Abstract
Objective
Uncontrolled intervention studies, including studies involving breast cancer survivors, have demonstrated improvements in vasomotor symptoms (VMS) following stellate ganglion blockade (SGB) with local anesthetic. This study presents the first randomized, sham-controlled trial of SGB for the treatment of VMS.
Methods
Participants included 40 postmenopausal women aged 30 to 70 years with moderate-to-severe VMS. The design was a randomized, sham-controlled trial comparing the effect of SGB versus sham injection on the frequency of total and moderate-to-severe VMS as measured by daily diaries. Image-guided SGB was performed with 0.5% bupivacaine 5 mL. Sham injection of saline was performed in the subcutaneous tissue in the neck. VMS were recorded at baseline and for six months thereafter. Objective VMS were recorded using ambulatory sternal skin conductance monitoring over a 24-hour period at baseline and 3-month follow-up.
Results
There were no significant group differences in overall VMS frequency, but the frequency of moderate-to-very severe VMS was reduced more in the active compared to sham treatment group, RR 0.50, CI 0.35–0.71, p<0.001. The frequency of objective VMS was also reduced to a greater degree in the SGB group compared to the sham group (RR 0.71, CI 0.64–0.99, p<0.05). There were no study-related serious adverse events.
Conclusions
SGB may provide an effective treatment for VMS in women who seek non-hormonal therapies due to safety concerns and personal preference. The finding that SGB significantly reduces objectively measured VMS provides further evidence of efficacy. A larger trial is warranted to confirm these findings.
Keywords: menopause, hot flashes, hot flushes, vasomotor symptoms, stellate ganglion injections, non-hormonal treatment
Introduction
Hot flashes and night sweats (i.e., vasomotor symptoms, VMS) affect 80% of women as they transition through menopause1. The severity of VMS is especially high for women who undergo surgical menopause or early menopause due to treatments for breast cancer. Hormone therapy (HT) is the most effective treatment for VMS.2 In meta-analysis of placebo-controlled trials, HT reduces VMS frequency by 75% and VMS severity by 87% compared to placebo.3 Many women, however, seek non-hormonal therapies for VMS due to safety concerns and personal preference. Gabapentin and clonidine are effective non-hormonal treatments for reducing VMS4,5, but their use is limited because of modest symptom improvement, undesirable side effects (pedal edema, weight gain and blurred vision with gabapentin and constipation, orthostatic hypotension and dry mouth with clonidine). In addition, these agents lack FDA approval for treatment of VMS. Botanical therapies (e.g., phytoestrogens, black cohosh) show relative inefficacy,6,7 and lifestyle interventions are at best marginally more effective than placebo in relieving VMS.8,9
In 2013, the Food and Drug Administration (FDA) approved, paroxetine, a selective serotonin reuptake inhibitor (SSRI; 7.5 mg), as the first non-hormonal treatment for VMS10. As with hormone therapy, about a third of women experience a relapse of vasomotor symptoms after discontinuing SSRIs11. The product label warns of a possible reduction in the effectiveness of tamoxifen when taken with paroxetine, of obvious concern for women being treated for breast cancer, in addition to an increased risk of bleeding and a risk of developing serotonin syndrome (i.e., confusion, rapid heart rate, and high blood pressure)11. Indeed, the use of SSRIs for treatment of VMS is limited by lower effectiveness of SSRIs when compared to HT, as well as side effects and relapse of symptoms following treatment discontinuation12. Identifying safe and effective non-hormonal treatments for VMS remains a priority in women’s health research.
Uncontrolled open-label intervention studies have demonstrated improvements in the frequency and intensity of VMS following stellate ganglion blockade (SGB) with local anesthetic, with effects ranging from 34–90% reduction 4 weeks to several months after blockade.13–16 Although the exact mechanism is not fully understood, treatment with SGB is based on the interruption of the sympathetic nervous system and may affect blood flow and modulate norepinephrine levels in thermoregulatory areas of the brain. This trial compared SGB with bupivacaine to a sham procedure involving saline injection on VMS in women with natural or surgical menopause over a 6-month follow-up. The primary outcomes were the frequency of total and moderate-to-very severe VMS reported at the end of the 6-month follow-up and the frequency of objective VMS measured using ambulatory skin conductance monitors at the end of the 3-month follow-up.
Methods
Patients
Participants underwent an informed consent process including provision of written informed consent before any study procedure and were compensated for their time and effort. Women aged 30 to 70 years of age with natural or surgical menopause and moderate-to-very severe VMS (defined as ≥ 25 reported VMS per week, criteria used in prior VMS studies7) willing to undergo fluoroscopy-guided SGB were recruited for this study. The study was advertised with IRB-approved flyers, Chicago Transit Authority (CTA) advertising, and the internet. Participants were initially screened by telephone to evaluate interest and eligibility criteria and consented in person. Participants were confirmed to have ≥ 25 VMS per week in paper diaries for a minimum of 2 weeks prior to randomization to treatment group. Exclusionary criteria included: American Society of Anesthesiologists (ASA) physical status score > 2 (indicating more than one systemic disease); anatomic abnormalities of the anterior neck or cervical spine; cardiac/pulmonary compromise; acute illness/infection; coagulopathy/ bleeding disorder; allergic reactions/contraindications to local anesthetic or contrast dye; use of oral or transdermal hormones; conditions or disorders that affect cognitive functioning (including stroke, severe brain injury, loss of consciousness, current use of SSRIs, SNRIs, or gabapentin); current or past diagnosis of psychosis; current diagnosis of depression, alcohol or substance abuse; and conditions that invalidate cognitive testing procedures (e.g. inability to write, speak, or read in English).
The Institutional Review Boards at Northwestern University and the University of Illinois at Chicago approved this study. The trial study was registered on www.clinicaltrials.gov (NCT00992914).
