, it was more costly than as-needed bevacizumab, but had an ICER <$100 000/QALY, compared with as-needed bevacizumab.
Base Model Excluding Anti-VEGF Systemic Side Effects
Because the difference in safety profile between intravitreal bevacizumab and intravitreal ranibizumab is incompletely understood, we performed a sensitivity analysis, rerunning the model after excluding the systemic side effects VTE, CVA, and MI. In this scenario over 20 years the expected costs for a single patient with newly diagnosed disease receiving monthly and as-needed bevacizumab, and monthly and as-needed ranibizumab, respectively, were $68 705, $54 339, $276 510, and $169 008; QALYs were 7.32, 7.30, 7.40, and 7.35. The ICER of monthly bevacizumab over as-needed bevacizumab was $678 250/QALY. The ICER of monthly ranibizumab over as-needed bevacizumab was $2 569 040/QALY. As-needed ranibizumab was dominated by monthly bevacizumab and monthly ranibizumab, meaning as-needed ranibizumab had fewer QALYs and higher cost per QALY than either monthly option (Table 4).
Sensitivity Analyses
Sensitivity analyses examine the impact of changes to model assumptions.
Varying the Ranibizumab Cost and the Risk for Systemic Side Effects
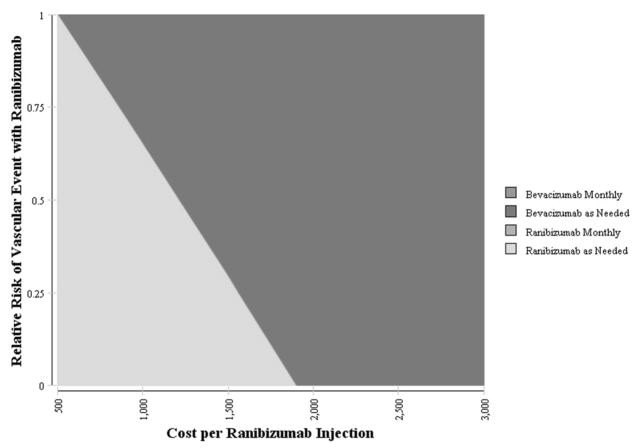
If the risks for serious systemic effects associated with ranibizumab injection were 50% lower than those observed in CATT, the injection cost would still need to be reduced to less than $1210 for as-needed ranibizumab to be the preferred treatment option at a WTP of $100 000/QALY. Likewise, if the risk for serious systemic effects with ranibizumab was 75% lower than that in CATT, the cost per ranibizumab injection would need to be $1556 for as-needed ranibizumab to confer the greatest value (Figure 3).
Figure 3. The Impact of the Cost per Ranibizumab Injection and the Risk of Serious Vascular Events Associated with Intravitreal Ranibizumab Use on the Preference for Therapy for Neovascular Age-Related Macular Degeneration.
Two-way sensitivity analysis varying the cost of each injection of ranibizumab and risk of serious vascular thrombotic events associated with ranibizumab on which is the preferred treatment option. The color reflects the treatment alternative which is most cost-effective treatment option given a willingness-to-pay of $100 000/QALY. For example, if the cost of each ranibizumab injection was reduced to $1000 and the relative risk of serious vascular events with ranibizumab were 50% lower than those reported in the CATT trial, as needed ranibizumab would be the preferred treatment alternative. Likewise, if the cost of each ranibizumab injection was reduced to $2000 and the risk of serious vascular thrombotic events was 0%, as-needed bevacizumab would be the preferred treatment alternative.
CATT = Comparison of Age-Related Macular Degeneration Treatment Trial
QALY = quality-adjusted life year
Varying the Utility of Severe Vision Loss
Our initial assumption was that the vision category of <20/200 had a utility of 0.61, which assumes vision of 20/200–20/400. In sensitivity analysis, we change the utility of the <20/200 category to 0.47 to represent the possibility of even worse vision, including no light perception. Under this assumption, monthly bevacizumab looks better than the other strategies because it had the fewest patients with vision <20/200 after 2 years. Monthly bevacizumab has an ICER of $156 360/QALY relative to as-needed bevacizumab. Under this assumption, the ranibizumab strategies have equivalent or worse health outcomes (and higher costs) compared with monthly bevacizumab.
Varying the Long-Term Effectiveness of Bevacizumab and Ranibizumab
In another sensitivity analysis, we simultaneously varied the long-term effectiveness of ranibizumab and bevacizumab to assess the effect on which is the preferred treatment option. If every patient receiving ranibizumab had no BCVA decline after year 2 and all patients receiving bevacizumab had vision decline by one category (e.g., from 20/12–20/20 to 20/25–20/40) each year, patients receiving as-needed ranibizumab would gain 0.9 discounted QALYs at a cost of $87 940, leading to an ICER of $97 340/QALY.
