Abstract
Background
Reduced sleep duration has been increasingly reported to predict obesity. However, timing and regularity of sleep may also be important. In this study, the cross-sectional association between objectively measured sleep patterns and obesity was assessed in two large cohorts of older individuals.
Methods
Wrist actigraphy was performed in 3053 men (mean age: 76.4 years) participating in the Osteoporotic Fractures in Men Study (MrOS) and 2985 women (mean age: 83.5 years) participating in the Study of Osteoporotic Fractures (SOF). Timing and regularity of sleep patterns were assessed across nights, as well as daytime napping.
Results
Greater night-to-night variability in sleep duration and daytime napping were associated with obesity independent of mean nocturnal sleep duration in both men and women. Each 1 hour increase in the variability of nocturnal sleep duration increased the odds of obesity 1.63-fold (95% CI [1.31-2.02]) among men and 1.22-fold (95% CI [1.01-1.47]) among women. Each 1 hour increase in napping increased the odds of obesity 1.23-fold (95%CI [1.12-1.37]) in men and 1.29-fold (95%CI [1.17-1.41]) in women. In contrast, associations between later sleep timing and night-to-night variability in sleep timing with obesity were less consistent.
Conclusions
In both older men and women, variability in nightly sleep duration and daytime napping were associated with obesity independent of mean sleep duration. These findings suggest that characteristics of sleep beyond mean sleep duration may play a role in weight homeostasis, highlighting the complex relationship between sleep and metabolism.
Keywords: sleep duration, sleep pattern, napping, variability, obesity, geriatrics
Introduction
Obesity is one of the most significant health problems in the United States today. From the late 1970s to 2010, the prevalence of obesity has more than doubled to greater than 35% among U.S. adults today.1,2 Though much of the focus of the ‘obesity epidemic’ has centered on young adults and children, this secular rise in obesity has also impacted older populations. Among those over the age of 60, the prevalence of obesity was 36.6% in men and 42.3% in women in a national survey from 2009-2010.1 Given the limitations in efficacy of currently available preventative and treatment strategies for obesity, increasing attention has been paid to novel obesity risk factors. Objectively measured short sleep has been associated with obesity in many populations including older adults.3 However, the importance of other aspects of sleep patterns, such as timing of sleep, regularity of sleep, and daytime napping remain relatively unexplored. A recent study of young adults found that individuals with later bedtimes consumed more calories, and were heavier.4 Another study in children found that a variable sleep schedule acts synergistically with short sleep to increase obesity risk.5 Thus far, these facets of sleep have not been evaluated in an older population. While daytime napping has been shown to increase risk of mortality in older adults,6 whether napping is associated with obesity has not yet been explored. In this analysis, we evaluated the relationships between obesity with three aspects of sleep: sleep variability, sleep timing, and daytime napping with the three separate hypotheses that those with a variable sleep schedule, those with later sleep times, and those with more time spent napping will be more likely to be obese and these associations will persist after accounting for potential differences in sleep duration.
Methods
Study Population
Men were participants in the prospective Osteoporotic Fractures in Men Study (MrOS). During the baseline examination from 2000 to 2002, 5994 community-dwelling men 65 years or older were enrolled at 6 clinical centers in the United States: Birmingham, Alabama; Minneapolis, Minnesota; Palo Alto, California; Pittsburgh, Pennsylvania; Portland, Oregon; and San Diego, California. The MrOS Sleep Study, an ancillary study of the parent MrOS cohort, was conducted between December 2003 and March 2005 and recruited 3135 of these participants for a comprehensive sleep assessment. Of these, 3053 had adequate actigraphy data as well as body mass index measured.
Women were participants in the prospective Study of Osteoporotic Fractures (SOF). During the baseline examination from 1986 to 1988, 9704 community-dwelling white women 65 years or older were enrolled from population-based listings in 4 areas of the United States: Baltimore, Maryland; Minneapolis, Minnesota; Pittsburgh, Pennsylvania; and Portland, Oregon. An additional 662 African-American women were enrolled between 1997 and 1998. Assessment of sleep occurred during the 8th examination in this cohort which took place from January 2002 to April 2004. A total of 4727 women (84% of survivors) participated, of whom 3676 had a clinical visit and 2985 had adequate actigraphy data and body mass index measured.
For both studies, individuals were not eligible to participate if they reported bilateral hip replacement or required the assistance of another person in ambulation at the baseline examination. Details of the cohorts have been previously published.7-9 The protocol for MrOS and SOF were approved by the institutional review boards at all of the participating institutions. All participants provided written informed consent.
Sleep Patterns
Data on sleep habits were obtained using wrist actigraphy (Sleepwatch-O, Ambulatory Monitoring, Inc., Ardsley NY) in both cohorts. The actigraph, which measures movement using a piezoelectric biomorph-ceramic cantilevered beam, was worn on the wrist of the non-dominant hand. Subjects were asked to wear the actigraph a minimum of 5 consecutive 24-hour periods in MrOS and 3 consecutive 24-hour periods in SOF. Data were collected continuously and stored in 1-minute epochs. The digital integration mode of analysis for this device, which has been validated against polysomnography in these cohorts,10,11 was used to distinguish sleep from wake. Action W-2 software (Ambulatory Monitoring, Inc.) was used to analyze the raw data,12 and the University of California San Diego (UCSD) scoring algorithm was used to determine sleep/wake status.13
While wearing the actigraph, participants completed sleep diaries which included time into and time out of bed and times the actigraph was removed. This information was used in editing the actigraphy data files to set intervals for when the participant got into bed, got out of bed, or took any naps as well as to delete time when the actigraph was removed from analyses. A standardized protocol to account for inaccurate or missing diaries was utilized and this protocol has been shown to provide consistent results across scorers.14 Sleep onset was defined as the beginning of the first 20-minute continuous block of sleep after the time marked as getting in bed while sleep offset was defined as the last minute scored as sleep before time out of bed.