Randomization and Masking
A computer-generated 1:1 block randomization scheme was used to assign participants to receive either a SGB with bupivacaine or a sham injection with saline. Randomization was performed by the injectionist immediately before the injection procedure by opening an opaque envelope to reveal the participant number and group assignment printed on an index card. Participants and all other study personnel were blinded to group assignment. Only the injectionist and statistician were unblinded at the conclusion of the study. One board certified anesthesiologist (DRW) with 15 years of injection experience performed all injections.
Procedures
At the time of the injection procedure, a 20-g angiocatheter was placed in the hand or arm for peripheral intravenous access as a safety precaution. Participants were positioned supine in cervical extension. The anterior neck was prepped with chlorhexidine and draped in the standard sterile manner. For active SGB, a right-sided SGB was performed. Using fluoroscopic guidance, the C6 vertebrae was identified and the skin overlying the tubercle was anesthetized using 2 mL of 1% lidocaine. Using digital pressure to laterally retract the carotid artery, a 22 g 1.5-inch needle was placed to make contact with the anterolateral portion of the C6 vertebra and then retracted 1–2 mm and secured; contrast material (iopamidol 1–2 mL) was injected with fluoroscopic guidance to confirm contrast dye spread in the prevertebral fascial plane and to rule out intravascular or intrathecal dye spread. 0.5% bupivacaine (5 mL) was injected and the needle was removed. For sham injection, the same positioning, monitoring, sterile preparation and technique were used with identical visual, auditory and tactile cues, except the needle was placed in the superficial tissues overlying the C6 tubercle. With fluoroscopic guidance, contrast material (iopamidol 1–2 mL) was injected to confirm contrast dye spread in the subcutaneous tissues, not in the plane of the stellate ganglion. Preservative-free saline (5 mL) was injected and the needle was then removed. Participants were transferred to a recovery area and monitored in a reclining position for at least 30 minutes to assess potential adverse effects of the injection. Presence of a Horner’s sign (miosis, ptosis, anhydrosis) was recorded and validated successful SGB.
Outcome Measures
The primary outcomes were the frequency of total and moderate-to-very severe VMS as measured by daily diaries and the frequency of objective VMS measured using ambulatory skin conductance monitors. Secondary outcomes included sleep quality, depression, and quality of life. Measures were performed in conjunction with cognitive assessments, prior to the injection treatment and again three months later. These measures are commonly evaluated in menopause studies.17–21 For a minimum of two weeks before the injection procedure and for six months thereafter, participants recorded the frequency and severity of daily VMS in a paper diary. Participants were instructed to rate each hot flash as “mild” (< 5 minutes, warm, red face, uncomfortable), “moderate” (< 15 minutes, warmth involving neck, ears, head, whole body, with perspiration, clammy skin, dry mouth, tense muscles, tachycardia, irritation, agitation, embarrassment), “severe” (< 20 minutes, warmth described as a raging furnace or burning up, weak, faint, headache, chest heaviness, extreme perspiration, prickling sensation over skin, heart irregularities, anxious, panic attacks) or “very severe” (< 45 minutes, boiling eruption, rolling perspiration, inability to breathe, faint/dizzy, leg/foot cramps, heart irregularities, difficulty functioning, distressed, nausea).22 Intensity of daytime hot flashes was calculated based on the equation: Intensity = Frequency*Severity = [(frequency of mild*1) + (frequency of moderate*2) + (frequency of severe*3) + (frequency of very severe*4)] and used as a secondary endpoint.23 Frequency of night sweats was also recorded daily via self-report the following morning. Baseline VMS frequency was calculated as the mean of the daily count totals on diaries during the first two screening weeks. Week 1 VMS frequency was calculated as the mean of the daily count totals reported in the first seven days after injection. VMS frequency at Months 1 to 6 was calculated as the mean of the daily count totals reported for the 30 days before each visit.
To measure VMS objectively, participants were fitted with an ambulatory sternal skin conductance monitor (Biolog Model 3991 x/2-HFI) featuring two skin conductance electrodes connected to the sternum by adhesive electrode pads (UFI, 1081-HFD). The monitor was placed inside a small pouch that was worn on a belt or slung over the shoulder. Both objective (i.e., > 2.0 μmho increase in 30 sec) and subjective (button press) VMS were recorded with the monitor according to standard procedures24. Participants were instructed to push two red buttons on the monitor when they experienced a hot flash. These events were time stamped to record the time of a subjective hot flash. Participants also kept a diary of the time, severity, and intensity of the hot flash. Raw hot flash data was transmitted from the monitor to a PC using the Biolog Interface Box. Time series of skin conductance data in μmho units were shown in a time-based graphical display showing subjective VMS (i.e., event markers) and objective VMS (see below) using specialized software (DPS v.1.5, UFI, Morro Bay, CA, USA). Raw objective hot flash data were analyzed by a combination of automated computer software and two trained data coders. According to standard procedures, once an objective hot flash was coded, no other VMS were coded for the next 15 minutes.25 Data were independently double-scored and double-entered into the database by coders blinded to treatment assignment. Frequency of objective and subjective VMS during sleeping and waking hours were scored based on reports in hot flash diaries of the time participants went to bed and the time they woke up while being monitored. Depressive symptoms were assessed by the Center for Epidemiological Studies – Depression Scale (CES-D), a 20-item self-report measure of depressive symptoms over the past week. A score ≥16 was considered severe. Sleep quality was assessed with the Modified Pittsburgh Sleep Quality Index (PSQI) for the preceding one month period.26 A total sleep score was calculated using an established scoring scheme, with higher scores indicating greater sleep disturbance.26 Quality of life was measured with the Utian Quality of Life (UQOL), a validated, subjective appraisal of life satisfaction and well-being.27 Participants rated 23 questions about four life domains -occupational, health, sexual, and emotional on a 5-point Likert scale (1 = “not true of me,” 5 = “very true of me”) for the preceding one month period. The outcome was a total score derived by summing the separate domain scores.