Varying the Drug Costs
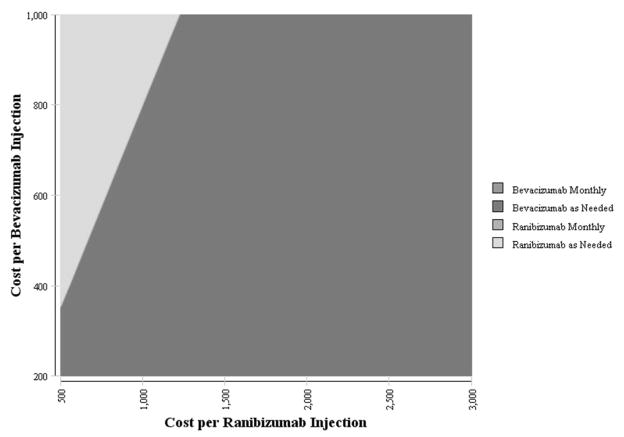
Because the BCVA and health outcomes associated with bevacizumab and ranibizumab are similar, the price premium for ranibizumab versus bevacizumab is only approximately $100–$300, by assuming a WTP of $100 000/QALY. For example, if the per-injection cost of bevacizumab is less than $355, bevacizumab would be the preferred option even if ranibizumab cost as little as $500 per injection (Figure 4).
Figure 4. The Impact of the Cost per Bevacizumab and Ranibizumab Injection on the Preference for Therapy for Neovascular Age-Related Macular Degeneration.
Two-way sensitivity analysis varying the cost of each injection of bevacizumab and ranibizumab on which is the preferred treatment option. The color reflects the treatment alternative which is most cost-effective treatment option given a willingness-to-pay of $100 000/QALY. For example, if the cost of each ranibizumab injection was reduced to $1500 and the cost of each bevacizumab injection was increased to $600, as needed bevacizumab would be the preferred treatment alternative. Likewise, if the cost of each ranibizumab injection was reduced to $750 and the cost of each bevacizumab injection was increased to $800, as needed ranibizumab would be the preferred treatment alternative.
QALY = quality-adjusted life year
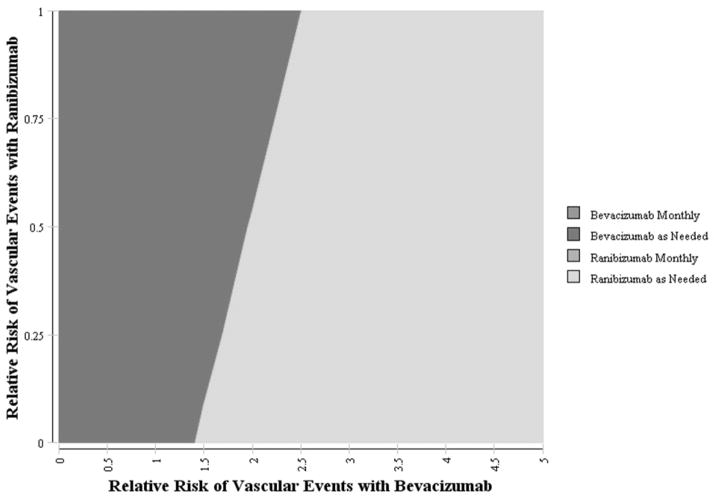
Varying the Risks for Adverse Events
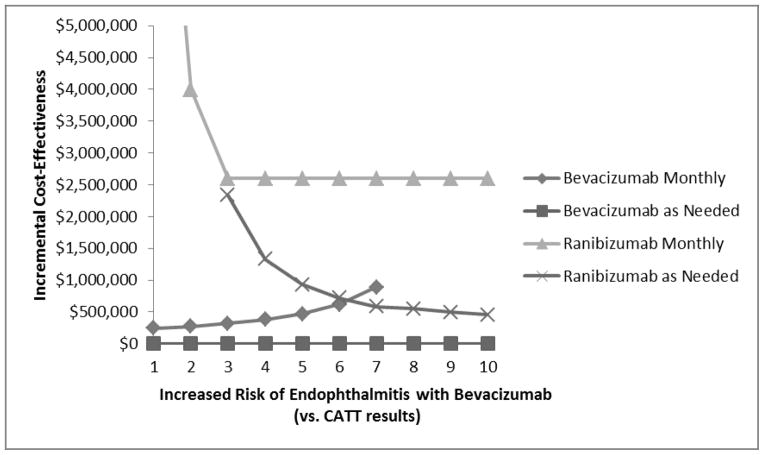
At a WTP of $100 000/QALY, the risk for all serious systemic adverse events (CVA, MI, VTE) would need to be about 2.5 times greater with bevacizumab than with ranibizumab for as-needed ranibizumab to be the preferred treatment (Figure 5). In varying the risk for endophthalmitis by using the CATT-reported rates, we find that even if the risk for endophthalmitis were 10 times greater with bevacizumab than the risk observed in CATT, as-needed bevacizumab would still confer the greatest value (Figure 6). If the annual risk for endophthalmitis with bevacizumab were 40% per person annually, the ICER of as-needed ranibizumab would be less than $100 000/QALY. This is primarily because ranibizumab is very expensive, and this patient population has a relatively limited life expectancy to benefit from averted endophthalmitis. Thus, for a patient receiving as-needed bevacizumab who gets on average 7 injections annually, the risk for endophthalmitis per bevacizumab injection would be approximately 7% to give a 40% risk over the course of the year.
