Abstract
Background. Unlike cytomegalovirus (CMV) infection and aging, human immunodeficiency virus (HIV) decreases the proportion of CD28−CD8+ T cells expressing CD57. Whether this abnormality predicts mortality in treated HIV infection and can be reversed by early antiretroviral therapy (ART) remains unknown.
Methods. We sampled recently HIV-infected individuals (<6 months) and HIV-uninfected controls and compared longitudinal changes in the proportion of CD28−CD8+ T cells expressing CD57 between those who initiated ART early (<6 months) vs later (≥2 years). We also assessed the relationship between this phenotype and mortality in a nested case-control study of ART-suppressed chronically infected individuals.
Results. Compared to HIV-uninfected controls (n = 15), individuals who were recently infected with HIV had lower proportions of CD28−CD8+ T cells expressing CD57 (P < .001), and these proportions increased during ART. The early ART group (n = 33) achieved normal levels, whereas the later ART group (n = 30) continued to have lower levels than HIV-uninfected controls (P = .02). Among 141 ART-suppressed participants in the SOCA study, those in the lowest quartile of CD28−CD8+ T cells expressing CD57 had 5-fold higher odds of mortality than those in the highest quartile (95% CI, 1.6–15.9, P = .007).
Conclusions. Abnormally low proportions of CD28−CD8+ T cells expressing CD57 predict increased mortality during treated HIV infection and may be reversed with early ART initiation.
Keywords: HIV, CD57, CD28, Immunosenescence, aging, mortality, antiretroviral therapy, immune activation
Despite effective antiretroviral therapy (ART), individuals infected with human immunodeficiency virus (HIV) remain at higher risk for mortality and morbidities commonly associated with aging than the general population [1]. Chronic immune activation is thought to mediate this increased risk [2]. Many have hypothesized that the chronic inflammatory state of HIV infection may drive T-cell senescence, as seen with chronic cytomegalovirus (CMV) infection and among elderly HIV-uninfected individuals [3–5], potentially increasing the risk of infections and malignancies, and further fueling the persistent inflammatory state. T-cell senescence is typically characterized by the accumulation of terminally differentiated CD8+ T cells with shortened telomeres, loss of the costimulatory molecule CD28, and increased expression of CD57, a marker of proliferative history and poor proliferative capacity [6]. Even during suppressive ART, HIV-infected individuals have increased frequencies of CD28−CD8+ T cells [7, 8], but the impact of HIV on CD57 expression on CD8+ T-cell subsets—particularly the effector memory CD8+ T-cell subsets that normally express CD57—is less well documented. We recently demonstrated that although CMV promotes terminal differentiation and the expression of CD57 on CD28−CD8+ T cells, consistent with the “immunosenescent” phenotype of aging, HIV is associated with enrichment for less well-differentiated transitional CD8+ T cells and abnormally low proportions of CD28−CD8+ T cells expressing CD57 [9, 10].
In the current study, we sought to determine whether abnormally low proportions of CD28−CD8+ T cells expressing CD57 (ie, the %CD57+ of CD28−CD8+ T cells) are evident during the first few months of HIV infection, whether early ART initiation (<6 months following estimated date of HIV infection) is capable of reversing this abnormality, and whether persistently low proportions of CD28−CD8+ T cells expressing CD57 predict mortality during treated HIV infection. Because several innate immune activation pathways in HIV infection may contribute to T-cell proliferative defects [11, 12], we also assessed whether soluble markers of innate immune activation were associated with low proportions of CD28−CD8+ T cells expressing CD57.
MATERIALS AND METHODS
Participants
Recently HIV-infected participants (<6 months since estimated date of infection) who subsequently initiated ART “early” (<6 months following infection) or “later” (≥2 years following infection) and who subsequently maintained ≥2 years of ART-mediated viral suppression, and risk-matched HIV-uninfected controls were sampled from the UCSF OPTIONS cohort as described elsewhere [13]. HIV-infected participants were followed for a median of 3 years during confirmed ART-mediated viral suppression (<40 copies/mL). Because CMV is an important determinant of CD57 expression [9] and because almost all HIV-infected participants were CMV coinfected, we restricted all analyses to HIV-infected and uninfected participants with confirmed asymptomatic CMV infection.
To assess the impact of CD8+ T-cell phenotypes on mortality, we performed a nested case-control study of ART-suppressed HIV-infected individuals in the Study of the Ocular Complications of AIDS (SOCA), a multicenter cohort of >2200 HIV-infected participants who initiated ART with an AIDS diagnosis. Cases were ART-suppressed participants with confirmed plasma HIV RNA levels <400 copies/mL who died of nonaccidental death and had peripheral blood mononuclear cells (PBMCs) and plasma samples available within 18 months of death. Controls were matched to cases 2:1 by age, gender, duration of viral suppression, history of CMV retinitis, and nadir CD4+ cell count. All OPTIONS and SOCA participants provided written informed consent, and this research was approved by the institutional review board of the University of California, San Francisco.