Mean nightly sleep duration was defined as the total minutes scored as sleep between sleep onset and sleep offset averaged over all nights of recording. Sleep timing was assessed by identifying the clock time halfway between the beginning and end of the sleep period for each night, termed the sleep midpoint. This midpoint time was averaged across all nights to obtain mean sleep midpoint, which has previously been shown to correlate with dim light melatonin onset, a physiologic marker of circadian phase, in a free-living population.15 Variability in nightly sleep duration and timing was assessed using the standard deviation in the nightly sleep duration and sleep midpoint respectively across all nights of recording. Only subjects with at least two nights of data were included in these variability analyses. Nap time was defined as minutes scored as sleep using the UCSD sleep/wake scoring algorithm in blocks of at least 5 minutes occurring outside of the main sleep interval. Episodes of shorter duration during the day were assumed to represent quiet wakefulness. Mean nap time was averaged across all days of recording.
Obesity
Body weight was measured with a standard balance beam or digital scale, height with a wall-mounted Harpenden stadiometer (Holtain, England), and these measurements were used to calculate body mass index (BMI). Obesity was defined as a BMI ≥ 30 kg/m2.
Covariates
All participants completed questionnaires, which included items about demographics, medical history, physical activity, smoking and alcohol use. Caffeine consumption was estimated based on self-report of the average daily number of cups of caffeinated coffee and tea, or cans of caffeinated soda consumed.16 Participants were asked to bring in all current medications used within the preceding 30 days. All prescription medications were entered into an electronic database and each medication was matched to its ingredient(s) based on the Iowa Drug Information Service (IDIS) Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA).17 The Geriatric Depression Scale (GDS) was used to assess depressive symptoms, and the standard cutoff of six or more symptoms was used to define depression.18 In MrOS, level of activity was assessed using the Physical Activity Scale for the Elderly (PASE),19 while in SOF, physical activity was assessed by asking women if they walked for exercise. Cognitive function was assessed using the Modified Mini-Mental State (3MS) examination in MrOS and the Mini-Mental State Examination (MMSE) in SOF.20,21 Participants completed the Medical Outcomes Study 12-item Short Form (SF-12), which included a question about self-reported health status compared to others of similar age (categorized as excellent/good versus fair/poor/very poor).22
In-home sleep studies using unattended polysomnography (Safiro unit; Compumedics, Melbourne, Australia) were performed in the MrOS cohort. The recording montage included C3/A2 and C4/A1 electroencephalography, bilateral electrooculography, submental electromyography, thoracic and abdominal respiratory effort, airflow (nasal-oral thermocouple and nasal pressure), finger pulse oximetry, electrocardiography, body position, and bilateral leg movements. Sleep staging was done using standard criteria.23 Apneas were defined as a complete or almost complete cessation of airflow for more than 10 seconds. Hypopneas were defined as a > 30% reduction in amplitude of either respiratory effort or airflow for more than 10 seconds associated with a ≥ 4% oxygen desaturation. The apnea hypopnea index (AHI) was computed as the number of apneas and hypopneas per hour of recorded sleep. Although sleep studies were conducted in a subsample of SOF as well, the proportion of women with polysomnography data was < 15% so adjustment for sleep apnea severity was not performed in this cohort.
Statistical Analyses
Each cohort was analyzed separately but in parallel fashion. Baseline characteristics were summarized by quartile of the sleep measure (mean sleep timing, variability in sleep duration, variability in sleep timing, and daytime napping) and differences were compared using analysis of variance for normally distributed continuous data, Kruskal-Wallis tests for continuous skewed data, and chi-square tests for homogeneity for categorical data.
The independent relationships between sleep measures (mean timing of sleep, variability in sleep duration, variability in sleep timing, and daytime napping) with body mass index and obesity risk were assessed with multivariable adjusted linear and logistic regression models. Three sets of nested models were used for these analyses. The first adjusted for age, race, and site only; the second additionally included habits, co-morbidities, and medications; and the third also included mean nocturnal sleep duration. In addition, in MrOS, further adjustment was made for sleep apnea severity as assessed by AHI. Covariates were selected based on being associated with both sleep duration and obesity in prior studies. Covariates included study site, age, race (Caucasian vs. non-Caucasian), level of education (< 12 years, 12-16 years, > 16 years), history of diabetes, stroke, coronary artery disease (angina, myocardial infarction, or coronary revascularization procedure), or congestive heart failure, use of antidepressants, use of benzodiazepines, smoking status (never, past, current), alcohol consumption (0-2 drinks/week, 3-13 drinks/week, ≥ 14 drinks/week), caffeine consumption (continuous), depression (GDS score ≥ 6), level of physical activity (continuous in MrOS and dichotomous in SOF), cognitive function (continuous 3MS score in MrOS and MMSE score in SOF), self-reported health status (dichotomized as those reporting good or excellent), and mean nocturnal sleep duration.
