Abstract
Tobacco dependence is the most preventable cause of death and is a chronic, relapsing disorder in which compulsive tobacco use persists despite known negative health consequences. All currently available cessation agents (nicotine, varenicline and bupropion) have limited efficacy and are associated with high relapse rates, revealing a need for more efficacious, alternative pharmacotherapies. The major alkaloid in tobacco, nicotine, activates nicotinic receptors (nAChRs) which increase brain extracellular dopamine producing nicotine reward leading to addiction. nAChRs are located primarily presynaptically and modulate synaptic activity by regulating neurotransmitter release. Subtype-selective nAChR antagonists that block reward-relevant mesocorticolimbic and nigrostriatal dopamine release induced by nicotine may offer advantages over current therapies. An innovative approach is to provide pharmacotherapies which are antagonists at nAChR subtypes mediating nicotine evoked dopamine release. In addition, providing multiple medications with a wider array of targets and mechanisms should provide more treatment options for individuals who are not responsive to the currently available pharmacotherapies. This review summarizes the currently available smoking cessation therapies and discusses emerging potential therapeutic approaches employing pharmacological agents which act as antagonists at nicotinic receptors.
Keywords: smoking cessation, nicotine, nicotinic acetylcholine receptor, pharmacotherapies
1. nAChRs and Smoking
1.1. Introduction
Tobacco dependence is the most preventable cause of death and is a chronic, relapsing disorder in which compulsive tobacco use persists despite its known negative health consequences [1–3]. Relapse typically occurs within the first month of cessation in ~80% of tobacco smokers attempting to quit, and after 6 months there is only a 3% success rate [4]. A strong correlation has been reported between tobacco smoking and mood disorders [4–7]. Clinically depressed individuals are more likely to smoke tobacco, to be nicotine dependent, and to have more difficulty with cessation and greater withdrawal symptoms [8–11]. In addition to depression, other neuropsychological diseases (e.g., schizophrenia and Tourette’s syndrome) are known to be comorbid with tobacco dependence [12–16]. This review summarizes currently available smoking cessation therapies and novel therapeutic approaches employing pharmacological agents which act at nicotinic acetylcholine receptors (nAChRs).
1.2. Diversity of nAChRs
nAChRs are members of the Cys-loop family of ligand-gated ion channel receptors, and consist of pentameric transmembrane proteins with diverse composition [17, 18]. Nicotine activates all known nAChR subtypes, with varying affinities [19–22]. Significant functional diversity is suggested by the identification of 12 genes encoding α2-α10 and β2-β4 subunits and based on results from in situ hybridization studies which reveal discrete, but overlapping, CNS distribution of mRNAs encoding these subunits [23–30]. Although nAChR subtype predominance does not necessarily reflect functional importance, the α4β2* subtype is predominant in the CNS and is probed by high affinity [3H]nicotine binding to brain membranes [31–33]. Immunoprecipitation studies indicate that more than two different subunits assemble to form functional receptors and individual neurons elaborate multiple subtypes [19, 34–38], further increasing complexity, diversity and challenges associated with the elucidation of the function of specific native nAChR subtypes. In addition to heteromeric nAChRs, homomeric nAChRs consist of α7, α8, α9 or α10 [30, 31, 39, 40]. α7* nAChRs are the second most abundant in brain [17, 30, 31], and are probed by [3H]methyllycaconitine binding to brain membranes [41–44]. The exact subunit composition, stoichiometry and arrangement of native nAChRs remains to be elucidated conclusively [45]. Nevertheless, evidence indicates that nAChR subunit composition has an important impact on pharmacological sensitivity, including agonist and antagonist affinity at the nAChR binding site [23, 46–49].
1.3. nAChRs and Neurotransmitter Release
Nicotine activation of nAChRs increases brain extracellular DA which mediates, at least in part, nicotine reward and leads to nicotine addiction [50, 51]. Mesocorticolimbic and nigrostriatal DA systems, including the nucleus accumbens (NAcc), medial prefrontal cortex (mPFC), striatum and associated circuitry, have been implicated in drug reward. The NAcc shell is believed to encode primary appetitive stimuli associated with unconditioned reward produced by nicotine [50, 52, 53]. mPFC encodes secondary conditioned stimuli associated with environmental cues paired with nicotine, and integration of motivational information from mPFC occurs in striatum leading to initiation and execution of movement in reward expectancy and detection [52, 54]. In these brain regions, nAChRs are located primarily presynaptically and modulate synaptic activity by regulating neurotransmitter release [21, 24, 39, 55–62].
Rat substantia nigra neurons express mRNA for α3, α4, α5, α6, α7, β2, β3 and β4 subunits [25, 26, 30, 63, 64] and express multiple subtypes that may be involved in nicotine-evoked striatal DA release. Studies using β2 knockout mice reveal that β2 is necessary for nicotine-evoked DA release [58, 65–70]. Subtype assignment of native nAChRs mediating nicotine-evoked DA release is based largely on inhibition of agonist-induced responses by subtype-selective antagonists, defined by their inhibitory activity in cell systems expressing nAChR subunits of known composition. A major role for α6 and β3 in nicotine-evoked DA release in striatum is based on both knockout and gain-of-function studies [71, 72]; these subunits are highly expressed in substantia nigra and ventral tegmental area (VTA; [25, 27, 64, 71, 73]. α-Conotoxin MII (α-CtxMII) inhibits nicotine-evoked [3H]DA release from striatal preparations [74–78]. Although α-CtxMII was thought to be a selective antagonist for α3-containing subtypes, the finding that 125I-α-CtxMII binding remains in α3 knockout mice, but is abolished in α6 knockouts, provides supports that α-CtxMII is a selective antagonist at α6-containing nAChRs [79– 81]. Novel α-Ctx peptides (e.g. α-CtxPIA) have higher selectivity for α6-containing over α3-containing nAChRs and inhibit nicotine-evoked [3H]DA release from rat striatum [82]. Although subtype-selective α-Ctx peptide antagonists represent useful pharmacological tools, mechanistic interpretations should be made with caution. For example, specific nAChR subtypes may display higher affinity for these α-Ctx peptides; however, one cannot rule out the possibility that these molecules also inhibit other nAChRs subtypes with lower potency, as well as other subtypes that have not yet been fully elucidated but contribute to the functional response. Further, it is unlikely these neurotoxin peptides will be developed into pharmacotherapies for tobacco cessation, in part due to their poor brain bioavailability and their susceptibility to cleavage by peptidases. Thus, these molecules will likely only be useful as pharmacologic tools.
Results from a comprehensive molecular genetics study in which an individual subunit gene (α4, α5, α7, β2, β3, and β4) has been deleted suggest that 6 different subtypes, including α-CtxMII-sensitive (α6β2β3*, α4α6β2β3*, α6β2* and α4α6β2*) and α-CtxMII-insensitive (α4β2* and α4α5β2*) subtypes, mediate nicotine-evoked DA release from mouse striatal synaptosomes, whereas deletion of β4 and α7 subunits had no effect [67, 83]. The α4α6β2β3* subtype constituted ~50% of α6-containing nAChRs on DA terminals of wild-type mice and has the highest sensitivity to nicotine of any native nAChR subtype [59, 84], strongly implicating α4α6β2β3* in nicotine-evoked DA release. Thus, different subtypes mediate nicotine-evoked DA release, suggesting that small molecule antagonists could differentially target these sites to selectively inhibit nicotine-evoked DA release and reward.
Although DA is of major interest in nicotine addiction, evidence exists that norepinephrine (NE) also plays a role [85–87]. Nicotine evokes NE release from rat hippocampal [88–92] and cortical [93, 94] synaptosomes and slices, and releases NE in hypothalamus as shown in in vivo microdialysis studies [93]. Nicotine-induced NE release modulates DA function, and thereby, may contribute indirectly to nicotine addiction [95]. α3, α4, α5, α6, α7, β2, β3 and β4 subunit mRNAs are expressed in locus coeruleus, providing potential subtype diversity in NE cell body and terminal regions [26, 27, 30, 96–98]. The nAChR subtype most associated with mediating nicotine-evoked NE release from hippocampal synaptosomes is α3β4*, based on inhibition (34%) by α-CtxAuIB, whereas α-CtxAuIB does not inhibit nicotine-evoked DA release from striatal synaptosomes [89, 99]. These same studies show that α-CtxMII does not inhibit nicotine-evoked NE release from rat hippocampus, suggesting that α6β2-containing nAChRs are not involved in this response. More recently, α-CtxBuIA (which distinguishes β2 from β4) was used to evaluate nAChRs (α6α4β2β3β4 and α6α4β2β3) contributing to nicotine-evoked NE release in mouse hippocampus; interestingly these subtypes were distinct from those mediating nicotine-evoked NE release in rat hippocampus, i.e., α3β4* and α×β4*, but not α6 [100]. These results indicate that nAChR subtypes mediating nicotine-evoked NE release include β4 in rat, whereas subtypes mediating this effect in mouse hippocampus include β2 and β4 subunits. Thus, species differences need to be taken into consideration with regards to the relative contribution of various subtypes in mediating nicotine-evoked NE release. Similarly, species differences have also been noted with respect to nicotine-evoked DA release. While α6-containing nAChRs comprise 30% of presynaptic nAChRs mediating nicotine-evoked DA release in mice, 70% of nAChRs mediating nicotine-evoked DA release are α6-containing in non-human primates [67, 77, 101, 102]. Another issue for consideration is the proportional amount of DA release mediated by α6β2-containing nAChRs differs among brain regions. For instance, while α-CtxMII only slightly diminished DA release evoked by electrical stimulation in the dorsal striatum, DA release in the nucleus accumbens was almost completely eliminated [103, 104].