Statistical Analysis
Baseline characteristics were compared between treatment groups using t-tests or chi-square tests for categorical variables. A modified intent-to-treat (ITT) analyses, whereby inclusion of all randomized participants who provided diary data, was performed using a series of mixed-effects regressions (random intercept only). This method is consistent with previous approaches used in randomized controlled trials of escitalopram and paroxetine for the treatment of vasomotor symptoms.10,28 Specifically, mixed-effects Poisson regressions were used for count data (e.g., mean daily count of subjective and objective VMS). Exponentiation of the unstandardized Beta coefficients from these models yields event rates/event rate ratios. Event rates provide information on the expected number of events that occur over a given period of time. From each model, an event rate ratio is computed for each group using the following equation: (Expected number of VMS at baseline – Expected number of VMS at a subsequent time point)/ (Expected number of VMS at baseline). The Ratio of Event Rate Ratios (RRR) provides a statistical comparison of the expected change in VMS from baseline to a subsequent time point between the SGB and sham-control group. Specifically, the ratio of event rate ratios simply divides the event rate ratio for the SGB group by the event rate ratio for the sham-control group.
Mixed-effects regression was used for continuous outcomes (e.g., subjective VMS intensity). For subjective VMS and menopausal symptoms, independent predictors included Treatment Group (active SGB vs. sham control), dummy variables for week 1 (vs. baseline), months 1–3 (vs. baseline), and months 4–6 (vs. baseline) as well as interactions between Treatment Group and each dummy variable. For menopausal symptoms, the dummy variables reflected week 3 (vs. baseline) and month 3 (vs. baseline). For objective VMS outcomes, independent predictors included Treatment Group, a dummy variable for month 3 (vs. baseline), and their interaction.
SAS statistical software version 9.2 (SAS Institute Inc., Cary, NC, USA) was used for statistical analyses. Significance was set at p<0.05.
Power for total subjective hot flashes was calculated using PASS 11.0 (Power Analysis and Sample Size) software and was based on a mixed design with one between subjects factor (Treatment: Sham-control, SGB) and one within subjects factor (Time: Baseline, Post-treatment) and a Geisser-Greenhouse Corrected F-test with 5% significance level, using data from a previously published pilot study.13 A sample size of 20 participants in each treatment group provided at least 80% power to test the two-way interaction between Treatment and Time.
Results
Two hundred and sixty-six women expressed interest in participation and contacted the site between February, 2009 and November, 2012 (see Figure 1). Of these, sixty one women enrolled and provided written informed consent. Twenty one women failed screening primarily due to insufficient number of hot flushes and 40 participants were randomized. Participants were followed for 6 months after injection procedures. There were no statistically significant group differences in baseline demographic characteristics, VMS symptoms, or menopausal symptoms (Table 1).
Figure 1.

Participant Flow Diagram.
Table 1.
Demographic and Clinical Characteristics by Treatment Group at Baseline.
| Treatment Group | |||
|---|---|---|---|
|
| |||
| SGB (n=20) | Sham-control (n=20) |
||
| M (SD) | M (SD) | p-value | |
| Demographics | |||
| Age | 51.70 (2.36) | 52.90 (4.09) | 0.26 |
| Body Mass Index (BMI) | 28.20 (5.45) | 28.05 (5.09) | 0.93 |
| Race, n (%) | 0.24 | ||
| White | 6 (30) | 11 (55) | |
| Black | 11 (55) | 8 (40) | |
| Hispanic | 3 (15) | 1 (5) | |
| Menopause, n (%) | 0.15 | ||
| Natural | 17 (85) | 13 (65) | |
| Surgical | 3 (15) | 7 (35) | |
| Postmenopause Status, n (%) | 0.75 | ||
| <5 years since last LMP | 12 (60) | 13 (65) | |
| Menopausal Symptoms | |||
| CES-D Depressive symptoms | 11.11 (7.06) | 12.40 (8.92) | 0.62 |
| PSQI Global Score | 8.63 (3.70) | 10.55 (4.87) | 0.18 |
| UQOL Total Score | 80.53 (9.77) | 82.26 (11.03) | 0.61 |
| Vasomotor Symptoms (Daily Count) | |||
| Subjective (via diaries) | |||
| Mild | 1.20 (1.46) | 1.99 (2.14) | 0.18 |
| Moderate-to-very severe | 4.75 (2.28) | 5.73 (3.66) | 0.31 |
| Night Sweats | 2.13 (1.74) | 2.07 (1.73) | 0.92 |
| Total† | 8.08 (3.08) | 9.89 (5.82) | 0.22 |
| Intensity | 12.52 (5.69) | 15.90 (11.18) | 0.24 |
| Objective (via monitor)‡ | |||
| Total | 15.07 (12.41) | 10.10 (9.94) | 0.22 |
| Awake | 12.32 (10.65) | 7.34 (7.68) | 0.14 |
| Asleep | 2.75 (2.12) | 2.75 (3.96) | 0.99 |
Note. LMP=last menstrual period. SGB= Stellate Ganglion Blockade. CES-D = Center for Epidemiological Studies – Depression Scale. PSQI=Pittsburgh Sleep Quality Index. UQOL=Utian Quality of Life Scale.
Total=mild, moderate, severe, very severe, and night sweats.
Valid objective data was available on 35/40 women (18 SGB; 17 sham-control). Refer to text for definition of intensity.
