Abstract
Background
Drug abuse (DA) is a clinically heterogeneous syndrome. Can we, in a large epidemiological sample, identify clinical features of DA cases that index genetic risk?
Method
Using registration in medical, legal or pharmacy records, we identified four kinds of relative pairs (n =935854) starting with a proband with DA: monozygotic co-twins; full siblings; half-siblings; and cousins. Using linear hazard regression, we examined the interaction between three clinical features of DA in the proband and risk for DA in these four relative pairs, ordered by degree of genetic relationship.
Results
Increased risk for DA in relatives was robustly predicted by early age at first registration, total number of registrations, and ascertainment in the criminal versus the medical or pharmacy registry. In multivariate models, all three of these variables remained significant and in aggregate strongly predicted DA risk in relatives. The risk for DA in siblings of DA probands in the highest decile of genetic risk predicted by our three indices was more than twice as great as that predicted in siblings of probands in the lowest decile of risk.
Conclusions
In an epidemiological sample, genetic risk for DA can be substantially indexed by simple clinical and historical variables.
Keywords: Age at first registration, drug abuse, genetic risk, Sweden
Introduction
Drug abuse (DA), the risk for which is strongly influenced by familial/genetic factors (Tsuang et al. 1996; Kendler & Prescott, 1998; Merikangas et al. 1998; Lynskey et al. 2002; Kendler et al. 2000, 2012b), is a clinically heterogeneous syndrome. Both for clinical and research purposes, it is of obvious interest to determine whether available clinical or historical information about affected individuals can usefully reflect their underlying genetic risk to DA. In both neuropsychiatric and medical disorders, age at onset is often related inversely, sometimes quite strongly, with level of genetic risk (Heston et al. 1981; McGue et al. 1992; Marenberg et al. 1994; Steele, 2002). In major depression, one of the most consistent indices of genetic risk is recurrence (Sullivan et al. 2000; Kendler et al. 2007). In our study of DA in Sweden using publicly available information, subjects can be ascertained through criminal, medical or pharmacy records, and it is of interest to determine whether the average genetic risk differs between subjects ascertained by these different means.
In this report, in a nationwide Swedish sample, we examine whether age at first DA registration (AFDAR), recurrence (indexed by the number of independent registrations for DA) and mode of ascertainment predict risk for DA in four classes of relatives: monozygotic (MZ) co-twins; full siblings; half-siblings; and cousins. Analogous to other psychiatric disorders, we predict that both early age at onset and high levels of recurrence will predict risk for DA in relatives with the strength of the prediction closely related to the degree of genetic relationship (i.e. MZ>siblings> half-siblings>cousins).
Method
We used the same data sources as we have utilized and described in our previous publications on DA in Sweden (Kendler et al. 2012a,b, 2013b, 2014). In short, we used linked data from multiple Swedish nationwide registries and healthcare data. Linking was achieved via the unique individual 10-digit personal identity (ID) number assigned at birth or immigration to all Swedish residents. In order to preserve confidentiality, this ID number was replaced by a serial number. The following sources were used to create our database: The Multi-Generation Register, providing information on family relations; the Swedish Hospital Discharge Register, containing all hospitalizations for all Swedish inhabitants from 1964 to 2010; the Swedish Prescribed Drug Register, containing all prescriptions in Sweden picked up by patients from 2005 to 2009; the Outpatient Care Register, containing information from all out-patient clinics from 2001 to 2010; the Primary Health Care Register, containing out-patient primary care data on diagnoses and time for diagnoses 2001–2007 for 1 million patients from Stockholm and middle Sweden; the Swedish Crime Register included national complete data on all convictions from 1973 to 2011; the Swedish suspicion register included national complete data on all individuals strongly suspected of crime from 1998 to 2011; and the Swedish Mortality Register, containing causes of death. MZ twins were identified through the Swedish twin registry, which is formed from a nearly complete registration of all twin births in the country (Lichtenstein et al. 2002).
We secured ethical approval for this study from the Regional Ethical Review Board of Lund University (no. 2008/409).
Definition of DA
DA was identified in the Swedish medical registries by International Classification of Diseases (ICD) codes [ICD-8: Drug dependence (304); ICD-9: Drug psychoses (292) and Drug dependence (304); ICD-10: Mental and behavioral disorders due to psychoactive substance use (F10–F19, which includes intoxication, harmful use, dependence and withdrawal), except those due to alcohol (F10) or tobacco (F17)]; in the Suspicion register by codes 3070, 5010, 5011 and 5012, which reflect crimes related to DA; and in the Crime register by references to laws covering narcotics (law 1968:64, paragraph 1, point 6) and driving offences (law 1951:649, paragraph 4, subsection 2 and paragraph 4A, subsection 2). DA was identified in individuals (excluding those suffering from cancer) in the Prescribed Drug Register, who had retrieved (on average) more than four defined daily doses per day for 12 months of either hypnotics and sedatives [Anatomical Therapeutic Chemical (ATC) Classification System N05C and N05BA] or opioids (ATC: N02A). We restricted the diagnosis of DA to individuals above the age of 10 years, except from the prescribed drug register where the age limit was set at 18 years.
