Abstract
The role of diet in hepatocellular carcinoma (HCC) and its typical precursor, chronic liver disease (CLD), is poorly understood. Following dietary recommendations has been shown to reduce risk of many cancers, but whether such diets are associated with HCC and CLD is unknown. We prospectively evaluated the association of two dietary indices, the Healthy Eating Index-2010 (HEI-2010) and the alternate Mediterranean Diet Score (aMED), with HCC incidence and CLD mortality in a large U.S. prospective cohort. We calculated the HEI-2010 and aMED scores for 494,942 participants in the National Institutes of Health-AARP Diet and Health study, based on typical diet assessed using a food frequency questionnaire between 1995 and 1996. Hazard ratios (HRs) and 95% confidence intervals (CIs) for quintiles of each index were estimated using Cox proportional hazards regression, after adjusting for alcohol intake, smoking, body mass index, diabetes, and other covariates. A total of 509 HCC cases (1995-2006) and 1053 CLD deaths (1995-2011) were documented during follow-up. Higher HEI-2010 scores, reflecting favorable adherence to dietary guidelines, were associated with lower risk of HCC (HR: 0.72, 95% CI: 0.53-0.97 for the highest quintile compared to lowest; P-trend=0.03), and lower mortality due to CLD (HR: 0.57; 95% CI: 0.46-0.71; P-trend<0.0001). High aMED scores were also associated with lower risk of HCC (HR: 0.62; 95% CI: 0.47-0.84; P-trend=0.0002) and lower risk of CLD mortality (HR: 0.52; 95% CI: (0.42-0.65; P-trend<0.0001). Conclusions: Adhering to dietary recommendations may reduce the risk of developing HCC and dying of CLD.
Keywords: diet, liver cancer, Healthy Eating Index-2010, alternate Mediterranean Diet Score, cohort study
Liver cancer is the sixth most frequently occurring cancer and second most common cause of cancer death worldwide, with an estimated 745,517 cancer deaths in 2012 (1) and increasing incidence rates in the U.S. and Europe (2-4). Hepatocellular carcinoma (HCC) accounts for the vast majority of liver cancers (2, 5). Despite the significant contribution of hepatitis B virus (HBV) and hepatitis C virus (HCV) in HCC and its typical precursor condition, chronic liver disease (CLD) (2, 5), a substantial proportion occurs in unexposed patients particularly in Western countries (5-7). Exposure to aflatoxins, excessive alcohol intake and pre-existing diabetes are well-defined risk factors (2, 8). However, the role of diet in HCC and CLD is poorly understood.
As foods are eaten in combination, distinguishing the role of one from another is difficult in standard single food- or nutrient-based analysis. Dietary pattern analysis overcomes this challenge by providing an overarching view of an individual's overall diet (9-11). Two recent studies found evidence for an association of certain dietary patterns with liver cancer and with a risk factor for liver cancer, nonalcoholic fatty liver disease (NAFLD), using a data-driven factor analysis (12-13). However, since data-driven dietary patterns may vary by methods of exposure assessment and analytic approaches, results using this approach can be difficult to interpret and reproduce (11). In contrast, index-based dietary patterns that evaluate predefined healthy diets are more readily compared across studies and are easier to interpret (10). The Healthy Eating Index (HEI), which is a measure of diet quality in terms of conformance with U.S. federal dietary guidance(14), and the alternate Mediterranean Diet Score (aMED), which reflects principles of the traditional Mediterranean diet adapted to the American population (9, 15), are among the most popular indices measuring healthy eating patterns. Better adherence to HEI and aMED has been associated with decreased risk of several incident cancers and total mortality (16-21). However, whether such diets are associated with HCC and CLD are unknown. Here, we prospectively investigated associations between the most recent version of the HEI (22) (HEI-2010) and aMED with risk of HCC incidence and CLD mortality in the National Institutes of Health (NIH)-AARP Diet and Health Study.
Materials and Methods
Study population
The NIH-AARP Diet and Health study began in 1995 and 1996, when a total of 566,398 AARP members aged 50 to 71 years residing in six states (California, Florida, Louisiana, New Jersey, North Carolina, and Pennsylvania) and two metropolitan areas (Atlanta, Georgia, and Detroit, Michigan) returned a dietary and lifestyle questionnaire. Proxy respondents (n=15,760) and those with prevalent cancer at baseline (n=51,234) were excluded, as were respondents with total energy intake exceeding twice the inter-quartile range of Box-Cox log-transformed intake (n=4,417), or those who died before their questionnaires were scanned (n=45). The resulting cohort included 494,942 participants: 295,283 men and 199,659 women. The study was approved by the Special Studies Institutional Review Board of the U.S. National Cancer Institute. All participants provided informed consent by virtue of questionnaire completion and return.
Exposure Assessment
At baseline, participants were asked about their frequency of dietary intake over the past 12 months with a 124-item food frequency questionnaire (FFQ). Participants reported their typical frequency of intake using 10 categories ranging from never to two or more times/day for solid foods and never to six or more times/day for beverages (23). The food items, portion sizes, and nutrient databases were constructed using the Department of Agriculture's 1994-1996 Continuing Survey of Food Intakes by Individuals (24). The FFQ was calibrated against two non-consecutive 24-hr dietary recalls in a subset of participants (25-26).
The HEI measures the overall compliance of study participants with the Dietary Guidelines for Americans, which have been the basis of nutrition policy for the U.S. Federal government (14). The most recent version, HEI-2010, maintains the major features of HEI-2005, and also captures several key changes, such as the addition of a recommendation for seafood and limitations on refined grains (Supplementary Table 1 and 2) (22). The 12 components of this index were scored for a total of 0 (non-adherence) to 100 points (optimal adherence). Components were determined per 1000 kcal in order to account for differences in energy intake (density method)(14, 22). For those nine adequacy components with a minimum intake recommendation, participants whose intakes met the dietary recommendations received higher scores. Of these nine components, six (total fruit, whole fruits, total vegetables, greens and beans, total protein foods, and seafood and plant proteins) were awarded 0-5 points each; three components (whole grains, dairy, and fatty acids) were awarded 0-10 points each. For those three moderation components with a maximum intake recommendation, higher scores indicate lower consumption. Two components (refined grains and sodium) were awarded 0-10 points each and one component (empty calories, including calories from solid fat, alcohol, and added sugar) was awarded 0-20 points. Scores of HEI-2010 were calculated using the code developed by the Division of Cancer Control and Population Sciences in National Cancer Institute (http://appliedresearch.cancer.gov/tools/hei/tools.html, retrieved Feb 12, 2014).