The mean (SD) daily frequency of total subjective VMS at baseline was 9.85 (8.58), with 63% VMS rated as moderate-to-very severe. The modified ITT analysis of mean daily count of all VMS (mild, moderate, severe, very severe, and night sweats) showed no significant treatment group differences in VMS frequency from baseline to Week 1 or to Month 1 to 3 post-injection (Table 2; Figure 2A). The sham-control group showed a significant placebo effect of 34% until three months post-injection. At Months 4–6, total VMS were notably reduced in the SGB group (34% reduction) compared to the sham-control group (18% reduction), but this difference did not reach statistical significance (p=0.10). In the modified ITT analysis of moderate-to-very severe VMS, SGB-treated women showed significantly greater reductions (52%) from baseline to Months 4–6 compared to the sham-control group (4%) (Figure 2B). This same pattern of effects was observed for VMS intensity (Figure 3). SGB-treated women showed significantly greater reduction (38%) from baseline to 4–6 months (B=−4.80, SE=1.23, p<0.001) compared to the sham-control group (8%) (B=−1.30, SE=1.28, p=0.31) (Treatment × Time: B=−3.50, SE=1.78, p=0.04).
Table 2.
Modified Intent to Treat Analysis: Estimated Event Rate Ratio and 95% Confidence Interval as a Function of Time for Subjective Vasomotor Symptoms for Women Randomized to Active Stellate Ganglion Blockade (SGB) or the sham-control.
| Treatment Group | Treatment Group × Time |
||
|---|---|---|---|
| SGB | Sham-control | ||
|
|
|||
| Primary Outcomes | RR (95%CI) | RR (95%CI) | RRR (95%CI) |
| Baseline to Week 1 | |||
| Total | 0.76 (0.61–0.96)* | 0.75 (0.61–0.93)** | 1.01 (0.74–1.39) |
| Moderate-to-very severe | 0.73 (0.54–1.00)‡ | 0.74 (0.56–0.98)* | 0.99 (0.65–1.50) |
| Baseline to Months 1–3 | |||
| Total | 0.68 (0.57–0.83)*** | 0.68 (0.56–0.80)*** | 1.03 (0.80–1.34) |
| Moderate-to-very severe | 0.62 (0.48–0.80)*** | 0.69 (0.55–0.87)** | 0.90 (0.64–1.27) |
| Baseline to Months 4–6 | |||
| Total | 0.66 (0.54–0.81)*** | 0.82 (0.69–0.98)* | 0.81 (0.62–1.03)T |
| Moderate-to-very severe | 0.48 (0.36–0.63)*** | 0.96 (0.77–1.20) | 0.50 (0.35–0.71)*** |
Note.
p<0.001;
p<0.01;
p<0.05;
p=0.05;
p=0.10; SGB=Stellate Ganglion Blockade; CI = Confidence Interval; RR = Event Rate Ratio; RRR=Ratio of Event Rate Ratios. Results are reflective of a mixed effects Poisson regression (random intercept only).
Figure 2.
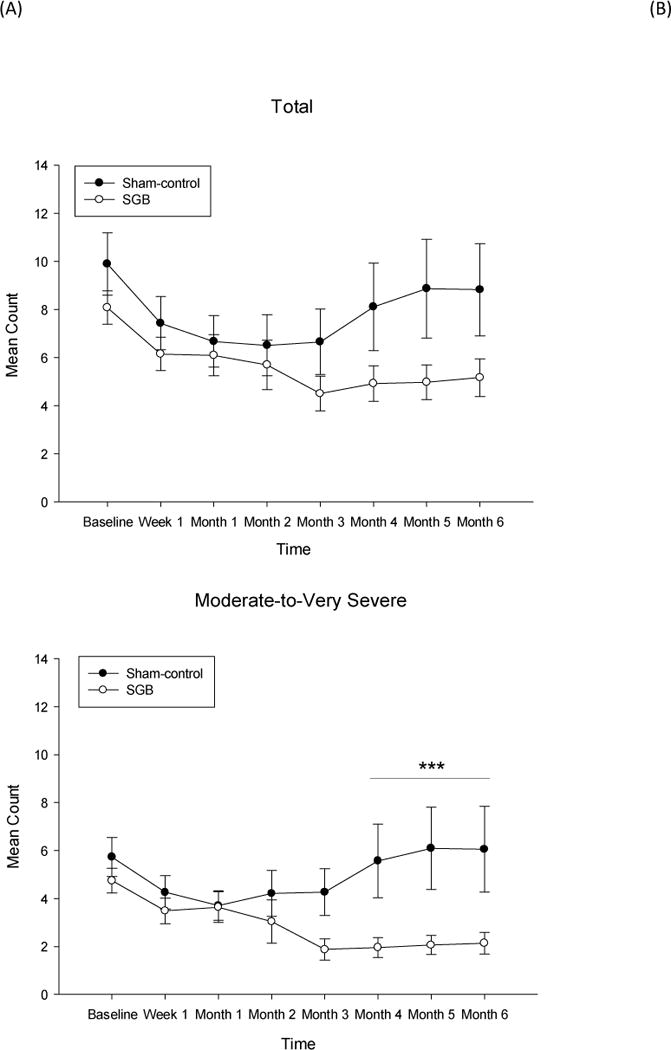
Modified Intent-to-Treat analysis of the mean daily count of (A) total subjective vasomotor symptoms and (B) moderate-to-very severe vasomotor symptoms.
Note. SGB=Stellate Ganglion Blockade. Total=mild, moderate, severe, very severe, and night sweats. Data points represent mean and standard error at each time point. Results are reflective of a mixed-effects Poisson regression model for count data. *** Treatment × Time (baseline to Months 4 to 6) Interaction p<0.001.
Figure 3.