1.4. Nicotine-Mediated Changes in nAChRs
Increases in nAChR expression as a function of repeated nicotine administration has been well described in the literature (for review see [105]). Nicotine-mediated increases in nAChR expression are subtype specific, with some nAChR subtypes resistant and some sensitive to up-regulation. Subtypes containing α2, α3 and α5 are not thought to be up-regulated by chronic nicotine administration [106, 107]. Repeated activation of α4-containing nAChRs results in receptor up-regulation [108, 109]. Subtypes containing β2 subunits are also up-regulated following repeated nicotine, and deletion of the β2 subunit eliminates receptor up-regulation [102]. This may be of particular importance given that β2 subunits are thought to be present in all nAChRs that mediate nicotine-evoked DA release [67, 84]. Conversely, the effect of repeated nicotine administration on α6-containing receptors is less clear, with studies showing up-regulation [110], down-regulation [111, 112] and no change [113, 114].
Additional regulatory response to repeated nicotine administration include altered subunit stoichiometry, specifically the stoichiometry of α4β2 nAChRs (α4(2)β2(3) and α4(3)β2(2)). The functional consequences of this altered stoichiometry have been shown to be variation in agonist (e.g., nicotine and acetylcholine) and antagonist (e.g., mecamylamine) sensitivities, rate of desensitization and calcium permeability [22, 115– 119]. The observation that partial deletion of the α4 and β2 subunit genes changes acetylcholine sensitivity of 86Rb+ efflux in cortex and thalamus supports the conclusion that α4β2 exists in different stoichiometries in native tissues [120]. Chronic nicotine has been shown to up-regulate the high sensitivity α4β2 isoform, and may also down-regulate midbrain α6-containing nAChRs [111]. Since the composition of nAChRs is more complex than initially thought, taken together with the observation that all subunits expressed in a subtype contribute to antagonist sensitivity, there may be opportunities to take advantage of this complexity and dynamic responsiveness to set the stage for discovery of subtype-selective nAChR antagonists, particularly antagonists targeted at the specific nAChR subtypes important for treating smoking cessation.
1.5. nAChR Desensitization
Nicotine both activates and desensitizes nAChRs [121–123]. Activation of nAChRs occurs when nicotine or endogenous acetylcholine binds at the interface of two α subunits or an α and β subunit, resulting in a conformational change that opens the channel pore and allows sodium and calcium influx [124, 125]. Desensitization is defined as a decline in response to nicotine following repeated exposure [126]. The kinetics of both receptor activation and desensitization are subtype-dependent [122, 127, 128]. For example, a recent study found that α7* nAChRs had faster activation than α4β2* containing receptors, and that nAChRs sensitive to cytisine (i.e., β4* nAChRs) had faster activation than all other subtypes [129, 130]. The β subunit has a strong influence on desensitization kinetics, with nAChRs that contain β2 subunits having a much faster rate of desensitization than nAChRs with β4 subunits [131]. α subunits have also been shown to modulate desensitization kinetics with α4 subunits associated with slowly desensitizing currents [132]. Further, inclusion of α5 subunits increases the speed with which receptors desensitize [133]. Studies using Xenopus oocytes to express different nAChR subunits found that the speed with which heteromeric nAChRs desensitize can be rank ordered from fast to slow as α3β2 > α4β2 > α3β4 > α4β4 [134].
Interestingly, nAChR desensitization, and the resulting up-regulation that occurs as a result of diminished nicotinic functional activity, is thought to play a role in tolerance and craving [102, 108, 123, 135]. A recent clinical trial found that typical tobacco use results in almost complete (88 – 95%) occupancy of α4β2* nAChRs, indicating that smokers maintain saturation of this nAChR subtype throughout the day. This study also purported that tobacco craving is only alleviated when receptor occupancy is at least 88%. Thus, when a sufficient percentage of previously desensitized nAChRs become unoccupied, and as a result recover to a responsive state, this leads to tobacco craving [136]. Several clinical studies using nicotine replacement to aide in tobacco smoking cessation support the above contention. Smokers administered a 21 mg nicotine patch 24 hr/day had significantly lower craving during the first two weeks following cessation than smokers administered a 15 mg nicotine patch for only 16 hr/day [137]. Moreover, smokers administered a 35 mg nicotine patch 24 hr/day exhibited reductions in both withdrawal symptoms and craving and a significantly reduced risk of lapse [138]. Since the patch provides a constant supply of nicotine to an individual, it is highly likely that these results are mediated, at least in part, through continuous nAChR desensitization. Thus, repeated nicotine administration may act as a functional antagonist by inactivating nAChRs [105, 139], implying that antagonist-induced inactivation of nAChRs has therapeutic potential for smoking cessation since the functional outcome is the same as agonist-induced desensitization.
2. nAChR Partial Agonists as Smoking Cessation Treatments
2.1 Introduction
Currently available cessation agents have been shown to have limited efficacy and are associated with high relapse rates [4, 140–142], revealing a need for alternative more efficacious pharmacotherapies. In targeting nAChRs mediating nicotine-evoked DA and NE release for medication development, either a subtype-selective agonists or antagonists can be developed. Each strategy has potential advantages and limitations. nAChR agonists (partial or full) are generally well-tolerated and produce good patient compliance, as they substitute for the reinforcing effect of tobacco use [4, 140–142]. Partial and full agonists at nAChRs also provide relief from withdrawal symptoms that typify abstinence [143]. While nicotine replacement therapy has been the mainstay of smoking cessation therapeutics, several new and potential smoking cessation therapeutics are nAChR partial agonists. Partial agonists mimic nicotine replacement therapy by alleviating withdrawal symptoms and craving resulting from smoking cessation, while simultaneously reducing both nicotine reinforcement and the repetitive nicotine-induced phasic DA release mediated by nAChRs [144, 145]. Compared with the full agonist, partial agonists may have lower abuse liability due to the less than maximal response with respect to neurotransmitter release [146]. However, the beneficial effects of agonist replacement therapy are less than optimal [147]. A potential disadvantage of nAChR agonist replacement therapy is that continued stimulation of nAChRs maintains the dependence induced by tobacco use. Thus, in the event of a relapse to tobacco use following nicotine replacement therapy, the reinforcing effect of nicotine self-administration may be reinstated rapidly.
2.2 Varenicline
Originally developed by Pfizer, Inc. in 1997 [148], varenicline (Chantix) is structurally to the plant alkaloid cytisine (discussed below), and one of only three smoking cessation therapeutics currently approved by the United States Food and Drug Administration (FDA). Initial in vivo binding studies found that varenicline has high affinity for the α4β2 nAChR subtype with little affinity for other subtypes [148]. Further, in rat brain slices, varenicline was found to release lower concentrations of DA release (40–60% of that released by nicotine) [149]. Collectively, these findings suggested that varenicline is a partial agonist at α4β2* nAChRs [148]. However, studies report that varenicline also is as a full agonist at α7 nAChRs expressed in cell systems [150]. In humans, maximal absorption of varenicline occurs within 3–4 hr of oral administration, and the drug has an elimination half-life of ~24 hr [151], primarily through renal excretion [152]. Further, steady-state conditions are established within 4 days of oral administration in healthy adults [151].
Varenicline fully substitutes for nicotine in preclinical drug discrimination studies and blocks nicotine self-administration in rats [149]. According to several recent reviews that summarize the results from Phase 2 and 3 clinical trials, varenicline generally increases the chances of a successful quit attempt 2- to 3-fold greater than placebo [153, 154]. A recent multicenter, randomized, double-blind placebo-controlled study found continuous abstinence rates of 44% for varenicline during 9–12 weeks after quitting, which was higher than the abstinence rates for patients treated with bupropion (30%) or placebo (18%) [155]. Further, while the majority of patients, regardless of therapeutic intervention, returned to a regular pattern of smoking during the 9–52 wk follow-up period, abstinence rates among patients that received varenicline (22%) were still higher than those for patients receiving bupropion (16%) or placebo (8%; [155]). In February, 2008, a public health advisory note was issued by the FDA, stating that patients taking varenicline experience serious neuropsychiatric symptoms, including behavior, agitation, depressed mood, suicidal ideation, and attempted and completed suicide [156]. However, the number of patients experiencing these behavioral changes are small, and an analysis of neuropsychiatric adverse events in the patients that participated in all nine completed, placebo-controlled clinical trials is currently being performed [145, 155]. Results from these analyses will be important for evaluating the continued used of varenicline as a smoking cessation therapeutic.