Figure 5. The Impact of the Rate of Serious Vascular Thrombotic Events with Bevacizumab and Ranibizumab on the Preference for Therapy for Age-Related Macular Degeneration.
Two-way sensitivity analysis demonstrating the impact of varying the rates of serious vascular thrombotic events associated with injections of bevacizumab and ranibizumab on which is the most cost-effective alternative. The color reflects the treatment alternative which is most cost-effective treatment option given a willingness-to-pay of $100 000/QALY. For example, if the rate of serious vascular thrombotic events from ranibizumab injections are 50% lower than those observed in the CATT trial and vascular thrombotic event rates with bevacizumab are 3 times higher than those in the CATT trial, as needed ranibizumab would be the preferred treatment alternative. Likewise, if the rate of serious vascular thrombotic events from ranibizumab injections are 25% lower than those observed in the CATT trial and vascular thrombotic event rates with bevacizumab are 2 times higher than those in the CATT trial, as needed injections of bevacizumab would still be the preferred treatment alternative. Rates reflected in the figure are multiples of the rates of all vascular events (VTE, CVA, and MI) as observed in the CATT trial. Note different scales on the X and Y axes.
CATT = Comparison of Age-Related Macular Degeneration Treatment Trial; VTE = venous thrombotic events; CVA = cerebrovascular disease; MI = myocardial infarction; QALY = quality-adjusted life year
Figure 6. The Impact of the Risk of Endophthalmitis with Bevacizumab on the Cost-Effectiveness of Ranibizumab Therapy for Neovascular Age-Related Macular Degeneration.
This figure shows the impact of varying the risk of endophthalmitis associated with bevacizumab use from the rates that were reported in the CATT Trial. As the risk of endophthalmitis increases with bevacizumab, the monthly strategy becomes less favorable because it has higher baseline risks of endophthalmitis relative to the as-needed dosing. The ranibizumab strategies become more favorable, but even if the risk of endophthalmitis is 10 times higher with bevacizumab than the risk reported in the CATT Trial, the ranibizumab therapies would have very high cost-effectiveness ratios: as-needed ranibizumab would cost $450 000/QALY and monthly ranibizumab cost over $2.5 million/QALY.
CATT = Comparison of Age-Related Macular Degeneration Treatment Trial; QALY = quality-adjusted life years
Varying the Cost of Bevacizumab and Bevacizumab-Associated Risk for Endophthalmitis
Compared with multi-use containers, single-use bevacizumab vials cost more but are associated with a lower risk for endophthalmitis. Thus, we explored the impact of simultaneously varying the cost per bevacizumab injection and the risk for endophthalmitis, assuming similar costs and endophthalmitis risk levels for ranibizumab as used in the base model. If the single-use vial completely eliminated the risk for endophthalmitis, a single-use bevacizumab vial could command a premium of as much as $35/injection above the cost of multi-use containers (Figure 7, available at http://aaojournal.org) for single-use vials to be considered cost-effective at a WTP of $100 000/QALY. At today’s prices, the risk for endophthalmitis would need to be 3 times greater with multi-use containers than with single-use vials for the latter to be the preferred treatment option.
Varying the Number of Ranibizumab Injections
If as-needed bevacizumab recipients received 7.05 intravitreal injections annually, as was done in CATT, as-needed ranibizumab recipients would need to receive no more than 1.3 injections/year to attain an ICER <$100 000/QALY.
Other Parameters
Additional sensitivity analyses explored the effects of varying other model parameters: life expectancy and age at onset of neovascular AMD (Figures 8 and 9, available at http://aaojournal.org). These did not substantially affect conclusions.
Probabilistic Sensitivity Analysis
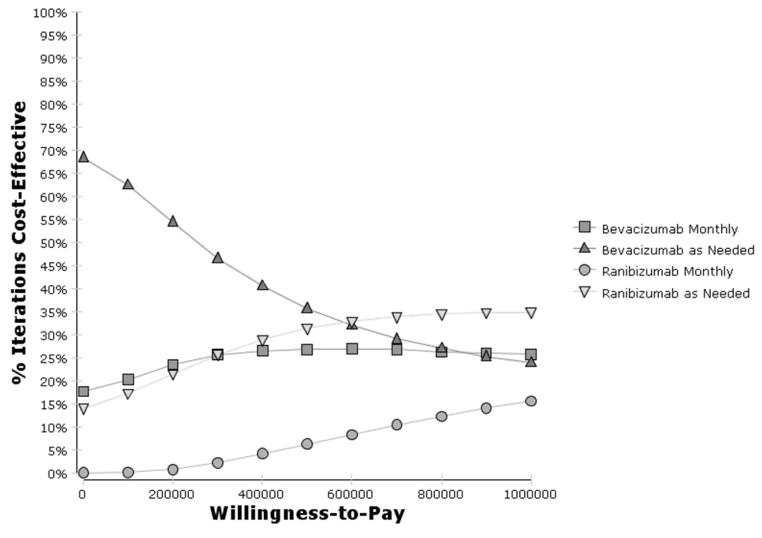
A cost-effectiveness acceptability curve was created by varying all model parameters simultaneously in 10 000 simulation iterations (Figure 10). Bevacizumab strategies are most likely to be cost-effective at WTP values less than $600 000/QALY. As-needed bevacizumab was the preferred therapy choice in 68% of simulations at a WTP threshold of $0/QALY and in 62% of simulations at a $100 000/QALY threshold. Monthly bevacizumab was preferred in 18–20% of simulations when WTP was less than $100 000/QALY. Due to uncertainty in all parameters, ranibizumab therapies were occasionally (14–17% of the time) cost-effective at less than $100 000/QALY. At a WTP of one-million dollars per QALY, ranibizumab therapies were cost-effective in about 50% of simulations.