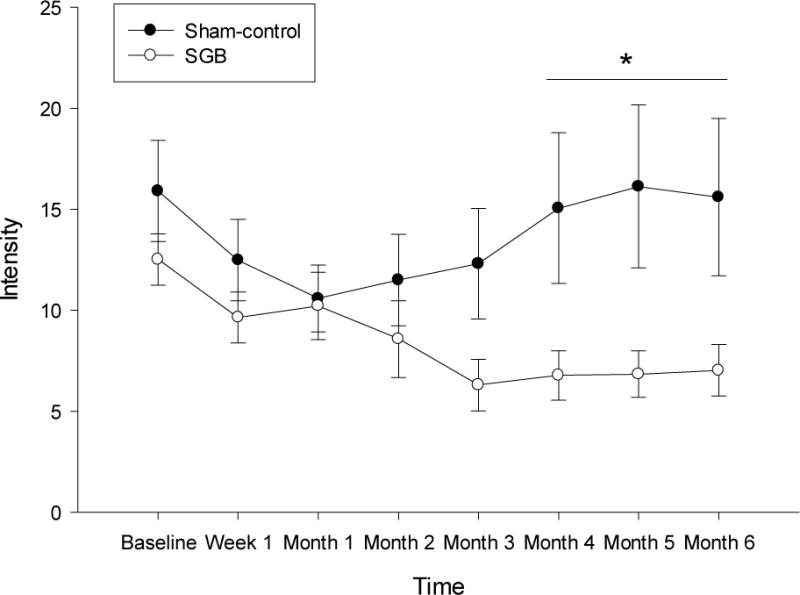
Modified Intent-to-Treat Analysis of Vasomotor Symptom Intensity (Frequency* Severity) as a function of treatment (Active SGB versus Placebo) over 12 months of Follow-up.
Note. SGB=Stellate Ganglion Blockade. Data points represent mean and standard error at each time point. Results are reflective of a mixed-effects regression model for continuous data. There were no treatment group differences on VMS intensity from baseline to 1 week (Treatment × Time: B=0.54, SE=2.09, p=0.80) or from baseline to 1–3 months (Treatment × Time: B=0.96, SE=1.74, p=0.58) post-injection. However, SGB-treated women showed significantly greater reductions in VMS intensity from baseline to 4–6 months (B=−4.80, SE=1.23, p<0.001) compared to the sham-control group (B=−1.30, SE=1.28, p=0.31) (Treatment × Time: B=−3.50, SE=1.78, p=0.04).
Valid objective VMS data were available for 18 women in the SGB group and 17 in the sham-control group. The total number of objective VMS from baseline to 3 months was reduced by 21% in the SGB group whereas the sham-control group showed no reduction, for a significant group difference, p<0.05 (See Table 3).
Table 3.
Estimated Mean Counts (95% confidence interval) and Estimated Event Rate Ratio and (95% confidence interval) as a Function of Time for Objective Vasomotor Symptoms for Women Randomized to Active Stellate Ganglion Blockade (SGB) or the sham control.
| Treatment Group | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| SGB (n=18) | Sham-Control (n=17) | ||||||
|
| |||||||
| Baseline | 3-months Post | Baseline | 3-months Post | ||||
| M (95%CI) | M (95%CI) | RR (95%CI) | M (95%CI) | M (95%CI) | RR (95%CI) | RRR (95%CI) | |
| Total | 7.21 (3.68–14.1) | 5.74 (2.92–11.3) | 0.79 (0.64–0·98)* | 5.60 (2.85–11.0) | 6.24 (3.12–12.5) | 1.11 (0.86–1.43) | 0.71 (0.64–0.99)* |
| Awake | 5.84 (2.93–11.6) | 4.33 (2.17–8.66) | 0.74 (0.59–0·94)* | 3.95 (1.97–7.96) | 4.48 (2.19–9.17) | 1.13 (0.84–1.52) | 0.66 (0.45–0.96)* |
| Sleep | 1.66 (0.84–3.29) | 1.66 (0.84–3.30) | 1.00 (0.63–1.60) | 1.36 (0.67–2.77) | 1.45 (0.69–3.04) | 1.06 (0.63–1.71) | 0.94 (0.48–1.83) |
Note.
p<0.05. SGB=Stellate Ganglion Blockade; CI= Confidence Interval. RRR=Ratio of Event Rate Ratios. Results are reflective of a mixed effects Poisson regression (random intercept only).
The SGB group showed trends towards improvement in depressive symptoms at Week 3 (B=−2.25, SE=1.31, p=0.09) and Month 3 (B=−2.45, SE=1.26, p=0.069), whereas the sham control group showed no improvement (Table 4; Figure 4). The group difference at Month 3, however did not meet statistical significance, p<0.10. There were no improvements in either treatment group on sleep or quality of life from baseline to Week 3 or Month 3.
Table 4.
Estimated means (SE) and estimated change scores across time for menopausal symptoms for women randomized to Stellate Ganglion Blockade (SGB) or placebo.
| Treatment Group | Treatment Group × Time (Δ baseline to 3 weeks) | Treatment Group × Time (Δ baseline to 3 months) | ||||
|---|---|---|---|---|---|---|
| SGB (n=20) | Sham-control (n=20) | |||||
|
| ||||||
| Δ Baseline to 3 weeks |
Δ Baseline to 3 months |
Δ Baseline to 3 weeks |
Δ Baseline to 3 months |
|||
| Outcomes | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) |
| Depressive Symptoms (CES-D) | −2.25 (1.31)T | −2.45 (1.26)‡ | 0.05 (1.38) | 1.06 (1.29) | −2.30 (1.91) | −3.51 (1.81)‡ |
| Utian Quality of Life (UQOL) | ||||||
| Total | 2.42 (1.91) | 0.53 (1.84) | −0.34 (2.01) | −1.85 (1.88) | 2.76 (2.77) | 2.39 (2.63) |
| Pittsburgh Sleep Quality Index (PSQI) | ||||||
| Global Score | 0.51 (0.79) | −0.97 (0.76) | −0.12 (0.86) | −0.59 (0.76) | 0.63 (1.17) | −0.38 (1.08) |
Note.
p=0.06;
p=0.09; B=Beta; SE=Standard Error of Beta; Δ=change in score between two time-points; SGB=Stellate Ganglion Blockade. CES-D = Center for Epidemiological Studies Depression Scale. PSQI and CES-D: higher is more severe symptoms; UQOL: higher is less severe symptoms.