2.3 Cytisine
As was discussed above, varenicline is a structural analog of cytisine, an alkaloid present in several plant species, including Cytisus laburnum [148]. Cytisine was originally characterized as a selective, partial agonist with high affinity for α4β2* nAChRs [157]. In support of this, cytisine has been found to evoke [3H]DA release from both striatal slices and synaptosomes in vitro, with a maximum effect ~50% of that found for nicotine [158, 159]. The partial agonist properties of cytisine have also been demonstrated in vivo, where it increases the rate of DA turnover in the nucleus accumbens with a maximum effect ~40% of that found for nicotine [148]. However, similar to varenicline, cytisine interacts with additional nAChR subtypes, including α4β4-and for α6-containing subtypes [160, 161]. Further, the efficacy of cytisine appears to vary depending on the species and experimental system used [162].
Behaviorally, cytisine decreases locomotor activity in drug naïve rats, with a maximum effect lower than that produced by nicotine [163]. Cytisine had no effect on locomotor activity in rats that received repeated nicotine administration (0.4 mg/kg/day for 21 days) [163], indicating the development of cross-tolerance. In drug discrimination studies, cytisine substituted for nicotine [164]. In a limited number of studies, cytisine has been shown to have reinforcing properties. Drug naïve mice self-administer cytisine intravenously [165], providing evidence for its reinforcing properties. In other studies, cytisine has been shown to produce conditioned place preference in rats [166]. A limited number of clinical trials assessing the usefulness of cytisine in smoking cessation have been reported. A recent uncontrolled clinical trial reported smoking cessation efficacy rates (~14%) for cytisine comparable with that obtained with nicotine replacement therapy [167].
2.4. Dianicline
Another partial agonist currently in development by Sanofi Aventis, Inc. for use as a smoking cessation therapeutic is dianicline. Dianicline (SSR591813) is similar in structure to both varenicline and cytisine, although mechanistic information is somewhat limited [168]. Dianicline has a high affinity for human α4β2 nAChRs and a low affinity for other nAChR subtypes expressed in Xenopus oocytes, HEK 293 cells and IMR-32 cells [168]. In electrophysiology studies, dianicline exhibited an Emax of 19% of the response to acetylcholine, indicating its action as a partial agonist [168]. In light of the recent studies demonstrating that varenicline and cytisine are not selective for α4β2* nAChRs, it is likely that dianicline will also lack selectivity for α4β2* due to its structural similarity with these two drugs. In drug discrimination studies, dianicline substituted for nicotine, although at doses that decreased the rate of responding [168]. In clinical trials, dianicline had a 16% success rate compared to 8% for placebo [169], however, in February, 2008, Sanofi Aventis announced that the development of dianicline was terminated [170].
2.5. Sazetidine-A
Sazetidine-A is a novel nAChR ligand reported to have high affinity and selectivity for α4β2 nAChRs [171, 172]. Affinity for α4β2 was 4-orders of magnitude higher than for α3β4 nAChR subtypes expressed in cell systems [171, 172], and prolonged incubation with this novel compound increased the density of α4β2 binding sites [171]. However, initial studies determined that sazetidine-A did not stimulate 86Rb+ efflux from cells stably expressing α4β2 nAChRs, suggesting that this compound may not activate nAChRs [171]. Interestingly, preincubation with sazetidine-A reduced nicotine-evoked 86Rb+ efflux that was not readily reversible [171], indicating that receptor desensitization had occurred. Thus, while sazetidine-A did not activate α4β2* nAChRs in this cell expression system, these receptors were up-regulated and desensitized. This mechanism of action has been termed “silent desensitization”, since desensitization occurs without receptor activation. Interestingly, co-incubation of sazetidine-A with nicotine did not result in inhibition of 86Rb+ efflux, whereas preincubation with sazetidine-A prior to incubation with nicotine inhibited completely the effect of nicotine. These results have been interpreted to indicate that sazetidine-A has low affinity for α4β2* nAChRs in the resting state, but high affinity in the desensitized state. Further, the results suggest sazetidine-A binds with high affinity to nAChRs that spontaneously convert to the desensitized state, trapping the receptors in this desensitized state and resulting in inhibition of channel function [171, 139].
Recent studies using more sensitive voltage clamp techniques reported that sazetidine-A induces current amplitudes in cells expressing α4β2 nAChRs similar to amplitudes induced by acetylcholine [172], suggesting that this novel nAChR ligand acts as a agonist. In this study, sazetidine-A was also found to potently evoke [3H]DA release from rat striatal slices and produced an Emax about 90% of that produced by nicotine. Sazetidine-A-evoked [3H]DA release was inhibited completely by both mecamylamine and dihydro-β-erythroidine (DHβE; [172]), indicating that the response is nAChR mediated. Further, the α6 nAChR selective antagonist α-CtxMII also inhibited sazetidine-A evoked [3H]DA release with an Imax of 50%. Collectively, these findings suggest that both α4β2* and α6* nAChRs mediate sazetidine-A evoked [3H]DA release. At 1000-fold higher concentrations, sazetidine-A also evoked [3H]NE release from rat hippocampal slices, with an Emax of ~50% of the maximal response to nicotine, suggesting a partial agonist action. Similarly, the response to sazetidine-A was inhibited completely by both mecamylamine and DHβE [172]. Furthermore, sazetidine-A substitutes completely for nicotine in the drug discrimination assay [173], also consistent with a nAChR agonist action. Thus, in contrast to the initial results obtained using the 86Rb+ efflux assay, sazetidine-A acts as a partial agonist at nAChRs in hippocampous and as a full agonist at nAChRs in striatum. Ass such, sazetidine-A may have utility as a smoking cessation agent similar to other nAChR agonists.
3. nAChR Antagonists as Smoking Cessation Treatments
3.1. Introduction
Given the availability of replacement therapies using either full or partial agonists or therapies that indirectly stimulate reward-relevant DA receptors, an innovative alternative approach is to provide pharmacotherapies which are antagonists at nAChR subtypes mediating neurotransmitter release associated with the reward produced by tobacco smoking. In this regard, subtype-selective nAChR antagonists that block reward-relevant mesolimbic DA release induced by nicotine may offer an advantage. Blockade of nAChRs mediating nicotine-evoked NE release may also be a viable target for preventing relapse, as NE neurotransmitter systems are implicated in nicotine seeking [175, 176]. Since relapse rates are high among smokers primarily due to the environmental cues that surround the experience of tobacco use [177, 178], it may be inevitable that many tobacco users will lapse (e.g., smoke one cigarette) during a quit attempt. In this regard, it may be advantageous to use a medication that effectively blocks the pleasurable effect of the initial lapse, thus discouraging a full-scale relapse back to a pattern of regular smoking. In addition, providing multiple medications with a wider array of mechanistic targets should enhance the clinical utility among individuals who are not responsive to the currently available agonist pharmacotherapies. For example, mecamylamine has been shown to have clinical efficacy in double blind, placebo controlled studies [3, 179, 180], but its nonselective inhibition of peripheral receptors has produced untoward peripheral side effects that have precluded its clinical development. The significance of this alternative approach is that it will lead to the development of subtype selective antagonists, which retain and/or enhance the efficacy of mecamylamine, while exhibiting reduced and/or negligible peripheral side effects, thus breaking the impasse for developing a clinically-useful nAChR antagonist.
3.2. Mecamylamine
Mecamylamine dose-dependently decreases nicotine self-administration, a behavioral task used to measure reward associated with drug administration in laboratory animals [181–189]. Pretreatment with mecamylamine also blocks performance in a progressive ratio (PR) model of nicotine self-administration [190]. Furthermore, cue-induced reinstatement of nicotine-seeking behavior, whereby re-introduction of an environmental cue associated with nicotine delivery reinstates extinguished nicotine seeking, is also blocked in rats by pretreatment with mecamylamine [191, 192].
Building upon the findings in the preclinical literature, several clinical studies have investigated the therapeutic potential of mecamylamine in regards to tobacco use cessation [193]. Mecamylamine, a noncompetitive antagonist at all known central and peripheral nAChRs, reverses both positive and negative subjective effects of intravenous nicotine in smokers [194]. Mecamylamine alone was reported also to be beneficial in reducing smoking satisfaction [195]. In a randomized, double-blind placebo-controlled study, mecamylamine combined with a nicotine transdermal patch was shown to improve smoking cessation outcome for up to one year compared to nicotine alone [180]. Since mecamylamine is an open channel blocker, these results suggest that the presence of the agonist augments access of mecamylamine to its binding site within the receptor channel pore. Due to the lack of selectivity at nAChRs including inhibition of peripheral nAChRs, the clinical utility of mecamylamine is limited by its anticholinergic side effects (e.g., constipation, hypotension; [196]). These studies provide precedence for the use of nAChR antagonists as tobacco use cessation agents. A selective drug, which is targeted at central nAChRs that specifically mediate nicotine-evoked DA release, would be predicted to retain the beneficial therapeutic effects of a nAChR antagonist while averting the peripherally-mediated side effects. Such an antagonist treatment may be especially useful for highly motivated individuals attempting to quit tobacco use.
3.3. Bupropion
The antidepressant, bupropion, has demonstrated benefit as a tobacco use cessation agent [141, 142, 175, 197–201]. In addition to its antidepressant activity, which presumably derives from its ability to inhibit DA and NE transport into the presynaptic nerve terminal, bupropion is an effective and well-tolerated tobacco use cessation agent [199, 202]. Similarly, reboxetine, a selective NE transporter (NET) inhibitor and effective antidepressant [203–205], decreases nicotine self-administration in rats [87] and inhibits nicotine-evoked [3H]NE release from superfused brain slices [206]. Taken together with the findings from the above studies with mecamylamine, these results provide rationale for determining if antagonists that selectively inhibit central nAChRs mediating nicotine-evoked DA and/or NE release will decrease nicotine self-administration and relapse.