Laboratory Methods
Immunophenotyping was performed on cryopreserved PBMCs as previously described [14]. Cells were thawed, washed, stained with LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Invitrogen) to exclude nonviable cells, and stained with fluorescently conjugated monoclonal antibodies: CD3-Pacific Blue, CD31-FITC, CCR7-PE-CY7, and CD28-PE-Cy5 (BD Pharmingen); CD8-QDOT605 and CD4-PE-Texas Red (Invitrogen); CD45RA-PE, (BD Bioscences); CD57-Alexa Fluor 647 (BioLegend); and CD27-APCeFluor780 (eBiosciences). For the mortality analysis in SOCA, CCR7-PE-CY7 was replaced with CCR7-Alexa Fluor 700 (BD Pharmingen), which improved CCR7 discrimination. Cells were then fixed in 0.5% formaldehyde, and data were acquired on a BD LSR II Flow cytometer (BD Biosciences), with ≥250 000 lymphocytes collected. CPT beads (BD bioscience) were used for instrument setup for each run and Rainbow beads (Spherotec) standardized instrument settings between runs. Control specimen aliquots were thawed with every run and used to confirm run-to-run reproducibility. Fluorescence Minus One (FMO) controls were also prepared on controls for each run to check that gates were set consistently between runs. Data were compensated and analyzed in FlowJo V9 (TreeStar). CD8+ T cells were defined as CD3+CD8+ cells, after standard lymphocyte, singlet and dead cell exclusion gates were applied. FMO controls were used to define positive gates for CCR7, CD27, CD28, and CD57 expression on CD8+ T cells. CD45RA expression was defined on a CD45 vs CD27 plot where the CD45RA gate was set high on CD27+ cells and according to the FMO on CD27− cells. The Boolean function in FlowJo was used to calculate the frequency of each of the 32 possible combinations of these markers. These Boolean populations were used to derive the primary populations analyzed in this study, including the %CD28−CD57+ CD8+ T cells and the proportion of CD28−CD8+ T cells expressing CD57 (%CD57+ of CD28−CD8+ T cells). Results from Boolean combinations were confirmed by gating directly visualized populations to validate the gating approach. Maturational subsets of CD28−CD8+ T cells were also derived from Boolean data and defined as transitional TTR (CD27+CCR7−CD45RA−), effector memory, TEM (CD27−CCR7−CD45RA−), and terminally differentiated effectors, TEMRA (CD27−CCR7−CD45RA+). Programmed cell death-1 (PD-1) expression on CD8+ T cells was defined in a separate staining panel [15].
Cryopreserved plasma was assessed by immunoassay for interleukin 6 (IL-6, R&D Systems), soluble CD14 (sCD14, R&D Systems), Intestinal Fatty Acid Binding Protein (I-FABP, Cell Sciences), and zonulin-1 (ALPCO) levels. Plasma tryptophan and kynurenine levels were assessed by high performance liquid chromatography-tandem mass spectrometry [16]. The extent of indoleamine 2,3-dioxygenase-1 (IDO-1) activity was assessed as the plasma kynurenine to tryptophan (K/T) ratio. Chronic asymptomatic CMV infection was confirmed as a positive CMV immunoglobulin G (IgG) titer, or for 2 individuals with previously available CMV-specific T-cell response data, >0.1% pp65/IE-specific IFN-γ+ CD8+ T-cell responses by cytokine flow cytometry (10-fold increase over limit of detection) as described elsewhere [17].
Statistical Methods
Cross-sectional pairwise comparisons between groups were performed using Wilcoxon rank-sum tests, and adjusted comparisons were assessed with linear regression. For longitudinal analyses among recently HIV-infected patients starting early vs later ART, participants were evaluated at initial diagnosis, pre-ART initiation (later ART only), at 1 year, and at their last observed time on suppressive ART (median of 3 years, Figure 1A) [13]. Longitudinal changes were assessed using linear mixed models with random intercepts. Age and proximal CD4+ T-cell count were included in multivariate analyses as potential confounders and adjusted models were restricted to men to exclude potential confounding by gender.
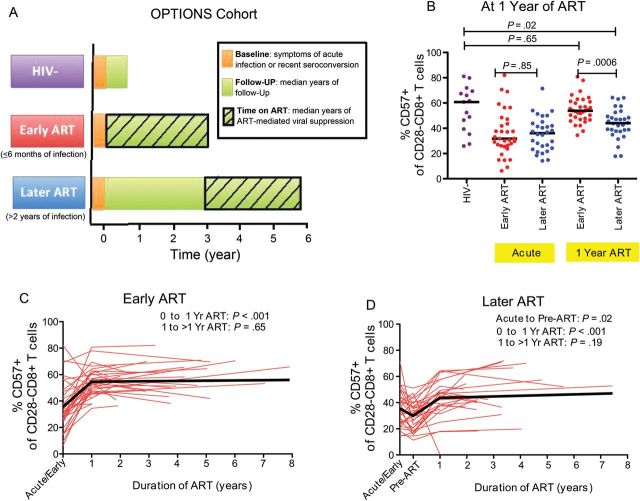
Figure 1.