Sample
The dataset was created by entering all first cousin, half-sibling, full sibling and MZ twin pairs in the Swedish population where both individuals in the pair were born between 1950 and 1993. Furthermore, we required that at least one individual in the pair was registered as DA. The individual in the pair first registered as DA was defined as the proband. The number of pairs can be seen in Table 1.
Table 1.
Characteristics of the sample studied
| Cousins | Half-siblings | Siblings | MZ twins | |
|---|---|---|---|---|
| Pairs, n | 607929 | 125259 | 202366 | 300 |
| Prevalence of drug abuse in relative, n (%) | 21465 (3.5) | 9135 (7.3) | 18969 (9.4) | 89 (29.7) |
| Medical register, n (%) | ||||
| Proband | 269101 (44) | 63955 (51) | 92374 (46) | 146 (49) |
| Relative | 7589 (35) | 3542 (39) | 6824 (36) | 27 (30) |
| Criminal register, n (%) | ||||
| Proband | 463251 (76) | 92317 (74) | 144283 (71) | 187 (62) |
| Relative | 17556 (82) | 7506 (82) | 15611 (82) | 70 (79) |
| Prescription register, n (%) | ||||
| Proband | 25576 (4) | 5561 (4) | 11030 (5) | 26 (9) |
| Relative | 758 (4) | 364 (6) | 851 (4) | 9 (10) |
| No. of registrations – proband | ||||
| 25th percentile | 1 | 1 | 1 | 1 |
| 50th percentile | 2 | 3 | 2 | 2 |
| 75th percentile | 7 | 9 | 7 | 4 |
| Mean AFDAR – proband, years (S.D.) | 25.7 (8.0) | 26.9 (9.0) | 27.6 (10.0) | 29.5 (10.4) |
| Median year of birth | ||||
| Proband | 1980 | 1977 | 1978 | 1976 |
| Relatives | 1978 | 1975 | 1977 | 1976 |
| Mean age difference, years (S.D.) | 7.6 (6.1) | 9.5 (5.5) | 4.7 (3.4) | 0 |
MZ, Monozygotic, S.D., standard deviation; AFDAR, age at first drug abuse registration.
Statistical methods
We looked at the interactions between three clinical features of the DA registration in the proband: (1) AFDAR; (2) number of registrations; and (3) type of registration, and genetic resemblance on the one hand and subsequent DA in relative to the proband on the other. In the database, each pair was assigned their degree of genetic resemblance (1 for MZ twins; 0.5 for full siblings; 0.25 for half-siblings; 0.125 for cousins).
We utilized Aalen’s linear hazard regression to investigate all pairs from the year of DA registration in the proband until (a) year of first registration of DA in the relative, (b) death in the relative or (c) end of follow-up (year 2011), whichever came first. As we have previously shown that siblings and cousins were significantly more similar in their history of DA if they were closer versus more distant in age (Kendler et al. 2013b, 2014), we controlled for the absolute age difference between the proband and the relative in all models. As we used Aalen’s linear hazards model, the results from the models will be interpreted at the additive scale as the excess number of cases. In all models we investigated the proportionality assumption. The key predictor variables in the models were the interaction terms between the clinical feature and genetic resemblance.
In the first analysis, we focused on age at onset, defined as AFDAR in the proband. In the model we investigated both the linear and quadratic effects of age at onset and their interactions with genetic resemblance. In the second analysis, we focused on the number of DA registrations in the proband. We investigated both the linear and quadratic effects of the number of DA registrations and their interactions with genetic resemblance. The quadratic term was not significant (p=0.45) and therefore was not included in the final model.
In the third analysis, we focused on the type of DA registration in the proband. We categorized the registrations into three groups: (1) medical registrations (the Swedish Hospital Discharge Register, the Out-patient Care Register, and the Primary Health Care Register); (2) criminal registrations (the Swedish Crime Register and the Swedish suspicion register); and (3) prescription registrations (the Swedish Prescribed Drug Register). In the models we used the criminal register as the reference group. We investigated the interaction between medical registration and genetic resemblance, and the interaction between prescription registration and genetic resemblance.
In the final analysis we included all clinical features and their interactions with genetic resemblance in the same model. As we wanted to compare their separate effects, we only investigated the linear effects of age registration and number of registrations. Furthermore, we translated the variable type of registration into a continuous variable based on their relative size of the interaction term between the contrasts and the nature of genetic relationship (1=prescription registration, 2=medical registration and 3=criminal registration). As a proband could be registered in several different types of registers, we selected the register in which the proband was first registered.