The aMED measures adherence to the Mediterranean diet and was adapted from the original Mediterranean diet score (MED) for the American population (9, 15). Each of nine components was energy adjusted by the density method and standardized to 2,500 calories for men and 2,000 calories for women. Participants received 1 point for intake above the cohort-wide median of seven healthy components (vegetables excluding potatoes, legumes, fruit, nuts, whole grains, fish, and ratio of monounsaturated to saturated fat), received a point for alcohol intake of 5-15g/day, and received a point for having a below median intake of red and processed meat. An aMED score of 0 meant that no guidelines were met, whereas a score of 9 meant that all guidelines were met.
Cohort Follow-up
Addresses of cohort members were updated by annual linkage to the U.S. Postal Service change of address database and direct responses from participants. Vital status was ascertained by periodic linkage of the cohort to the Social Security Administration Death Master File, follow-up searches of the National Death Index, and responses to other mailings.
Identification of Deaths Due to CLD
Cause of death was ascertained through linking cohort participants to the National Death Index Plus maintained by the National Center for Health Statistics. The underlying cause of death from death certificates was documented using the International Classification of Diseases (ICD)-9 and ICD-10 codes. Deaths were available through December 31, 2011. We used the same definition of CLD as the National Center for Health Statistics and classified participants who were given specific underlying cause of death codes for CLD, liver fibrosis and cirrhosis, alcoholic liver disease, and chronic hepatitis (ICD-9: 571.0, 571.2–571.6, 571.8, and 571.9; ICD-10: K70, K73, and K74)(27). Classification of CLD in the National Death Index was validated against the electronic medical records from members of the Kaiser Permanente Medical Care Program of Northern California and was found to have a high specificity (89%) (28).
Identification of incident primary HCC
Participants with incident HCC were ascertained by linking cohort membership to cancer registries of the eight baseline states plus Arizona, Nevada, and Texas, states where study participants often relocated. We estimated a sensitivity of 90% and a specificity of nearly 100% using this approach for cancer assessment (29). Incident cases were defined using codes compatible with HCC (topography code C22.0 and morphology codes of 8000, 8010, 8140, 8170-8175, or 8190) (30), based on the International Classification of Diseases for Oncology 3rd edition (31). In a sensitivity analysis, we applied a more conservative definition by restricting our analysis to HCC cases with morphology codes between 8170 and 8175. Incident cancer cases were available through December 31, 2006. Participants identified both as having an incident HCC and as dying from CLD were classified in our study as having had incident HCC only, as HCC incidence was the more specific diagnosis and occurred before CLD death. We did not assess liver cancer mortality because death certificates can erroneously miscode metastatic lesions to the liver (a common site for cancer metastasis) as primary liver tumors.
Statistical Analysis
Participants were classified into quintiles of HEI-2010 and aMED scores (0-2, 3, 4, 5, or 6-9) respectively. We calculated the Spearman's correlation between the two indices, since they were not normally distributed. For HCC, follow-up time extended from the return date of the baseline questionnaire (between 1995 and 1996) to date of diagnosis of HCC, date of death, movement out of the study areas, or end of follow-up (December 31, 2006), whichever came first. For CLD, follow-up time was calculated from the questionnaire return date to the date of death or end of follow-up (December 31, 2011), whichever came first. Age- and sex-adjusted, as well as multivariate-controlled hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using Cox-regression models. We tested the proportional hazards assumption by including an interaction term of time and quintiles of each index and did not find significant deviations. We conducted multivariable models adjusting for age (continuous), sex, alcohol intake (0, >0-0.5, >0.5-1, >1-2, >2-4, or >4 drinks/day), smoking (never, former ≤1, former >1, current ≤1, or current >1 pack/day), body mass index (BMI, <18.5. 18.5-24.9, 25.0-29.9, 30.0-34.9, or ≥35.0 kg/m2), education (less than high school, completion of high school, some post-high school, completion of college, or completion of graduate school), race (non-Hispanic White, non-Hispanic Black, Hispanic, or others), diabetes (yes or no), usual activity throughout the day (sit without walking much, sit but walk fair amount, stand/walk a lot without lifting, lift/carry light loads or climb stairs/hills often, or do heavy work/carry loads), vigorous physical activity (never, rarely, 1-3 times/month, 1-2, 3-4, or ≥5 times/wk), and total energy intake (continuous). An indicator variable was created for each covariate with missing data (missing rate was very low, with ≤3.8% for all covariates). Linear trend across quintiles were tested by assigning participants the median score of each quintile.
We performed a number of exploratory and sensitivity analyses. Associations for specific components were adjusted for modified scores that lacked that component. Indices lacking the alcohol component were also examined. We conducted stratified analysis by sex, alcohol intake, BMI, cigarette smoking, and diabetes, and evaluated the P for heterogeneity (Pheterogeneity) among subgroups using Q statistics. As associations were similar in men and women, we focused on the overall results. Lag analyses were performed by excluding the first 5 years’ follow-up. We also performed analyses restricted to the 91% of our participants who were non-Hispanic Whites, and after excluding participants reporting poor health, coronary heart disease, or diabetes at baseline. To facilitate comparisons with previous studies of other cancers (18-21), we also examined results for the previous HEI-2005 index. Finally, we adjusted our findings for nonsteroidal anti-inflammatory drugs (NSAIDs) (32). However, as such adjustment had no effect on our analysis and NSAIDS were available for only a subset of cohort participants (300,000), we did not adjust for NSAIDS in our main analysis.