Impact of early vs later initiation of ART on CD57 expression in recently HIV-infected individuals. Study design of acutely infected HIV-positive individuals and HIV-uninfected controls (A). The proportion of CD28−CD8+ T cells expressing CD57 was compared between HIV-uninfected individuals (purple) and HIV-infected individuals initiating ART “early,” <6 months of infection (red), or “later,” ≥2 years after initial infection (blue), at acute HIV diagnosis and after 1 year of ART (B). Changes in the proportion of CD28−CD8+ T cells expressing CD57 among recently HIV-infected individuals initiating ART early (C) and later (D) were also assessed over time. Individual participant trajectories shown with red lines. Linear mixed model estimated mean changes shown in thick black lines. Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus.
Predictors of mortality were assessed using conditional logistic regression among participants with available immunologic marker data; 1:1 matching was allowed if only 1 control had complete data. Potential confounding by proximal CD4+ T-cell count was evaluated in adjusted models.
Relationships between continuous variables were assessed with Spearman rank order correlation coefficients. Variables in logistic and/or linear regression models were transformed as necessary to satisfy model assumptions. A 2-tailed P value <.05 was considered significant for all analyses. Statistical analyses were performed using STATA version 12 (StataCorp).
RESULTS
Abnormally Low Proportions of CD28−CD8+ T cells Expressing CD57 Are Evident During Acute/early HIV Infection
We first assessed whether abnormally low proportions of CD28−CD8+ T cells expressing CD57 would be evident during acute/early HIV infection in the OPTIONS cohort. Participants diagnosed during acute/early infection—both those who would subsequently start ART <6 months after infection (n = 33) or ≥2 years after infection (n = 30)—were initially assessed at a median of 2.4 months after the estimated date of infection (as estimated by de-tuned enzyme-linked immunosorbent assay [13]) and compared to 15 risk-matched HIV-uninfected participants (Table 1). Most were men with a median age of 37–39 years. All participants were confirmed to be CMV-infected. The early (<6 months) and later (≥2 years) ART groups had similar median CD4+ T-cell counts upon diagnosis (533 and 567 cells/mm3, respectively), but the early ART group had a higher median plasma HIV RNA level than the later ART group (5.1 vs 4.1 log10 copies/mL). Median proportions of CD28−CD8+ T cells expressing CD57 were abnormally low at the time of diagnosis in both the early (32%) and later (35%) ART groups as compared to HIV-uninfected controls (61%, P ≤ .001 for both comparisons, Figure 1B).
Table 1.
Characteristics of HIV-uninfected Controls and Recently HIV-infected Individuals Starting Their First ART Regimen Early (<6 months) or Later (≥2 years) After Infection in the OPTIONS Cohort
| Median (IQR) | Median (IQR) | Median (IQR) | |
|---|---|---|---|
| HIV−a | HIV+ Early ART+a | HIV+ Later ART−a | |
| Characteristic | (n = 15) | (n = 33) | (n = 30) |
| Age, years | 39 (30–43) | 38 (34–42) | 37 (34–43) |
| Male gender, no., (%) | 14 (93%) | 32 (97%) | 30 (100%) |
| Duration of HIV infection at diagnosis, months | NA | 2.4 (0.8–2.4) | 2.4 (2.4–2.4) |
| CD4+ count during acute/early HIV infection, cells/mm3 | NA | 533 (434–742) | 567 (502–704) |
| HIV RNA level during acute/early infection, log10copies/ mL | NA | 5.1 (4.5–5.7) | 4.1 (3.4–4.7) |
| CD4+ count pre-ART, cells/mm3 | NA | 533 (434–742) | 339 (248–437) |
| CD4+ count after year 1 of ART, cells/mm3 | NA | 799 (614–946) | 488 (408–600) |
| Duration of HIV infection at maximal follow-up, years | NA | 2.9 (1.9–4.6) | 5.9 (4.7–7.3) |
| Duration of ART at maximal follow up, years | NA | 3.1 (1.9–4.5) | 2.6 (2.0–3.9) |
Abbreviations: ART, antiretroviral therapy; CMV, cytomegalovirus; HIV, human immunodeficiency virus; IQR, interquartile range; NA, not applicable.
a All CMV+.