The statistical analyses were performed using SAS 9.3 (SAS Institute, Inc., USA; Butler & Heron, 2008) and the R-package 2.14.1 (The R Foundation for Statistical Computing; 2011).
Results
Nature of sample
The key descriptive features of the sample used in this report, which includes a total of 935854 relative pairs, are outlined in Table 1. The prevalence of DA in the population (born 1950 to 1993) was 3.4%. The frequency and 95% confidence intervals (CIs) of DA in the co-relative of our proband with DA increased with increasing genetic relationship: cousin 3.5% (95% CI 3.5–3.6); half-sibling 7.3% (95% CI 7.2–7.4); full sibling 9.4% (95% CI 9.2–9.5); and MZ co-twin 29.7% (95% CI 24.8–35.1). The prevalence for DA differed significantly (p<0.0001) between all relative groups. Across all groups, probands and affected relatives were most commonly detected with DA from the criminal registry, and least commonly from the prescription register. AFDAR was in the mid-20 to late-20 years and the median number of registrations was 2 or 3.
Age at onset
We first modeled risk as a hazard function beginning with the linear effects of AFDAR, genetic relationship between the pair and their interaction, with age difference as a control variable. However, we detected significant quadratic effects of AFDAR on DA risk in relatives and so present the model seen in Table 2. We found a strong interaction between genetic relationship in the relative pair, and both the linear and quadratic effects of AFDAR. As seen in Fig. 1, the magnitude of the decline in risk in the co-relative as a function of increasing AFDAR in the proband predicted by this model is robustly related to the degree of genetic relationship in the relative pair. That is, it is strongest in MZ twins and declines in magnitudes from siblings to half-siblings to cousins. The decline is also not linear but is steeper at younger ages and becomes flatter for AFDARs above ~30 years. More specifically, the difference in the number of new cases of DA per 10000 person years if the proband was registered at age 15 years versus age 40 years was predicted to equal 348 for MZ co-twins, 170 for siblings, 80 for half-siblings and 35 for cousins.
Table 2.
Results of a linear hazard model predicting risk of drug abuse in relatives as a function of the linear and quadratic effects of the proband’s AFDAR, genetic relationship between proband and relative, age differences and the interaction between AFDAR and genetic relationship
| β coefficient | (robust S.E.) | z | p | |
|---|---|---|---|---|
| AFDAR, centered at mean | 0.0000465 | (0.0000172) | 2.71 | 0.007 |
| Genetic relationship | 0.0168 | (0.00116) | 14.5 | <0.001 |
| Age difference in relative pair | −0.000141 | (0.00000737) | −19.1 | <0.001 |
| AFDAR2 | −0.00000366 | (0.000000838) | −4.73 | <0.001 |
| AFDAR×genetic relationship | −0.00154 | (0.000109) | −14.1 | <0.001 |
| AFDAR2×genetic relationship | 0.0000454 | (0.000004) | 11.4 | <0.001 |
AFDAR, Age at first drug abuse registration; S.E., standard error.
Fig. 1.

Predicted results from the linear hazard model presented in full in Table 2. This figure depicts the predicted excess number of cases of drug abuse per 10000 person years in monozygotic (MZ) co-twins, full siblings, half-siblings and cousins of probands with drug abuse as a function of the proband’s age at first registration for drug abuse.
Number of DA registrations
As seen in Table 3, we next modeled risk as a linear function of the number of DA registrations, age differences, genetic relationship and the interaction between genetic relationship in the relative pair and number of registrations. (We examined for but found no quadric effect of the number of registrations on risk.) As seen in Fig. 2, we found a significant interaction with the increase in the number of new cases in relatives as a function of increasing number of DA registrations in the proband predicted by this model as related to their degree of genetic relationship. We can quantify this by examining the absolute difference in number of new DA cases per 10000 person years expected in relatives if the proband had only one versus 10 DA registrations. These equal 16 in MZ co-twins, eight in siblings, three in half-siblings and one in cousins.
Table 3.
Results of a linear hazard model predicting risk of drug abuse in relatives as a function of the number of drug abuse registrations of the proband, the genetic relationship between proband and relative, age differences and the interaction between number of registrations and genetic relationship
| β coefficient | (robust S.E.) | z | p | |
|---|---|---|---|---|
| Number of registrations | −0.00000607 | (0.00000316) | −1.90 | 0.057 |
| Genetic relationship | 0.0164 | (0.000277) | 57.9 | <0.001 |
| Age difference in relative pair | −0.000156 | (0.00000482) | −31.3 | <0.001 |
| Number of registrations×genetic relationship | 0.000197 | (0.000015) | 12.5 | <0.001 |
S.E., Standard error.