Bupropion inhibits the function of both the DA transporter (DAT) and the NET, which likely contributes to its efficacy as a tobacco use cessation agent. Bupropion inhibits [3H]DA uptake (IC50=2 µM) into striatal synaptosomes and [3H]NE uptake (IC50=5 µM) into hypothalamic synaptosomes [3, 179, 180, 207, 208]. Increased extracellular DA and NE concentrations may substitute for nicotine-evoked neurotransmitter release as a result of tobacco smoking. However, nicotine reinforcement has been associated primarily with increased DA release [3, 50, 51, 179, 180, 209]. Bupropion dose-dependently increases presynaptic vesicular DA uptake and redistributes vesicular monoamine transporter protein [210]. Important from the current perspective, bupropion acts as a nAChR antagonist, inhibiting (IC50=11 µM) nAChR agonist-induced 86Rb+ efflux from cells expressing α3β4 ganglionic nAChRs, from human clonal cells expressing muscle-type nAChRs (IC50=1.5 µM; [211], and from Xenopus oocytes expressing rat α3β2 (IC50=1.3 µM), α4β2 (IC50=8 µM) and α7 (IC50=60 µM) nAChRs [212]. 86Rb+ efflux models K+ efflux and is a functional assay for nAChRs [213–218]. Bupropion inhibition of nAChR function is not surmounted by increasing agonist concentration, and bupropion does not displace [3H]nicotine binding to native nAChRs, consistent with allosteric inhibition [211, 212]. Bupropion metabolites also inhibit nAChR function [219]. We evaluated the ability of bupropion to specifically inhibit native nAChRs mediating nicotine-evoked [3H]DA and [3H]NE release from rat striatal and hippocampal slices [220]. Bupropion inhibited nicotine-evoked [3H]DA and [3H]NE release (IC50=1.3 and 0.32 µM, respectively), indicating that bupropion acts as a nAChR antagonist at subtypes mediating this response. DAT and NET were not mediating the bupropion-induced inhibition of nicotine-evoked neurotransmitter release since the superfusion buffer included saturating concentrations of nomifensine or desipramine. Bupropion concentrations that inhibit DAT and NET did not inhibit field stimulation-evoked [3H]DA release, suggesting mediation by nAChRs. Bupropion-induced decreases in smoking may result from one or both mechanisms (i.e., nAChR antagonism and DAT/NET inhibition), both of which may contribute to its smoking cessation and antidepressant efficacy.
3.4. UCI-30002
Another novel antagonist currently being examined for potential use as a smoking cessation therapeutic is UCI-30002 [221]. UCI-30002 is a positive allosteric modulator of GABAA receptors [222]; however, since GABAA and nAChRs are both members of the ligand-gated ion channel superfamily, it was hypothesized that this novel compound may have efficacy as an allosteric modulator at α4β2* nAChRs [221]. UCI-30002 inhibited nicotine-evoked currents in Xenopus oocytes expressing neuronal α4β2, α7 and α3β4 nAChR subtypes [221]. Further, UCI-30002 also inhibited nicotine-evoked currents in oocytes expressing muscle-type nAChRs. Thus, UCI-30002 may act as a negative allosteric modulator of nAChRs similar to its action at GABAA receptors [221], although additional evidence is needed to support this mechanism of action.
With regards to behavioral effects, UCI-30002 inhibited high-dose, nicotine-induced seizures [221]. More importantly, UCI-30002 decreased nicotine self-administration on both a fixed ratio 5 (FR5) and a PR schedule [221], suggesting that this compound decreases nicotine reward. Further, UCI-30002 did not alter food-maintained responding, indicating that it specifically decreased responding for nicotine [221]. While additional studies are needed, these results are promising and suggest that UCI-30002 may have potential as smoking cessation therapeutic. Thus, negative allosteric modulators of nAChRs may constitute an unexplored target for the development of novel therapeutics to treat nicotine addiction.
3.5 bPiDDB and r-bPiDDB
Development of antagonists selective for nAChRs mediating nicotine-evoked DA and NE release should retain therapeutic efficacy as smoking cessation agents without producing peripheral side effects. Unfortunately, no compounds are available for clinical use that have this profile. In this regard, N-n-alkylnicotinium analogs with C7–C12 N-nalkyl groups potently inhibit nicotine-evoked [3H]DA release from rat striatal slices in an orthosteric manner and inhibit high affinity [3H]nicotine binding to rat brain membranes [223, 224]. Structurally-related N-n-pyridinium analogs with C10–C20 N-n-alkyl groups were also potent inhibitors of nicotine-evoked [3H]DA release, and longer chain analogs (C15 and C20) showed incomplete maximal inhibition (Imax=50%), supporting the involvement of more than one nAChR subtype in this effect of nicotine [225]. These pyridinium analogs had little affinity for the [3H]nicotine binding site [225], indicating enhanced selectivity for nAChRs mediating nicotine-evoked DA release.
The related bis-quaternary ammonium compounds, hexamethonium and decamethonium, which are considered to be simplified analogs of d-tubocurarine, have been used to differentiate muscle and ganglionic nAChRs [226–228]. The bis-quaternary ammonium structural framework was utilized to enhance nAChR subtype selectivity and afford a new class of N,N’-alkane-diyl-bis-3-picolinium (bAPi) analogs [229–231]. These polar and charged analogs were predicted to have poor brain bioavailability following systemic administration. However, previous work with structurally-related polar mono-nicotinium analogs revealed good affinity for the blood-brain barrier (BBB) choline transporter and active transport into brain [232]. A lead analog, N,N’-dodecane-1,12-diyl-bis-3-picolinium dibromide (C12, bPiDDB), was evaluated for inhibition of nicotine-evoked DA release and for their ability to inhibit the discriminative stimulus and/or locomotor stimulant centrally-mediated effects of nicotine. bPiDDB exhibited little affinity for α4β2* and α7* high affinity ligand binding sites, nor for nAChRs modulating DA transporter function, but potently inhibited nicotine-evoked [3H]DA release (IC50=2 nM; Imax=64%; [231]), bPiDDB did not inhibit electrically-evoked [3H]DA release, suggesting specific nAChR inhibitory effects and a lack of toxicity to DA neurons. Schild analysis suggested that bPiDDB interacts in an orthosteric manner at nAChRs mediating nicotine-evoked [3H]DA release. To determine if bPiDDB interacted with α-CtxMII-sensitive α6β2-containing nAChRs, slices were exposed concomitantly to maximally-effective concentrations of bPiDDB (10 nM) and α-CtxMII (1 nM). Inhibition of nicotine-evoked [3H]DA release was not different with the combination compared with either antagonist alone, suggesting that bPiDDB interacts with α6β2-containing nAChRs. These results support the interpretation that similar to α-CtxMII, the lead analog bPiDDB is a selective high potency antagonist at a subset of nAChR subtypes containing α6 and β2, and likely inhibits α6β2*, α6β2β3*, α4α6β2* and/or α4α6β2β3*. Furthermore, bPiDDB exhibited high affinity for the blood-brain barrier choline transporter in vivo and [14C]bPiDDB was a substrate for the choline transporter [233], suggesting brain bioavailability. In microdialysis studies using rats, bPiDDB decreased extracellular DA levels in nucleus accumbens following systemic nicotine [234] and decreased intravenous nicotine self-administration, but not sucrose maintained responding [235]. Surprisingly, in contrast to mecamylamine and DHβE, bPiDDB did not block the discriminative stimulus effect of nicotine [231]. Since mecamylamine and DHBE block the full complement of β2-containing nAChRs mediating nicotine-evoked DA release, whereas bPiDDB blocks only a subset of β2-containing nAChRs (i.e., those also containing α6) and only partially inhibits nicotine-evoked [3H]DA release, these results suggest that inhibition of all β2-containing nAChRs and/or complete inhibition of nicotine-evoked DA release may be required to block the nicotine cue.
In contrast to the discriminative stimulus effect of nicotine, DA systems are critically involved in mediating the locomotor stimulant effect of nicotine. Locomotor sensitization produced by repeated nicotine administration is also associated with increased nicotine-evoked DA release in NAcc [236, 237]. Both mecamylamine and DHβE block the nicotine-induced hyperactivity in nicotine-sensitized rats. Similarly, bPiDDB decreased nicotine-induced hyperactivity [231]. Since bPiDDB did not reduce locomotor activity when co-administered with saline in the nicotine-sensitized rats, the bPiDDB-induced decrease in nicotine-induced hyperactivity was not likely due to nonspecific motor impairment. The bPiDDB-induced decrease in nicotine-induced hyperactivity likely reflects a specific blockade of nAChRs mediating the nicotine-evoked DA release. Future experiments should determine whether bPiDDB would be useful for preventing cue-dependent relapse to tobacco smoking in animal models and clinical populations.