Early but not Later ART Initiation Appears to Normalize the Proportion of CD28−CD8+ T cells Expressing CD57
Since recent reports suggest that immune activation markers may be significantly improved in recently HIV-infected individuals who initiate ART within the first 6 months of infection [13, 18], we hypothesized that early ART initiation might restore higher proportions of CD28−CD8+ T cells expressing CD57 than later ART initiation. In unadjusted linear mixed models, both early and later ART groups experienced substantial increases in the mean proportion of CD28−CD8+ T cells expressing CD57 from the last pre-ART specimen to year 1 of ART (early: 31% to 54%; later: 30% to 44%; P < .001 for both), but there was no evidence for further increases after the first year in either group (slopes not different from zero, P = .65 and P = .19, respectively, Figures 1C and D). After 1 year of ART, the later ART group continued to have a lower median proportion of CD28−CD8+ T cells expressing CD57 than HIV-uninfected controls (44% vs 61%, P = .02), whereas the early ART group achieved similar levels as HIV-negative controls (54% vs 61%, P = .65, Figure 1B). The early ART group continued to have a mean 10% higher proportion of CD28−CD8+ T cells expressing CD57 than the later ART group, even after a median of 3 years of ART (P = .008). This difference remained significant even after adjusting for age, proximal CD4+ T-cell count, and restricting the analysis to men (P = .007).
A Low Proportion of CD28−CD8+ T cells Expressing CD57 Predicts Increased Mortality During Suppressive ART
We next assessed the clinical implications of low proportions of CD28−CD8+ T cells expressing CD57 in a nested case-control study of 51 ART-suppressed SOCA participants who subsequently died and 90 controls matched for duration of viral suppression, age, gender, history of CMV retinitis, and nadir CD4+ T-cell count (Table 2). The median time between the specimen collection and the date of death was 5 months. Although there was no evidence for an association between the %CD28− CD8+ T cells and mortality, and only a marginal association between the %CD28−CD57+ CD8+ T cells and mortality, a low proportion of CD28−CD8+ T cells expressing CD57 was strongly associated with an increased odds of mortality. For each quartile decrease in the proportion of CD28−CD8+ T cells expressing CD57, there was a 1.77-fold increased odds of death (95% confidence interval [CI], 1.22–2.58, P = .003, Table 3), which remained significant even after adjusting for proximal CD4+ T cell count (odds ratio [OR]: 1.83, 95% CI, 1.23–2.72, P = .003). Compared to those in the highest quartile, those in the lowest quartile also had an estimated 5-fold greater odds of death (95% CI, 1.55–15.91, P = .007). To assess whether this effect was likely to be mediated primarily by an expansion of CD28−CD57−CD8+ T cells, we evaluated the prognostic significance of absolute CD28−CD57−CD8+ T-cell counts as well. The absolute CD28−CD57−CD8+ T-cell count failed to predict mortality (OR per quartile decrease, 0.78, 95% CI, .57–1.07, P = .12), suggesting that expansion of these cells per se is unlikely to mediate the observed association between the proportion of CD28−CD8+ T cells expressing CD57 and mortality. The prognostic significance of low CD57 was also greater on CD8+ T cells with greater degrees of terminal differentiation. For example, compared to participants in the highest quartile, those in the lowest quartile of %CD57+ of CD8+ TEMRA cells had a nearly 8-fold increased odds of death (95% CI, 2.2–27.8, P = .002).
Table 2.
Characteristics of ART-suppressed Cases Who Died and Matched Controls in the SOCA Cohort
| Median (IQR) |
||
|---|---|---|
| HIV+ Deathsa | HIV+ Non-Deathsa | |
| Characteristic | (n = 51) | (n = 90) |
| Age, years | 47 (41–55) | 44 (39–50) |
| Male gender, no., (%) | 42 (82%) | 75 (83%) |
| Pre-ART nadir CD4+ count, cells/mm3 | 25 (9–97) | 37 (10–74) |
| Pre-ART HIV RNA level, log10 copies/mL | 5.3 (4.9–5.7) | 5.2 (4.5–5.7) |
| Proximal CD4+ count, cells/mm3 | 283 (169–490) | 387 (245–582) |
| Proximal HIV RNA level, copies/mL | ≤400 | ≤400 |
Abbreviations: ART, antiretroviral therapy; CMV, cytomegalovirus; HIV, human immunodeficiency virus; IQR, interquartile range; SOCA, Study of the Ocular Complications of AIDS.
a All CMV+.
Table 3.