Fig. 2.

Predicted results from the linear hazard model presented in full in Table 3. This figure depicts the predicted excess number of cases of drug abuse per 10000 person years in monozygotic (MZ) co-twins, full siblings, half-siblings and cousins of probands with drug abuse as a function of the number of the proband’s registrations for drug abuse.
Source of DA ascertainment
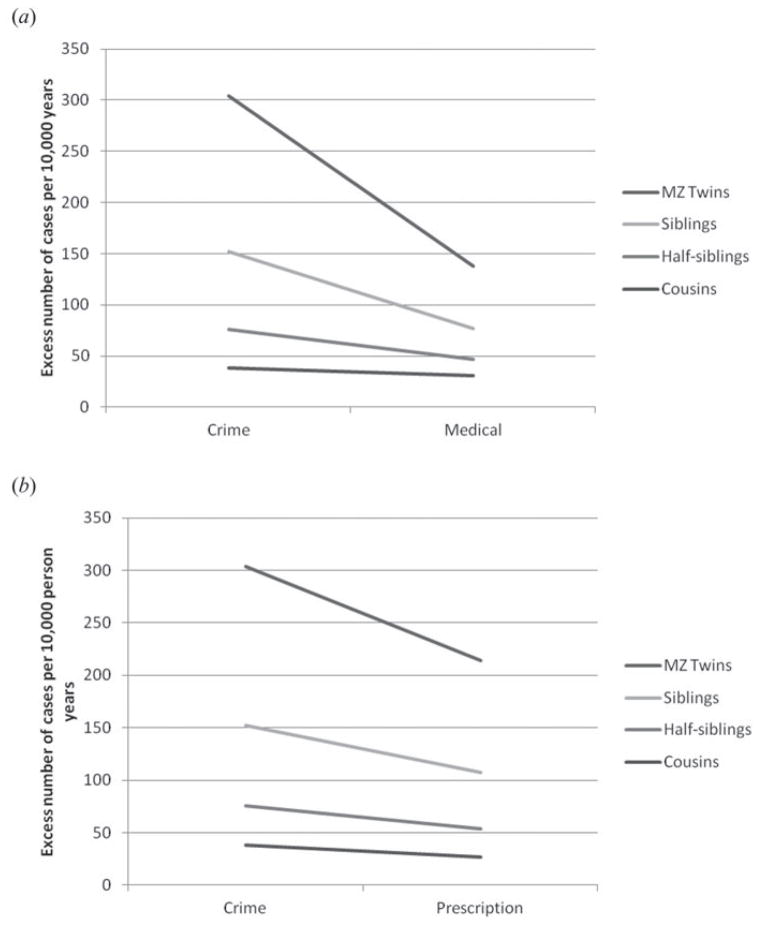
Table 4 presents our model where we compare risk in relatives as a function of source of DA registration with the reference group being those detected in the criminal register. We see significant interactions between both contrasts and the nature of genetic relationship such that risk in relatives is lower when the proband was ascertained for DA in the medical or the prescription registers versus the crime register. These results are illustrated, respectively, in Fig. 3(a, b). To quantify these effects, if the proband is registered in the criminal versus the medical register, the model predicts the following number of new cases of DA per 10000 person years: MZ co-twins 166; siblings 75; half siblings 30; and cousins 11. The parallel figures for the criminal versus the prescription registry were: MZ twins 90; siblings 45; half siblings 22; and cousins 11.
Table 4.
Results of a linear hazard model predicting risk of drug abuse in relatives as a function of the source of registration of the proband (medical or prescription with crime registration as the reference), the genetic relationship between proband and relative, age differences and the interaction between source of registration and genetic relationship
| β coefficient | (robust S.E.) | z | p | |
|---|---|---|---|---|
| Medical register | 0.00158 | (0.000111) | 14.2 | <0.001 |
| Prescription register | 0.0000228 | (0.000238) | 0.10 | 0.924 |
| Genetic relationship | 0.0304 | (0.000485) | 62.6 | <0.001 |
| Age difference in relative pair | −0.000156 | (0.00000496) | −31.4 | <0.001 |
| Medical register×genetic relationship | −0.0182 | (0.00053) | −34.3 | <0.001 |
| Prescription register×genetic relationship | −0.00903 | (0.00102) | −8.85 | <0.001 |
S.E., Standard error.
Fig. 3.
