SUMMARY
Sirtuins are a class of NAD+-dependent deacetylases, such as deacetylases, that have a wide array of biological functions. Recent studies have suggested that reduced sirtuin action is correlated with Type 2 diabetes. Both overnutrition and aging, which are two major risk factors for diabetes, lead to decreased sirtuin function and result in abnormal glucose and lipid metabolism. Therefore, restoring normal levels of sirtuin action in Type 2 diabetes may be a promising method of treating diabetes. This article reviews the biological functions of three of the seven mammalian sirtuins – SIRT1, SIRT3 and SIRT6 – that have demonstrated prominent metabolic roles and early potential for drug targeting. Clinical trials investigating the use of sirtuin activators for treating diabetes are already underway and show promise as alternatives to current diabetes therapies. Thus, further research into sirtuin activators is warranted and may lead to a new class of safe, effective diabetes treatments.
Type 2 diabetes is endemic with over 366 million adults estimated to have Type 2 diabetes, and is estimated to grow to 552 million by 2030 [1]. Worldwide, diabetes expenditures exceeded US$465 billion in 2011, accounting for 11% of total healthcare costs in adults [1]. Strikingly, even with considerable investment, the WHO lists diabetes as one of the world’s top ten causes of death [201]. Furthermore, the WHO estimates that deaths from diabetes-related complications will increase by two-thirds between the years 2008 and 2030 [202]. Clearly, new and better ways to treat and/or prevent Type 2 diabetes are required.
Type 2 diabetes is a complicated, multifactorial disease, and the cause is probably a combination of both genetic and environmental factors. In fact, the cause of Type 2 diabetes could be different for each patient. An abundance of genome-wide array studies have identified several different genes associated with Type 2 diabetes incidence, as well as genes that are related to the response and effectiveness of current diabetes drugs in individual patients [2]. These studies suggest that a customized medical approach and/or combination therapies may be needed to effectively treat Type 2 diabetes.
Therefore, in order to leverage this strategy to treat diabetes, a wide range of diabetes drugs are needed. While several current diabetes therapies are effective (metformin, insulin and insulin secretagogues, among others), these drugs only treat the general symptoms of diabetes, and several side effects are associated with their use, including weight gain, gastrointestinal effects (nausea and diarrhea) and hypoglycemia [3]. In addition, considering the above statistics, these drugs have clearly not been effective enough at curtailing mortality associated with diabetes. Newer classes of antidiabetic drugs approved within the past 15 years include thiazolidinediones, GLP-1 receptor agonists and DPP-IV inhibitors. These drugs have increased the therapeutic options available to clinicians. However, thiazolidinedione availability has recently been restricted owing to increased risk of cardiovascular events; and the potential for safe long-term use of GLP-1 receptor agonists and DPP-IV inhibitors remains to be seen. Therefore, continued research into new classes of diabetes drugs is required.
One emerging class of proteins that is associated with Type 2 diabetes and its risk factors is the sirtuins. Sirtuins are highly conserved proteins with a wide array of biological functions in organisms from bacteria to humans. There are seven members in the mammalian sirtuin family (SIRT1–7), each containing the conserved sirtuin core domain that confers NAD+-dependent deacylase activity (Figure 1). The sirtuins are divided into four phylogenetic classes [4] that seem to correlate with their specific deacylase activity [5]. SIRT1–3 are class I sirtuins that show strong deacetylase activity [6], whereas SIRT4, a class II sirtuin, has ADP-ribosyltransferase activity [7]. SIRT5 is a class III sirtuin that displays weak deacetylase activity and has recently been shown to have desuccinylase [8–10] and demalonylase [9] activity. Class IV sirtuins include SIRT6 and SIRT7. SIRT6 has been reported to have deacetylase [11,12] and ADP-ribosyltransferase [13] activity, and the enzymatic activity of SIRT7 was recently shown to be a highly specific deacetylase [14].
Figure 1.
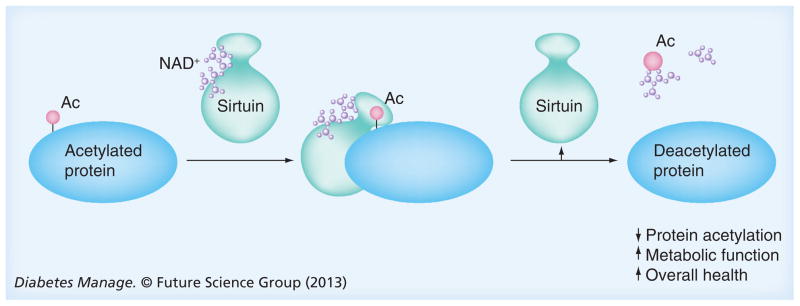
Sirtuin deacetylase activity
Ac: Acetyl group.
Metabolic effects of sirtuins
The most well-studied sirtuin with effects on metabolism is SIRT1. The initial excitement for SIRT1 surrounded its homology to the yeast Sir2 protein, which was shown to extend lifespan when overexpressed in yeast [15]. Sir2 homologs in Caenorhabditis elegans [16] and Drosophila melanogaster [17] initially showed similar life-extending effects. Given that SIRT1 is the closest mammalian homolog to yeast Sir2 [4], there was much hope that SIRT1 could also extend lifespan in humans. However, the life-extending effects of SIRT1 in C. elegans and D. melanogaster have recently been called into question [18]. Furthermore, it has not been definitively demonstrated whether SIRT1 is required or sufficient for increasing longevity in mammals, since some studies demonstrate SIRT1 is associated with increased lifespan [19,20], while others show no effects [21,22]. In contrast to the uncertain role on lifespan extension, what is more clear is the effect SIRT1 has on protecting against age-related disease, including Type 2 diabetes. SIRT1 is expressed in several metabolically active tissues, including liver [23–25], muscle [24,26], adipose tissue [23,24], heart [24–26], pancreas [24,27] and brain [23–25,28]. Interestingly, SIRT1 levels decrease in mice upon diet-induced obesity [29,30], as well as during aging [31], two of the most prevalent risk factors for Type 2 diabetes. Humans with morbid obesity also display decreased SIRT1 expression in visceral adipose tissue [32]. These findings suggest that decreased SIRT1 expression may be one mechanism linking obesity and aging to the development of Type 2 diabetes. Indeed, mice with a moderate whole-body decrease in SIRT1 develop obesity and insulin resistance [33] and have elevated lipid accumulation in the liver, as well as increased inflammation in adipose tissue when fed a high-fat diet [34]. Complementary to these findings, transgenic mice with a whole body overexpression of SIRT1 are leaner and more metabolically active, eat less, have decreased fasting serum insulin, glucose and cholesterol, and have improved glucose tolerance compared with control animals [35]. Even mice with a moderate elevation of SIRT1 still show improved fasting serum insulin and glucose levels, as well as improved glucose tolerance when fed a high-fat diet [24,36]. The molecular mechanisms behind these antidiabetic effects of SIRT1 have been intensely studied and have recently been reviewed [37–39]. Together, these studies suggest that SIRT1 is a potential drug target for both the treatment of diabetic symptoms, as well as possible prevention of diet- and age-induced diabetes.