Relationship Between CD57 Expression on CD8+ T-Cell Subsets and Mortality in ART-suppressed SOCA Participants
| T-Cell Subset | Analysis | 4th Quartile | 3rd Quartile |
2nd Quartile |
1st Quartile |
OR per Quartile Decrease |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |||
| % CD28− | No. of Cases/Controls | 15/20 | 11/24 | 13/22 | 12/24 | |||||
| of CD8+ T cells | Primarya | 1.0 | 0.62 (.23, 1.65) | .34 | 0.80 (.30, 2.15) | .66 | 0.64 (.24, 1.70) | .37 | 0.90 (.66, 1.22) | .49 |
| Adjustedb | 1.0 | 0.66 (.24, 1.83) | .42 | 0.89 (.32, 2.44) | .82 | 0.85 (.31, 2.38) | .76 | 0.98 (.71, 1.36) | .90 | |
| % CD28−CD57+ | No. of Cases/Controls | 8/27 | 14/21 | 13/22 | 16/20 | |||||
| of CD8+ T cells | Primarya | 1.0 | 2.57 (.88, 7.53) | .09 | 2.33 (.75, 7.27) | .14 | 3.31 (1.05, 10.46) | .04 | 1.38 (.98, 1.94) | .07 |
| Adjustedb | 1.0 | 3.48 (1.02, 11.88) | .05 | 2.71 (.81, 9.11) | .11 | 5.80 (1.53, 21.98) | .01 | 1.56 (1.07, 2.29) | .02 | |
| % CD57+ | No. of Cases/Controls | 7/28 | 9/26 | 17/18 | 18/18 | |||||
| of CD28−CD8+ T cells | Primarya | 1.0 | 1.14 (.35, 3.58) | .82 | 4.41 (1.37,14.24) | .01 | 4.97 (1.55, 15.91) | .007 | 1.77 (1.22, 2.58) | .003 |
| Adjustedb | 1.0 | 0.98 (.30, 3.21) | .98 | 4.81 (1.36, 16.98) | .02 | 5.48 (1.59, 18.89) | .007 | 1.83 (1.23, 2.72) | .003 | |
| By T-Cell Differentiation Phenotype: | ||||||||||
| % CD57+ | No. of Cases/Controls | 13/23 | 9/26 | 14/20 | 15/21 | |||||
| of CD28−CD27+CCR7− | Primarya | 1.0 | 0.61 (.22, 1.66) | .33 | 1.22 (.48, 3.08) | .68 | 1.38 (.53, 3.60) | .51 | 1.17 (.86, 1.58) | .33 |
| CD45RA− CD8+ T cells | Adjustedb | 1.0 | 0.63 (.22, 1.78) | .38 | 1.26 (.48, 3.36) | .64 | 1.67 (.61, 4.55) | .32 | 1.23 (.89, 1.69) | .21 |
| % CD57+ | No. of Cases/Controls | 9/30 | 12/23 | 13/18 | 17/19 | |||||
| of CD28−CD27−CCR7− | Primarya | 1.0 | 1.49 (.50, 4.42) | .47 | 2.18 (.79, 6.03) | .14 | 2.89 (1.05, 7.98) | .04 | 1.43 (1.04, 1.97) | .03 |
| CD45RA− CD8+ T cells | Adjustedb | 1.0 | 1.35 (.43, 4.29) | .61 | 2.10 (.73, 6.08) | .17 | 2.99 (1.05, 8.50) | .04 | 1.45 (1.04, 2.02) | .03 |
| % CD57+ | No. of Cases/Controls | 4/31 | 11/24 | 18/17 | 18/18 | |||||
| of CD28−CD27−CCR7− | Primarya | 1.0 | 2.94 (.87, 9.94) | .08 | 7.85 (2.20, 27.97) | .001 | 7.81 (2.19, 27.77) | .002 | 1.90 (1.31, 2.75) | .001 |
| CD45RA+ CD8+ T cells | Adjustedb | 1.0 | 2.48 (.71, 8.66) | .16 | 7.66 (2.04, 28.82) | .003 | 7.85 (2.17, 28.40) | .002 | 1.94 (1.31, 2.86) | .001 |
Abbreviations: CI, confidence interval; CMV, cytomegalovirus; OR, odds ratio; SOCA, Study of the Ocular Complications of AIDS.
a Primary matched case-control analysis controlling for age, gender, duration of viral suppression, presence of CMV retinitis, and nadir CD4+ cell count.
b Analyses also adjusted for proximal CD4+ count.
Immunologic Correlates of Low %CD57+ of CD28−CD8+ T Cells During ART
Because low proportions of CD28−CD8+ T cells expressing CD57 might reflect a decreased proliferative history of these cells, we hypothesized that other known determinants of T-cell proliferative defects might be associated with this phenotype among ART-suppressed SOCA participants. Higher plasma K/T ratio, a marker of IDO-1 induction, was weakly associated with low proportions of CD28−CD8+ T cells expressing CD57 (ρ = −0.16, P = .04, Supplementary Figure 1A). Low proportions of CD28−CD8+ T cells expressing CD57 were also modestly correlated with other markers of innate immune activation including plasma sCD14 (ρ = −0.24, P = .002, Supplementary Figure 1B) and IL-6 levels (ρ = −0.17, P = .03, Supplementary Figure 1C). However, there was no evidence for a relationship between the proportion of CD28−CD8+ T cells expressing CD57 and the %PD-1+ CD8+ T cells (ρ = −0.09, P = .39), the %CD38+HLA−DR+ CD8+ T cells (ρ = 0.02, P = .82), or plasma markers of gut epithelial barrier integrity, namely, zonulin-1 (ρ = 0.03, P = .70) or I-FABP levels (ρ= −0.07, P = .39).