Evidence for effect modification by mean nocturnal sleep duration on the relationship between sleep duration variability and daytime napping with obesity risk was assessed by stratifying the cohorts into four nocturnal sleep duration categories (≤ 5 hrs, >5 to <7 hrs, 7 to <8 hrs, and ≥ 8 hrs) based on our prior analyses of these cohorts.3 Statistical significance of the interactions were assessed by testing the significance of an interaction term (categorical average sleep duration * average napping time or sleep duration variability, F test with 3 degrees of freedom) when added to the multivariable model.
All analyses were performed using SAS statistical software (version 9.2, SAS Institute, Inc., Cary, North Carolina).
Results
A total of 3053 men (mean age 76.4 y) and 2985 women (mean age 83.5 y) had actigraphic data of sufficient quality to be included in these analyses (98% and 93% of the sample studied respectively). The mean ± SD number of days of usable actigraphy data was 5.2 ± 0.9 in the male cohort and 4.1 ± 0.8 in the female cohort. The mean BMI in the male cohort was 27.2 ± 3.8 kg/m2 with an obesity prevalence of 20.4%. Among women, the mean BMI was 27.0 ± 5.0 kg/m2 and obesity prevalence was 24.8%.
The distribution of demographics, social habits, medications, co-morbidities, mood and cognitive function by the key sleep measures – variability in sleep duration, midpoint of sleep timing, variability in sleep timing, and nap time – are displayed in Supplementary Tables 1-4 respectively. High variability in nightly sleep duration was associated in both men and women with minority race, higher rates of diabetes, coronary artery disease, and heart failure, greater use of antidepressants, reduced cognitive function, reduced self-reported health status, and lower mean nightly sleep duration. Later sleep timing was associated in both men and women with minority race, higher rate of diabetes, greater use of antidepressants and benzodiazepines, reduced self-reported health status, less exercise, and lower mean nightly sleep duration. High variability of sleep timing was associated in both men and women with minority race, higher rates of diabetes and heart failure, greater use of antidepressants, reduced cognitive function, reduced self-reported health status, and lower mean nightly sleep duration. Increased time napping was associated in both men and women with older age, higher rates of diabetes, stroke, heart failure, and depression, greater use of antidepressants, lower use of alcohol and caffeine, reduced cognitive function, reduced self-reported health status, and less exercise. In addition, greater napping was associated with reduced mean nightly sleep duration in women.
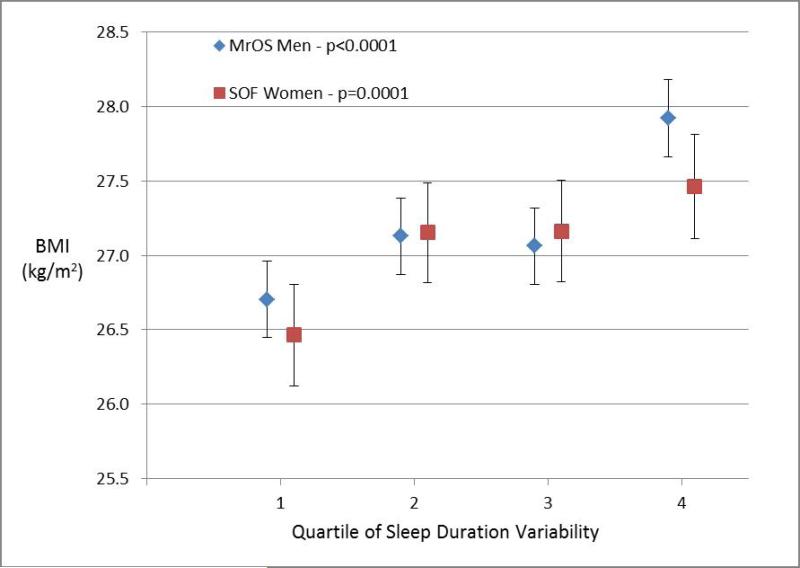
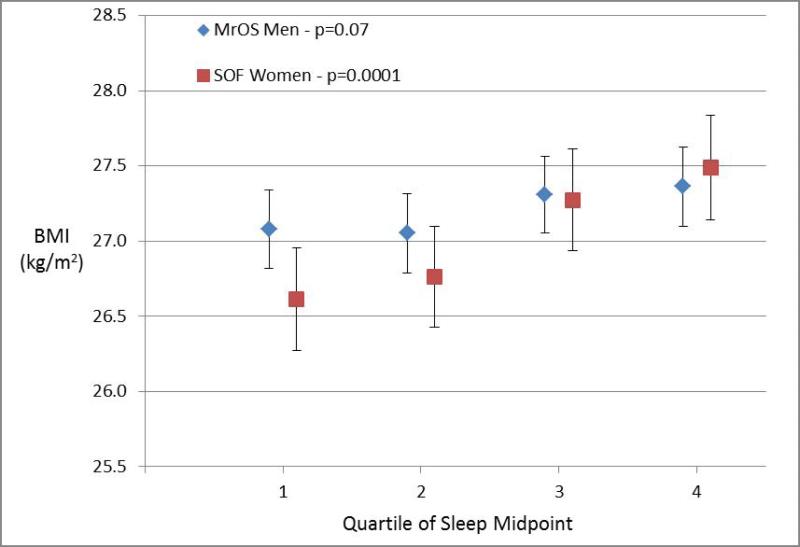