While a large body of literature supports the important role that SIRT1 plays in metabolism, more recently SIRT3 and SIRT6 have gained attention in their respective roles in maintaining metabolic homeostasis. SIRT3 is expressed in metabolic tissues, including the liver, brain, kidney, skeletal muscle [40,41] and brown adipose tissue [42]. One study identified SIRT3 as a major mitochondrial deacetylase and found that a number of key mitochondrial enzymes were hyperacetylated in the liver, brown adipose tissue, heart and brain of SIRT3-deficient mice[43]. While these mice had no metabolic abnormalities under basal conditions, under metabolic stresses, such as prolonged fasting and high-fat diet feeding, SIRT3-deficient mice had several metabolic defects. Under fasting conditions, SIRT3 is normally upregulated in the liver and brown adipose tissue [44]. However, when SIRT3-deficient mice were fasted for 24 h, they did not have upregulated SIRT3 and this resulted in decreased levels of fatty acid oxidation and increased triglycerides in the liver [44]. In contrast to fasting, long-term high-fat feeding has been shown to downregulate levels of SIRT3 mRNA, protein and activity in the mouse liver [45–49], leading to hyperacetylation and decreased activity of mitochondrial proteins [46,47] involved in oxidative metabolism [46,47]. Interestingly, unlike chronic high-fat feeding, acute high-fat feeding led to increased SIRT3 expression. The molecular basis for this is not known. Physiologically, when mice with a total loss of SIRT3 were chronically fed a high-fat diet, they showed accelerated obesity, glucose intolerance, insulin resistance, hyperlipidemia and steatohepatitis compared with wild-type mice fed a high-fat diet [46]. A more detailed review of the molecular mechanisms behind these physiological effects can be found in several recent reviews [37–39]. Collectively, these findings point to the important role SIRT3 plays in maintaining metabolic homeostasis, leading to speculation that patients with symptoms of the metabolic syndrome or Type 2 diabetes may have decreased levels of SIRT3. Thus, a therapeutic intervention that activates SIRT3 may be an efficacious strategy for Type 2 diabetes treatment.
In addition to SIRT1 and SIRT3, SIRT6 has also been linked to metabolism, specifically in maintaining normal glucose homeostasis. SIRT6 is located in the nucleus and plays a role in telomere maintenance [11], DNA repair [48,49] and aging [48,49]. One study reported that SIRT6 knockout mice are smaller than normal mice and develop considerable metabolic abnormalities including lack of subcutaneous fat, severe hypoglycemia and premature death [48]. SIRT6 was identified as a corepressor of the transcription factor Hif1α [12], which is an important regulator of responses to nutritional stress [50]. The hypoglycemia observed in SIRT6 knockout mice may be attributed to an increase in Hif1α activity, which results in increased glucose uptake and diminished mitochondrial respiration [12]. It was further shown that deficiency of SIRT6 led to low circulating insulin levels but enhanced insulin signaling, which also contributes to hypoglycemia [51]. Interestingly, when transgenic mice overexpressing SIRT6 were fed a high-fat diet [52] or aged to 19 months [53], they had improved glucose tolerance, which was associated with enhanced glucose-stimulated insulin secretion compared with wild-type controls [52]. These results indicate that SIRT6 has an important role in maintaining normal glucose homeostasis and more work is required to determine the full metabolic role of SIRT6 and uncover its potential as an antidiabetic therapeutic.
Compared with SIRT1, SIRT3 and SIRT6, less is known about the metabolic roles of the other sirtuins. SIRT2 has been shown to deacetylate and prevent degradation of PEPCK [54], the rate-limiting enzyme in gluconeogenesis, as well as deacetylate and activate FOXO1 [55], a transcription factor regulating both glucose and lipid metabolic genes. Two studies have demonstrated moderate effects of SIRT4 on metabolism [7,56]. SIRT4 knockout mice have increased amino acid-stimulated plasma insulin levels, moderately lower fasted blood glucose and slightly improved glucose tolerance [7]. Furthermore, knockdown of SIRT4 in mice via tail vein injections of an shRNA adenovirus resulted in increased expression of genes involved in fatty acid oxidation in liver and muscle and also slightly decreased blood glucose levels [56]. SIRT5 has been shown to deacetylate [57] and desuccinylate [8] CPS1, leading to enhanced urea cycle function. Effects of SIRT7 on metabolism have not yet been reported. While it is tempting to speculate that these other sirtuins could be antidiabetic drug targets, more studies need to be carried out to understand the biological pathways regulated by SIRT2, SIRT4, SIRT5 and SIRT7.
Genetic polymorphisms in sirtuin genes
In humans, a number of single nucleotide polymorphisms (SNPs) associated with obesity and Type 2 diabetes have been identified in the SIRT1 and SIRT3 genes, suggesting that sirtuins may play a role in the development of these conditions. In a study of obese and lean subjects of Belgian Caucasian origin, the SNP rs7069102 located in intron 4 of SIRT1 was associated with a significant increase in visceral fat area in obese men carrying one or two copies of the minor allele compared with obese men with the wild-type allele [58]. This same SIRT1 SNP (as well as SNPs rs7895833 and rs2273773) were found to be associated with high body fat and blood pressure in a study of 1279 Japanese patients [59]. In a study of 3501 Pima Indians, who have a high incidence of Type 2 diabetes (44%), two SNPs in SIRT1 (rs10509291 and rs7896005) were associated with Type 2 diabetes but not BMI [60]. Participants in the study carrying the at-risk alleles in these SNPs had a reduced acute insulin response to glucose, which may account for an increased risk of Type 2 diabetes [60].
In addition to being associated with an increase in obesity, some SNPs have been identified that are associated with leanness, suggesting that genetic variation of SIRT1 may protect against obesity and, in turn, protect against Type 2 diabetes. For example, the G allele of rs7895833 was associated with a lower BMI in a study of two independent Dutch populations [61]. In another study, a number of SIRT1 SNPs were identified that were associated with obesity in morbidly obese adults; however, there was a greater frequency of the minor allele in the control lean population compared with the case population, suggesting that the minor allele of these SNPs may have a protective effect against obesity [62]. While specific SIRT1 SNPs may directly influence BMI, there is evidence that SIRT1 genetic variations may also affect the outcome of treatment. In a controlled lifestyle intervention study of Caucasians at risk for Type 2 diabetes, patients carrying the minor allele of SNP rs12413112 did not respond as well to the lifestyle interventions of decreased fat intake and increased exercise compared with those with the wild-type allele as measured by fasting plasma glucose, insulin sensitivity and liver fat [63]. These results indicate that understanding genetic variations of SIRT1 may provide insight as to what type of treatment a prediabetic or diabetic patient will best respond to.
Some SNPs and their associated effects have also been identified in the human SIRT3 gene. In 2003, it was reported that the TT genotype of the G477T marker of SIRT3 increases survival in the elderly [64], but associations with obesity or diabetes were not examined. An additional study by Dransfeld et al. identified two SNPs within the functional domain of the SIRT3 protein in a cohort of 640 Caucasians, although they did not determine the functionality associated with these polymorphisms [65]. In 2011, Hirschey et al. independently identified and characterized one of the SNPs identified by Dransfeld et al., rs11246020 (change of valine to isoleucine in the sirtuin conserved domain of SIRT3), in a Caucasian cohort with fatty liver disease and found that this polymorphism is correlated with an increased risk of developing the metabolic syndrome [46]. Recombinantly expressed SIRT3 with this point mutation had decreased activity compared with the wild-type protein, suggesting that this reduction in SIRT3 function may predispose a patient to developing the metabolic syndrome [46]. Given the gap in knowledge of SIRT3 polymorphisms and their associated effects, this remains an important field of study with a strong need to identify the most efficacious individualized treatment for patients with metabolic syndrome or Type 2 diabetes.