(a) Predicted results from the linear hazard model presented in full in Table 4. This figure depicts the predicted excess number of cases of drug abuse per 10000 person years in monozygotic (MZ) co-twins, full siblings, half-siblings and cousins of probands with drug abuse as a function of the proband’s registration for drug abuse in the crime versus medical register. (b) Predicted results from the linear hazard model presented in full in Table 4. This figure depicts the predicted excess number of cases of drug abuse per 10000 person years in monozygotic co-twins, full siblings, half-siblings and cousins of probands with drug abuse as a function of the proband’s registration for drug abuse in the crime versus prescription register.
All three indices of genetic risk to DA in a single model
Table 5 presents the results of z-transformations of the three indices of genetic risk as predictors of risk for DA in relatives. Each of the indices continues to have a statistically robust interaction with level of genetic relatedness. As assessed by the z score of the interaction, when jointly analysed, the source of registration is the most powerful index of genetic risk (z=37.2) followed by AFDAR (z=31.6). The number of registrations is a rather distant third (z=10.5).
Table 5.
Results of a linear hazard model predicting risk of drug abuse in relatives as a function of z-transformed scores for the source of registration of the proband (coded as 1=medical, 2=prescription, 3=crime), AFDAR and the number of registrations, genetic relationship between proband and relative, age differences and the interaction between source of registration, AFDAR and number of registrations and the genetic relationship
| β coefficient | (robust S.E.) | z | p | |
|---|---|---|---|---|
| Source of registration | −0.000707 | (0.0000518) | −11.9 | <0.001 |
| AFDAR | −0.0000422 | (0.000054) | −0.68 | 0.498 |
| Number of registrations | −0.000118 | (0.0000455) | −2.10 | 0.036 |
| Genetic relationship | 0.0242 | (0.000304) | 67.0 | <0.001 |
| Age difference in relative pair | −0.000148 | (0.00000477) | −28.6 | <0.001 |
| AFDAR×genetic relationship | −0.0082 | (0.000222) | −31.6 | <0.001 |
| Number of registrations×genetic relationship | 0.00257 | (0.000216) | 10.5 | <0.001 |
| Source of registration×genetic relationship | 0.0105 | (0.00024) | 37.2 | <0.001 |
AFDAR, Age at first drug abuse registration; S.E., standard error.
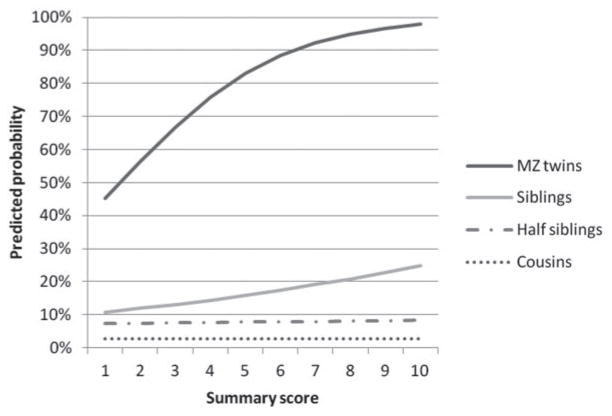
Finally, we aggregated the z scores for the three clinical features and constructed one summary measure. We then divided this summary measure into 10 equally sized groups and then performed a logistic regression analysis with the summary measure, on the one hand, and DA in the relative on the other for each relative group. As seen in Fig. 4, the difference in risk for DA in the close relatives of DA probands (MZ co-twins and siblings) classified as being at low versus high genetic risk was quite substantial. The predicted risk for DA in the MZ co-twin and siblings of a DA individual ranked in the lowest, fifth and highest deciles of genetic risk were, respectively, 45.2, 83.2 and 97.9% and 10.8, 15.9 and 24.8%. The differences in more distant relatives were more modest, with predicted risk for DA in half-siblings and cousins of DA probands in the lowest, fifth and highest deciles of genetic risk of, respectively, 7.3, 7.8 and 8.4% and 2.78, 2.80 and 2.83%.
Fig. 4.
Predicted risk for drug abuse as calculated by logistic regression in monozygotic (MZ) co-twins, full siblings, half-siblings and cousins of probands with drug abuse as a function of the proband’s aggregate genetic risk estimated from the model presented in full in Table 5. The z scores for the three clinical features were aggregated into one summary measure and divided into 10 equally sized groups. The figure depicts the predicted probability of drug abuse in probands for the 10 groups.
Discussion
The goal of this report was to determine, in a Swedish national epidemiological sample, whether three clinical and historical features of DA ascertained through official registries were related to genetic risk as assessed by the prevalence of DA in four classes of relatives: cousins; half-siblings; full siblings; and MZ co-twins. We indeed found that risk to relatives was predicted by early AFDAR, higher numbers of drug registrations and by registration via the crime versus the pharmacy or medical registries. As would be expected if these measures indexed genetic risk, this association varied as a function of the genetic relationship of the relative pair being strongest in MZ pairs, intermediate in full and half-siblings, and weakest in first cousins. Finally, when put into a multivariate model, all three predictors were significantly related to risk in relatives, and significantly interacted with the degree of genetic relationship. Source of DA registration was the most powerful predictor followed by AFDAR, with the number of registrations the least robust predictor.