Results
Among 494,942 participants, the mean age was 62.0 years and 40.3% were women. The minimum score of HEI-2010 was 18 and the maximum was 98. The aMED scores ranged between 0 and 9. HEI-2010 and aMED scores were also significantly correlated (r=0.60, P<0.0001). Men tended to adhere to aMED better than to HEI-2010. Participants with higher scores in HEI-2010 or aMED tended to be older and better-educated. They were also more likely to engage in vigorous physical activity, report low overall caloric intake, and were less likely to be current smokers, or perform heavy activities at work (Table 1).
Table 1.
Baseline characteristics of the participants by quintiles of diet-index scores, NIH-AARP Diet and Health Study
| Healthy Eating Index-2010 |
Alternate Mediterranean Diet Score |
|||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 3 | Quintile 5 | Quintile 1 | Quintile 3 | Quintile 5 | |
| n | 98,988 | 98,988 | 98,988 | 86,535 | 108,525 | 110,377 |
| Score median | 52 | 68 | 79 | 2 | 4 | 6 |
| Age, year, mean (SD) | 61.3 (5.5) | 62.0 (5.3) | 62.8 (5.2) | 61.5 (5.5) | 62.0 (5.4) | 62.4 (5.3) |
| Men (%) | 66.7 | 59.7 | 52.6 | 56.9 | 57.4 | 66.7 |
| BMI, kg/m2, mean (SD) | 27.5 (5.1) | 27.3 (4.8) | 26.3 (4.5) | 27.5 (5.2) | 27.2 (4.9) | 26.5 (4.3) |
| Race (non-Hispanic white, %) | 92.6 | 92.3 | 92.4 | 93.9 | 91.9 | 92.3 |
| Current smoking (%) | 23.3 | 10.5 | 6.0 | 22.3 | 11.9 | 5.6 |
| Heavy alcohol intake (>3 drinks/day, %) | 6.6 | 8.3 | 6.2 | 16.0 | 6.6 | 2.5 |
| Usual activity (heavy work, %) | 4.5 | 2.7 | 2.1 | 4.0 | 2.8 | 2.3 |
| Vigorous physical activity (≥5 times/wk, %) | 13.5 | 19.0 | 26.5 | 13.6 | 18.5 | 26.0 |
| Education (college graduate, %) | 28.7 | 41.0 | 47.7 | 29.4 | 38.7 | 50.3 |
| Total calories intake, kcal/day, mean (SD) | 2,060 (952) | 1,805 (770) | 1,669 (657) | 2,019 (940) | 1,807 (790) | 1,744 (693) |
| Diabetes (%) | 7.3 | 9.8 | 9.3 | 8.1 | 9.7 | 8.3 |
We identified 509 incident cases of HCC in 4,806,205 person-years of follow-up and 1,053 CLD deaths in 6,685,736 person-years. We found an inverse association between HEI-2010 scores and both HCC incidence (the highest quintile compared with the lowest: HR=0.72, 95% CI=0.53-0.97, Ptrend=0.03) and CLD mortality (HR=0.57, 95% CI=0.46-0.71, Ptrend<0.0001), in multivariate adjusted models (Table 2). Similarly, higher scores in aMED were associated with a lower risk of HCC (HR=0.62, 95% CI=0.47-0.84, Ptrend=0.0002) and CLD mortality (HR=0.52, 95% CI=0.42-0.65, Ptrend<0.0001). In a sensitivity analysis, we restricted our HCC endpoint to those 435 cases with an morphology code between 8170 and 8175 and observed similar results (HEI-2010: HR=0.78, 95% CI=0.56-1.09, Ptrend=0.06; aMED: HR=0.64; 95% CI=0.46-0.87; Ptrend=0.001).
Table 2.
Hazard ratios (HRs) and 95% confidence intervals (CIs) for hepatocellular carcinoma (HCC) incidence and chronic liver disease (CLD) mortality by quintiles of the Healthy Eating Index-2010 (HEI-2010) and the alternate Mediterranean Diet Score (aMED)
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P-trend* | |
|---|---|---|---|---|---|---|
| HEI-2010 | n=98,988 | n=98,989 | n=98,988 | n=98,989 | n=98,988 | |
| HCC | ||||||
| No. of Cases | 128 | 107 | 115 | 87 | 72 | |
| Age and sex-adjusted HR (95% CI) | 1.00 | 0.82 (0.64-1.06) | 0.88 (0.69-1.14) | 0.67 (0.51-0.88) | 0.55 (0.41-0.74) | <0.0001 |
| Multivariate-adjusted HR* (95% CI) | 1.00 | 0.86 (0.66-1.11) | 0.96 (0.74-1.25) | 0.78 (0.59-1.04) | 0.72 (0.53-0.97) | 0.03 |
| CLD | ||||||
| No. of Cases | 295 | 246 | 205 | 175 | 132 | |
| Age and sex-adjusted HR (95% CI) | 1.00 | 0.81 (0.69-0.96) | 0.67 (0.56-0.80) | 0.57 (0.47-0.69) | 0.42 (0.34-0.52) | <0.0001 |
| Multivariate-adjusted HR* (95% CI) | 1.00 | 0.84 (0.71-1.00) | 0.74 (0.62-0.89) | 0.68 (0.56-0.82) | 0.57 (0.46-0.71) | <0.0001 |
| aMED | n=86,535 | n=91,596 | n=108,525 | n=97,909 | n=110,377 | |
| HCC | ||||||
| No. of Cases | 125 | 112 | 103 | 83 | 86 | |
| Age and sex-adjusted HR (95% CI) | 1.00 | 0.82 (0.64-1.06) | 0.62 (0.48-0.80) | 0.53 (0.40-0.70) | 0.44 (0.34-0.59) | <0.0001 |
| Multivariate-adjusted HR* (95% CI) | 1.00 | 0.88 (0.68-1.13) | 0.70 (0.54-0.91) | 0.64 (0.48-0.86) | 0.62 (0.47-0.84) | 0.0002 |
| CLD | ||||||
| No. of Cases | 318 | 239 | 225 | 149 | 122 | |
| Age and sex-adjusted HR (95% CI) | 1.00 | 0.69 (0.59-0.82) | 0.54 (0.45-0.64) | 0.38 (0.31-0.46) | 0.26 (0.21-0.32) | <0.0001 |
| Multivariate-adjusted HR* (95% CI) | 1.00 | 0.85 (0.72-1.01) | 0.76 (0.64-0.91) | 0.62 (0.50-0.76) | 0.52 (0.42-0.65) | <0.0001 |
Multivariable models were adjusted for age, sex, race, smoking (never, former ≤1, former >1, current ≤1, or current >1 pack/day), alcohol intake (0, >0-0.5, >0.5-1, >1-2, >2-4, or >4 drinks/day), education, BMI (<18.5, 18.5-24.9, 25.0-29.9, 30.0-34.9, or ≥35.0 kg/m2), diabetes, usual activity throughout the day, vigorous physical activity, and total energy intake.