First generation of sirtuin activators: resveratrol
The potential of the sirtuins for effective treatment or even prevention of diabetes has resulted in significant efforts into finding novel therapeutics that can activate sirtuins. Among these, a naturally occurring compound called resveratrol is the most well studied. Resveratrol was first isolated from the white hellebore plant (Veratrum grandiflorum) in 1940 [66]. In 1963, Nonomura et al. extracted resveratrol from root extracts used in traditional Japanese and Chinese medicine that, among other things, was used to treat hyperlipidemia [67]. Indeed, resveratrol was shown to reduce liver lipid accumulation in rats fed a high-fat diet [68]. When resveratrol was discovered in red wine [69], there was a great interest in determining whether resveratrol was the compound that conferred the health benefits of red wine and was responsible for the so-called French paradox, whereby the French have high consumption rates of saturated fats yet have one of the lowest rates of cardiovascular disease. In 2003, resveratrol was the top hit on a screen to identify SIRT1 activators [70]. Owing to the link between sirtuins and lifespan, the ability of resveratrol to extend lifespan in mice was tested. While administration to mice did not extend lifespan [71], resveratrol restored normal lifespan to mice fed a high-calorie diet [72]. These early studies demonstrated that resveratrol had many antidiabetic effects in rodents fed a high-fat diet, including reduced circulating glucose levels [72–74], reduced circulating insulin levels [72,73,75,76], improved glucose tolerance [72,75] and increased insulin sensitivity [75,77]. Even in rodent models of diabetes induced by the β-cell toxin streptozotocin, resveratrol potently reduced blood glucose levels [78–80]. Resveratrol also reduced hepatic lipid accumulation in rodents fed high-calorie diets [76,81,82]. Thus, data collected from rodent models support resveratrol as an antidiabetic treatment.
In humans, despite the widespread availability of oral resveratrol supplements, the data regarding the effectiveness of resveratrol in humans are not fully understood. In a systematic review that compiled the opinions of experts who gathered at the first International Conference on Resveratrol and Health (Resveratrol 2010), Vang et al. concluded that the evidence in humans is not sufficient to justify administering resveratrol to humans for disease treatment [83]. The authors describe only two clinical studies by Elliott et al. [84] that reported decreased fasting glucose, decreased postprandial glucose and decreased postprandial insulin levels in Type 2 diabetic patients treated with resveratrol, but experimental details were not disclosed. Even with such limited human data, Vang et al. point out that the data in animal models are promising and more clinical trials should be completed. More recently, additional small-scale studies on resveratrol have been performed to assess its effects on diabetes in humans. A report by Yoshino et al. showed that resveratrol treatment (75 mg/day for 12 weeks) to nonobese women with normal glucose tolerance (n = 15 in each group) had no effects on insulin sensitivity [85]. When Poulsen et al. treated obese but otherwise healthy men with resveratrol (500 mg, three-times per day for 4 weeks; n = 12 in each group), they also reported no effects on body composition and insulin sensitivity [86]. However, Timmers et al. performed a crossover study on obese but otherwise healthy men (similar to Poulsen et al.) and found that resveratrol treatment (150mg, once daily for 29 days; n = 11) mildly increased intramyocellular lipid levels but decreased hepatic lipids, decreased circulating glucose, insulin and leptin, and improved insulin sensitivity as assessed by the homeostatic model assessment of insulin resistance [87]. The reason for the discrepancy between the Poulsen et al. and Timmers et al. studies is unknown but may involve different study designs, different doses of resveratrol and the low number of subjects tested. In contrast to these studies on obese, healthy individuals, recent studies on glucose intolerant individuals demonstrated more effective results for resveratrol. For example, Brasnyo et al. treated 19 Type 2 diabetic men with resveratrol (5 mg, twice daily for 4 weeks) and reported modest effects on decreasing postprandial glucose levels and improving insulin sensitivity as measured by the homeostatic model assessment of insulin resistance and by increased phophosphorylation of Akt in platelets [88]. Furthermore, resveratrol treatment (1–2 g/day for 4 weeks; n = 10) to elderly patients with impaired glucose tolerance resulted in no changes to fasting glucose or insulin levels but improved glucose tolerance and increased insulin sensitivity [89].
In reviewing the metabolic effects of resveratrol in several animal and human studies (Table 1), it is evident that in both rodent and human studies, resveratrol has little effect on metabolism in nondiabetic, nonobese subjects. This may be a positive aspect of resveratrol since the risk of hypoglycemia is low in a normal metabolic state. Furthermore, in conditions of positive energy balance, such as high-fat feeding or obesity, there is a trend for higher doses of resveratrol to decrease body weight. However, this could be an indirect effect of resveratrol since at high doses in clinical studies, mild gastrointestinal symptoms, such as nausea and diarrhea, have been reported [90]. Interestingly, in more metabolically stressed subjects, such as rodents fed a high-fat diet or humans with diagnosed diabetic pathologies, the evidence strongly points to an effect of resveratrol on reducing glucose levels and/or increasing insulin sensitivity. While these data are promising, more clinical data from larger studies are clearly needed in order to determine the appropriate dosing and to assess the long-term safety and efficacy of using resveratrol for diabetes treatment. To this end, more than 20 studies are currently listed on the clinical trials database provided by the US NIH [203] that are investigating the effects of resveratrol on the diabetic condition.
Table 1.
Metabolic effects of resveratrol in various metabolic states.