Figure 10. Probability that Bevacizumab and Ranibizumab Therapy for Neovascular Age-Related Macular Degeneration Will be Cost-Effective Given Uncertainty in All Parameters.
Cost-effectiveness acceptability curves derived from 10 000 iterations of Monte Carlo simulations simultaneously varying all model parameters. Note that the x-axis goes up to $1 million per QALY. Bevacizumab strategies are most likely to be cost-effective at willingness-to-pay values of less than $600 000/QALY. Ranibizumab therapies (more likely, as needed ranibizumab) however, are about 14% likely to be cost-effective at a willingness-to-pay of $0/QALY, about 17% likely to be cost-effective at a willingness-to-pay of $100 000/QALY, and about 50% likely to be cost-effective at a willingness-to-pay of $1 million/QALY. Bevacizumab as needed therapy is highly likely to be cost-effective, although there still is a reasonable chance (15–30%) that monthly bevacizumab therapy would be considered cost-effective at all willingness-to-pay levels.
QALY = quality-adjusted life year
DISCUSSION
As health policymakers aim to curtail rising health care costs, treatments that confer the greatest relative value must be identified. Using data from CATT, we find that, compared with as-needed dosing of bevacizumab, the ICERs of monthly bevacizumab and monthly ranibizumab for neovascular AMD are $242 357/QALY and $10 708 377/QALY, respectively. Furthermore, as-needed ranibizumab was dominated by as-needed bevacizumab, meaning that as-needed ranibizumab is more costly and less effective. Sensitivity analyses highlight the impact of varying the model parameters, including the proportion of patients who experience serious systemic side effects from these agents, the number of injections administered, the cost per injection of each agent, and patient’s life expectancy on the ICER of the treatment options. Finally, when each parameter was simultaneously varied in a probabilistic sensitivity analysis, as-needed bevacizumab was preferred in nearly two-thirds of the simulations by using a WTP of $100 000/QALY; monthly bevacizumab was preferred in another 18–20% of simulations.
Previous cost-effectiveness analyses have compared some older treatments for neovascular AMD, such as FALP,13,43,44 PDT with verteporfin,13,45–49 pegaptanib,13,15–17 or ranibizumab,14,18–20 with supportive care, or compared PDT with verteporfin versus pegaptanib or ranibizumab.15,19,21–23 Little, however, has been known about the relative cost-effectiveness of the two most commonly used interventions today. We know of only two other studies that directly compared the cost-effectiveness of bevacizumab and ranibizumab; neither analysis used data from head-to-head trials. Raftery and colleagues25 found that monthly injections of bevacizumab for predominantly classic and occult choroidal neovascularization conferred considerably more value relative to monthly ranibizumab at a cost per QALY of more than £100 000 ($161 840 United States dollars (USD)). They also reported that ranibizumab would need to be at least 2.5 times as efficacious as bevacizumab to be the preferred treatment option, and doubling the rates of serious ocular side effects associated with bevacizumab use had little effect on the findings. The other study, by Patel and colleagues,26 drew effectiveness data for ranibizumab and bevacizumab from different sources. Since those sources suggested that bevacizumab was much more likely to improve vision, the researchers found bevacizumab to be dominant. Neither study considered severe systemic side effects. Although these studies did not incorporate CATT data, their findings that bevacizumab was considerably more cost-effective than ranibizumab are similar to ours, generated using head-to-head vision and side-effects data from CATT.
Two-year results were recently reported by the other large RCT: Inhibit VEGF in Age-related choroidal Neovascularization (IVAN).50 The change in BCVA and safety outcomes from IVAN are similar to CATT’s and show bevacizumab to be noninferior in efficacy to ranibizumab. Although these outcomes are nonstatistically different from CATT, if anything, IVAN shows bevacizumab in a slightly better light (better visual improvement, fewer safety events) than ranibizumab; thus, if we recreated our analyses using IVAN data, bevacizumab would probably seem even more cost-effective, compared with ranibizumab, than we are reporting.