Among the components of the two scores, greens and beans, seafood and plant proteins, and fatty acids in the HEI-2010, and ratio of monounsaturated/saturated fat and alcohol in the aMED were significantly associated with lower risk of HCC and CLD mortality (Table 3-4). In addition, whole grains, dairy, and total protein foods in the HEI-2010 and whole grains, fish, and nuts in the aMED were inversely associated with deaths from CLD, whereas empty calories in the HEI-2010, legumes in the aMED, and vegetables in both HEI-2010 and aMED scores were inversely associated with HCC incidence (Table 3-4). In contrast, the fruit component of the two indices was significantly associated with increased risk of HCC and with increased CLD mortality.
Table 3.
Association of components in Health Eating Index-2010 with HCC incidence and CLD mortality
| Criteria for maximum score* | HCC, HR†(95% CI) | CLD, HR†(95% CI) | |
|---|---|---|---|
| Total fruit | ≥0.8 cups/1,000 kcal | 1.08 (1.00-1.16) | 1.09 (1.04-1.15) |
| Whole fruits (not juice) | ≥0.4 cups/1,000 kcal | 1.04 (0.97-1.11) | 1.05 (1.01-1.10) |
| Total vegetables | ≥1.1 cups/1,000 kcal | 0.89 (0.82-0.96) | 0.95 (0.90-1.01) |
| Greens and beans | ≥0.2 cups/1,000 kcal | 0.92 (0.87-0.97) | 0.95 (0.91-0.98) |
| Whole grains | ≥1.5 ounces/1,000 kcal | 0.97 (0.93-1.01) | 0.90 (0.88-0.93) |
| Dairy | ≥1.3 cups/1,000 kcal | 1.03 (0.99-1.06) | 0.96 (0.94-0.98) |
| Total protein foods | ≥2.5 ounces/1,000 kcal | 0.93 (0.85-1.03) | 0.86 (0.81-0.91) |
| Seafood and plant proteins | ≥0.8 ounces/1,000 kcal | 0.92 (0.86-0.98) | 0.90 (0.86-0.94) |
| Fatty acids | (total poly- and monounsaturated fatty acids)/(saturated fatty acids) ≥2.5 | 0.94 (0.91-0.97) | 0.95 (0.93-0.97) |
| Refined grains | ≤1.8 ounces/1,000 kcal | 1.03 (0.99-1.07) | 1.08 (1.05-1.11) |
| Sodium | ≤1.1 gram/1,000 kcal | 1.04 (0.99-1.07) | 1.04 (0.99-1.07) |
| Empty calories‡ | ≤19% kcal | 0.98 (0.96-1.00) | 0.99 (0.98-1.01) |
1 ounce = 28.35 g; 1 cup = 0.24 L.
HR for one point change of each component. Multivariable models were adjusted for age, sex, race, smoking (never, former ≤1, former >1, current ≤1, or current >1 pack/day), alcohol intake (0, >0-0.5, >0.5-1, >1-2, >2-4, or >4 drinks/day), education, BMI (<18.5, 18.5-24.9, 25.0-29.9, 30.0-34.9, or ≥35.0 kg/m2), diabetes, usual activity throughout the day, vigorous physical activity, total energy intake, and a modified score that lacked the particular component.
Calories from solid fats, alcohol, and added sugars; threshold for counting alcohol is >13 grams/1,000 kcal.
Table 4.
Association of components in the alternate Mediterranean Diet Score with HCC incidence and CLD mortality
| Criteria for optimal score (1)* | HCC, HR† (95% CI) | CLD, HR† (95% CI) | |
|---|---|---|---|
| Whole grains | ≥median: 1.09 ounces | 0.87 (0.72-1.04) | 0.72 (0.63-0.82) |
| Vegetables (no white potatoes) | ≥median: 1.86 cups | 0.79 (0.65-0.95) | 0.90 (0.79-1.03) |
| Fruit | ≥median: 2.30 cups | 1.17 (0.97-1.40) | 1.15 (1.01-1.31) |
| Fish | ≥median: 0.60 ounces | 0.92 (0.77-1.11) | 0.82 (0.73-0.93) |
| Red and processed meat | <median: 2.45 ounces | 0.93 (0.77-1.13) | 0.90 (0.79-1.03) |
| Legumes | ≥median: 0.08 cups | 0.81 (0.68-0.97) | 0.93 (0.82-1.05) |
| Nuts | ≥median: 0.30 ounces | 1.00 (0.83-1.19) | 0.85 (0.75-0.96) |
| Ratio of monounsaturated: saturated fat | ≥median: 1.24 | 0.78 (0.65-0.93) | 0.80 (0.71-0.91) |
| Alcohol | 5-15g/day | 0.72 (0.55-0.96) | 0.56 (0.45-0.70) |
1 ounce = 28.35 g; 1 cup = 0.24 L. Each component was awarded 1 or 0. 1 point was awarded for intake meeting the optimal criteria listed in the table. Otherwise, 0 point was award.