| Metabolic state | Species | Dose | Effects | Ref. |
|---|---|---|---|---|
| Normal, lean | Mouse | Mixed in with food, ad libitum feeding for 20 weeks (est. dose is 200 mg/kg/day) | No change in fasting glucose or insulin; no effect on glucose tolerance; no change in insulin sensitivity; no effect on hepatic lipids; no effect on body weight | [106] |
| Normal, lean | Mouse | 4 g/kg of food, fed ad libitum for 16 weeks (est. dose is 400 mg/kg/day) | No significant change in body weight | [75] |
| Normal, lean | Rat | 6 mg/l in drinking water (est. dose is 1 mg/kg/day) | No effect on fasting glucose, total cholesterol or triglycerides; elevated HDL, reduced oxidized LDL; no effect on body weight | [74] |
| Normal, lean | Rat | 10 mg/kg/day for 8 weeks via oral gavage | No change in fasting glucose, insulin, leptin, triglycerides, cholesterol or FFA; no change in insulin sensitivity; no effect on hepatic lipids; no effect on body weight or abdominal fat | [107] |
| Normal, lean, middle-aged | Nonhuman primate | 200 mg/kg/day for 21 or 33 months | No effects on fasting glucose or insulin; improved glucose tolerance; increased insulin sensitivity; no effect on body weight | [108] |
| Normal, nonobese, middle-aged | Human | 75 mg/day for 12 weeks | No change in plasma glucose, insulin, leptin, triglycerides, cholesterol or FFA; no effect on insulin sensitivity; no effect on body weight or adiposity | [85] |
| High-fat diet for 19 weeks | Mouse | 79.2 ng/day for 5 weeks via intracerebroventricular infusion | Decreased serum glucose and insulin; reduced hepatic glucose production; no effect on body weight | [73] |
| High-fat diet for 6 months starting at 1-year old | Mouse | 0.04% in food, fed ad libitum for 6 months (est. dose is 22.4 mg/kg/day) | Decreased fed and fasting insulin; decreased fasting glucose; increased fasting cholesterol; improved glucose tolerance; reduced hepatic lipids; no effect on body weight | [72] |
| High-fat diet for 24 weeks | Mouse | Mixed in with food, ad libitum feeding for 20 weeks (est. dose is 200 mg/kg/day) | No change in fasting glucose; reduced serum insulin and leptin; improved glucose tolerance; increased insulin sensitivity; decreased hepatic lipid accumulation; no effect on body weight | [106] |
| High-fat diet for 24 weeks | Mouse | 400 mg/kg/day for 16 weeks via oral gavage | Reduced fasting blood glucose; decreased plasma insulin and FFA; normalized glucose tolerance; improved insulin sensitivity; normalized body weight | [109] |
| High-fat diet for 16 weeks | Mouse | 4 g/kg of food, fed ad libitum for 16 weeks (est. dose is 400 mg/kg/day) | Decreased serum insulin; no change in serum glucose or lipids; increased insulin sensitivity; reduced body weight | [75] |
| High-fat diet for 26 weeks | Mouse | 4 g/kg of food, fed ad libitum for 26 weeks | Decreased serum insulin, leptin and triglycerides; improved glucose tolerance; decreased hepatic cholesterol; reduced body weight and adiposity | [110] |
| High-cholesterol, high-fructose diet for 15 weeks with no body weight gain | Rat | 1 mg/kg/day for 15 days or 15 weeks via oral gavage | No change in fasting glucose; decreased fasting insulin, cholesterol and triglycerides; improved insulin-stimulated glucose uptake; no effect on body weight | [77] |
| High-fat diet for 45 days | Rat | 6 mg/l in drinking water for 15 days (est. dose is 1 mg/kg/day) | Reduced fasting glucose; no effect on fasting cholesterol or triglycerides; reduced oxidized LDL; no effect on body weight | [74] |
| High-fat diet for 16 weeks | Rat | 100 mg/kg/day for 10 weeks via oral administration | Decreased fasting insulin; improved insulin sensitivity; reduced hepatic lipid accumulation; decreased visceral obesity | [76] |
| High-fat diet for 8 weeks | Rat | 100 mg/kg/day via intragastric administration | No effect on fasting glucose, triglycerides or FFA; reduced fasting insulin; improved insulin sensitivity; reduced body weight | [111] |
| Nondiabetic, obese | Human | 150 mg/day for 29 days | Decreased circulating glucose, insulin, leptin and triglycerides; improved insulin sensitivity; increased intramyocellular lipids; decreased hepatic lipids; no effect on body weight | [87] |
| Nondiabetic, obese | Human | 1.5 g/day for 4 weeks | No effect on plasma glucose, insulin, leptin, cholesterol or triglycerides; no changes to insulin sensitivity; no effect on hepatic or intramyocellular lipids; no effect on body weight or adiposity | [86] |
| Elderly, overweight to obese, impaired glucose tolerance | Human | 1–2 g/day for 4 weeks | No changes to fasting glucose or insulin; improved glucose tolerance; increased insulin sensitivity; no change in body weight | [89] |
| Type 2 diabetes | Human | 10 mg/day for 4 weeks | Decreased postprandial glucose levels; improved insulin sensitivity | [88] |
| Type 2 diabetes | Human | 5 g/day | Decreased fasting glucose; decreased postprandial glucose and insulin | [84] |
Studies are organized by metabolic state and species. Doses within a species are ordered from lowest to highest.
Est.: Estimated; FFA: Free fatty acid; HDL: High-density lipoprotein; LDL: Low-density lipoprotein.
The promising studies on resveratrol have shown that activating sirtuins may be a viable way to treat diabetes. It is encouraging that resveratrol is well tolerated in patients with few adverse effects [85,87,90] aside from mild gastrointestinal complaints (nausea and diarrhea) at high doses [90], although long-term studies have not been completed. Importantly, few cases of hypoglycemia have been reported with resveratrol treatment, even in rodents or humans that had normal blood glucose levels prior to therapy. While promising, many of the glucose lowering and insulin sensitizing effects of resveratrol in humans have been mild, possibly due to the fact that the bioavailability of unaltered resveratrol taken orally in humans may be quite low [91,92]. Furthermore, it has recently been called into question whether resveratrol directly or indirectly activates SIRT1 [93]. Regardless of the molecular mechanisms, resveratrol has been shown in many studies to activate SIRT1 and elicit anti-diabetic effects. However, indirect activation of SIRT1 leaves open the possibility that resveratrol may have several nonspecific effects. This further highlights the need for next-generation sirtuin activators that are more specific and more effective than resveratrol.
The next generation of sirtuin activators
The development of more effective sirtuin activators is currently underway. Recently, it was reported that AS101, a tellurium compound developed at Bar-Ilan University (Ramat Gan, Israel), increases SIRT1 protein expression and activity and shows promise as an antidiabetic drug [94]. In a rat model of Type 2 diabetes, treatment with AS101 before clinical hyperglycemia resulted in increased insulin sensitivity and decreased blood glucose levels, and treatment with AS101 after the development of Type 2 diabetes partially restored normal glucose homeostasis [94]. Another group at Sirtris, a Glaxosmithkline company (MA, USA), focuses specifically on developing small molecule drugs that target sirtuins in order to treat diseases of aging, and is the leading pharmaceutical company pursuing the discovery of small molecules that activate sirtuins. In 2007, Sirtris published a report describing the antidiabetic effects of SRT1720, a small molecule activator of SIRT1 that is structurally unrelated to resveratrol but 1000-fold more potent [95]. Initial studies indicated that diet-induced obese mice treated with SRT1720 had reduced fed glucose levels through 10 weeks of treatment with similar results observed in a genetic mouse model of Type 2 diabetes [95], indicating the potential to treat Type 2 diabetes with activators of SIRT1. Further studies reported that SRT1720 protects against diet-induced obesity and insulin resistance by the induction of fatty acid oxidation [96]. Recently, Minor et al. described increased healthspan of obese mice treated with SRT1720 as a result of reduced liver steatosis, increased insulin sensitivity, enhanced locomotive activity, and normalization of gene expression profiles and markers of inflammation and apoptosis [19]. While SRT1720 remains in the preclinical testing phase due to controversy surrounding off target effects [97], three other SIRT1 small molecule activators developed at Sirtris (SRT2104, SRT2379 and SRT3025) are currently undergoing clinical trials. Recently, the results of Phase I clinical trials that examined the tolerability and pharmacokinetics of SRT2104 oral dosing in healthy volunteers were published and reported no adverse reactions [98]. In this study, Hoffmann et al. referred to unpublished data that show improved glucose and insulin homeostasis in diet-induced obese mice and genetically obese ob/ob mice when treated with SRT2104 [98]. Results of the clinical studies indicated that SRT2104 was well tolerated at doses up to 3.0 g; however, a lack of solubility of the small molecule prevented optimal absorption [98]. While no serious adverse reactions were reported in the studies described, Phase II clinical trials of SRT2104 are necessary to demonstrate the efficacy of SRT2104 as a SIRT1 activator in humans.