Figure 4.
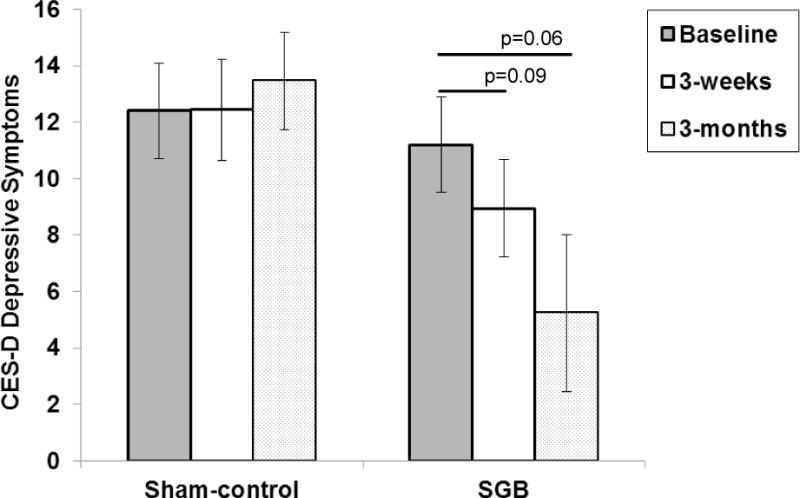
Changes in depressive symptoms for women randomized to SGB or sham-control.
Note. Data points represent mean and standard error at each time point. SGB=Stellate Ganglion Blockade. CES-D= Center for Epidemiological Studies Depression Scale.
There were no study-related serious adverse events. All participants who underwent SGB developed a Horner’s syndrome (miosis, ptosis, anhydrosis) immediately after the injection, confirming successful sympathetic blockade. No sham-controls exhibited a Horner’s syndrome. Only one injection was given to those in the SGB group. Two women in the placebo group opted to receive a second SGB injection three months after the first inject as allowed by study protocol.
Discussion
In this randomized sham-controlled trial, SGB led to a 52% reduction in the diary-reported frequency of moderate-to-very- severe VMS. In addition, a 38% reduction in VMS intensity (a measure that reflects both frequency and severity) was found in the SGB group as compared to sham controls, findings that were durable through the 6-month follow up. As expected, the sham control group showed a notable initial improvement in reported VMS frequency (akin to a placebo effect), but the effect of SGB was significantly greater and longer lasting. SGB reduced the frequency of all VMS regardless of severity by 19% more in the treatment group compared to the control group, but this difference was not statistically significant. Objective measures of hot flash frequency were 21% lower in women who received SGB as compared to sham.
These findings are consistent with non-randomized studies of SGB on women with severe VMS and a history of breast cancer. In an observational study in 13 breast cancer survivors with severe VMS, SGB reduced total VMS by nearly 90% during a 12-week follow-up period.13 SGB decreased mean hot flash frequency by 44% at six weeks in 10 breast cancer survivors who failed conventional VMS treatment.14 SGB led to a 47% decrease in hot flash scores at 24 weeks in 34 women with non-recurrent early stage postmenopausal breast cancer and severe VMS with a positive effect on sleep observed over the 24-week follow up.15 In another uncontrolled trial of SGB in 19 post-menopausal women, a 34% decrease in hot flash scores was seen at 4 weeks following SGB in “responders”, though 10 of the 19 women were “non-responders” with 0–11% reduction in hot flash scores. Quality of life and sleep measures were also significantly improved in “responders”.16 In contrast to these prior studies, SGB did not improve sleep quality in the present study, but a trend toward an improvement in depressive symptoms following SGB was seen that warrants further investigation, especially given evidence that SGB improved affective symptoms in patients with post-traumatic stress disorder.29
Stellate ganglion injections are considered safe when performed by experienced practitioners. In the current study, none of the participants experienced any serious adverse events. Severe injury related to stellate ganglion injections is rare when performed by experienced, skilled practitioners. The published incidence of complications with SGB, predating the common use of image guidance is 1.7 per 1,000 procedures and is related to intravascular injection of local anesthetic, resulting in temporary seizures related to local anesthetic.30 It follows that image-guided injections would have far fewer complications, as critical vascular or neural structures can be visualized in real time and thus can be avoided. However, safety concerns may evolve in the future if inadequately trained or inexperienced practitioners perform SGB, given the close proximity of critical structures like the vertebral artery, internal carotid artery, inferior thyroid artery, and spinal nerves.
The mechanism by which VMS occur with menopause is not well understood, and the mechanism by which SGB modulates these symptoms bears further study. Though the hypothalamus has long been considered to be the central thermoregulatory center, functional MRI studies have confirmed that the brain stem is activated immediately before a hot flash whereas activity in the insula only rises after the experience of the hot flash.31 One hypothesis is that the sympathetic nervous system induces activity in those regions. SGB causes increased blood flow in the head, neck, upper extremity and trunk via temporary sympatholysis. Blood flow changes to thermoregulatory regions of the brain could decrease VMS. Anatomic studies reveal connections between the stellate ganglion and thermoregulatory regions of the brain, specifically the insular cortex, via second and third-order synapses.32 Alternatively, SGB may modulate nerve growth factor (NGF) and norepinephrine, which increases centrally before and during a hot flash.33
To our knowledge, this is the first randomized sham-controlled trial of SGB for VMS. Unlike prior studies, no participants in the active arm underwent more than one SGB during the study. All patients in the SGB group exhibited a Horner’s sign immediately after the injection, confirming a successful sympathetic block, whereas failure to identify a Horner’s sign occurred in 5–22% in prior studies.14,15 Nonetheless, our study has several limitations. The sample size was only 40 women, though this is the largest clinical study of SGB for the treatment of VMS to date. A larger sample would likely provide the statistical power necessary to see an effect of SGB on total VMS given the sizeable placebo effect. Although women in the sham control group showed a 34% placebo effect on reported VMS from baseline to Month 3, objective monitoring showed no improvement in VMS in the sham control group over that same time frame compared to a 21% reduction in objective VMS in the SGB group. Other trials also report a lack of improvement in objective VMS in women randomized to placebo despite an improvement in subjective VMS.34,35 Further studies of SGB in women with moderate-to-very severe VMS resulting from the natural, surgical or pharmaceutical initiation of menopausal symptoms are needed to assess important clinical characteristics of SGB such as duration of symptom relief as well as the comparative effectiveness of other doses and types of sympatholytic agents. Nonetheless, further robust studies showing the benefit of SGB in symptomatic women will corroborate this and earlier observational reports that SGB is an effective and non-hormonal intervention for women seeking relief from VMS that are adversely affecting health and well-being.