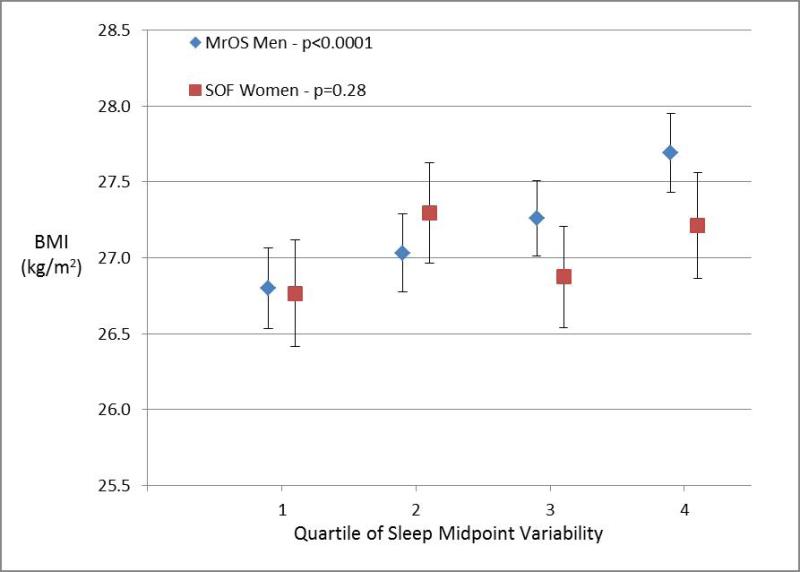
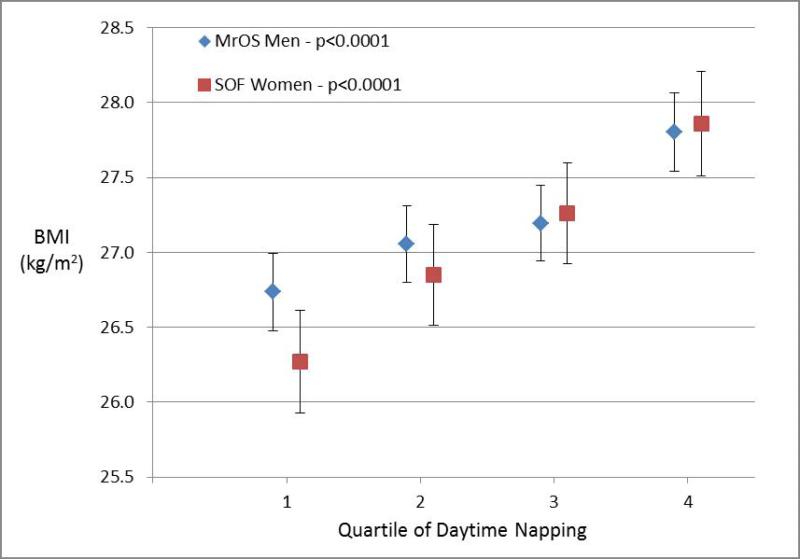
Figures 1-4 display the multivariable adjusted association between BMI and each of the four sleep measures (sleep duration variability, sleep timing, sleep timing variability, and daytime napping). With increasing variability in sleep duration and daytime napping, there was a consistent increase in mean BMI in both men and women with highly significant tests for trend (p ≤ 0.0001 for all four analyses). The association between BMI and sleep timing and variability in timing was less robust. Mean BMI was greatest in the quartile with latest sleep midpoint in both men and women but while the test for trend was highly significant in women, the relationship was much weaker in men. In contrast, with regards to the sleep midpoint variability, there was a clear linear dose-response relationship with increasing variability in timing associated with increased BMI in men (p<0.0001) but no evidence of a relationship in women.
Figure 1. Body Mass Index by Sleep Measures.
Mean body mass index (BMI) plotted by quartile of sleep measure among men in MrOS and women in SOF cohorts adjusting for age, site, race, education, history of diabetes, stroke, coronary artery disease, heart failure, antidepressant use, benzodiazepine use, smoking, alcohol, caffeine, exercise, depression, cognitive function, self-reported health, and mean nocturnal sleep duration. The association with sleep duration variability is shown in Panel 1a, sleep midpoint in Panel 1b, sleep midpoint variability in Panel 1c, and time spent napping in Panel 1d. P-values are based on a test of trend.
All four sleep measures (variability in sleep duration, daytime napping, sleep midpoint, and variability in sleep midpoint) were associated with an increased likelihood of obesity in analyses adjusted for age, demographic characteristics, co-morbidities, and medication use (Table 1). After further adjustment for mean nocturnal sleep duration, greater sleep duration variability and daytime napping remained significantly associated with obesity in both cohorts. Each hour increase in nocturnal sleep duration variability was associated with a 63% increase in obesity odds among men and 22% increase in obesity odds among women, while each additional hour spent napping was associated with 23% and 29% increases in obesity odds among men and women respectively. The results for sleep timing were less consistent. Neither sleep midpoint nor variability in sleep midpoint was significantly associated with obesity after adjusting for nocturnal sleep duration among men in MrOS. In contrast, later sleep timing and greater variability in sleep timing remained associated with obesity in the fully-adjusted analyses among women in the SOF cohort.