In addition to the drug candidates described above, supplementation by NAD+ precursors is another potential therapeutic strategy for activation of sirtuins. In 2007, it was reported that exogenous nicotinamide riboside, a vitamin precursor of NAD+, extends the lifespan of yeast by elevating NAD+ and activating Sir2 [99]. Importantly, this result was observed in the absence of calorie restriction, suggesting that NAD+ precursors might directly activate mammalian sirtuins. Indeed, nicotinamide riboside supplemented to mice led to increased levels of NAD+ in tissues and was accompanied by a slight improvement in glucose tolerance and a reduction in insulin secretion [100]. Additionally, supplementation by another NAD+ precursor, nicotinamide mononucleotide, also demonstrated the potential of these compounds to activate sirtuins. In a high-fat diet-induced Type 2 diabetes mouse model, treatment with nicotinamide mononucleotide restored the diet-induced depletion of NAD+ levels to normal and increased glucose tolerance, which was, in part, due to activation of SIRT1 [101]. The improvement in glucose tolerance observed in these two studies indicates that NAD+ precursors could hold promise as novel sirtuin activators to manage and treat Type 2 diabetes.
The therapeutic strategies to activate sirtuins described above are mainly focused on activating SIRT1 owing to the fact that it is the best understood sirtuin. However, there remain a number of strategies yet to be explored that may activate SIRT1, SIRT3 and/or SIRT6. Since SIRT3 and SIRT6 are also NAD+-dependent enzymes, the activity of these sirtuins when treated with NAD+ precursors should be explored as a potential method to treat metabolic disorders. Additionally, as the mechanisms of sirtuin regulation become better understood, a number of targets of activation will certainly come to the forefront of efforts to discover novel therapeutics. For example, targeting transcriptional regulators or mechanisms of post-translational modifications of the sirtuins may provide efficacious methods to specifically activate the sirtuins.
Possible trade-offs of sirtuin activation
Another important point to consider in the development of new therapeutics is the potential trade-offs of developing broad-based sirtuin activators. For SIRT1 in particular, several studies have shown that the positive metabolic effects of SIRT1 may be restricted to specific cell types. For example, mice with a whole body overexpression of SIRT1, while leaner and more glucose tolerant than controls [35], displayed an increase in anxious behaviours due to increased direct activation of monoamine oxidase A in the brain [102]. Furthermore, overexpression of SIRT1 in neurons expressing neuron-specific enolase caused memory deficits [103]. Interestingly, while activation of SIRT1 in POMC- and SF1-expressing neurons in the hypothalamus appears to have antiobesity effects, SIRT1 action in hypothalamic AgRP-expressing neurons may actually increase food intake [104]. In addition, overexpression of SIRT1 in the forebrain of mice leads to increased adiposity and impaired glucose tolerance [105]. Given these varying cell-specific effects of SIRT1 in the brain, perhaps the best pharmacological activator of SIRT1 may be one that does not cross the blood–brain barrier.
Conclusion & future perspective
The data on sirtuins and their antidiabetic potential are compelling, and on-going studies will determine whether sirtuin activators will be an effective therapeutic against diabetes. While it appears that sirtuins may not extend lifespan in humans as first predicted, it is becoming clear that sirtuins may improve quality of life and extend healthspan by combatting age-related defects, such as the metabolic abnormalities associated with Type 2 diabetes. The evidence from human SNP studies and high-fat fed rodent models suggest that dysfunction in various sirtuins may be a molecular mechanism linking overnutrition and diabetes. Thus, targeting sirtuins may be an effective method of treating or possibly even preventing the development of diabetes due to aging or unhealthy lifestyle.
Currently, more than 30 clinical trials are investigating the safety and efficacy of resveratrol and other SIRT1 activators for use in treating diabetes [203]. While drug discovery of activators of SIRT3 and SIRT6 is still in its infancy, the review of animal models presented here highlights the therapeutic potential of activators of SIRT3 and SIRT6 in treating the metabolic abnormalities associated with Type 2 diabetes. Together, the fact that levels of SIRT1 and SIRT3 decrease with aging and high-fat feeding, decreased SIRT1 and SIRT3 activity is related to metabolic abnormalities consistent with Type 2 diabetes, and human SNPs that result in decreased sirtuin activity are related to the development of metabolic syndrome, it is tempting to speculate that sirtuin activity may be a mechanistic link between aging and overnutrition and Type 2 diabetes. If true, sirtuin activators could be effective diabetes treatments. While it is too early to tell, drugs aimed at preventing the decline in sirtuin activity associated with aging or overnutrition could offset the continued rise in incidence and mortality associated with diabetes.
Practice Points.
Sirtuins are a family of highly conserved NAD+-dependent deacetylases.
Reduced sirtuin expression and/or activity is correlated with development of Type 2 diabetes.
SIRT1, SIRT3 and SIRT6 have currently been shown to have the most robust effects on metabolism.
Mice lacking any one of these sirtuins show characteristics of Type 2 diabetes, including insulin resistance and glucose intolerance, especially when fed a high-fat diet.
Single nucleotide polymorphisms associated with obesity and Type 2 diabetes have been identified in the SIRT1 and SIRT3 genes, suggesting that sirtuins may play a role in the development of these conditions in humans.
Recent clinical trials show that resveratrol, a SIRT1 activator, has moderate glucose-lowering and insulin-sensitizing effects in patients with diabetes, but not in healthy patients.
The resveratrol clinical trials demonstrate that SIRT1 is a promising target for future antidiabetic therapies; however, more specific and more effective activators are required.
Several compounds targeting SIRT1 have been developed and are entering clinical trials.
Activation of SIRT1 in certain neurons in the brain may lead to increased food intake, glucose intolerance and increased anxiety. Thus, the best activator of sirtuin action may be one that does not cross the blood–brain barrier.
Currently, over 30 clinical trials are in progress to investigate the possibility of resveratrol and other SIRT1 activators for use in treating diabetes.