HR for one point change of each component. Multivariable models were adjusted for age, sex, race, smoking (never, former ≤1, former >1, current ≤1, or current >1 pack/day), alcohol intake (0, >0-0.5, >0.5-1, >1-2, >2-4, or >4 drinks/day), education, BMI (<18.5, 18.5-24.9, 25.0-29.9, 30.0-34.9, or ≥35.0 kg/m2), diabetes, usual activity throughout the day, vigorous physical activity, total energy intake, and a modified score that lacked the particular component.
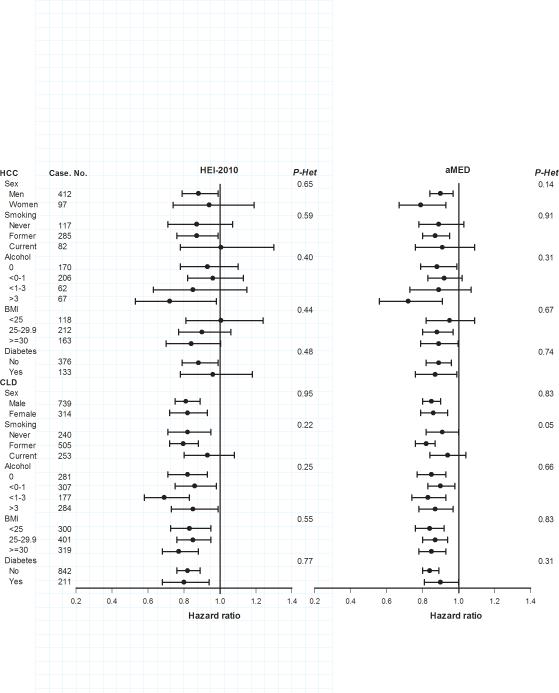
Stratified analyses by sex, smoking, alcohol intake, BMI and diabetes yielded generally similar findings across subgroups of each variable, with no statistical evidence for heterogeneity (Figure 1). Associations for HEI-2010 and HCC appeared stronger for men than women, however this apparent difference was not statistically significant (Pheterogeneity=0.65). Similarly, although the HRs for both indices with HCC were lowest among those who consumed ≥3 drinks of alcohol per day, such differences across all alcohol categories were not statistically significant (Pheterogeneity=0.40 for HEI-2010 and 0.31 for aMED) (Figure 1).
Figure 1. Associations of the HEI-2010 and aMED score with incident hepatocellular carcinoma (HCC) incidence and chronic liver disease (CLD) mortality.
Hazard ratios are the risk of HCC incidence or CLD mortality associated with an increase of 10 points of HEI-2010 or 1 point of aMED, after adjusting for age, sex, race, smoking, alcohol intake, education, BMI, diabetes, usual activity throughout the day, vigorous physical activity, and total energy intake (except the variable serving as the stratifier). P values for heterogeneity (P-Het) were calculated using Q statistics.
As alcohol is a noted risk factor for hepatic diseases, we modified each index by removing the alcohol component and found similar associations. The HR (95% CI) for the highest quintile of modified HEI-2010 was 0.72 (0.53-0.97) for HCC (Ptrend=0.03) and 0.58 (0.46-0.72) for CLD (Ptrend<0.0001). The HR (95% CI) for the highest quintile of modified aMED was 0.64 (0.47-0.86) for HCC (Ptrend=0.0006) and 0.53 (0.42-0.67) for CLD (Ptrend<0.0001).
In other sensitivity analyses, excluding the first 5 years’ follow-up did not change the findings appreciably. Similarly, restricting analyses to non-Hispanic Whites, or excluding those reporting poor health, coronary heart disease, or diabetes did not materially alter the associations (data not shown).
Scores of HEI-2005 and HEI-2010 were closely correlated (Spearman r=0.82, P<0.0001) and results were similar for both indices (Supplementary Table 3-4).
Discussion
In this large prospective cohort, a higher score in the HEI-2010 or aMED, reflecting better adherence to established dietary guidelines for Americans, was associated with reduced risk of developing HCC or dying from CLD. Our study is the first prospective study evaluating these associations.
Few previous reports on dietary pattern in liver cancer and liver diseases are available (12-13, 33-34). Using a data-driven approach, a recent Chinese study found reduced liver cancer risk in association with a vegetable-based dietary pattern (12). An Australian study showed an increased risk of NAFLD among persons with a Western dietary pattern, and a decreased risk among persons with a healthy dietary pattern (13). Of the few studies examining index-based patterns (33-35), one found that scores for a healthy eating index including 10 components based on the method of Kennedy et al.(36), were higher in healthy controls than in patients with NAFLD (P=0.01) (33). A second study observed that the traditional Mediterranean diet was inversely associated with severity of NAFLD (34). A third study found that adherence to the traditional Mediterranean diet appears to be protective against HCC (35). Such findings are complementary to the current analysis, although each of these previous studies had a small sample size and employed a cross-sectional case-control design. The only other prospective study to date found a lower risk of liver cancer among subjects adhering to the World Cancer Research Fund/American Institute for Cancer Research lifestyle guidelines, although such guidelines include maintaining a healthy weight and physical activity in addition to diet (37).
Maintaining a diet in accord with health recommendations may benefit chronic liver disease and liver cancer through several possible pathways. For example, chronic inflammation is a major hallmark of hepatic carcinogenesis (5, 38) and oxidative stress may also be important (39). Higher diet quality scores have been correlated with lower concentrations of inflammatory biomarkers and less free radical production (9, 40-41). Thus, among other mechanisms, having a healthy diet could potentially modify inflammation and oxidative stress pathways and in this way have a beneficial effect on liver carcinogenesis.