Although the RCTs provide strong evidence of noninferior efficacy between ranibizumab and bevacizumab, some contend that providers should use ranibizumab instead of bevacizumab because intravitreal bevacizumab carries an increased risk for serious systemic side effects.51 The evidence for an elevated side-effect risk often comes from comparisons of systemic use, not intravitreal injection, of these agents to treat patients with colon and gastric cancers.52,53 The CATT trial monitored participants for serious systemic side effects, such as CVA, MI, and VTE, and found no major differences in the agents’ adverse event rates; however, CATT was inadequately powered to determine with certainty whether any potentially significant differences in safety exist between the two agents. In addition, CATT did identify higher rates of serious adverse events with bevacizumab use that were previously unreported with anti-VEGF treatment. Because these are described in insufficient detail to analyze their costs and HRQL impact, we excluded them in the analysis. Future research could explore whether these events could affect conclusions about the relative cost-effectiveness of bevacizumab and ranibizumab. Finally, because participants in the monthly arms were randomly assigned to as-needed treatment after 1 year, there is a particular lack of power for monthly outcomes in the second year. Recent studies have found that serum levels of VEGF in patients with neovascular AMD may differ between ranibizumab and bevacizumab users. Carneiro and colleagues54 found that after 3 monthly injections, serum VEGF concentrations were significantly lower in bevacizumab-treated patients than in ranibizumab users, despite the groups’ similar VEGF levels at baseline. Thus, bevacizumab may have more effects on the cardiovascular system than ranibizumab does. In our sensitivity analysis exploring the impact of potential differences in the agents’ risk for serious systemic side effects, bevacizumab recipients would need to experience 3 times as many serious vascular events per year as ranibizumab recipients for ranibizumab to be the preferred treatment. Also debatable is how often anti-VEGF agents must be administered to maintain their effectiveness. In CATT, vision improved slightly more in monthly anti-VEGF users than in as-needed users.11,12 In general, the expense per injection is high (especially with ranibizumab); thus, future confirmation that visual gains are similar between patients receiving monthly injections and those receiving less frequent injections could substantially affect the relative value of each treatment option. We find that monthly bevacizumab is considerably less cost-effective than as-needed bevacizumab ($242 357/QALY), whereby it misses the $100 000/QALY cutoff often used to designate whether an intervention is acceptable.42 However, monthly bevacizumab would be preferred over as-needed bevacizumab if only the injection costs, without accompanying costs for visits and OCT scans, are considered.
With a recent U.S. outbreak of fungal meningitis associated with the use of medications from compounding pharmacies, the very small, albeit real, increased risk for serious ocular side effects such as endophthalmitis with use of bevacizumab from compounding pharmacies is a pertinent consideration for providers. Ranibizumab, in contrast, comes directly from the drug manufacturer. In one of our two-way sensitivity analyses, we simultaneously varied the cost per bevacizumab injection and endophthalmitis risk to capture the increased cost associated with the presumably safer, single-use vials of bevacizumab rather than the cheaper bevacizumab packaged in multiuse containers. We find that the risk for endophthalmitis would need to be 3 times greater with multiuse containers than with single-use vials for the latter to become the preferred treatment at today’s prices.
We evaluated which treatment option confers the greatest value from a societal perspective. Because health care resources are not unlimited, there is an opportunity cost associated with choosing therapies that are relatively costly or less effective. For example, Raftery and coworkers note that a year of treatment with monthly ranibizumab at £1000 (roughly $1615 USD) per injection costs £300 million ($485 million), whereas monthly bevacizumab would cost £8 million ($13 million) annually, resulting in a £292 million ($472 million) savings.25 These sorts of analyses can be performed from other perspectives, such as those of (a) Medicare patients, many of whom lack the supplemental insurance to cover a co-pay of 20% of the cost of injections; (b) eye-care providers who must expend resources to safely store these medications; and (c) the manufacturer, Genentech, who sells both these anti-VEGF drugs. The inputs to the model would vary considerably depending on whose perspective is considered.
Our study has several limitations. The CATT trial compared the efficacy, need for additional injections, and side effects from injections over only 2 years. Extrapolating the CATT findings beyond year 2 is challenging because little is known about the longer-term natural history of neovascular AMD among ranibizumab or bevacizumab users. Although we performed sensitivity analyses to address the uncertainty of the various model parameters beyond year 2, varying model inputs beyond the ranges used in our analyses could affect our findings. In addition, participants in a clinical trial such as CATT may differ systematically from other patients in their health behavior, affecting the generalizability of the findings.
Another limitation involves the assumption that BCVA is an acceptable surrogate for the impact of neovascular AMD on overall HRQL. Visual needs vary among patients, and different BCVA levels could differentially affect patients’ overall HRQL. Unfortunately, CATT collected no additional information on HRQL that we could incorporate in our models. Finally, although increased anti-VEGF use can worsen geographic atrophy, we could not incorporate this into our models because the frequency and extent of this effect among recipients of the different treatments are unclear.