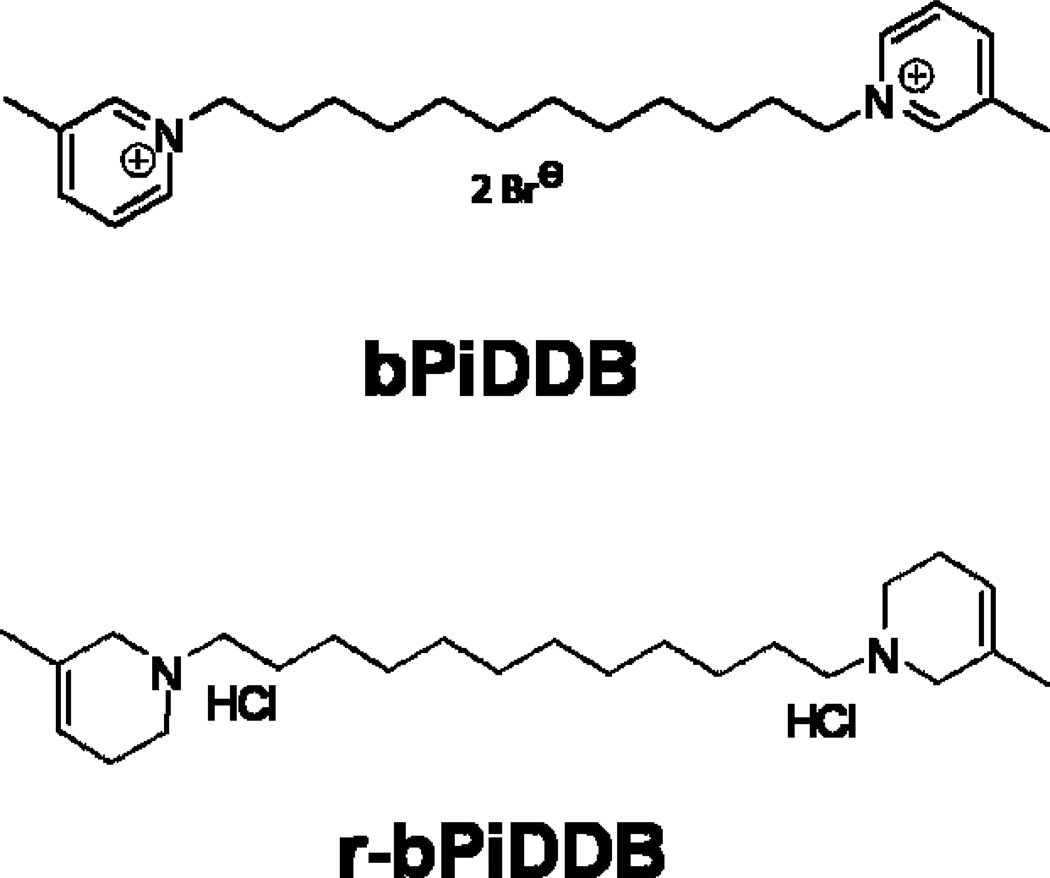
The bPiDDB molecule is able to access brain by the blood-brain barrier choline transporter even though it is a bis-quaternary ammonium with insignificant lipophilic character, and thus, is unable to permeate cell membranes by passive diffusion. Pharmacokinetic analysis in the rat indicates that bPiDDB has good brain bioavailability when administered via the subcutaneous route and reaches behaviorally-relevant concentrations in brain with no indication of toxicity [238]. Nevertheless, when given by the oral route, bPiDDB has poor plasma and brain bioavailability, and thus, may be categorized as a poorly drugable molecule. As part of a structural optimization program to improve the drugability of bis-quaternary ammonium analogs that act as subtype-selective nAChR antagonists, we identified a structural analog of bPiDDB in which the two quaternary ammonium groupings (3-picolinium headgroups) were converted into tertiary amino groupings (3-methyl-1,2,5,6-tetrahydropyridines) through a simple chemical reduction procedure, affording r-bPiDDB, a highly lipophilic molecule with greatly improved drugability.
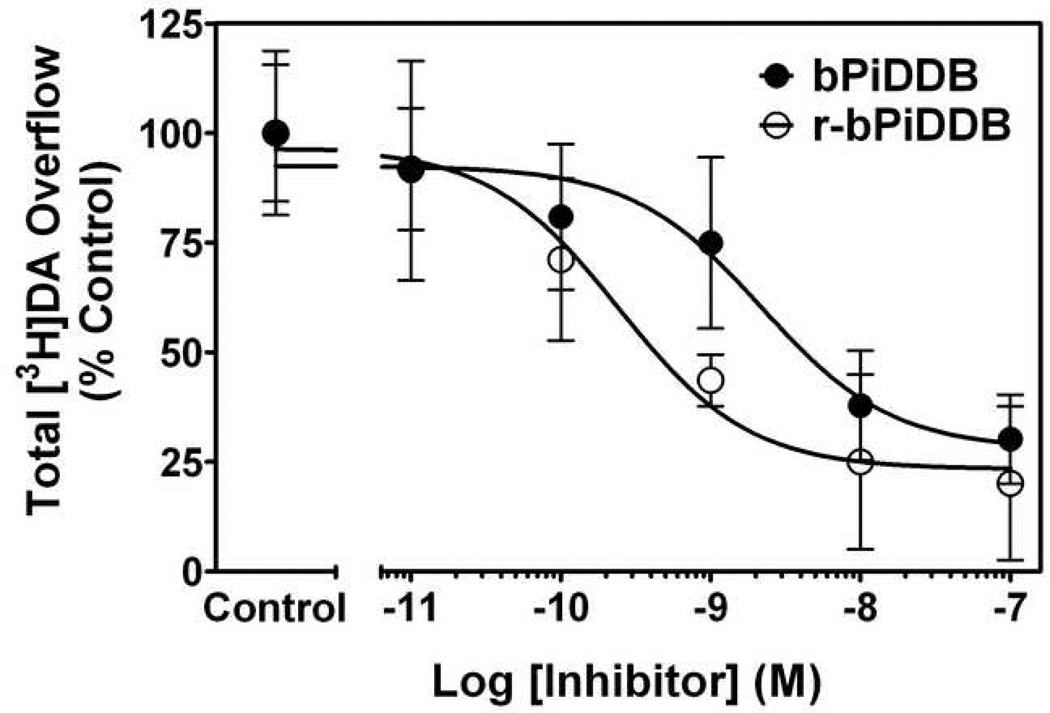
r-bPiDDB has physicochemical properties that predict good bioavailability by the oral route and it is a potent inhibitor of nicotine-evoked [3H]DA release from superfused rat striatal slices (IC50 = 0.29 nM, Imax = 74%), and thus, had 10-fold greater potency than bPiDDB (Fig. 2). These results suggest that the two quaternary ammonium head groups in the bPiDDB molecule may not be a structural requirement for nAChR antagonism and are replaceable with more lipophilic, non-quaternary tertiary amino headgroups that could be protonated at physiological pH, allowing more efficient partitioning through biological membranes
Fig. 2. Concentration dependence of bPiDDB and r-bPiDDB inhibition of nicotine-evoked [3H]DA overflow from superfused rat striatal slices.
Superfusion buffer contained nomifensine (10 µM) and pargyline (10 µM) throughout the experiment. Striatal slices were superfused in the absence (control) or presence of bPiDDB or r-bPiDDB for 36 min and then for an additional 36 min with nicotine (10 µM) added to the buffer. Control represents [3H]DA overflow in response to nicotine (total [3H]DA overflow as a percentage of tissue-3H content for; mean ± S.E.M.). The concentration response curves were generated by nonlinear regression. Data are expressed as percentage of control; n = 6 rats/group.
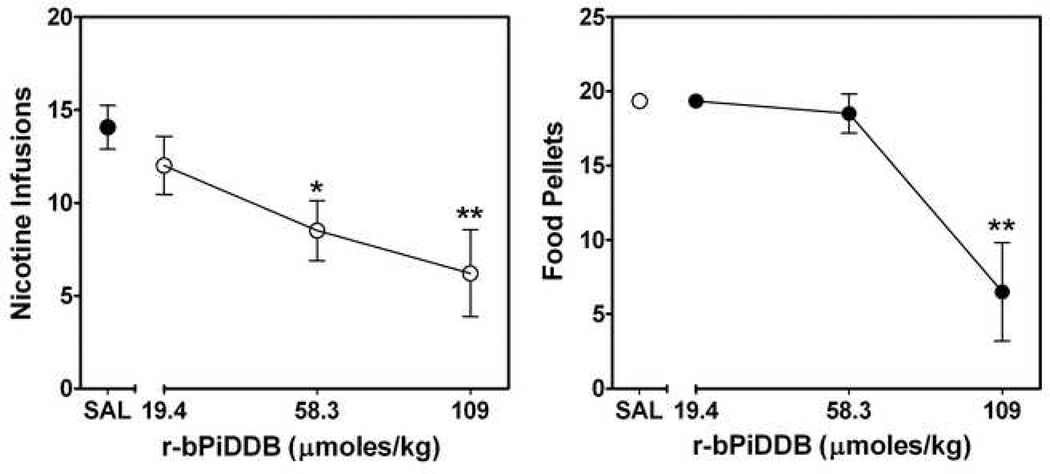
Similar to bPiDDB, which was effective in decreasing nicotine self-administration in the rat [235] r-bPiDDB, over a broad dose range, also exhibited effectiveness in the nicotine self-administration model (Fig. 3). Importantly, some specificity of effect was obtained, as a dose of r-bPiDDB (58.3 µmoles/kg) that significantly decreased nicotine self-administration did not alter responding for food reinforcement (Fig. 3). Further, the ability of r-bPiDDB to decrease nicotine self-administration was retained without any loss of effect following 7 repeated daily treatments (data not shown). Additional structurally-related bis-tertiary amino analogs are being evaluated to optimize the specificity of effect on nicotine self-administration.