There are precedents for our finding that AFDAR is inversely related to genetic risk for DA. Several molecular genetic variants have been shown to be associated with early onset of DA (Vanyukov et al. 1995), or of alcohol and tobacco initiation (Schlaepfer et al. 2008). More directly relevant, twin studies have shown that genetic influences on alcohol use disorders were greatest in those with an early onset of drinking (Agrawal et al. 2009), and that early-onset cannabis use indexed increased genetic risk for nicotine dependence (Agrawal et al. 2008). It has been suggested that early age at onset can be used as an index of high genetic risk for DA (Heath et al. 2002).
We have had two prior hints in our work with this sample that criminal registration for DA might be associated with increased familial/genetic risk. First, in our adoption study (Kendler et al. 2012b), a history of criminal conviction in biological parents increased the risk of DA in adoptees, controlling for the parental risk for DA. Second, in a latent class analysis of DA in the Swedish population, two of the identified six classes (high-frequency medical criminal and low-frequency pure criminal) contained virtually all those cases ascertained through the criminal registry and had the highest and third highest risk for DA in siblings (Kendler et al. 2013a). While this will need to be confirmed by further work, we would speculate that a larger proportion of cases of DA identified through the criminal registry than the medical or prescription registers fits the pattern of type B or type II alcoholism (Cloninger, 1987; Babor et al. 1992) (which has been successfully applied to DA; Ball et al. 1995), typified by a range of clinical features including early age at onset, prominent antisocial behavior, high psychiatric co-morbidity, male predominance, and high genetic loading. This study differs, however, from any previous analyses in this sample in that it combines a range of available indices of genetic risk for DA and examined their performance in multivariate analyses.
It is useful to outline two possible conceptual frameworks in which to understand the association between AFDAR and number of registrations and genetic risk to DA. The first would be a multifactorial stress–diathesis model for DA. Assume two hypothetical individuals – M and H – who begin life with, respectively, a moderate and high genetic diathesis to DA. Assume that psychosocial stressors (which increase risk for DA; Sinha, 2001) are, to a first approximation, randomly distributed across time. Because of differences in their diathesis, individual M will require more severe stress to develop DA than individual H. Under these assumptions, we would expect on average that individual M will be older when he has confronted the severe stressors needed to precipitate DA than will individual H when he experiences the more modest levels of stress needed to develop DA for the first time. This would predict the observed inverse association between AFDAR and genetic risk. Similarly, once affected with DA, individual M is, during his lifetime, likely to confront fewer adversities that can ‘push him’ past the threshold to cause a relapse than would individual H. This would predict the observed positive association between number of registrations and genetic risk.
Gene–environment correlation provides an alternative pathway through which high genetic risk for DA would cause both early onset and multiple relapses. Prior evidence suggests that individuals at high genetic risk for DA – like individual H – are more likely to seek out deviant peers, who provide access to and support for early drug use and abuse than individuals like M (Kendler et al. 2008; Gillespie et al. 2009). Such a relationship could then explain the association between AFDAR and genetic risk. Furthermore, if individuals at high genetic risk are more prone to returning to deviant social environments after remission, this might explain the observed relationship between genetic risk and number of relapses or episodes of DA.
Limitations
These results should be interpreted in the context of three potentially important methodological limitations. First, this study was confined to one Scandinavian country and we cannot be certain that our findings would generalize to other cultural and ethnic groups. Second, subjects with DA were ascertained from medical, legal and pharmacy records. Contrary to standard epidemiological surveys, this approach has the advantage of ascertaining cases independent of subject cooperation or accurate recall and reporting. However, this method undoubtedly produces both false-negative and false-positive diagnoses. An epidemiological study of DA conducted in neighboring Norway reported rates of drug use and abuse (Kraus et al. 2003; Hibell et al. 2007), assessed using Diagnostic and Statistical Manual of Mental Disorders, third edition, revised (DSM-III-R) criteria (APA, 1987), quite similar to those found using our registry-based methods (Kringlen et al. 2001), suggesting that it is unlikely that we have substantially under-ascertained DA.
Third, we were only able to assess age at onset of DA indirectly through AFDAR. The time period between the true onset of DA and first detection by our methods is likely to be variable. In general, this increased variability should attenuate rather than exaggerate the association with risk of illness in relatives. Similarly, our measure of recurrence – number of registrations – is surely imperfect. An individual might have multiple registrations during a single prolonged period of DA or might have episodes of DA that go undetected by our methods. Again, the imperfection of this measure is more likely to bias downward rather than upward the observed association with risk of DA in relatives.