The benefits of a healthy eating habit may reflect a combined role of diverse foods and nutrients. Unfortunately, few data on individual foods and liver cancer are available (5, 42). Although the goal of the current analysis was to examine the overall diet, we also conducted exploratory analyses to assess individual components. In our study, the component of greens and beans in HEI-2010 was associated with lower risks of HCC and CLD mortality, and the component of vegetables in both HEI-2010 and aMED was associated inversely with HCC, suggesting a favorable effect of vegetable intake. Previous studies on vegetable intake and HCC have been inconsistent (12). Fatty acids (the ratio of total unsaturated/saturated fatty acids ≥2.5, HEI-2010) (as well as saturated fat ≤7% kcal, HEI-2005) and the ratio of monounsaturated/saturated fat (aMED) were significantly associated with decreased risk of both HCC incidence and CLD mortality in the current study, similar to a previous publication from our cohort (30), although few other reports are available. We also found evidence that other dietary components may be associated with HCC and CLD mortality. The newly added component in HEI-2010, seafood and plant proteins was inversely associated with both HCC and CLD mortality. Also, the whole grains component of both HEI-2010 and aMED was inversely associated with CLD mortality. Surprisingly, the fruit components of the two indices were associated positively with both HCC and CLD mortality, in contrast to prior suggestions of a possible beneficial role of fruit (42). Although explanations for this observation are unclear, increased fructose consumption has been associated with liver fibrosis (43-44) and fruits are major sources of fructose. As we examined multiple aspects of diet in these exploratory analyses, some associations may be due to chance. Therefore, these results require replication and should be interpreted cautiously. It is important to note that although we examined a one point change in each component, such a change does not necessarily correspond to the same amount of food in HEI-2010 and aMED, giving different scoring standards.
Consistent with other reports (1), men had higher liver cancer rates than women in our study. However, similar associations in men and women were generally found for each score and endpoint. Although associations appeared stronger for HEI-2010 and HCC in men, this apparent difference was not statistically significant and our study had many fewer HCC cases in women, limiting power in this group.
Dietary pattern could be a surrogate for overall healthy behaviors. However, associations persisted across levels of alcohol intake, cigarette smoking, BMI, and diabetes. As alcohol is an important contributor to these diseases(2, 5), we examined indices lacking the alcohol component and results were similar. Together these data suggest that associations for the healthy eating indices are independent of other major lifestyle factors, such as alcohol and smoking.
We lacked information on prevalent chronic liver disease in our cohort. In sensitivity analyses, however, we observed generally similar associations after excluding endpoints occurring in the first five years of follow-up and after excluding those groups at higher risk of chronic liver disease including those reporting poor health, diabetes, and heavy alcohol intake at baseline. Nevertheless, it is possible that changes in diet related to underlying chronic liver disease could have affected our observed associations. We also were also unable to assess possible differences in the associations of dietary patterns with chronic liver disease incidence and progression. Future studies of dietary patterns within populations with well-defined liver disease are needed.
Our study has several strengths. A detailed FFQ and large sample size allowed for a comprehensive exploration of dietary patterns with sufficient statistical power to examine subgroups. The prospective study design reduced the likelihood of recall bias and permitted the assessment of dietary patterns well before HCC diagnosis or death from CLD.
Our study also had several limitations. First, as in all observational studies, we cannot rule out the possibility of residual confounding by unmeasured or insufficiently adjusted covariates. In particular, we lacked information on HBV and HCV infection. However, the prevalence of HBV and HCV infection in US Whites, which make up the vast majority of our cohort, is particularly low. Using data from the nationally-representative National Health and Nutrition Examination Survey (NHANES, 1999-2008), only 0.11% of non-Hispanic Whites aged 6 years or older had chronic HBV infection and 2.8% had been exposed to HBV (45). A second study using NHANES data found a 1.3% prevalence of anti-HCV among Whites in 2010 (46). Future work, however, is needed to determine whether viral infections modify the association between diet and HCC and CLD. Second, as discussed above, we lacked information on prevalent and incident chronic liver disease. Third, index-based dietary patterns were derived based on current knowledge and may not capture all the aspects of a healthy diet. Fourth, a majority of the study participants were non-Hispanic Whites, better educated, and had healthier lifestyles than the general U.S. population, so extrapolation to other populations or ethnic groups requires caution.
In summary, we observed an inverse association between adherence to the 2010 US dietary guidelines (HEI-2010) and principle of Mediterranean diet for Americans (aMED) and HCC incidence and CLD mortality. Our findings suggest that adhering to dietary recommendations may contribute to lower risk of these diseases.
Supplementary Material
Acknowledgment
Cancer incidence data from the Atlanta metropolitan area were collected by the Georgia Center for Cancer Statistics, Department of Epidemiology, Rollins School of Public Health, Emory University. Cancer incidence data from California were collected by the California Department of Health Services, Cancer Surveillance Section. Cancer incidence data from the Detroit metropolitan area were collected by the Michigan Cancer Surveillance Program, Community Health Administration, State of Michigan. The Florida cancer incidence data used in this report were collected by the Florida Cancer Data System (FCDS) under contract with the Florida Department of Health (FDOH). Cancer incidence data from Louisiana were collected by the Louisiana Tumor Registry, Louisiana State University Medical Center in New Orleans. Cancer incidence data from New Jersey were collected by the New Jersey State Cancer Registry, Cancer Epidemiology Services, New Jersey State Department of Health and Senior Services. Cancer incidence data from North Carolina were collected by the North Carolina Central Cancer Registry. Cancer incidence data from Pennsylvania were supplied by the Division of Health Statistics and Research, Pennsylvania Department of Health, Harrisburg, Pennsylvania. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions. Cancer incidence data from Arizona were collected by the Arizona Cancer Registry, Division of Public Health Services, Arizona Department of Health Services. Cancer incidence data from Texas were collected by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services. Cancer incidence data from Nevada were collected by the Nevada Central Cancer Registry, Center for Health Data and Research, Bureau of Health Planning and Statistics, State Health Division, State of Nevada Department of Health and Human Services.
The authors thank the participants in the NIH-AARP Diet and Health Study for their cooperation. The authors thank Sigurd Hermansen and Kerry Grace Morrissey from Westat Inc. (Rockville, MD) for study outcomes ascertainment and management and Leslie Carroll at Information Management Services (Silver Spring, MD) for data support and analysis.