Conclusions
SGB may provide an effective treatment for VMS in women who seek non-hormonal therapies due to safety concerns and personal preference. The finding that SGB significantly reduces objectively measured VMS provides further evidence of efficacy. A larger trial is warranted to confirm these findings.
Acknowledgments
The authors would like to thank Dr. Eugene Lipov for his collegial support in our initial discussions of study design for this clinical research.
Funding/Support: Funding was provided by the Department of Obstetrics and Gynecology, Northwestern University. Dr. Rubin’s effort was supported by grant number K12HD055892 from the National Institute of Child Health and Human Development and the National Institutes of Health Office of Research on Women’s Health.
Footnotes
Disclosures: David Walega, Leah Rubin, and Suzanne Banuvar have no conflicts of interest. Lee Shulman has received honoraria from Shionogi for consulting and speaking engagements about non-hormonal treatment from vulvovaginal atrophy and from Noven Pharmaceuticals for speaking engagements concerning hormonal treatments in symptomatic menopausal women. Pauline Maki has received honoraria from Noven Pharmaceuticals and DepoMed for consulting about non-hormonal treatments for vasomotor symptoms.
References
- 1.Kronenberg F. Hot flashes: epidemiology and physiology. Ann N Y Acad Sci. 1990;592:52–86. doi: 10.1111/j.1749-6632.1990.tb30316.x. discussion 123–133. [DOI] [PubMed] [Google Scholar]
- 2.de Villiers TJ, Gass ML, Haines CJ, et al. Global Consensus Statement on menopausal hormone therapy. Maturitas Apr. 2013;74(4):391–392. doi: 10.1016/j.maturitas.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Maclennan AH, Broadbent JL, Lester S, Moore V. Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes. Cochrane Database Syst Rev. 2004;(4):CD002978. doi: 10.1002/14651858.CD002978.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandya KJ, Morrow GR, Roscoe JA, et al. Gabapentin for hot flashes in 420 women with breast cancer: a randomised double-blind placebo-controlled trial. Lancet. 2005 Sep 3–9;366(9488):818–824. doi: 10.1016/S0140-6736(05)67215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandya KJ, Raubertas RF, Flynn PJ, et al. Oral clonidine in postmenopausal patients with breast cancer experiencing tamoxifen-induced hot flashes: a University of Rochester Cancer Center Community Clinical Oncology Program study. Ann Intern Med. 2000 May 16;132(10):788–793. doi: 10.7326/0003-4819-132-10-200005160-00004. [DOI] [PubMed] [Google Scholar]
- 6.Geller SE, Shulman LP, van Breemen RB, et al. Safety and efficacy of black cohosh and red clover for the management of vasomotor symptoms: a randomized controlled trial. Menopause. 2009 Nov-Dec;16(6):1156–1166. doi: 10.1097/gme.0b013e3181ace49b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newton KM, Reed SD, LaCroix AZ, Grothaus LC, Ehrlich K, Guiltinan J. Treatment of vasomotor symptoms of menopause with black cohosh, multibotanicals, soy, hormone therapy, or placebo: a randomized trial. Ann Intern Med. 2006 Dec 19;145(12):869–879. doi: 10.7326/0003-4819-145-12-200612190-00003. [DOI] [PubMed] [Google Scholar]
- 8.Morrow PK, Mattair DN, Hortobagyi GN. Hot flashes: a review of pathophysiology and treatment modalities. Oncologist. 2011;16(11):1658–1664. doi: 10.1634/theoncologist.2011-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher TE, Chervenak JL. Lifestyle alterations for the amelioration of hot flashes. Maturitas. 2012 Mar;71(3):217–220. doi: 10.1016/j.maturitas.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Simon JA, Portman DJ, Kaunitz AM, et al. Low-dose paroxetine 7.5 mg for menopausal vasomotor symptoms: two randomized controlled trials. Menopause. 2013 Oct;20(10):1027–1035. doi: 10.1097/GME.0b013e3182a66aa7. [DOI] [PubMed] [Google Scholar]
- 11.Joffe H, Guthrie KA, Larson J, et al. Relapse of vasomotor symptoms after discontinuation of the selective serotonin reuptake inhibitor escitalopram: results from the menopause strategies: finding lasting answers for symptoms and health research network. Menopause. 2013 Mar;20(3):261–268. doi: 10.1097/GME.0b013e31826d3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA. 2006 May 3;295(17):2057–2071. doi: 10.1001/jama.295.17.2057. [DOI] [PubMed] [Google Scholar]
- 13.Lipov EG, Joshi JR, Sanders S, et al. Effects of stellate-ganglion block on hot flushes and night awakenings in survivors of breast cancer: a pilot study. Lancet Oncol. 2008 Jun;9(6):523–532. doi: 10.1016/S1470-2045(08)70131-1. [DOI] [PubMed] [Google Scholar]
- 14.Pachman DR, Barton D, Carns PE, et al. Pilot evaluation of a stellate ganglion block for the treatment of hot flashes. Support Care Cancer. 2011 Jul;19(7):941–947. doi: 10.1007/s00520-010-0907-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haest K, Kumar A, Van Calster B, et al. Stellate ganglion block for the management of hot flashes and sleep disturbances in breast cancer survivors: an uncontrolled experimental study with 24 weeks of follow-up. Ann Oncol. 2012 Jun;23(6):1449–1454. doi: 10.1093/annonc/mdr478. [DOI] [PubMed] [Google Scholar]