Table 1.
Odds Ratios for Association between Obesity and Sleep Measures
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| MrOS Men | |||
| Sleep Duration Variability (per hr) | 1.77 (1.44-2.17) | 1.71 (1.39-2.12) | 1.63 (1.31-2.02) |
| Sleep Midpoint (per hr) | 1.13 (1.03-1.23) | 1.10 (1.01-1.21) | 1.04 (0.94-1.14) |
| Sleep Midpoint Variability (per hr) | 1.73 (1.36-2.20) | 1.66 (1.29-2.13) | 1.28 (0.98-1.66) |
| Daytime Napping (per hr) | 1.31 (1.20-1.44) | 1.25 (1.13-1.38) | 1.23 (1.12-1.37) |
| SOF Women | |||
| Sleep Duration Variability (per hr) | 1.38 (1.17-1.63) | 1.30 (1.08-1.56) | 1.22 (1.01-1.47) |
| Sleep Midpoint (per hr) | 1.18 (1.09-1.28) | 1.16 (1.06-1.27) | 1.11 (1.01-1.22) |
| Sleep Midpoint Variability (per hr) | 1.60 (1.28-1.98) | 1.54 (1.21-1.96) | 1.32 (1.03-1.69) |
| Daytime Napping (per hr) | 1.35 (1.24-1.46) | 1.31 (1.19-1.44) | 1.29 (1.17-1.41) |
Model 1 adjusted for age, race and site.
Model 2 adjusted for Model 1 plus alcohol, smoking, caffeine, education, history of diabetes, stroke, coronary artery disease, congestive heart failure, use of antidepressants, use of benzodiazepines, exercise, cognitive function, depression, and self-reported health status.
Model 3 adjusted for Model 2 plus mean nightly sleep duration.
Findings were not substantially impacted by accounting for sleep apnea. After further adjustment for AHI in MrOS, each hour increase in variability of nocturnal sleep duration was associated with an obesity odds ratio of 1.50 [95% CI: 1.20-1.89], each hour increase in sleep midpoint with an odds ratio of 1.03 [0.93-1.14], each hour increase in sleep midpoint variability with an odds ratio of 1.30 [0.98-1.72], and each hour increase in napping with an odds ratio of 1.25 [1.13-1.40].
Formal tests for interaction found no evidence for effect modification of the sleep duration variability – obesity relationship by mean nocturnal sleep duration in either the MrOS or SOF cohorts. In contrast, the impact of daytime napping on obesity risk did appear to differ by mean nocturnal sleep duration (Table 2). A similar pattern was seen in both cohorts such that additional napping appeared to have no effect on obesity risk among those with nocturnal sleep duration ≤ 5 hours, the greatest impact in those sleeping >5 to <8 hours, and an intermediate effect in those sleeping ≥ 8 hours at night. This interaction was statistically significant among women in SOF and of borderline significance among men in MrOS.
Table 2.
Odds Ratios for Association between Obesity and Daytime Napping by Nocturnal Sleep Duration.
| Daytime Napping (per hr) | Mean Nocturnal Sleep Duration | Interaction p-value | |||
|---|---|---|---|---|---|
| ≤ 5 hrs | >5 to <7 hrs | 7 to <8 hrs | ≥ 8 hrs | ||
| MrOS Men | 0.99 (0.78-1.25) | 1.29 (1.12-1.48) | 1.38 (1.09-1.75) | 1.22 (0.79-1.87) | 0.097 |
| SOF Women | 0.98 (0.75-1.27) | 1.48 (1.28-1.71) | 1.23 (1.03-1.48) | 1.23 (0.94-1.62) | 0.008 |
All models adjusted for age, race, site, alcohol, smoking, caffeine, education, history of diabetes, stroke, coronary artery disease, congestive heart failure, use of antidepressants, use of benzodiazepines, exercise, cognitive function, depression, and self-reported health status. p-value is test for interaction across sleep duration categories.
Discussion
An increasing body of literature implicates poor sleep as an independent risk factor for obesity, with evidence coming from longitudinal cohorts as well as small interventional studies.24-26 While the focus of most of this work has been on duration of sleep, the aspects of sleep most relevant to obesity risk are unclear. In this work, we identified both high variability in night-to-night sleep duration as well as increased time napping during the day to be strongly associated with obesity independent of mean nightly sleep duration. The evidence for an independent association between obesity and both later sleep timing and high variability in sleep timing was less robust. However, there did appear to be a consistent relationship between greater levels of obesity and later sleep times in women.
Our work has many strengths. We utilized actigraphy as an objective marker of sleep rather than reliance on self-report. We studied an older age group that limits the influence of socioeconomic factors such as work schedule or child care responsibilities on sleep habits. While medical co-morbidities may influence sleep patterns, we attempted to control for the most influential co-morbidities (depression, heart disease, diabetes, and stroke) as well as medication use. Finally, by testing our hypotheses in two separate cohorts, we were able to assess the robustness of the identified associations.