Fig. 3. Nicotine self-administration (left) and food-maintained responding (right) in rats following acute treatment with varying doses of r-bPiDDB.
Results are expressed as the number of nicotine infusions or sucrose pellets earned (mean ± S.E.M.) during a 60-min operant conditioning session. n = 4–10/ dose. Nicotine self-administration was decreased dose-dependently by r-bPiDDB [F3,24=5.17, p<0.01]. r-bPiDDB did not decrease food-maintained responding significantly, except for the highest dose of r-bPiDDB [F3,24=5.17, p<0.01]. * represents a significant difference compared to saline (SAL) control group, *p < 0.05, **p < 0.01.
Taken together, these findings suggest that the two quaternary ammonium head groups in the bis-quaternary ammonium series of analogs are not a structural requirement for nAChR antagonism, and that neurochemical and behavioral activity can be retained and improved by simple conversion of the quaternary ammonium headgroups in bPiDDB to their chemically reduced bis-tertiary amino equivalents, as in r-bPiDDB. The two tertiary amino groups in r-bPiDDB can be predominantly protonated at physiological pH, since r-bPiDDB is predicted to have pKa values in the range 9–9.5, providing cationic moieties at physiological pH that may interact with the nAChR binding site in a similar manner to the azaaromatic quaternary ammonium headgroups in the bPiDDB molecule. Thus, this approach may result in better lead candidates for drug development, since the reduced bis-tertiary amino equivalent analogs likely will be potent, behaviorally-active and orally bioavailable due to their physicochemical properties.
Fig. 1. The structures of N,N-dodecane-1,12-diyl-bis-picolinium dibromide (bPiDDB; top) and reduced-bPiDDB (r-bPiDDB; bottom).
ACKNOWLEDGMENTS
The research reported in this review was supported by NIH grant U19 DA17548, T32 DA007304 and F31 DA023853.
The University of Kentucky holds patents on bPiDDB and r-bPiDDB. A potential royalty stream to L.P.D. and P.A.C. may occur consistent with University of Kentucky policy.
Nonstandard Abbreviations
- bPiDDB
N,N’-dodecane-1,12-diyl-bis-3-picolinium dibromide
- r- bPiDDB
reduced-bPiDDB
* indicates putative nAChR subtype assignment
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.George TP, O'Malley SS. Current pharmacological treatments for nicotine dependence. Trends Pharmacol Sci. 2004;25:42–48. doi: 10.1016/j.tips.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Le Foll B, Goldberg SR. Effects of nicotine in experimental animals and humans: an update on addictive properties. Handb Exp Pharmacol. 2009:335–367. doi: 10.1007/978-3-540-69248-5_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rose JE. Disrupting nicotine reinforcement: from cigarette to brain. Ann NY Acad Sci. 2008;1141:233–256. doi: 10.1196/annals.1441.019. [DOI] [PubMed] [Google Scholar]
- 4.Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Ann Rev Pharmacol Toxicol. 2009;49:57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glassman AH, Helzer JE, Covey LS, Cottler LB, Stetner F, Tipp JE, et al. Smoking, smoking cessation, and major depression. JAMA. 1990;264:1546–1549. [PubMed] [Google Scholar]
- 6.Pomerleau CS, Marks JL, Pomerleau OF. Who gets what symptom? Effects of psychiatric cofactors and nicotine dependence on patterns of smoking withdrawal symptomatology. Nicotine Tob Res. 2000;2:275–280. doi: 10.1080/14622200050147547. [DOI] [PubMed] [Google Scholar]
- 7.Pomerleau OF, Burmeister M, Madden P, Long JC, Swan GE, Kardia SL. Genetic research on complex behaviors: an examination of attempts to identify genes for smoking. Nicotine Tob Res. 2007;9:883–901. doi: 10.1080/14622200701485125. [DOI] [PubMed] [Google Scholar]
- 8.Berlin I, Covey LS. Pre-cessation depressive mood predicts failure to quit smoking: the role of coping and personality traits. Addiction. 2006;101:1814–1821. doi: 10.1111/j.1360-0443.2006.01616.x. [DOI] [PubMed] [Google Scholar]
- 9.Covey LS. Tobacco cessation among patients with depression. Prim Care. 1999;26:691–706. doi: 10.1016/s0095-4543(05)70124-x. [DOI] [PubMed] [Google Scholar]
- 10.Covey LS, Glassman AH, Jiang H, Fried J, Masmela J, LoDuca C, et al. A randomized trial of bupropion and/or nicotine gum as maintenance treatment for preventing smoking relapse. Addiction. 2007;102:1292–1302. doi: 10.1111/j.1360-0443.2007.01887.x. [DOI] [PubMed] [Google Scholar]
- 11.Covey LS, Glassman AH, Stetner F. Major depression following smoking cessation. Am J Psychiatry. 1997;154:263–265. doi: 10.1176/ajp.154.2.263. [DOI] [PubMed] [Google Scholar]
- 12.Dursun SM, Kutcher S. Smoking, nicotine and psychiatric disorders: evidence for therapeutic role, controversies and implications for future research. Med Hypotheses. 1999;52:101–109. doi: 10.1054/mehy.1997.0623. [DOI] [PubMed] [Google Scholar]
- 13.Fagerstrom K, Aubin HJ. Management of smoking cessation in patients with psychiatric disorders. Curr Med Res Opin. 2009;25:511–518. doi: 10.1185/03007990802707568. [DOI] [PubMed] [Google Scholar]
- 14.McEvoy JP, Allen TB. The importance of nicotinic acetylcholine receptors in schizophrenia, bipolar disorder and Tourette's syndrome. Curr Drug Targets CNS Neurol Disord. 2002;1:433–442. doi: 10.2174/1568007023339210. [DOI] [PubMed] [Google Scholar]
- 15.Mobascher A, Winterer G. The molecular and cellular neurobiology of nicotine abuse in schizophrenia. Pharmacopsychiatry. 2008;41(Suppl 1):S51–S59. doi: 10.1055/s-2008-1081463. [DOI] [PubMed] [Google Scholar]
- 16.Prochaska JJ, Hall SM, Bero LA. Tobacco use among individuals with schizophrenia: what role has the tobacco industry played? Schizophr Bull. 2008;34:555–567. doi: 10.1093/schbul/sbm117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anand R, Conroy WG, Schoepfer R, Whiting P, Lindstrom J. Neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes have a pentameric quaternary structure. J Biol Chem. 1991;266:11192–11198. [PubMed] [Google Scholar]
- 18.Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2009;56:237–246. doi: 10.1016/j.neuropharm.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 19.Gotti C, Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol. 2004;74:363–396. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Luetje CW. Getting past the asterisk: the subunit composition of presynaptic nicotinic receptors that modulate striatal dopamine release. Mol Pharmacol. 2004;65:1333–1335. doi: 10.1124/mol.65.6.1333. [DOI] [PubMed] [Google Scholar]
- 21.McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Ann Rev Physiol. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- 22.Parker SL, Fu Y, McAllen K, Luo J, McIntosh JM, Lindstrom JM, et al. Up-regulation of brain nicotinic acetylcholine receptors in the rat during long-term self-administration of nicotine: disproportionate increase of the alpha6 subunit. Mol Pharmacol. 2004;65:611–622. doi: 10.1124/mol.65.3.611. [DOI] [PubMed] [Google Scholar]
- 23.Chavez-Noriega LE, Crona JH, Washburn MS, Urrutia A, Elliott KJ, Johnson EC. Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors h alpha 2 beta 2, h alpha 2 beta 4, h alpha 3 beta 2, h alpha 3 beta 4, h alpha 4 beta 2, h alpha 4 beta 4 and h alpha 7 expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1997;280:346–356. [PubMed] [Google Scholar]
- 24.Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Ann Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- 25.Deneris ES, Connolly J, Rogers SW, Duvoisin R. Pharmacological and functional diversity of neuronal nicotinic acetylcholine receptors. Trends Pharmacol Sci. 1991;12:34–40. doi: 10.1016/0165-6147(91)90486-c. [DOI] [PubMed] [Google Scholar]
- 26.Dineley-Miller K, Patrick J. Gene transcripts for the nicotinic acetylcholine receptor subunit, beta4, are distributed in multiple areas of the rat central nervous system. Brain Res Mol Brain Res. 1992;16:339–344. doi: 10.1016/0169-328x(92)90244-6. [DOI] [PubMed] [Google Scholar]
- 27.Le Novere N, Zoli M, Changeux JP. Neuronal nicotinic receptor alpha 6 subunit mRNA is selectively concentrated in catecholaminergic nuclei of the rat brain. Eur J Neurosci. 1996;8:2428–2439. doi: 10.1111/j.1460-9568.1996.tb01206.x. [DOI] [PubMed] [Google Scholar]