As treatment paradigms for patients with neovascular AMD continue to rapidly evolve, some of these analyses will need to be revisited in the future. Such research should explore the incremental cost-effectiveness of newer medications, such as aflibercept (Regeneron Pharmaceuticals, Tarrytown, NY), relative to ranibizumab and bevacizumab. Other newer treatments aimed at reducing the need for frequent intravitreal injections are currently in development or testing; for example, a Phase III clinical trial is testing injection of viral vectors coding for a molecule with anti-VEGF properties that is secreted in the eye (Genzyme, Cambridge, MA), and an implant currently in a Phase II trial slowly secretes anti-VEGF medications (Neurotech Pharmaceuticals, Cumberland, RI). If these or other new treatments yield greater improvements in BCVA, provide longer stabilization of disease, or cost less than existing options, they may confer greater value. In addition to exploring new medications as they become available, researchers could conduct cost-effectiveness analyses to compare newer treatment algorithms, such as “treat and extend,” with more-established protocols. Finally, since our probabilistic sensitivity analysis found that ranibizumab was the preferred treatment in 14–17% of Monte Carlo simulations, additional research should explore the effects of varying some of the model parameters.
In conclusion, among the four treatment options for neovascular AMD tested in CATT, bevacizumab with as-needed dosing confers the greatest value. Ranibizumab dosed monthly or as needed confers considerably less value than bevacizumab, mainly because of ranibizumab’s considerably higher per-injection cost. Monthly bevacizumab treatment is preferred over as-needed bevacizumab therapy if providers bill for the injection alone, without accompanying visit or OCT charges. Insurers and health policymakers should consider endorsing the use of intravitreal bevacizumab over other treatments as first-line therapy for neovascular AMD, as this may curtail some of the rapidly rising costs of managing patients with this condition.
Supplementary Material
Acknowledgments
Funding/support: National Eye Institute K23 Mentored Clinician Scientist Award (1K23EY019511-01); Grant Number P30DK092926 from the National Institute of Diabetes and Digestive and Kidney Diseases; Research to Prevent Blindness “Physician Scientist” Award; and an unrestricted grant from Research to Prevent Blindness
Footnotes
Financial Disclosures: The authors have no proprietary or commercial interest in any material discussed in this manuscript. Paul P. Lee, MD, JD, is a consultant to Genentech; however, this company was not involved in the conception, design, or conduct of this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Advisory Eye Council. Vision Research, a National Plan, 1994–1998. Washington, D.C: U.S. Department of Health and Human Services; 1993. pp. xx–xx. NIH publication 93–3186. [Google Scholar]
- 2.Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–72. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1992;99:933–43. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 4.Hassell JB, Lamoureux EL, Keeffe JE. Impact of age related macular degeneration on quality of life. Br J Ophthalmol. 2006;90:593–6. doi: 10.1136/bjo.2005.086595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scilley K, Jackson GR, Cideciyan AV, et al. Early age-related maculopathy and self-reported visual difficulty in daily life. Ophthalmology. 2002;109:1235–42. doi: 10.1016/s0161-6420(02)01060-6. [DOI] [PubMed] [Google Scholar]
- 6.Macular Photocoagulation Study Group. Argon laser photocoagulation for senile macular degeneration. Results of a randomized clinical trial. Arch Ophthalmol. 1982;100:912–8. doi: 10.1001/archopht.1982.01030030920003. [DOI] [PubMed] [Google Scholar]
- 7.Macular Photocoagulation Study Group. Argon laser photocoagulation for neovascular maculopathy. Five-year results from randomized clinical trials. Arch Ophthalmol. 1991;109:1109–14. [PubMed] [Google Scholar]
- 8.Wormald R, Evans J, Smeeth L, Henshaw K. Photodynamic therapy for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2005;(3):CD002030. doi: 10.1002/14651858.CD002030.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Rosenfeld PJ, Brown DM, Heier JS, et al. MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 10.Brown DM, Kaiser PK, Michels M, et al. ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 11.CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Martin DF, Maguire MG, Fine SL, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388–98. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown GC, Brown MM, Brown HC, et al. A value-based medicine comparison of interventions for subfoveal neovascular macular degeneration. Ophthalmology. 2007;114:1170–8. doi: 10.1016/j.ophtha.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Smith DH, Fenn P, Drummond M. Cost effectiveness of photodynamic therapy with verteporfin for age related macular degeneration: the UK case. Br J Ophthalmol. 2004;88:1107–12. doi: 10.1136/bjo.2003.023986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colquitt JL, Jones J, Tan SC, et al. Ranibizumab and pegaptanib for the treatment of age-related macular degeneration: a systematic review and economic evaluation. Health Technol Assess. 2008;12:iii–iv. ix–201. doi: 10.3310/hta12160. [DOI] [PubMed] [Google Scholar]