DISCUSSION
Our group recently demonstrated that HIV infection—unlike CMV infection—results in abnormally low proportions of CD28−CD8+ T cells expressing the proliferative history marker, CD57 [9]. The prognostic significance of this phenotype and the degree to which it is associated with later ART initiation has remained unclear. In the current study, we demonstrated that this CD8+ T-cell abnormality is evident within the first few months of HIV infection. Furthermore, we show that early ART initiation (<6 months following HIV infection) is associated with reversal of this defect, whereas delayed ART initiation (≥2 years following infection) may result in irreversibly low proportions of CD28−CD8+ T cells expressing CD57. We also demonstrated that low proportions of CD28−CD8+ T cells expressing CD57 strongly predict increased mortality among ART-suppressed HIV-infected participants with advanced disease. Low proportions of CD28−CD8+ T cells expressing CD57 were also modestly associated with several innate immune activation markers, though interestingly not with CD8+ T-cell activation or PD-1 expression, suggesting a potentially novel link between adaptive and innate immune defects in HIV infection and possible targets for interventions.
Although we did not assess phenotypic characteristics of HIV-specific CD8+ T cells in our study, our finding of abnormally low proportions of CD28−CD8+ T cells expressing CD57 during early HIV infection is consistent with earlier studies demonstrating qualitative and functional defects in HIV-specific CD8+ T cells during acute HIV infection, which may be attenuated by early administration of ART [19, 20]. Although there is expansion of intermediately differentiated and Ki-67+ effector CD8+ T cells during acute HIV infection, these cells do not necessarily successfully complete multiple rounds of proliferation and are typically not capable of maintaining control of viremia [21, 22]. Consistent with this prior work, our study demonstrates that abnormally low proportions of CD28−CD8+ T cells expressing CD57 are evident within the first few months of HIV infection, long before the onset of advanced immunodeficiency and at a time when functional HIV-specific CD8+ T-cell defects first become apparent. Although we cannot address whether this phenotypic defect directly contributes to the failure of HIV-specific T cells to control viremia, prior studies suggested that greater CD57+CD8+ T cells are associated with lower levels of viremia during early HIV infection [23]. The fact that this defect is largely reversible with early, but not delayed, initiation of ART is also consistent with recent data suggesting that monocyte and T-cell activation approach normal levels when ART is initiated early in disease but not when ART is delayed by more than a few years [13, 18]. Collectively, these data add further support to current treatment guidelines recommending the early initiation of ART during the course of HIV infection [24].
Low proportions of CD28−CD8+ T cells expressing CD57 strongly predicted mortality in ART-suppressed participants in our study. Those in the lowest quartile had a 5-fold higher odds of death than those in the highest quartile, with even stronger associations observed when evaluating the proportion of CD8+ TEMRA cells expressing CD57. A low proportion of CD28−CD8+ T cells expressing CD57 was by far the strongest T-cell phenotypic predictor of mortality in the SOCA cohort tested to date and much stronger than any other cellular marker assessed in other recently reported studies evaluating predictors of clinical events in treated HIV infection [25, 26]. This result is particularly intriguing because higher, not lower, frequencies of CD28−CD57+ CD8+ T cells predict mortality in HIV-uninfected elderly populations [27]. These disparate results suggest that there may be fundamentally distinct immunologic pathways mediating the functional T-cell defects that persist during treated HIV infection and those that characterize the aging process.
Although it remains unclear how low proportions of CD28−CD8+ T cells expressing CD57 relate to CD8+ T-cell function in HIV infection, several lines of evidence suggest that this phenotypic defect may reflect an impaired ability of effector CD8+ T cells to terminally differentiate and successfully complete multiple rounds of proliferation in vivo. CD8+ T cells at any given maturational stage that express CD57 have shorter telomere length and decreased T-cell receptor excision circle (TREC) content compared to those that lack CD57, suggesting that CD57 is a marker of proliferative history [10]. Future functional studies directly measuring T-cell proliferation (ie, by carboxyfluorescein succinimidyl ester, CFSE, or nonisotopic labeling), proliferative history (ie, telomere length), and/or vaccine responsiveness are needed to formally test this hypothesis. An inability of effector CD8+ T cells to complete multiple rounds of cell division and terminally differentiate may well have functional consequences as terminally differentiated CD8+ T cells have superior antiviral activity against HIV-1 [28].