While human studies have not yet been performed, data from animal models suggest that therapies targeted at activating SIRT3 and SIRT6 may have beneficial effects in patients with Type 2 diabetes.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Unwin N, Whiting D, Guariguata L, Ghyoot G, Gan D, editors. International Diabetes Federation Diabetes Atlas. 5. International Diabetes Federation; Brussels, Belguim: 2011. [Google Scholar]
- 2.Mannino GC, Sesti G. Individualized therapy for Type 2 diabetes: clinical implications of pharmacogenetic data. Mol Diagn Ther. 2012;16(5):285–302. doi: 10.1007/s40291-012-0002-7. [DOI] [PubMed] [Google Scholar]
- 3.Inzucchi SE. Oral antihyperglycemic therapy for Type 2 diabetes: scientific review. JAMA. 2002;287(3):360–372. doi: 10.1001/jama.287.3.360. [DOI] [PubMed] [Google Scholar]
- 4.Frye RA. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun. 2000;273(2):793–798. doi: 10.1006/bbrc.2000.3000. [DOI] [PubMed] [Google Scholar]
- 5.Hirschey MD. Old enzymes, new tricks: sirtuins are NAD+-dependent de-acylases. Cell Metab. 2011;14(6):718–719. doi: 10.1016/j.cmet.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 2003;11(2):437–444. doi: 10.1016/s1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 7.Haigis MC, Mostoslavsky R, Haigis KM, et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126(5):941–954. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 8.Du J, Zhou Y, Su X, et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science. 2011;334(6057):806–809. doi: 10.1126/science.1207861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng C, Lu Z, Xie Z, et al. The first identification of lysine malonylation substrates and its regulatory enzyme. Mol Cell Proteomics. 2011;10(12):M111 012658. doi: 10.1074/mcp.M111.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer F, Gertz M, Suenkel B, Lakshminarasimhan M, Schutkowski M, Steegborn C. Sirt5 deacylation activities show differential sensitivities to nicotinamide inhibition. PLoS One. 2012;7(9):e45098. doi: 10.1371/journal.pone.0045098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michishita E, Mccord RA, Berber E, et al. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature. 2008;452(7186):492–496. doi: 10.1038/nature06736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong L, D’urso A, Toiber D, et al. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell. 2010;140(2):280–293. doi: 10.1016/j.cell.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liszt G, Ford E, Kurtev M, Guarente L. Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. J Biol Chem. 2005;280(22):21313–21320. doi: 10.1074/jbc.M413296200. [DOI] [PubMed] [Google Scholar]
- 14.Barber MF, Michishita-Kioi E, Xi Y, et al. SIRT7 links H3K18 deacetylation to maintenance of oncogenic transformation. Nature. 2012;487(7405):114–118. doi: 10.1038/nature11043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaeberlein M, Mcvey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13(19):2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410(6825):227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 17.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci USA. 2004;101(45):15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burnett C, Valentini S, Cabreiro F, et al. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 2011;477(7365):482–485. doi: 10.1038/nature10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19▪.Minor RK, Baur JA, Gomes AP, et al. SRT1720 improves survival and healthspan of obese mice. Sci Rep. 2011;1:70. doi: 10.1038/srep00070. Describes the metabolic benefits from targeted SIRT1 activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S, Bi X, Czarny-Ratajczak M, et al. Telomere maintenance genes SIRT1 and XRCC6 impact age-related decline in telomere length but only SIRT1 is associated with human longevity. Biogerontology. 2012;13(2):119–131. doi: 10.1007/s10522-011-9360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flachsbart F, Croucher PJ, Nikolaus S, et al. Sirtuin 1 (SIRT1) sequence variation is not associated with exceptional human longevity. Exp Gerontol. 2006;41(1):98–102. doi: 10.1016/j.exger.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Herranz D, Munoz-Martin M, Canamero M, et al. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun. 2010;1:3. doi: 10.1038/ncomms1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen HY, Miller C, Bitterman KJ, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305(5682):390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 24.Banks AS, Kon N, Knight C, et al. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8(4):333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakamoto J, Miura T, Shimamoto K, Horio Y. Predominant expression of Sir2alpha, an NAD-dependent histone deacetylase, in the embryonic mouse heart and brain. FEBS Lett. 2004;556(1–3):281–286. doi: 10.1016/s0014-5793(03)01444-3. [DOI] [PubMed] [Google Scholar]
- 26.Price NL, Gomes AP, Ling AJ, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15(5):675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bordone L, Motta MC, Picard F, et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006;4(2):e31. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zakhary SM, Ayubcha D, Dileo JN, et al. Distribution analysis of deacetylase SIRT1 in rodent and human nervous systems. Anat Rec (Hoboken) 2010;293(6):1024–1032. doi: 10.1002/ar.21116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiao L, Shao J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J Biol Chem. 2006;281(52):39915–39924. doi: 10.1074/jbc.M607215200. [DOI] [PubMed] [Google Scholar]
- 30.Deng XQ, Chen LL, Li NX. The expression of SIRT1 in nonalcoholic fatty liver disease induced by high-fat diet in rats. Liver Int. 2007;27(5):708–715. doi: 10.1111/j.1478-3231.2007.01497.x. [DOI] [PubMed] [Google Scholar]
- 31.Han L, Zhou R, Niu J, Mcnutt MA, Wang P, Tong T. SIRT1 is regulated by a PPAR{gamma}-SIRT1 negative feedback loop associated with senescence. Nucleic Acids Res. 2010;38(21):7458–7471. doi: 10.1093/nar/gkq609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.dos Costa CS, Hammes TO, Rohden F, et al. SIRT1 transcription is decreased in visceral adipose tissue of morbidly obese patients with severe hepatic steatosis. Obes Surg. 2010;20(5):633–639. doi: 10.1007/s11695-009-0052-z. [DOI] [PubMed] [Google Scholar]
- 33.Purushotham A, Xu Q, Li X. Systemic SIRT1 insufficiency results in disruption of energy homeostasis and steroid hormone metabolism upon high-fat-diet feeding. FASEB J. 2012;26(2):656–667. doi: 10.1096/fj.11-195172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu F, Gao Z, Zhang J, et al. Lack of SIRT1 (Mammalian Sirtuin 1) activity leads to liver steatosis in the SIRT1+/− mice: a role of lipid mobilization and inflammation. Endocrinology. 2010;151(6):2504–2514. doi: 10.1210/en.2009-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35▪.Bordone L, Cohen D, Robinson A, et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6(6):759–767. doi: 10.1111/j.1474-9726.2007.00335.x. Demonstrates that mice overexpressing SIRT1 are leaner and more glucose tolerant than control mice. [DOI] [PubMed] [Google Scholar]
- 36.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci USA. 2008;105(28):9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong XC. Sirtuin biology and relevance to diabetes treatment. Diabetes Manag (Lond) 2012;2(3):243–257. doi: 10.2217/dmt.12.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13(4):225–238. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baur JA, Ungvari Z, Minor RK, Le Couteur DG, De Cabo R. Are sirtuins viable targets for improving healthspan and lifespan? Nat Rev Drug Discov. 2012;11(6):443–461. doi: 10.1038/nrd3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Onyango P, Celic I, McCaffery JM, Boeke JD, Feinberg AP. SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria. Proc Natl Acad Sci USA. 2002;99(21):13653–13658. doi: 10.1073/pnas.222538099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang YH, Chen YH, Zhang CY, Nimmakayalu MA, Ward DC, Weissman S. Cloning and characterization of two mouse genes with homology to the yeast Sir2 gene. Genomics. 2000;69(3):355–369. doi: 10.1006/geno.2000.6360. [DOI] [PubMed] [Google Scholar]