- 16.Javitt JC, Zlateva GP, Earnshaw SR, et al. Cost-effectiveness model for neovascular age-related macular degeneration: comparing early and late treatment with pegaptanib sodium based on visual acuity. Value Health. 2008;11:563–74. doi: 10.1111/j.1524-4733.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 17.Wolowacz SE, Roskell N, Kelly S, et al. Cost effectiveness of pegaptanib for the treatment of age-related macular degeneration in the UK. Pharmacoeconomics. 2007;25:863–79. doi: 10.2165/00019053-200725100-00005. [DOI] [PubMed] [Google Scholar]
- 18.Brown MM, Brown GC, Brown HC, Peet J. A value-based medicine analysis of ranibizumab for the treatment of subfoveal neovascular macular degeneration. Ophthalmology. 2008;115:1039–45. doi: 10.1016/j.ophtha.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 19.Common Drug Review. Ranibizumab (Lucentis–Novartis Pharmaceuticals Canada Inc.), Indication--age-related macular degeneration (AMD) Ottowa, ON: Canadian Agency for Drugs and Technologies in Health; 2008. [Accessed October 20, 2013]. Overview of CDR clinical and pharmacoeconomic reports. Available at: http://www.cadth.ca/media/cdr/relatedinfo/cdr_trans_Lucentis_overview_Jul-30-08_e.pdf. [Google Scholar]
- 20.Hurley SF, Matthews JP, Guymer RH. Cost-effectiveness of ranibizumab for neovascular age-related macular degeneration. [Accessed October 20, 2013];Cost Eff Resour Alloc [serial online] 2008 6:12. doi: 10.1186/1478-7547-6-12. Available at: http://www.resource-allocation.com/content/6/1/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown A, Hodge W, Kymes S, et al. Management of neovascular age-related macular degeneration: systematic drug class review and economic evaluation [technology report number 110] Ottawa, ON: Canadian Agency for Drugs and Technologies in Health; 2008. [Accessed May 31, 2013]. pp. xx–xx. Available at: http://www.cadth.ca/en/products/health-technology-assessment/publication/813. [Google Scholar]
- 22.Fletcher EC, Lade RJ, Adewoyin T, Chong NV. Computerized model of cost-utility analysis for treatment of age-related macular degeneration. Ophthalmology. 2008;115:2192–8. doi: 10.1016/j.ophtha.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez-Pastor LJ, Ortega A, Garcia-Layana A, Giraldez J. Cost-effectiveness of ranibizumab compared with pegaptanib in neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2010;248:467–76. doi: 10.1007/s00417-009-1156-9. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell P, Annemans L, White R, et al. Cost effectiveness of treatments for wet age-related macular degeneration. Pharmacoeconomics. 2011;29:107–31. doi: 10.2165/11585520-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 25.Raftery J, Clegg A, Jones J, et al. Ranibizumab (Lucentis) versus Bevacizumab (Avastin): modelling cost effectiveness. Br J Ophthalmol. 2007;91:1244–6. doi: 10.1136/bjo.2007.116616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel JJ, Mendes MA, Bounthavong M, et al. Cost-utility analysis of bevacizumab versus ranibizumab in neovascular age-related macular degeneration using a Markov model. J Eval Clin Pract. 2012;18:247–55. doi: 10.1111/j.1365-2753.2010.01546.x. [DOI] [PubMed] [Google Scholar]
- 27.Dooren JC, Whalen J. Study compares Lucentis, Avastin. The Wall Street Journal. 2011 Apr 28; [Google Scholar]
- 28.Medicare payments for drugs used to treat wet age-related macular degeneration (OEI-03-10-00360) Department of Health and Human Services, Office of Inspector General; 2012. [Accessed October 20, 2013]. Available at: https://oig.hhs.gov/oei/reports/oei-03-10-00360.pdf. [Google Scholar]
- 29.Drummond MF, Sculpher MJ, Torrance GW, et al. Methods for the Economic Evaluation of Health Care Programmes. 3. Oxford: Oxford University Press; 2005. pp. xx–xx. [Google Scholar]
- 30.Lalwani GA, Flynn HW, Jr, Scott IU, et al. Acute-onset endophthalmitis after clear corneal cataract surgery (1996–2005): clinical features, causative organisms, and visual acuity outcomes. Ophthalmology. 2008;115:473–6. doi: 10.1016/j.ophtha.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Endophthalmitis Vitrectomy Study Group. Results of the Endophthalmitis Vitrectomy Study: a randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol. 1995;113:1479–96. [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. [Accessed May 31, 2013];CDC WONDER. Underlying Cause of Death, 1999–2010 [database online] Revised 2012. Available at: http://wonder.cdc.gov/ucd-icd10.html.