We also observed modest associations between low proportions of CD28−CD8+ T cells expressing CD57 and markers of innate immune activation that have previously been associated with T-cell proliferative defects including plasma K/T ratio (a marker of IDO-1 induction) and sCD14 (a marker of monocyte activation). HIV induces IDO-1 in activated dendritic cells and monocytes, and the enzyme catabolizes tryptophan into kynurenine and other immunologically active metabolites that suppress T-cell proliferation and alter T-cell differentiation [11, 29]. Chronic HIV-associated microbial translocation may also suppress T-cell proliferation via increased monocyte PD-1 expression and IL-10 production [12]. Interestingly, while soluble monocyte activation markers (sCD14) were associated with lower proportions of CD28−CD8+ T cells expressing CD57, specific markers of gut epithelial barrier integrity were not, raising the possibility that drivers of monocyte activation other than the translocation of bacterial products may be contributing to this CD8+ T-cell abnormality in vivo. Targeted interventional studies may also be helpful in evaluating whether these innate immune activation pathways are causally associated with this phenotypic CD8+ T-cell abnormality. Finally, future studies evaluating the association between HIV reservoirs in lymphoid tissues and this CD8+ T-cell abnormality may be important, as this defect could conceivably be a cause or consequence of viral persistence during treated HIV disease.
Our study has limitations that deserve mention. First, our comparison of patients recently infected with HIV who initiated ART early vs later was not a randomized comparison, and we cannot exclude the possibility that individuals choosing to initiate ART earlier were healthier. However, the early ART group had comparable CD4+ T-cell counts and in fact higher baseline plasma HIV RNA levels than the later ART group, suggesting that healthier status at diagnosis is unlikely to explain the superior recovery of CD57 expression during ART in those starting early. Second, the HIV-infected participants included in the mortality analysis had very advanced AIDS prior to ART initiation. Thus, it is unclear whether the observed mortality association will be generalizable to less advanced populations of HIV-infected individuals, particularly because we demonstrated a strong impact of earlier ART initiation on reversing this abnormality. However, few cohort studies of less advanced ART-suppressed HIV-infected individuals that also stored viably cryopreserved PBMCs have sufficient numbers of participant deaths to address these questions at the present time. The relatively short period between biomarker measurement and death in our study (a median of 5 months) may also raise the possibility that low proportions of CD28−CD8+ T cells expressing CD57 are simply a consequence of a soon-to-be-fatal illness rather than a cause. This seems less likely, however, because the most common cause of death (among known causes) in our sample was cardiovascular disease (Supplementary Table 1). Finally, because this was an observational study, we cannot establish that low proportions of CD28−CD8+ T cells expressing CD57 are causally associated with mortality. It remains possible that this phenotypic abnormality is simply a marker for innate immune activation or some other immunologic process that is driving disease. Future pathway and/or mediation analyses will be important to address this possibility.
In summary, we found that although HIV leads to an expansion of CD28− CD8+ T cells, fewer of these cells express CD57. This CD8+ T-cell abnormality is evident within the first few months of HIV infection and can be largely reversed by early ART initiation, but not when ART is delayed by even a few years. Furthermore, this CD8+ T-cell defect is the strongest phenotypic T-cell predictor of mortality identified to date during treated HIV infection, suggesting a potentially important pathway to be studied and a possible target for novel interventions.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors wish to acknowledge the participation of all the study participants who contributed to this work as well as the clinical research staff of the OPTIONS and SOCA cohorts who made this research possible. The authors also wish to acknowledge the helpful scientific discussions facilitated by the Cleveland Immunopathogenesis Consortium.