Differences in findings between the two cohorts should not be attributed solely to differences in gender as other differences exist between the two cohorts. For example, the men in MrOS were on average 7 years younger than the women in SOF. In addition, the differing protocols resulted in more days of recording in the MrOS cohort which may have led to better assessments of night-to-night variability. Nevertheless, while potential differences in the relationship between timing of sleep and obesity were apparent between the two cohorts, the association of daytime napping and sleep duration variability with obesity was strikingly similar. In addition, the pattern of interaction between daytime napping and nocturnal sleep duration on obesity risk was strikingly similar between the two cohorts such that the impact of napping was greatest in those with average nocturnal sleep durations and least in those with short nocturnal sleep durations.
It should be noted that our results are consistent with cross-sectional findings from a cohort of schoolchildren where irregular sleep schedules were also associated with increased obesity risk independent of mean sleep duration.5 However, it should be noted that this does not necessarily imply that individuals who are chronically sleep deprived should not extend their sleep when provided the opportunity. A study of Hong Kong schoolchildren found the impact of reduced sleep during school nights on obesity risk was mitigated by catch up sleep on weekends and holidays.27 Thus the beneficial impact of increasing average sleep duration may outweigh any adverse impact of irregularity.
There are a number of potential explanations for the associations identified in this analysis. High night-to-night variability in sleep duration may identify individuals who are repeatedly in a state of sleep debt due to insufficient sleep on some nights and then compensate for this with catch-up sleep on subsequent nights. This pattern is commonly seen in individuals who work or go to school full time who limit sleep on work/school days and then compensate on weekends. Such individuals may be exposed to the weight-promoting effects of insufficient sleep on the days following curtailed sleep with no compensatory weight loss on days following compensatory sleep. In support of this hypothesis, children who avoid catch-up sleep on the weekends have greater levels of physical activity.28 Of note, the difference between weekday and weekend sleep duration was only 8 minutes in MrOS and 3 minutes in SOF suggesting that night-to-night variability in sleep duration in our cohorts was likely due to factors other than work.
Irregularity of sleep-wake habits may also lead to, or be a marker of, irregularity in eating patterns. Individuals who do not wake up at the same time every day are likely to have more variability in when and whether or not they eat breakfast. Irregular eating patterns have been associated with metabolic syndrome,29 while both snacking between meals and skipping breakfast have been found to predict increased weight gain.30-33 Greater night to night variability in sleep duration, sleep timing, and daytime napping could also all represent a more irregular sleep-wake rhythm. This may reflect a weaker circadian output from the master clock in the suprachiasmatic nucleus but could also represent greater dyssynchrony between daily activities and the underlying circadian rhythm. In either case, this circadian dysregulation may lead to metabolic consequences given the close relationship between the molecular clock and metabolic processes. Circadian dysfunction induced through genetic or environmental manipulation has been shown in animal models to induce metabolic alterations leading to obesity.34-36 In humans, shiftworking has been associated with increased obesity.37 In addition, in younger populations, circadian dyssynchrony is commonly induced by work/school schedules and is referred to as social jetlag. Social jetlag is associated with poorer health including a greater risk of overweight/obesity independent of short sleep duration.38,39
A delayed circadian phase as evidenced by a later sleep timing may influence obesity risk by increasing the relative proportion of calories eaten at night as opposed to in the morning. Those with later sleep times are more likely to skip breakfast and snack after dinner.4 In a diabetic population, later sleep midpoint on weekends was associated with a greater proportion of calories eaten at dinner, which in turn predicted worse glycemic control.40 Night eating syndrome is an extreme form of obesity associated with nocturnal snacking and night eating symptoms have been found to correlate with an evening chronotype.41,42
Another possibility is that irregular sleep patterns may simply serve as a marker for a chaotic lifestyle associated with less regular mealtimes, greater proportion of meals eaten outside of the house, and less regular opportunities to exercise. Further research on the correlation in patterns of sleep, eating, and exercise behaviors is needed in naturalistic settings.
Clearly some of the postulated mechanisms support a causal role of irregular sleep patterns (e.g., via dampening of the intrinsic circadian oscillator) on obesity risk while others suggest it is only a marker (e.g., predicting a chaotic lifestyle) for the true causal risk factor. Further research to understand the biological mechanisms for the identified associations are needed to better assess the potential for interventions targeting the regularization of sleep patterns to be used to combat obesity.
The strong findings regarding daytime napping reveal the complexity of the sleep – obesity relationship in that reduced nocturnal sleep is associated with obesity while increased diurnal sleep is associated with obesity. This apparent contradiction may again reflect an irregular sleep schedule with either a weakened circadian rhythm or greater desynchrony of the circadian rhythm. The interaction analyses suggests a complex relationship where daytime napping may be beneficial (or at least not harmful) in those whose nocturnal sleep is substantially restricted in that napping in such a situation allows for a more normal amount of total sleep in a 24-hour period. However, in those obtaining sufficient sleep at night, daytime napping is more strongly associated with obesity. In this situation, the adverse effects of napping on daytime eating or exercise behaviors are no longer counteracted by any beneficial impact of further increases in 24-hour sleep time. Since actigraphy is not well validated to detect daytime naps, it is also possible that the association between obesity and daytime naps may actually reflect periods of quiet wakefulness and lower levels of physical activity. In order to limit this bias, only periods of more than 5 consecutive minutes scored as sleep were considered as naps. However, other factors not considered in this work, such as timing of the naps may also impact the impact of napping on weight and obesity risk.