- 42.Shi T, Wang F, Stieren E, Tong Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem. 2005;280(14):13560–13567. doi: 10.1074/jbc.M414670200. [DOI] [PubMed] [Google Scholar]
- 43.Lombard DB, Alt FW, Cheng HL, et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27(24):8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirschey MD, Shimazu T, Goetzman E, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464(7285):121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bao J, Scott I, Lu Z, et al. SIRT3 is regulated by nutrient excess and modulates hepatic susceptibility to lipotoxicity. Free Radic Biol Med. 2010;49(7):1230–1237. doi: 10.1016/j.freeradbiomed.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46▪▪.Hirschey MD, Shimazu T, Jing E, et al. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol Cell. 2011;44(2):177–190. doi: 10.1016/j.molcel.2011.07.019. Demonstrates that aberrant SIRT3 action may contribute to the development of metabolic syndrome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kendrick AA, Choudhury M, Rahman SM, et al. Fatty liver is associated with reduced SIRT3 activity and mitochondrial protein hyperacetylation. Biochem J. 2011;433(3):505–514. doi: 10.1042/BJ20100791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48▪▪.Mostoslavsky R, Chua KF, Lombard DB, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124(2):315–329. doi: 10.1016/j.cell.2005.11.044. Demonstrates that SIRT6 knockout mice have severe metabolic abnormalities. [DOI] [PubMed] [Google Scholar]
- 49.Lombard DB, Schwer B, Alt FW, Mostoslavsky R. SIRT6 in DNA repair, metabolism and ageing. J Intern Med. 2008;263(2):128–141. doi: 10.1111/j.1365-2796.2007.01902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iyer NV, Kotch LE, Agani F, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12(2):149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiao C, Kim HS, Lahusen T, et al. SIRT6 deficiency results in severe hypoglycemia by enhancing both basal and insulin-stimulated glucose uptake in mice. J Biol Chem. 2010;285(47):36776–36784. doi: 10.1074/jbc.M110.168039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kanfi Y, Peshti V, Gil R, et al. SIRT6 protects against pathological damage caused by diet-induced obesity. Aging Cell. 2010;9(2):162–173. doi: 10.1111/j.1474-9726.2009.00544.x. [DOI] [PubMed] [Google Scholar]
- 53.Kanfi Y, Naiman S, Amir G, et al. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483(7388):218–221. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- 54.Jiang W, Wang S, Xiao M, et al. Acetylation regulates gluconeogenesis by promoting PEPCK1 degradation via recruiting the UBR5 ubiquitin ligase. Mol Cell. 2011;43(1):33–44. doi: 10.1016/j.molcel.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jing E, Gesta S, Kahn CR. SIRT2 regulates adipocyte differentiation through FoxO1 acetylation/deacetylation. Cell Metab. 2007;6(2):105–114. doi: 10.1016/j.cmet.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nasrin N, Wu X, Fortier E, et al. SIRT4 regulates fatty acid oxidation and mitochondrial gene expression in liver and muscle cells. J Biol Chem. 2010;285(42):31995–32002. doi: 10.1074/jbc.M110.124164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakagawa T, Lomb DJ, Haigis MC, Guarente L. SIRT5 deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell. 2009;137(3):560–570. doi: 10.1016/j.cell.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peeters AV, Beckers S, Verrijken A, et al. Association of SIRT1 gene variation with visceral obesity. Hum Genet. 2008;124(4):431–436. doi: 10.1007/s00439-008-0567-8. [DOI] [PubMed] [Google Scholar]
- 59.Shimoyama Y, Suzuki K, Hamajima N, Niwa T. Sirtuin 1 gene polymorphisms are associated with body fat and blood pressure in Japanese. Transl Res. 2011;157(6):339–347. doi: 10.1016/j.trsl.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 60.Dong Y, Guo T, Traurig M, et al. SIRT1 is associated with a decrease in acute insulin secretion and a sex specific increase in risk for Type 2 diabetes in Pima Indians. Mol Genet Metab. 2011;104(4):661–665. doi: 10.1016/j.ymgme.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zillikens MC, Van Meurs JB, Rivadeneira F, et al. SIRT1 genetic variation is related to BMI and risk of obesity. Diabetes. 2009;58(12):2828–2834. doi: 10.2337/db09-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clark SJ, Falchi M, Olsson B, et al. Association of sirtuin 1 (SIRT1) gene SNPs and transcript expression levels with severe obesity. Obesity (Silver Spring) 2012;20(1):178–185. doi: 10.1038/oby.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weyrich P, Machicao F, Reinhardt J, et al. SIRT1 genetic variants associate with the metabolic response of Caucasians to a controlled lifestyle intervention – the TULIP Study. BMC Med Genet. 2008;9(1):100. doi: 10.1186/1471-2350-9-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rose G, Dato S, Altomare K, et al. Variability of the SIRT3 gene, human silent information regulator Sir2 homologue, and survivorship in the elderly. Exp Gerontol. 2003;38(10):1065–1070. doi: 10.1016/s0531-5565(03)00209-2. [DOI] [PubMed] [Google Scholar]
- 65.Dransfeld CL, Alborzinia H, Wolfl S, Mahlknecht U. SIRT3 SNPs validation in 640 individuals, functional analyses and new insights into SIRT3 stability. Int J Oncol. 2010;36(4):955–960. doi: 10.3892/ijo_00000574. [DOI] [PubMed] [Google Scholar]
- 66.Takaoka MJ. Of the phenolic substances of white hellebore (Veratrum grandiflorum Loes. fil.) J Faculty Sci Hokkaido Imp Univ. 1940;3:1–16. [Google Scholar]
- 67.Nonomura S, Kanagawa H, Makimoto A. Constituents of polygonaceous plants. I Studies on the components of Ko-jo-kon (Polygonum cuspidatum Sieb et Zucc.) Yakugaku Zasshi. 1963;83(10):988–990. [PubMed] [Google Scholar]
- 68.Arichi H, Kimura Y, Okuda H, Baba K, Kozawa M, Arichi S. Effects of stilbene components of the roots of Polygonum cuspidatum Sieb. et Zucc on lipid metabolism. Chem Pharm Bull (Tokyo) 1982;30(5):1766–1770. doi: 10.1248/cpb.30.1766. [DOI] [PubMed] [Google Scholar]
- 69.Siemann EH, Creasy LL. Concentration of the phytoalexin resveratrol in wine. Am J Enol Vitic. 1992;43:49–52. [Google Scholar]
- 70▪.Howitz KT, Bitterman KJ, Cohen HY, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425(6954):191–196. doi: 10.1038/nature01960. First report of resveratrol as an activator of SIRT1. [DOI] [PubMed] [Google Scholar]
- 71.Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8(2):157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramadori G, Gautron L, Fujikawa T, Vianna CR, Elmquist JK, Coppari R. Central administration of resveratrol improves diet-induced diabetes. Endocrinology. 2009;150(12):5326–5333. doi: 10.1210/en.2009-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rocha KK, Souza GA, Ebaid GX, Seiva FR, Cataneo AC, Novelli EL. Resveratrol toxicity: effects on risk factors for atherosclerosis and hepatic oxidative stress in standard and high-fat diets. Food Chem Toxicol. 2009;47(6):1362–1367. doi: 10.1016/j.fct.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 75.Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127(6):1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 76.Shang J, Chen LL, Xiao FX, Sun H, Ding HC, Xiao H. Resveratrol improves non-alcoholic fatty liver disease by activating AMP-activated protein kinase. Acta Pharmacol Sin. 2008;29(6):698–706. doi: 10.1111/j.1745-7254.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- 77.Deng JY, Hsieh PS, Huang JP, Lu LS, Hung LM. Activation of estrogen receptor is crucial for resveratrol-stimulating muscular glucose uptake via both insulin-dependent and -independent pathways. Diabetes. 2008;57(7):1814–1823. doi: 10.2337/db07-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Palsamy P, Subramanian S. Resveratrol, a natural phytoalexin, normalizes hyperglycemia in streptozotocin-nicotinamide induced experimental diabetic rats. Biomed Pharmacother. 2008;62(9):598–605. doi: 10.1016/j.biopha.2008.06.037. [DOI] [PubMed] [Google Scholar]