- 28.Luetje CW, Piattoni M, Patrick J. Mapping of ligand binding sites of neuronal nicotinic acetylcholine receptors using chimeric alpha subunits. Mol Pharmacol. 1993;44:657–666. [PubMed] [Google Scholar]
- 29.Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, et al. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- 31.Flores CM, Davila-Garcia MI, Ulrich YM, Kellar KJ. Differential regulation of neuronal nicotinic receptor binding sites following chronic nicotine administration. J Neurochem. 1997;69:2216–2219. doi: 10.1046/j.1471-4159.1997.69052216.x. [DOI] [PubMed] [Google Scholar]
- 32.Whiting P, Lindstrom J. Purification and characterization of a nicotinic acetylcholine receptor from rat brain. Proc Natl Acad Sci U S A. 1987;84:595–599. doi: 10.1073/pnas.84.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zoli M, Moretti M, Zanardi A, McIntosh JM, Clementi F, Gotti C. Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J Neurosci. 2002;22:8785–8789. doi: 10.1523/JNEUROSCI.22-20-08785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conroy WG, Vernallis AB, Berg DK. The alpha 5 gene product assembles with multiple acetylcholine receptor subunits to form distinctive receptor subtypes in brain. Neuron. 1992;9:679–691. doi: 10.1016/0896-6273(92)90031-8. [DOI] [PubMed] [Google Scholar]
- 35.Forsayeth JR, Kobrin E. Formation of oligomers containing the beta3 and beta4 subunits of the rat nicotinic receptor. J Neurosci. 1997;17:1531–1538. doi: 10.1523/JNEUROSCI.17-05-01531.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicke A, Wonnacott S, Lewis RJ. Alpha-conotoxins as tools for the elucidation of structure and function of neuronal nicotinic acetylcholine receptor subtypes. Eur J Biochem. 2004;271:2305–2319. doi: 10.1111/j.1432-1033.2004.04145.x. [DOI] [PubMed] [Google Scholar]
- 37.Poth K, Nutter TJ, Cuevas J, Parker MJ, Adams DJ, Luetje CW. Heterogeneity of nicotinic receptor class and subunit mRNA expression among individual parasympathetic neurons from rat intracardiac ganglia. J Neurosci. 1997;17:586–596. doi: 10.1523/JNEUROSCI.17-02-00586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turner JR, Kellar KJ. Nicotinic cholinergic receptors in the rat cerebellum: multiple heteromeric subtypes. J Neurosci. 2005;25:9258–9265. doi: 10.1523/JNEUROSCI.2112-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 40.McIntosh JM, Plazas PV, Watkins M, Gomez-Casati ME, Olivera BM, Elgoyhen AB. A novel alpha-conotoxin, PeIA, cloned from Conus pergrandis, discriminates between rat alpha9alpha10 and alpha7 nicotinic cholinergic receptors. J Biol Chem. 2005;280:30107–30112. doi: 10.1074/jbc.M504102200. [DOI] [PubMed] [Google Scholar]
- 41.Davies AR, Hardick DJ, Blagbrough IS, Potter BV, Wolstenholme AJ, Wonnacott S. Characterisation of the binding of [3H]methyllycaconitine: a new radioligand for labelling alpha 7-type neuronal nicotinic acetylcholine receptors. Neuropharmacology. 1999;38:679–690. doi: 10.1016/s0028-3908(98)00221-4. [DOI] [PubMed] [Google Scholar]
- 42.Mogg AJ, Whiteaker P, McIntosh JM, Marks M, Collins AC, Wonnacott S. Methyllycaconitine is a potent antagonist of alpha-conotoxin-MII-sensitive presynaptic nicotinic acetylcholine receptors in rat striatum. J Pharmacol Exp Ther. 2002;302:197–204. doi: 10.1124/jpet.302.1.197. [DOI] [PubMed] [Google Scholar]
- 43.Orr-Urtreger A, Goldner FM, Saeki M, Lorenzo I, Goldberg L, De Biasi M, et al. Mice deficient in the alpha7 neuronal nicotinic acetylcholine receptor lack alpha-bungarotoxin binding sites and hippocampal fast nicotinic currents. J Neurosci. 1997;17:9165–9171. doi: 10.1523/JNEUROSCI.17-23-09165.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schoepfer R, Conroy WG, Whiting P, Gore M, Lindstrom J. Brain alpha-bungarotoxin binding protein cDNAs and MAbs reveal subtypes of this branch of the ligand-gated ion channel gene superfamily. Neuron. 1990;5:35–48. doi: 10.1016/0896-6273(90)90031-a. [DOI] [PubMed] [Google Scholar]
- 45.Lukas RJ, Changeux JP, Le Novere N, Albuquerque EX, Balfour DJ, Berg DK, et al. International Union of Pharmacology. XX. Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacol Rev. 1999;51:397–401. [PubMed] [Google Scholar]
- 46.Cachelin AB, Rust G. Beta-subunits co-determine the sensitivity of rat neuronal nicotinic receptors to antagonists. Pflugers Arch. 1995;429:449–451. doi: 10.1007/BF00374164. [DOI] [PubMed] [Google Scholar]
- 47.Harvey SC, Luetje CW. Determinants of competitive antagonist sensitivity on neuronal nicotinic receptor beta subunits. J Neurosci. 1996;16:3798–3806. doi: 10.1523/JNEUROSCI.16-12-03798.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harvey SC, Maddox FN, Luetje CW. Multiple determinants of dihydro-beta-erythroidine sensitivity on rat neuronal nicotinic receptor alpha subunits. J Neurochem. 1996;67:1953–1959. doi: 10.1046/j.1471-4159.1996.67051953.x. [DOI] [PubMed] [Google Scholar]
- 49.Papke RL, Dwoskin LP, Crooks PA. The pharmacological activity of nicotine and nornicotine on nAChRs subtypes: relevance to nicotine dependence and drug discovery. J Neurochem. 2007;101:160–167. doi: 10.1111/j.1471-4159.2006.04355.x. [DOI] [PubMed] [Google Scholar]
- 50.Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology. 1992;107:285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- 51.Rahman S, Zhang Z, Papke RL, Crooks PA, Dwoskin LP, Bardo MT. Region-specific effects of N,N'-dodecane-1,12-diyl-bis-3-picolinium dibromide on nicotine-induced increase in extracellular dopamine in vivo. Br J Pharmacol. 2008;153:792–804. doi: 10.1038/sj.bjp.0707612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mathieu-Kia AM, Kellogg SH, Butelman ER, Kreek MJ. Nicotine addiction: insights from recent animal studies. Psychopharmacology. 2002;162:102–118. doi: 10.1007/s00213-002-1096-0. [DOI] [PubMed] [Google Scholar]
- 54.Martin-Soelch C, Leenders KL, Chevalley AF, Missimer J, Kunig G, Magyar S, et al. Reward mechanisms in the brain and their role in dependence: evidence from neurophysiological and neuroimaging studies. Brain Res. 2001;36:139–149. doi: 10.1016/s0165-0173(01)00089-3. [DOI] [PubMed] [Google Scholar]
- 55.Azam L, Winzer-Serhan UH, Chen Y, Leslie FM. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J Comp Neurol. 2002;444:260–274. doi: 10.1002/cne.10138. [DOI] [PubMed] [Google Scholar]
- 56.Clarke PB, Pert A. Autoradiographic evidence for nicotine receptors on nigrostriatal and mesolimbic dopaminergic neurons. Brain Res. 1985;348:355–358. doi: 10.1016/0006-8993(85)90456-1. [DOI] [PubMed] [Google Scholar]
- 57.Collins AC, Salminen O, Marks MJ, Whiteaker P, Grady SR. The road to discovery of neuronal nicotinic cholinergic receptor subtypes. Handb Exp Pharmacol. 2009:85–112. doi: 10.1007/978-3-540-69248-5_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grady SR, Murphy KL, Cao J, Marks MJ, McIntosh JM, Collins AC. Characterization of nicotinic agonist-induced [3H]dopamine release from synaptosomes prepared from four mouse brain regions. J Pharmacol Exp Ther. 2002;301:651–660. doi: 10.1124/jpet.301.2.651. [DOI] [PubMed] [Google Scholar]
- 59.Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, Collins AC, et al. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem Pharmacol. 2007;74:1235–1246. doi: 10.1016/j.bcp.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quarta D, Ciruela F, Patkar K, Borycz J, Solinas M, Lluis C, et al. Heteromeric nicotinic acetylcholine-dopamine autoreceptor complexes modulate striatal dopamine release. Neuropsychopharmacology. 2007;32:35–42. doi: 10.1038/sj.npp.1301103. [DOI] [PubMed] [Google Scholar]
- 61.Teng L, Crooks PA, Sonsalla PK, Dwoskin LP. Lobeline and nicotine evoke [3H]overflow from rat striatal slices preloaded with [3H]dopamine: differential inhibition of synaptosomal and vesicular [3H]dopamine uptake. J Pharmacol Exp Ther. 1997;280:1432–1444. [PubMed] [Google Scholar]