Financial support: This research was supported by the Intramural Research Program of Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health.
List of Abbreviations
- aMED
Alternate Mediterranean Diet Score
- BMI
body mass index
- CI
confidence interval
- FFQ
food frequency questionnaire
- HR
hazard ratio
- HEI
Healthy Eating Index
Footnotes
Authors’ Disclosures of Potential Conflicts of Interest: Dr. Albert R. Hollenbeck is a retired employee of AARP, and serves as advisor in the National Science Foundation Fellowship Review Panel between Dec, 2013 and Jan, 2014, Love/Avon Army of Women Scientific Advisory Panel (volunteer), and Board of Directors, Society of Psychologists in Management (elected volunteer). The other authors indicated no potential conflicts of interest.
Reference
- 1.Ferlay JSI, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] International Agency for Research on Cancer; Lyon, France: 2013. Available from http://globocan.iarc.fr. [Google Scholar]
- 2.McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis. 2011;15:223–243. vii–x. doi: 10.1016/j.cld.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Center MM, Jemal A. International trends in liver cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2011;20:2362–2368. doi: 10.1158/1055-9965.EPI-11-0643. [DOI] [PubMed] [Google Scholar]
- 4.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 6.Di Bisceglie AM, Lyra AC, Schwartz M, Reddy RK, Martin P, Gores G, et al. Hepatitis C-related hepatocellular carcinoma in the United States: influence of ethnic status. Am J Gastroenterol. 2003;98:2060–2063. doi: 10.1111/j.1572-0241.2003.t01-1-07641.x. [DOI] [PubMed] [Google Scholar]
- 7.Yu L, Sloane DA, Guo C, Howell CD. Risk factors for primary hepatocellular carcinoma in black and white Americans in 2000. Clin Gastroenterol Hepatol. 2006;4:355–360. doi: 10.1016/j.cgh.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 8.Wang C, Wang X, Gong G, Ben Q, Qiu W, Chen Y, et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer. 2012;130:1639–1648. doi: 10.1002/ijc.26165. [DOI] [PubMed] [Google Scholar]
- 9.Fung TT, McCullough ML, Newby PK, Manson JE, Meigs JB, Rifai N, et al. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2005;82:163–173. doi: 10.1093/ajcn.82.1.163. [DOI] [PubMed] [Google Scholar]
- 10.Moeller SM, Reedy J, Millen AE, Dixon LB, Newby PK, Tucker KL, et al. Dietary patterns: challenges and opportunities in dietary patterns research an Experimental Biology workshop, April 1, 2006. J Am Diet Assoc. 2007;107:1233–1239. doi: 10.1016/j.jada.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Vaughan TL. Diet and upper gastrointestinal cancers: in search of dark matter. Clin Gastroenterol Hepatol. 2013;11:1137–1139. doi: 10.1016/j.cgh.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, Xiang YB, Li HL, Yang G, Cai H, Ji BT, et al. Vegetable-based dietary pattern and liver cancer risk: Results from the Shanghai Women's and Men's Health Studies. Cancer Sci. 2013 doi: 10.1111/cas.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oddy WH, Herbison CE, Jacoby P, Ambrosini GL, O'Sullivan TA, Ayonrinde OT, et al. The Western dietary pattern is prospectively associated with nonalcoholic fatty liver disease in adolescence. Am J Gastroenterol. 2013;108:778–785. doi: 10.1038/ajg.2013.95. [DOI] [PubMed] [Google Scholar]
- 14.Guenther PM, Kirkpatrick SI, Reedy J, Krebs-Smith SM, Buckman DW, Dodd KW, et al. The Healthy Eating Index-2010 Is a Valid and Reliable Measure of Diet Quality According to the 2010 Dietary Guidelines for Americans. J Nutr. 2014 doi: 10.3945/jn.113.183079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–1290. doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 16.Samieri C, Sun Q, Townsend MK, Chiuve SE, Okereke OI, Willett WC, et al. The association between dietary patterns at midlife and health in aging: an observational study. Ann Intern Med. 2013;159:584–591. doi: 10.7326/0003-4819-159-9-201311050-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez-Garcia E, Rodriguez-Artalejo F, Li TY, Fung TT, Li S, Willett WC, et al. The Mediterranean-style dietary pattern and mortality among men and women with cardiovascular disease. Am J Clin Nutr. 2014;99:172–180. doi: 10.3945/ajcn.113.068106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–1018. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arem H, Reedy J, Sampson J, Jiao L, Hollenbeck AR, Risch H, et al. The Healthy Eating Index 2005 and risk for pancreatic cancer in the NIH-AARP study. J Natl Cancer Inst. 2013;105:1298–1305. doi: 10.1093/jnci/djt185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bosire C, Stampfer MJ, Subar AF, Park Y, Kirkpatrick SI, Chiuve SE, et al. Index-based Dietary Patterns and the Risk of Prostate Cancer in the NIH-AARP Diet and Health Study. Am J Epidemiol. 2013;177:504–513. doi: 10.1093/aje/kws261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li WQ, Park Y, Wu JW, Ren JS, Goldstein AM, Taylor PR, et al. Index-based Dietary Patterns and Risk of Esophageal and Gastric Cancer in a Large Cohort Study. Clin Gastroenterol Hepatol. 2013;11:1130–1136. e1132. doi: 10.1016/j.cgh.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guenther PM, Casavale KO, Reedy J, Kirkpatrick SI, Hiza HA, Kuczynski KJ, et al. Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet. 2013;113:569–580. doi: 10.1016/j.jand.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schatzkin A, Subar AF, Thompson FE, Harlan LC, Tangrea J, Hollenbeck AR, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions : the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154:1119–1125. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 24.Tippett KS, Cypel YS. Design and operation: continuing survey of food intakes by individuals and diet and health knowledge survey, 1994-1996. U.S. Department of Agriculture; Beltsville, MD: 1998. No. 96-1. [Google Scholar]