- 33.Frick KD, Gower EW, Kempen JH, Wolff JL. Economic impact of visual impairment and blindness in the United States. Arch Ophthalmol. 2007;125:544–50. doi: 10.1001/archopht.125.4.544. [DOI] [PubMed] [Google Scholar]
- 34.Mahan CE, Borrego ME, Woersching AL, et al. Venous thromboembolism: annualised United States models for total, hospital-acquired and preventable costs utilising long-term attack rates. Thromb Haemost. 2012;108:291–302. doi: 10.1160/TH12-03-0162. [DOI] [PubMed] [Google Scholar]
- 35.MacDougall DA, Feliu AL, Boccuzzi SJ, Lin J. Economic burden of deep-vein thrombosis, pulmonary embolism, and post-thrombotic syndrome. Am J Health Syst Pharm. 2006;63(suppl):S5–15. doi: 10.2146/ajhp060388. [DOI] [PubMed] [Google Scholar]
- 36.Freeman JV, Zhu RP, Owens DK, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med. 2011;154:1–11. doi: 10.7326/0003-4819-154-1-201101040-00289. [DOI] [PubMed] [Google Scholar]
- 37.Brown MM, Brown GC, Sharma S, Landy J. Health care economic analyses and value-based medicine. Surv Ophthalmol. 2003;48:204–23. doi: 10.1016/s0039-6257(02)00457-5. [DOI] [PubMed] [Google Scholar]
- 38.Aaberg TM, Jr, Flynn HW, Jr, Schiffman J, Newton J. Nosocomial acute-onset postoperative endophthalmitis surgery. A 10-year review of incidence and outcomes. Ophthalmology. 1998;105:1004–10. doi: 10.1016/S0161-6420(98)96000-6. [DOI] [PubMed] [Google Scholar]
- 39.Bajaj PS, Veenstra DL. A risk–benefit analysis of factor V Leiden testing to improve pregnancy outcomes: a case study of the capabilities of decision modeling in genomics. Genet Med. 2013;15:374–81. doi: 10.1038/gim.2012.139. [DOI] [PubMed] [Google Scholar]
- 40.Red Book Drug References [database] Truven Health Analytics Inc; [Google Scholar]
- 41.Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. pp. xx–xx. [Google Scholar]
- 42.Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What is the price of life and why doesn’t it increase at the rate of inflation? Arch Intern Med. 2003;163:1637–41. doi: 10.1001/archinte.163.14.1637. [DOI] [PubMed] [Google Scholar]
- 43.Brown GC, Brown MM, Sharma S, et al. Incremental cost effectiveness of laser photocoagulation for subfoveal choroidal neovascularization. Ophthalmology. 2000;107:1374–80. doi: 10.1016/s0161-6420(00)00169-x. [DOI] [PubMed] [Google Scholar]
- 44.Busbee BG, Brown MM, Brown GC, Sharma S. CME review: a cost-utility analysis of laser photocoagulation for extrafoveal choroidal neovascularization. Retina. 2003;23:279–87. doi: 10.1097/00006982-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Larouche K, Rochon S, Wickens M, translators. Evaluation of photodynamic therapy for the treatment of exudative age-related macular degeneration (ARMD) with subfoveal neovascularization. [Accessed October 20, 2013];Montreal: Agence d’ évaluation des technologies et des modes d’ intervention en santé. 2005 :xx–xx. Available from: http://collections.banq.qc.ca/ark:/52327/bs52604.
- 46.Brown GC, Brown MM, Campanella J, Beauchamp GR. The cost-utility of photodynamic therapy in eyes with neovascular macular degeneration--a value-based reappraisal with 5-year data. Am J Ophthalmol. 2005;140:679–87. doi: 10.1016/j.ajo.2005.04.061. [DOI] [PubMed] [Google Scholar]
- 47.Hopley C, Salkeld G, Mitchell P. Cost utility of photodynamic therapy for predominantly classic neovascular age related macular degeneration. Br J Ophthalmol. 2004;88:982–7. doi: 10.1136/bjo.2003.039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meads C, Salas C, Roberts T, et al. Clinical effectiveness and cost-utility of photodynamic therapy for wet age-related macular degeneration: a systematic review and economic evaluation. Health Technol Assess. 2003;7:v–vi. 1–98. doi: 10.3310/hta7090. [DOI] [PubMed] [Google Scholar]
- 49.Sharma S, Brown GC, Brown MM, et al. The cost-effectiveness of photodynamic therapy for fellow eyes with subfoveal choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology. 2001;108:2051–9. doi: 10.1016/s0161-6420(01)00764-3. [DOI] [PubMed] [Google Scholar]
- 50.IVAN Study Investigators. Chakravarthy U, Harding SP, Rogers CA, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119:1399–411. doi: 10.1016/j.ophtha.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 51.Recent publicly-funded CATT trial highlights serious safety concerns around unlicensed ocular bevacizumab use versus Lucentis [press release] Basel, Switzerland: Novartis; May 1, 2012. [Accessed August 22, 2013]. Available from: http://www.novartis.com/newsroom/media-releases/en/2012/1607728.shtml. [Google Scholar]
- 52.Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–5. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 53.Shah MA, Ilson D, Kelsen DP. Thromboembolic events in gastric cancer: high incidence in patients receiving irinotecan- and bevacizumab-based therapy [letter] J Clin Oncol. 2005;23:2574–6. doi: 10.1200/JCO.2005.81.908. [DOI] [PubMed] [Google Scholar]
- 54.Carneiro AM, Costa R, Falcão MS, et al. Vascular endothelial growth factor plasma levels before and after treatment of neovascular age-related macular degeneration with bevacizumab or ranibizumab [report online] [Accessed October 20, 2013];Acta Ophthalmol. 2012 90:e25–30. doi: 10.1111/j.1755-3768.2011.02240.x. Available at: http://onlinelibrary.wiley.com/doi/10.1111/j.1755-3768.2011.02240.x/pdf. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.