- 16.van Gastel P, Kallewaard JW, van der Zanden M, de Boer H. Stellate-ganglion block as a treatment for severe postmenopausal flushing. Climacteric. 2013 Feb;16(1):41–47. doi: 10.3109/13697137.2012.709889. [DOI] [PubMed] [Google Scholar]
- 17.Bromberger JT, Matthews KA, Schott LL, et al. Depressive symptoms during the menopausal transition: the Study of Women’s Health Across the Nation (SWAN) J Affect Disord. 2007 Nov;103(1–3):267–272. doi: 10.1016/j.jad.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006 Apr;63(4):375–382. doi: 10.1001/archpsyc.63.4.375. [DOI] [PubMed] [Google Scholar]
- 19.Carpenter JS, Guthrie KA, Larson JC, et al. Effect of escitalopram on hot flash interference: a randomized, controlled trial. Fertil Steril. 2012 Jun;97(6):1399–1404. e1391. doi: 10.1016/j.fertnstert.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carpenter JS, Burns DS, Wu J, et al. Paced respiration for vasomotor and other menopausal symptoms: a randomized, controlled trial. J Gen Intern Med. 2013 Feb;28(2):193–200. doi: 10.1007/s11606-012-2202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Painovich JM, Shufelt CL, Azziz R, et al. A pilot randomized, single-blind, placebo-controlled trial of traditional acupuncture for vasomotor symptoms and mechanistic pathways of menopause. Menopause. 2012 Jan;19(1):54–61. doi: 10.1097/gme.0b013e31821f9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finck G, Barton DL, Loprinzi CL, Quella SK, Sloan JA. Definitions of hot flashes in breast cancer survivors. J Pain Symptom Manage. 1998 Nov;16(5):327–333. doi: 10.1016/s0885-3924(98)00090-6. [DOI] [PubMed] [Google Scholar]
- 23.Sloan JA, Loprinzi CL, Novotny PJ, Barton DL, Lavasseur BI, Windschitl H. Methodologic lessons learned from hot flash studies. J Clin Oncol. 2001 Dec 1;19(23):4280–4290. doi: 10.1200/JCO.2001.19.23.4280. [DOI] [PubMed] [Google Scholar]
- 24.Freedman RR. Laboratory and ambulatory monitoring of menopausal hot flashes. Psychophysiology. 1989 Sep;26(5):573–579. doi: 10.1111/j.1469-8986.1989.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 25.Carpenter JS, Andrykowski MA, Freedman RR, Munn R. Feasibility and psychometrics of an ambulatory hot flash monitoring device. Menopause. 1999 Fall;6(3):209–215. doi: 10.1097/00042192-199906030-00006. [DOI] [PubMed] [Google Scholar]
- 26.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989 May;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 27.Utian WH, Janata JW, Kingsberg SA, Schluchter M, Hamilton JC. The Utian Quality of Life (UQOL) Scale: development and validation of an instrument to quantify quality of life through and beyond menopause. Menopause. 2002 Nov-Dec;9(6):402–410. doi: 10.1097/00042192-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011 Jan 19;305(3):267–274. doi: 10.1001/jama.2010.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mulvaney SW, McLean B, de Leeuw J. The use of stellate ganglion block in the treatment of panic/anxiety symptoms with combat-related post-traumatic stress disorder; preliminary results of long-term follow-up: a case series. Pain Pract. 2010 Jul-Aug;10(4):359–365. doi: 10.1111/j.1533-2500.2010.00373.x. [DOI] [PubMed] [Google Scholar]
- 30.Wulf H, Maier C. Complications and side effects of stellate ganglion blockade. Results of a questionnaire survey. Anaesthesist. 1992 Mar;41(3):146–151. [PubMed] [Google Scholar]
- 31.Diwadkar VA, Murphy ER, Freedman RR. Temporal Sequencing of Brain Activations During Naturally Occurring Thermoregulatory Events. Cereb Cortex. 2013 Jun 19; doi: 10.1093/cercor/bht155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Westerhaus MJ, Loewy AD. Central representation of the sympathetic nervous system in the cerebral cortex. Brain Res. 2001 Jun 8;903(1–2):117–127. doi: 10.1016/s0006-8993(01)02453-2. [DOI] [PubMed] [Google Scholar]
- 33.Lipov EG, Joshi JR, Sanders S, Slavin KV. A unifying theory linking the prolonged efficacy of the stellate ganglion block for the treatment of chronic regional pain syndrome (CRPS), hot flashes, and posttraumatic stress disorder (PTSD) Med Hypotheses. 2009 Jun;72(6):657–661. doi: 10.1016/j.mehy.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Carpenter JS, Storniolo AM, Johns S, et al. Randomized, double-blind, placebo-controlled crossover trials of venlafaxine for hot flashes after breast cancer. Oncologist. 2007 Jan;12(1):124–135. doi: 10.1634/theoncologist.12-1-124. [DOI] [PubMed] [Google Scholar]
- 35.Maki PM, Rubin LH, Fornelli D, et al. Effects of botanicals and combined hormone therapy on cognition in postmenopausal women. Menopause. 2009 Nov-Dec;16(6):1167–1177. doi: 10.1097/gme.0b013e3181ace484. [DOI] [PMC free article] [PubMed] [Google Scholar]