Financial support. This work was supported in part by the NIAID (R56AI100765, R21 AI087035, RO1 AI087145, K24AI069994, P01AI076174), the NEI (U10 EY008057), the UCSF/Gladstone Institute of Virology and Immunology CFAR (grant P30 AI027763), the UCSF Clinical and Translational Research Institute Clinical Research Center (UL1 RR024131 and KL2TR000143), the Center for AIDS Prevention Studies (P30 MH62246), and the CFAR Network of Integrated Systems (R24 AI067039). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–55. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunt PW. HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep. 2012;9:139–47. doi: 10.1007/s11904-012-0118-8. [DOI] [PubMed] [Google Scholar]
- 3.Effros RB, Fletcher CV, Gebo K, et al. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis. 2008;47:542–53. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desai S, Landay A. Early immune senescence in HIV disease. Curr HIV/AIDS Rep. 2010;7:4–10. doi: 10.1007/s11904-009-0038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deeks SG, Verdin E, McCune JM. Immunosenescence and HIV. Curr Opin Immunol. 2012;24:501–6. doi: 10.1016/j.coi.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Dock JN, Effros RB. Role of CD8T cell replicative senescence in human aging and in HIV-mediated immunosenescence. Aging Dis. 2011;2:382–397. [PMC free article] [PubMed] [Google Scholar]
- 7.Kalayjian RC, Landay A, Pollard RB, et al. Age-related immune dysfunction in health and in human immunodeficiency virus (HIV) disease: association of age and HIV infection with naive CD8+ cell depletion, reduced expression of CD28 on CD8+ cells, and reduced thymic volumes. J Infect Dis. 2003;187:1924–33. doi: 10.1086/375372. [DOI] [PubMed] [Google Scholar]
- 8.Tassiopoulos K, Landay A, Collier AC, et al. CD28-negative CD4+ and CD8+ T-cells in ART-naive HIV-infected adults enrolled in ACTG studies. J Infect Dis. 2012;205:1730–8. doi: 10.1093/infdis/jis260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SA, Sinclair E, Hatano H, et al. Impact of HIV on CD8+ T Cell CD57 Expression Is Distinct from That of CMV and Aging. PLoS ONE. 2014;9:e89444. doi: 10.1371/journal.pone.0089444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenchley JM, Karandikar NJ, Betts MR, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–20. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 11.Boasso A, Herbeuval JP, Hardy AW, et al. HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood. 2007;109:3351–9. doi: 10.1182/blood-2006-07-034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Said EA, Dupuy FP, Trautmann L, et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat Med. 2010;16:452–9. doi: 10.1038/nm.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain V, Hartogensis W, Bacchetti P, et al. Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. J Infect Dis. 2013;208:1202–11. doi: 10.1093/infdis/jit311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan RC, Sinclair E, Landay AL, et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis. 2011;203:452–63. doi: 10.1093/infdis/jiq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatano H, Jain V, Hunt PW, et al. Cell-based measures of viral persistence are associated with immune activation and programmed cell death protein 1 (PD-1)-expressing CD4+ T cells. J Infect Dis. 2012;208:50–6. doi: 10.1093/infdis/jis630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Favre D, Mold J, Hunt PW, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2:1–12. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naeger DM, Martin JN, Sinclair E, et al. Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PLoS ONE. 2010;5:e8886. doi: 10.1371/journal.pone.0008886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burdo TH, Lentz MR, Autissier P, et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis. 2011;204:154–63. doi: 10.1093/infdis/jir214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oxenius A, Price DA, Easterbrook PJ, et al. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc Natl Acad Sci U S A. 2000;97:3382–7. doi: 10.1073/pnas.97.7.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pantaleo G, Demarest JF, Schacker T, et al. The qualitative nature of the primary immune response to HIV infection is a prognosticator of disease progression independent of the initial level of plasma viremia. Proc Natl Acad Sci U S A. 1997;94:254–8. doi: 10.1073/pnas.94.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Harthi L, MaWhinney S, Connick E, et al. Immunophenotypic alterations in acute and early HIV infection. Clinical immunology. 2007;125:299–308. doi: 10.1016/j.clim.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roos MT, van Lier RA, Hamann D, et al. Changes in the composition of circulating CD8+ T cell subsets during acute Epstein-Barr and human immunodeficiency virus infections in humans. J Infect Dis. 2000;182:451–8. doi: 10.1086/315737. [DOI] [PubMed] [Google Scholar]
- 23.Lieberman J, Trimble LA, Friedman RS, et al. Expansion of CD57 and CD62L-CD45RA+ CD8 T lymphocytes correlates with reduced viral plasma RNA after primary HIV infection. Aids. 1999;13:891. doi: 10.1097/00002030-199905280-00004. [DOI] [PubMed] [Google Scholar]
- 24.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. 2013. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf . Accessed January 2014.
- 25.Tenorio A, Zheng E, Bosch R, et al. Soluble markers of inflammation & coagulation, but not T-cell activation, predict non-AIDS-defining events during suppressive antiretroviral therapy. In the Program and Abstracts of the 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, GA: 2013. Abstract 790. [Google Scholar]
- 26.Hunt P, Rodriguez B, Shive C, et al. Gut epithelial barrier dysfunction, inflammation, and coagulation predict higher mortality during treated HIV/AIDS. In the Program and Abstracts from the 19th Conference on Retroviruses and Opportunistic Infections; Seattle, WA: Abstract 278. [Google Scholar]
- 27.Wikby A, Ferguson F, Forsey R, et al. An immune risk phenotype, cognitive impairment, and survival in very late life: impact of allostatic load in Swedish octogenarian and nonagenarian humans. J Gerontol A Biol Sci Med Sci. 2005;60:556–65. doi: 10.1093/gerona/60.5.556. [DOI] [PubMed] [Google Scholar]
- 28.Northfield JW, Loo CP, Barbour JD, et al. Human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T(EMRA) cells in early infection are linked to control of HIV-1 viremia and predict the subsequent viral load set point. J Virol. 2007;81:5759–65. doi: 10.1128/JVI.00045-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196:459–68. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.