Because this work was cross-sectional, reverse causation cannot be excluded as a possible explanation for our findings. Obesity has been associated with an increase in sleep pressure,43,44 and thus it is possible that the abnormal sleep patterns observed are a result rather than cause of increased adiposity. In addition, residual confounding may be possible. For example, the measures of physical activity available in these cohorts, particularly SOF, were relatively inexact. Nevertheless, interventional studies in animals suggest circadian disruption can lead to alterations in carbohydrate metabolism and weight supporting irregular sleep as a causal risk factor for obesity.45
Prior work highlighting the relevance of regular sleep patterns for metabolic health has focused on children.5 Our work suggests that regularity of sleep is important in older individuals as well. This population has less external constraints on sleep scheduling due to work/school requirements and so is at greater risk for irregular sleep patterns. This age group is also at greater risk of obesity complications such as diabetes and cardiovascular disease. Thus, future research on the development of sleep interventions as a means to prevent or treat obesity should include older individuals.
Supplementary Material
Acknowledgements
In addition to the support below, this work was supported by National Institutes of Health grant HL098297. The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AR045580, U01 AR045614, U01 AR045632, U01 AR045647, U01 AR045654, U01 AR045583, U01 AG018197, U01 AG027810, and UL1 TR000128. The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: HL071194, HL070848, HL070847, HL070842, HL070841, HL070837, HL070838, and HL070839. The Study of Osteoporotic Fractures (SOF) is supported by National Institutes of Health funding. The National Institute on Aging (NIA) provides support under the following grant numbers: R01 AG005407, R01 AR35582, R01 AR35583, R01 AR35584, R01 AG005394, R01 AG027574, and R01 AG027576.
Footnotes
Supplementary information is available at the International Journal of Obesity's website.
Conflict of Interest
The authors declare no conflicts of interest.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960-1994. Int J Obes Relat Metab Disord. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 3.Patel SR, Blackwell T, Redline S, Ancoli-Israel S, Cauley JA, Hillier TA, et al. The association between sleep duration and obesity in older adults. Int J Obes (Lond) 2008;32:1825–34. doi: 10.1038/ijo.2008.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring) 2011;19:1374–81. doi: 10.1038/oby.2011.100. [DOI] [PubMed] [Google Scholar]
- 5.Spruyt K, Molfese DL, Gozal D. Sleep duration, sleep regularity, body weight, and metabolic homeostasis in school-aged children. Pediatrics. 2011;127:e345–52. doi: 10.1542/peds.2010-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stone KL, Ewing SK, Ancoli-Israel S, Ensrud KE, Redline S, Bauer DC, et al. Self-reported sleep and nap habits and risk of mortality in a large cohort of older women. J Am Geriatr Soc. 2009;57:604–11. doi: 10.1111/j.1532-5415.2008.02171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummings SR, Black DM, Nevitt MC, Browner WS, Cauley JA, Genant HK, et al. Appendicular bone density and age predict hip fracture in women. The Study of Osteoporotic Fractures Research Group. JAMA. 1990;263:665–8. [PubMed] [Google Scholar]
- 8.Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials. 2005;26:557–68. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Blackwell T, Redline S, Ancoli-Israel S, Schneider JL, Surovec S, Johnson NL, et al. Comparison of sleep parameters from actigraphy and polysomnography in older women: the SOF study. Sleep. 2008;31:283–91. doi: 10.1093/sleep/31.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blackwell T, Ancoli-Israel S, Redline S, Stone KL. Factors that may influence the classification of sleep-wake by wrist actigraphy: the MrOS Sleep Study. J Clin Sleep Med. 2011;7:357–67. doi: 10.5664/JCSM.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Action-W User's Guide, Version 2.0. Ambulatory Monitoring, Inc.; Ardsley, NY: [Google Scholar]
- 13.Girardin JL, Kripke DF, Mason WJ, Elliot JA, Youngstedt SD. Sleep estimation from wrist movement quantified by different actigraphic modalities. J Neurosci Methods. 2001;105:185–91. doi: 10.1016/s0165-0270(00)00364-2. [DOI] [PubMed] [Google Scholar]
- 14.Blackwell T, Ancoli-Israel S, Gehrman PR, Schneider JL, Pedula KL, Stone KL. Actigraphy scoring reliability in the study of osteoporotic fractures. Sleep. 2005;28:1599–605. doi: 10.1093/sleep/28.12.1599. [DOI] [PubMed] [Google Scholar]
- 15.Martin SK, Eastman CI. Sleep logs of young adults with self-selected sleep times predict the dim light melatonin onset. Chronobiol Int. 2002;19:695–707. doi: 10.1081/cbi-120006080. [DOI] [PubMed] [Google Scholar]
- 16.Barone JJ, Roberts HR. Caffeine consumption. Food Chem Toxicol. 1996;34:119–29. doi: 10.1016/0278-6915(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 17.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–11. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 18.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–73. [Google Scholar]