- 79.Hamadi N, Mansour A, Hassan MH, Khalifi-Touhami F, Badary O. Ameliorative effects of resveratrol on liver injury in streptozotocin-induced diabetic rats. J Biochem Mol Toxicol. 2012;26(10):384–392. doi: 10.1002/jbt.21432. [DOI] [PubMed] [Google Scholar]
- 80.Huang JP, Huang SS, Deng JY, Chang CC, Day YJ, Hung LM. Insulin and resveratrol act synergistically, preventing cardiac dysfunction in diabetes, but the advantage of resveratrol in diabetics with acute heart attack is antagonized by insulin. Free Radic Biol Med. 2010;49(11):1710–1721. doi: 10.1016/j.freeradbiomed.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 81.Poulsen MM, Larsen JO, Hamilton-Dutoit S, et al. Resveratrol up-regulates hepatic uncoupling protein 2 and prevents development of nonalcoholic fatty liver disease in rats fed a high-fat diet. Nutr Res. 2012;32(9):701–708. doi: 10.1016/j.nutres.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 82.Bujanda L, Hijona E, Larzabal M, et al. Resveratrol inhibits nonalcoholic fatty liver disease in rats. BMC Gastroenterol. 2008;8:40. doi: 10.1186/1471-230X-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83▪▪.Vang O, Ahmad N, Baile CA, et al. What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS One. 2011;6(6):e19881. doi: 10.1371/journal.pone.0019881. Provides an extensive overview of the effects of resveratrol, including limited clinical trials investigating metabolic effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Elliott PJ, Walpole S, Morelli L, et al. Resveratrol/SRT-501. Drugs Fut. 2009;34(4):291. [Google Scholar]
- 85.Yoshino J, Conte C, Fontana L, et al. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012;16(5):658–664. doi: 10.1016/j.cmet.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Poulsen MM, Vestergaard PF, Clasen BF, et al. High-dose resveratrol supplementation in obese men: an investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes. 2012;62(4):1186–1195. doi: 10.2337/db12-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Timmers S, Konings E, Bilet L, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14(5):612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brasnyo P, Molnar GA, Mohas M, et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in Type 2 diabetic patients. Br J Nutr. 2011;106(3):383–389. doi: 10.1017/S0007114511000316. [DOI] [PubMed] [Google Scholar]
- 89.Crandall JP, Oram V, Trandafirescu G, et al. Pilot study of resveratrol in older adults with impaired glucose tolerance. J Gerontol A Biol Sci Med Sci. 2012;67(12):1307–1312. doi: 10.1093/gerona/glr235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brown VA, Patel KR, Viskaduraki M, et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010;70(22):9003–9011. doi: 10.1158/0008-5472.CAN-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Walle T, Hsieh F, Delegge MH, Oatis JE, Jr, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32(12):1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 92.Goldberg DM, Yan J, Soleas GJ. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin Biochem. 2003;36(1):79–87. doi: 10.1016/s0009-9120(02)00397-1. [DOI] [PubMed] [Google Scholar]
- 93.Revelations into resveratrol’s mechanism. Nat Med. 2012;18(4):500–501. [Google Scholar]
- 94.Halperin-Sheinfeld M, Gertler A, Okun E, Sredni B, Cohen HY. The tellurium compound, AS101, increases SIRT1 level and activity and prevents Type 2 diabetes. Aging (Albany NY) 2012;4(6):436–447. doi: 10.18632/aging.100468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Milne JC, Lambert PD, Schenk S, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of Type 2 diabetes. Nature. 2007;450(7170):712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Feige JN, Lagouge M, Canto C, et al. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8(5):347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 97.Pacholec M, Bleasdale JE, Chrunyk B, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285(11):8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hoffmann E, Wald J, Lavu S, et al. Pharmacokinetics and tolerability of SRT2104, a first-in-class small molecule activator of SIRT1, after single and repeated oral administration in man. Br J Clin Pharmacol. 2012;75(1):186–196. doi: 10.1111/j.1365-2125.2012.04340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Belenky P, Racette FG, Bogan KL, McClure JM, Smith JS, Brenner C. Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+ Cell. 2007;129(3):473–484. doi: 10.1016/j.cell.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 100.Canto C, Houtkooper RH, Pirinen E, et al. The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15(6):838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14(4):528–536. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Libert S, Pointer K, Bell EL, et al. SIRT1 activates MAO-A in the brain to mediate anxiety and exploratory drive. Cell. 2011;147(7):1459–1472. doi: 10.1016/j.cell.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kakefuda K, Fujita Y, Oyagi A, et al. Sirtuin 1 overexpression mice show a reference memory deficit, but not neuroprotection. Biochem Biophys Res Commun. 2009;387(4):784–788. doi: 10.1016/j.bbrc.2009.07.119. [DOI] [PubMed] [Google Scholar]
- 104.Coppari R. Metabolic actions of hypothalamic SIRT1. Trends Endocrinol Metab. 2012;23(4):179–185. doi: 10.1016/j.tem.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu D, Qiu Y, Gao X, Yuan XB, Zhai Q. Overexpression of SIRT1 in mouse forebrain impairs lipid/glucose metabolism and motor function. PLoS One. 2011;6(6):e21759. doi: 10.1371/journal.pone.0021759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jeon BT, Jeong EA, Shin HJ, et al. Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes. 2012;61(6):1444–1454. doi: 10.2337/db11-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rivera L, Moron R, Zarzuelo A, Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol. 2009;77(6):1053–1063. doi: 10.1016/j.bcp.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 108.Marchal J, Blanc S, Epelbaum J, Aujard F, Pifferi F. Effects of chronic calorie restriction or dietary resveratrol supplementation on insulin sensitivity markers in a primate, Microcebus murinus. PLoS One. 2012;7(3):e34289. doi: 10.1371/journal.pone.0034289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang J, Chen L, Zheng J, et al. The protective effect of resveratrol on islet insulin secretion and morphology in mice on a high-fat diet. Diabetes Res Clin Pract. 2012;97(3):474–482. doi: 10.1016/j.diabres.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 110.Zhou M, Wang S, Zhao A, et al. Transcriptomic and metabonomic profiling reveal synergistic effects of quercetin and resveratrol supplementation in high fat diet fed mice. J Proteome Res. 2012;11(10):4961–4971. doi: 10.1021/pr3004826. [DOI] [PubMed] [Google Scholar]
- 111.Chen LL, Zhang HH, Zheng J, et al. Resveratrol attenuates high-fat diet-induced insulin resistance by influencing skeletal muscle lipid transport and subsarcolemmal mitochondrial beta-oxidation. Metabolism. 2011;60(11):1598–1609. doi: 10.1016/j.metabol.2011.04.002. [DOI] [PubMed] [Google Scholar]
Websites
- 201.WHO. The top 10 causes of death. Fact Sheet No. 310. www.who.int/mediacentre/factsheets/fs310/en/index.html.
- 202.WHO. Diabetes. Fact Sheet No. 312. www.who.int/mediacentre/factsheets/fs312/en/index.html.
- 203.ClinicalTrials.gov. www.clinicaltrials.gov.