- 25.Midthune D, Schatzkin A, Subar AF, Thompson FE, Freedman LS, Carroll RJ, et al. Validating an FFQ for intake of episodically consumed foods: application to the National Institutes of Health-AARP Diet and Health Study. Public Health Nutr. 2011;14:1212–1221. doi: 10.1017/S1368980011000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson FE, Kipnis V, Midthune D, Freedman LS, Carroll RJ, Subar AF, et al. Performance of a food-frequency questionnaire in the US NIH-AARP (National Institutes of Health-American Association of Retired Persons) Diet and Health Study. Public Health Nutr. 2008;11:183–195. doi: 10.1017/S1368980007000419. [DOI] [PubMed] [Google Scholar]
- 27.Kung HC, Hoyert DL, Xu J, Murphy SL. Deaths: final data for 2005. Natl Vital Stat Rep. 2008;56:1–120. [PubMed] [Google Scholar]
- 28.Manos MM, Leyden WA, Murphy RC, Terrault NA, Bell BP. Limitations of conventionally derived chronic liver disease mortality rates: Results of a comprehensive assessment. Hepatology. 2008;47:1150–1157. doi: 10.1002/hep.22181. [DOI] [PubMed] [Google Scholar]
- 29.Michaud DS, Midthune D, Hermansen S, Leitzmann M, Harlan LC, Kipnis V, et al. Comparison of cancer registry case ascertainment with SEER estimates and self-reporting in a subset of the NIH-AARP diet and health study. J Registry Manag. 2005;32:70–75. [Google Scholar]
- 30.Freedman ND, Cross AJ, McGlynn KA, Abnet CC, Park Y, Hollenbeck AR, et al. Association of meat and fat intake with liver disease and hepatocellular carcinoma in the NIH AARP cohort. J Natl Cancer Inst. 2010;102:1354–1365. doi: 10.1093/jnci/djq301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fritz AG, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, et al. 3rd edn. World Health Organization; Geneva: 2000. International classification of disease for oncology: ICD-O. [Google Scholar]
- 32.Sahasrabuddhe VV, Gunja MZ, Graubard BI, Trabert B, Schwartz LM, Park Y, et al. Nonsteroidal anti-inflammatory drug use, chronic liver disease, and hepatocellular carcinoma. J Natl Cancer Inst. 2012;104:1808–1814. doi: 10.1093/jnci/djs452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hashemi Kani A, Alavian SM, Esmaillzadeh A, Adibi P, Azadbakht L. Dietary Quality Indices and Biochemical Parameters Among Patients With Non Alcoholic Fatty Liver Disease (NAFLD). Hepat Mon. 2013;13:e10943. doi: 10.5812/hepatmon.10943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kontogianni MD, Tileli N, Margariti A, Georgoulis M, Deutsch M, Tiniakos D, et al. Adherence to the Mediterranean diet is associated with the severity of non-alcoholic fatty liver disease. Clin Nutr. 2013 doi: 10.1016/j.clnu.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Turati F, Trichopoulos D, Polesel J, Bravi F, Rossi M, Talamini R, et al. Mediterranean diet and hepatocellular carcinoma. J Hepatol. 2014;60:606–611. doi: 10.1016/j.jhep.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 36.Kennedy ET, Ohls J, Carlson S, Fleming K. The Healthy Eating Index: design and applications. J Am Diet Assoc. 1995;95:1103–1108. doi: 10.1016/S0002-8223(95)00300-2. [DOI] [PubMed] [Google Scholar]
- 37.Romaguera D, Vergnaud AC, Peeters PH, van Gils CH, Chan DS, Ferrari P, et al. Is concordance with World Cancer Research Fund/American Institute for Cancer Research guidelines for cancer prevention related to subsequent risk of cancer? Results from the EPIC study. Am J Clin Nutr. 2012;96:150–163. doi: 10.3945/ajcn.111.031674. [DOI] [PubMed] [Google Scholar]
- 38.Karin M, Lawrence T, Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 2006;124:823–835. doi: 10.1016/j.cell.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 39.Moy KA, Jiao L, Freedman ND, Weinstein SJ, Sinha R, Virtamo J, et al. Soluble receptor for advanced glycation end products and risk of liver cancer. Hepatology. 2013;57:2338–2345. doi: 10.1002/hep.26264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.George SM, Neuhouser ML, Mayne ST, Irwin ML, Albanes D, Gail MH, et al. Postdiagnosis diet quality is inversely related to a biomarker of inflammation among breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2010;19:2220–2228. doi: 10.1158/1055-9965.EPI-10-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim JY, Yang YJ, Yang YK, Oh SY, Hong YC, Lee EK, et al. Diet quality scores and oxidative stress in Korean adults. Eur J Clin Nutr. 2011;65:1271–1278. doi: 10.1038/ejcn.2011.120. [DOI] [PubMed] [Google Scholar]
- 42.World Cancer Research Fund / American Institute for Cancer Research . Food, Nutrition, Physical Activity and the Prevention of Cancer: a Global Perspective. AICR; Washington, DC: 2007. [Google Scholar]
- 43.Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson RJ, et al. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1961–1971. doi: 10.1002/hep.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kohli R, Kirby M, Xanthakos SA, Softic S, Feldstein AE, Saxena V, et al. High-fructose, medium chain trans fat diet induces liver fibrosis and elevates plasma coenzyme Q9 in a novel murine model of obesity and nonalcoholic steatohepatitis. Hepatology. 2010;52:934–944. doi: 10.1002/hep.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ioannou GN. Hepatitis B virus in the United States: infection, exposure, and immunity rates in a nationally representative survey. Ann Intern Med. 2011;154:319–328. doi: 10.7326/0003-4819-154-5-201103010-00006. [DOI] [PubMed] [Google Scholar]
- 46.Ditah I, Ditah F, Devaki P, Ewelukwa O, Ditah C, Njei B, et al. The Changing Epidemiology of Hepatitis C Virus Infection in the United States: National Health and Nutrition Examination Survey 2001 through 2010. J Hepatol. 2013 doi: 10.1016/j.jhep.2013.11.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.