Conclusion
In a large epidemiological sample of the Swedish population, we were able to identify three relatively simple clinical indices of DA that independently strongly reflected the genetic risk to DA especially when summed into an aggregate measure. Individuals who were first registered at a young age, had many registrations and were registered in the criminal registry were at particularly high genetic risk. These results suggest that the heterogeneity of the DA syndrome can, for clinical and research purposes, be meaningfully indexed by simple clinical measures. Our results also have particular implications for gene-finding efforts if the goal is to identify DA probands at high genetic risk. However, for a complex trait like DA, it cannot be assured that higher heritability would translate into greater ease of gene finding.
Acknowledgments
This study was funded by grant RO1 no. DA030005 from the National Institute of Drug Abuse, the Swedish Research Council (2012-2378), the ALF project grant, Lund, Sweden, the Swedish Council for Information on Alcohol and Other Drugs (CAN), and the Swedish Research Council for Health, Working Life and Welfare (reg. no. 2013-1836).
Footnotes
Declaration of Interest
None.
References
- Agrawal A, Lynskey MT, Pergadia ML, Bucholz KK, Heath AC, Martin NG, Madden PA. Early cannabis use and DSM-IV nicotine dependence: a twin study. Addiction. 2008;103:1896–1904. doi: 10.1111/j.1360-0443.2008.02354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Sartor CE, Lynskey MT, Grant JD, Pergadia ML, Grucza R, Bucholz KK, Nelson EC, Madden PA, Martin NG, Heath AC. Evidence for an interaction between age at first drink and genetic influences on DSM-IV alcohol dependence symptoms. Alcohol Clinical Experimental Research. 2009;33:2047–2056. doi: 10.1111/j.1530-0277.2009.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders. 3. American Psychiatric Association; Washington, DC: 1987. revised. [Google Scholar]
- Babor TF, Hofmann M, DelBoca FK, Hesselbrock V, Meyer RE, Dolinsky ZS, Rounsaville B. Types of alcoholics, I. Evidence for an empirically derived typology based on indicators of vulnerability and severity. Archives of General Psychiatry. 1992;49:599–608. doi: 10.1001/archpsyc.1992.01820080007002. [DOI] [PubMed] [Google Scholar]
- Ball SA, Carroll KM, Babor TF, Rounsaville BJ. Subtypes of cocaine abusers: support for a type A–type B distinction. Journal of Consulting and Clinical Psychology. 1995;63:115–124. doi: 10.1037//0022-006x.63.1.115. [DOI] [PubMed] [Google Scholar]
- Butler RJ, Heron J. The prevalence of infrequent bedwetting and nocturnal enuresis in childhood. A large British cohort. Scandinavian Journal of Urology and Nephrology. 2008;42:257–264. doi: 10.1080/00365590701748054. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–416. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- Gillespie NA, Neale MC, Jacobson K, Kendler KS. Modeling the genetic and environmental association between peer group deviance and cannabis use in male twins. Addiction. 2009;104:420–429. doi: 10.1111/j.1360-0443.2008.02457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Martin NG, Lynskey MT, Todorov AA, Madden PA. Estimating two-stage models for genetic influences on alcohol, tobacco or drug use initiation and dependence vulnerability in twin and family data. Twin Research. 2002;5:113–124. doi: 10.1375/1369052022983. [DOI] [PubMed] [Google Scholar]
- Heston LL, Mastri AR, Anderson VE, White J. Dementia of the Alzheimer type. Clinical genetics, natural history, and associated conditions. Archives of General Psychiatry. 1981;38:1085–1090. doi: 10.1001/archpsyc.1981.01780350019001. [DOI] [PubMed] [Google Scholar]
- Hibell B, Guttormsson U, Ahlstrom S, Balakireva O, Bjarnason T, Kokkevi A, Kraus L. The 2007 ESPAD Report: Substance Use Among Students in 35 European Countries. The Swedish Council for Information on Alcohol and Other Drugs (CAN); Stockholm: 2007. [Google Scholar]
- Kendler KS, Gatz M, Gardner CO, Pedersen NL. Clinical indices of familial depression in the Swedish Twin Registry. Acta Psychiatrica Scandinavica. 2007;115:214–220. doi: 10.1111/j.1600-0447.2006.00863.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Jacobson K, Myers JM, Eaves LJ. A genetically informative developmental study of the relationship between conduct disorder and peer deviance in males. Psychological Medicine. 2008;38:1001–1011. doi: 10.1017/S0033291707001821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Neale MC, Prescott CA. Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. Archives of General Psychiatry. 2000;57:261–269. doi: 10.1001/archpsyc.57.3.261. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Maes H, Sundquist K, Lichtenstein P, Ohlsson H, Sundquist JA. Genetic and family and community environmental effects on drug abuse in adolescence: a Swedish national twin and sibling study. American Journal of Psychiatry. 2012a doi: 10.1176/appi.ajp.2013.12101300. Published online 30 September 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Ohlsson H, Sundquist K, Sundquist J. A latent class analysis of drug abuse in a national Swedish sample. Psychological Medicine. 2013a;43:2169–2178. doi: 10.1017/S0033291713000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Ohlsson H, Sundquist K, Sundquist J. Within-family environmental transmission of drug abuse: a Swedish national study. Archives of General Psychiatry. 2013b;70:235–242. doi: 10.1001/jamapsychiatry.2013.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Ohlsson H, Sundquist K, Sundquist J. Environmental influences on familial resemblance for drug abuse in first-cousin pairs: a Swedish national study. Psychological Medicine. 2014;44:371–379. doi: 10.1017/S0033291713000846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Cannabis use, abuse, and dependence in a population-based sample of female twins. American Journal of Psychiatry. 1998;155:1016–1022. doi: 10.1176/ajp.155.8.1016. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Sundquist K, Ohlsson H, Palmer K, Maes H, Winkleby MA, Sundquist J. Genetic and familial–environmental influences on risk for drug abuse: a national Swedish adoption study. Archives of General Psychiatry. 2012b;69:690–697. doi: 10.1001/archgenpsychiatry.2011.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus L, Augustin R, Frischer M, Kummler P, Uhl A, Wiessing L. Estimating prevalence of problem drug use at national level in countries of the European Union and Norway. Addiction. 2003;98:471–485. doi: 10.1046/j.1360-0443.2003.00326.x. [DOI] [PubMed] [Google Scholar]
- Kringlen E, Torgersen S, Cramer V. A Norwegian psychiatric epidemiological study. American Journal of Psychiatry. 2001;158:1091–1098. doi: 10.1176/appi.ajp.158.7.1091. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, de Faire U, Floderus B, Svartengren M, Svedberg P, Pedersen NL. The Swedish Twin Registry: a unique resource for clinical, epidemiological and genetic studies. Journal of Internal Medicine. 2002;252:184–205. doi: 10.1046/j.1365-2796.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Heath AC, Nelson EC, Bucholz KK, Madden PA, Slutske WS, Statham DJ, Martin NG. Genetic and environmental contributions to cannabis dependence in a national young adult twin sample. Psychological Medicine. 2002;32:195–207. doi: 10.1017/s0033291701005062. [DOI] [PubMed] [Google Scholar]
- Marenberg ME, Risch N, Berkman LF, Floderus B, de Faire U. Genetic susceptibility to death from coronary heart disease in a study of twins. New England Journal of Medicine. 1994;330:1041–1046. doi: 10.1056/NEJM199404143301503. [DOI] [PubMed] [Google Scholar]
- McGue M, Pickens RW, Svikis DS. Sex and age effects on the inheritance of alcohol problems: a twin study. Journal of Abnormal Psychology. 1992;101:3–17. doi: 10.1037//0021-843x.101.1.3. [DOI] [PubMed] [Google Scholar]
- Merikangas K, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, Zhang H, O’Malley SS, Rounsaville BJ. Familial transmission of substance use disorders. Archives of General Psychiatry. 1998;55:973–979. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- Schlaepfer IR, Hoft NR, Collins AC, Corley RP, Hewitt JK, Hopfer CJ, Lessem JM, McQueen MB, Rhee SH, Ehringer MA. The CHRNA5/A3/B4 gene cluster variability as an important determinant of early alcohol and tobacco initiation in young adults. Biological Psychiatry. 2008;63:1039–1046. doi: 10.1016/j.biopsych.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berlin) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Steele CM. Cancer of the breast and female reproductive tract. In: Rimoin DL, Connor JM, Pyeritz RE, Korf BR, editors. Emery and Rimoin’s Principles and Practice of Medical Genetics. 2. Vol. 4. Churchill Livingstone; London: 2002. pp. 2352–2384. [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. American Journal of Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, Meyer JM, Toomey R, Faraone SV, Eaves L. Genetic influences on DSM-III-R drug abuse and dependence: a study of 3,372 twin pairs. American Journal of Medical Genetics. 1996;67:473–477. doi: 10.1002/(SICI)1096-8628(19960920)67:5<473::AID-AJMG6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Vanyukov MM, Moss HB, Yu LM, Tarter RE, Deka R. Preliminary evidence for an association of a dinucleotide repeat polymorphism at the MAOA gene with early onset alcoholism/substance abuse. American Journal of Medical Genetics. 1995;60:122–126. doi: 10.1002/ajmg.1320600207. [DOI] [PubMed] [Google Scholar]