- 19.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 20.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–8. [PubMed] [Google Scholar]
- 21.Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Arch Gen Psychiatry. 1983;40:812. doi: 10.1001/archpsyc.1983.01790060110016. [DOI] [PubMed] [Google Scholar]
- 22.Ware JE, Kosinski M, Keller SD. SF-12: How to score the SF-12 Physical and Mental Health Summary Scores. 3rd ed QualityMetric Incorporated; Lincoln, RI: 1998. [Google Scholar]
- 23.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. US Government Printing Office; Washington, DC: 1968. [Google Scholar]
- 24.Carter PJ, Taylor BJ, Williams SM, Taylor RW. Longitudinal analysis of sleep in relation to BMI and body fat in children: the FLAME study. BMJ. 2011;342:d2712. doi: 10.1136/bmj.d2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5:253–61. doi: 10.1038/nrendo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–53. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wing YK, Li SX, Li AM, Zhang J, Kong AP. The effect of weekend and holiday sleep compensation on childhood overweight and obesity. Pediatrics. 2009;124:e994–e1000. doi: 10.1542/peds.2008-3602. [DOI] [PubMed] [Google Scholar]
- 28.Stone MR, Stevens D, Faulkner GE. Maintaining recommended sleep throughout the week is associated with increased physical activity in children. Prev Med. 2013;56:112–7. doi: 10.1016/j.ypmed.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 29.Sierra-Johnson J, Unden AL, Linestrand M, Rosell M, Sjogren P, Kolak M, et al. Eating meals irregularly: a novel environmental risk factor for the metabolic syndrome. Obesity (Silver Spring) 2008;16:1302–7. doi: 10.1038/oby.2008.203. [DOI] [PubMed] [Google Scholar]
- 30.Timlin MT, Pereira MA, Story M, Neumark-Sztainer D. Breakfast eating and weight change in a 5-year prospective analysis of adolescents: Project EAT (Eating Among Teens). Pediatrics. 2008;121:e638–45. doi: 10.1542/peds.2007-1035. [DOI] [PubMed] [Google Scholar]
- 31.van der Heijden AA, Hu FB, Rimm EB, van Dam RM. A prospective study of breakfast consumption and weight gain among U.S. men. Obesity (Silver Spring) 2007;15:2463–9. doi: 10.1038/oby.2007.292. [DOI] [PubMed] [Google Scholar]
- 32.Woo J, Cheung B, Ho S, Sham A, Lam TH. Influence of dietary pattern on the development of overweight in a Chinese population. Eur J Clin Nutr. 2008;62:480–7. doi: 10.1038/sj.ejcn.1602702. [DOI] [PubMed] [Google Scholar]
- 33.Coakley EH, Rimm EB, Colditz G, Kawachi I, Willett W. Predictors of weight change in men: results from the Health Professionals Follow-up Study. Int J Obes Relat Metab Disord. 1998;22:89–96. doi: 10.1038/sj.ijo.0800549. [DOI] [PubMed] [Google Scholar]
- 34.Shi SQ, Ansari TS, McGuinness OP, Wasserman DH, Johnson CH. Circadian disruption leads to insulin resistance and obesity. Curr Biol. 2013;23:372–81. doi: 10.1016/j.cub.2013.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barclay JL, Husse J, Bode B, Naujokat N, Meyer-Kovac J, Schmid SM, et al. Circadian desynchrony promotes metabolic disruption in a mouse model of shiftwork. PLoS One. 2012;7:e37150. doi: 10.1371/journal.pone.0037150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–5. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Lorenzo L, De Pergola G, Zocchetti C, L'Abbate N, Basso A, Pannacciulli N, et al. Effect of shift work on body mass index: results of a study performed in 319 glucose-tolerant men working in a Southern Italian industry. Int J Obes Relat Metab Disord. 2003;27:1353–8. doi: 10.1038/sj.ijo.0802419. [DOI] [PubMed] [Google Scholar]
- 38.Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol. 2012;22:939–43. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 39.Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23:497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- 40.Reutrakul S, Hood MM, Crowley SJ, Morgan MK, Teodori M, Knutson KL, et al. Chronotype is independently associated with glycemic control in type 2 diabetes. Diabetes Care. 2013;36:2523–9. doi: 10.2337/dc12-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harb A, Levandovski R, Oliveira C, Caumo W, Allison KC, Stunkard A, et al. Night eating patterns and chronotypes: a correlation with binge eating behaviors. Psychiatry Res. 2012;200:489–93. doi: 10.1016/j.psychres.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Colles SL, Dixon JB, O'Brien PE. Night eating syndrome and nocturnal snacking: association with obesity, binge eating and psychological distress. Int J Obes (Lond) 2007;31:1722–30. doi: 10.1038/sj.ijo.0803664. [DOI] [PubMed] [Google Scholar]
- 43.Guan Z, Vgontzas AN, Bixler EO, Fang J. Sleep is increased by weight gain and decreased by weight loss in mice. Sleep. 2008;31:627–33. doi: 10.1093/sleep/31.5.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vgontzas AN, Bixler EO, Tan TL, Kantner D, Martin LF, Kales A. Obesity without sleep apnea is associated with daytime sleepiness. Arch Intern Med. 1998;158:1333–7. doi: 10.1001/archinte.158.12.1333. [DOI] [PubMed] [Google Scholar]
- 45.McDonald RJ, Zelinski EL, Keeley RJ, Sutherland D, Fehr L, Hong NS. Multiple effects of circadian dysfunction induced by photoperiod shifts: Alterations in context memory and food metabolism in the same subjects. Physiol Behav. 2013;118:14–24. doi: 10.1016/j.physbeh.2013.04.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.