- 62.Wonnacott S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- 63.Arroyo-Jimenez MM, Bourgeois JP, Marubio LM, Le Sourd AM, Ottersen OP, Rinvik E, et al. Ultrastructural localization of the alpha4-subunit of the neuronal acetylcholine nicotinic receptor in the rat substantia nigra. J Neurosci. 1999;19:6475–6487. doi: 10.1523/JNEUROSCI.19-15-06475.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Charpantier E, Barneoud P, Moser P, Besnard F, Sgard F. Nicotinic acetylcholine subunit mRNA expression in dopaminergic neurons of the rat substantia nigra and ventral tegmental area. Neuroreport. 1998;9:3097–3101. doi: 10.1097/00001756-199809140-00033. [DOI] [PubMed] [Google Scholar]
- 65.Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Lena C, et al. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci. 2003;23:7820–7829. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- 67.Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, et al. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- 68.Scholze P, Orr-Urtreger A, Changeux JP, McIntosh JM, Huck S. Catecholamine outflow from mouse and rat brain slice preparations evoked by nicotinic acetylcholine receptor activation and electrical field stimulation. Br J Pharmacol. 2007;151:414–422. doi: 10.1038/sj.bjp.0707236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Whiteaker P, Marks MJ, Grady SR, Lu Y, Picciotto MR, Changeux JP, et al. Pharmacological and null mutation approaches reveal nicotinic receptor diversity. Eur J Pharmacol. 2000;393:123–135. doi: 10.1016/s0014-2999(00)00052-2. [DOI] [PubMed] [Google Scholar]
- 70.Grady SR, Meinerz NM, Cao J, Reynolds AM, Picciotto MR, Changeux JP, et al. Nicotinic agonists stimulate acetylcholine release from mouse interpeduncular nucleus: a function mediated by a different nAChR than dopamine release from striatum. J Neurochem. 2001;76:258–268. doi: 10.1046/j.1471-4159.2001.00019.x. [DOI] [PubMed] [Google Scholar]
- 71.Cui C, Booker TK, Allen RS, Grady SR, Whiteaker P, Marks MJ, et al. The beta3 nicotinic receptor subunit: a component of alpha-conotoxin MII-binding nicotinic acetylcholine receptors that modulate dopamine release and related behaviors. J Neurosci. 2003;23:11045–11053. doi: 10.1523/JNEUROSCI.23-35-11045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Drenan RM, Grady SR, Whiteaker P, McClure-Begley T, McKinney S, Miwa JM, et al. In vivo activation of midbrain dopamine neurons via sensitized, high-affinity alpha 6 nicotinic acetylcholine receptors. Neuron. 2008;60:123–136. doi: 10.1016/j.neuron.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goldner FM, Dineley KT, Patrick JW. Immunohistochemical localization of the nicotinic acetylcholine receptor subunit alpha6 to dopaminergic neurons in the substantia nigra and ventral tegmental area. Neuroreport. 1997;8:2739–2742. doi: 10.1097/00001756-199708180-00019. [DOI] [PubMed] [Google Scholar]
- 74.Cartier GE, Yoshikami D, Gray WR, Luo S, Olivera BM, McIntosh JM. A new alpha-conotoxin which targets alpha3beta2 nicotinic acetylcholine receptors. J Biol Chem. 1996;271:7522–7528. doi: 10.1074/jbc.271.13.7522. [DOI] [PubMed] [Google Scholar]
- 75.Grady S, Marks MJ, Wonnacott S, Collins AC. Characterization of nicotinic receptor-mediated [3H]dopamine release from synaptosomes prepared from mouse striatum. J Neurochem. 1992;59:848–856. doi: 10.1111/j.1471-4159.1992.tb08322.x. [DOI] [PubMed] [Google Scholar]
- 76.Kaiser S, Wonnacott S. alpha-bungarotoxin-sensitive nicotinic receptors indirectly modulate [3H]dopamine release in rat striatal slices via glutamate release. Mol Pharmacol. 2000;58:312–318. doi: 10.1124/mol.58.2.312. [DOI] [PubMed] [Google Scholar]
- 77.Kaiser SA, Soliakov L, Harvey SC, Luetje CW, Wonnacott S. Differential inhibition by alpha-conotoxin-MII of the nicotinic stimulation of [3H]dopamine release from rat striatal synaptosomes and slices. J Neurochem. 1998;70:1069–1076. doi: 10.1046/j.1471-4159.1998.70031069.x. [DOI] [PubMed] [Google Scholar]
- 78.Schulz DW, Zigmond RE. Neuronal bungarotoxin blocks the nicotinic stimulation of endogenous dopamine release from rat striatum. Neurosci Lett. 1989;98:310–316. doi: 10.1016/0304-3940(89)90420-5. [DOI] [PubMed] [Google Scholar]
- 79.Champtiaux N, Han ZY, Bessis A, Rossi FM, Zoli M, Marubio L, et al. Distribution and pharmacology of alpha 6-containing nicotinic acetylcholine receptors analyzed with mutant mice. J Neurosci. 2002;22:1208–1217. doi: 10.1523/JNEUROSCI.22-04-01208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McIntosh JM, Azam L, Staheli S, Dowell C, Lindstrom JM, Kuryatov A, et al. Analogs of alpha-conotoxin MII are selective for alpha6-containing nicotinic acetylcholine receptors. Mol Pharmacol. 2004;65:944–952. doi: 10.1124/mol.65.4.944. [DOI] [PubMed] [Google Scholar]
- 81.Whiteaker P, Peterson CG, Xu W, McIntosh JM, Paylor R, Beaudet AL, et al. Involvement of the alpha3 subunit in central nicotinic binding populations. J Neurosci. 2002;22:2522–2529. doi: 10.1523/JNEUROSCI.22-07-02522.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Azam L, McIntosh JM. Effect of novel alpha-conotoxins on nicotine-stimulated [3H]dopamine release from rat striatal synaptosomes. J Pharmacol Exp Ther. 2005;312:231–237. doi: 10.1124/jpet.104.071456. [DOI] [PubMed] [Google Scholar]
- 83.Gotti C, Moretti M, Clementi F, Riganti L, McIntosh JM, Collins AC, et al. Expression of nigrostriatal alpha 6-containing nicotinic acetylcholine receptors is selectively reduced, but not eliminated, by beta 3 subunit gene deletion. Mol Pharmacol. 2005;67:2007–2015. doi: 10.1124/mol.105.011940. [DOI] [PubMed] [Google Scholar]
- 84.Salminen O, Drapeau JA, McIntosh JM, Collins AC, Marks MJ, Grady SR. Pharmacology of alpha-conotoxin MII-sensitive subtypes of nicotinic acetylcholine receptors isolated by breeding of null mutant mice. Mol Pharmacol. 2007;71:1563–1571. doi: 10.1124/mol.106.031492. [DOI] [PubMed] [Google Scholar]
- 85.Fu Y, Matta SG, Brower VG, Sharp BM. Norepinephrine secretion in the hypothalamic paraventricular nucleus of rats during unlimited access to self-administered nicotine: An in vivo microdialysis study. J Neurosci. 2001;21:8979–8989. doi: 10.1523/JNEUROSCI.21-22-08979.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Picciotto MR, Corrigall WA. Neuronal systems underlying behaviors related to nicotine addiction: neural circuits and molecular genetics. J Neurosci. 2002;22:3338–3341. doi: 10.1523/JNEUROSCI.22-09-03338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rauhut AS, Mullins SN, Dwoskin LP, Bardo MT. Reboxetine: attenuation of intravenous nicotine self-administration in rats. J Pharmacol Exp Ther. 2002;303:664–672. doi: 10.1124/jpet.303.2.664. [DOI] [PubMed] [Google Scholar]
- 88.Amtage F, Neughebauer B, McIntosh JM, Freiman T, Zentner J, Feuerstein TJ, et al. Characterization of nicotinic receptors inducing noradrenaline release and absence of nicotinic autoreceptors in human neocortex. Brain Res Bull. 2004;62:413–423. doi: 10.1016/j.brainresbull.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 89.Clarke PB, Reuben M. Release of [3H]-noradrenaline from rat hippocampal synaptosomes by nicotine: mediation by different nicotinic receptor subtypes from striatal [3H]-dopamine release. Br J Pharmacol. 1996;117:595–606. doi: 10.1111/j.1476-5381.1996.tb15232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leslie FM, Gallardo KA, Park MK. Nicotinic acetylcholine receptor-mediated release of [3H]norepinephrine from developing and adult rat hippocampus: direct and indirect mechanisms. Neuropharmacology. 2002;42:653–661. doi: 10.1016/s0028-3908(02)00019-9. [DOI] [PubMed] [Google Scholar]
- 91.Sacaan AI, Dunlop JL, Lloyd GK. Pharmacological characterization of neuronal acetylcholine gated ion channel receptor-mediated hippocampal norepinephrine and striatal dopamine release from rat brain slices. J Pharmacol Exp Ther. 1995;274:224–230. [PubMed] [Google Scholar]
- 92.Sershen H, Balla A, Lajtha A, Vizi ES. Characterization of nicotinic receptors involved in the release of noradrenaline from the hippocampus. Neuroscience. 1997;77:121–130. doi: 10.1016/s0306-4522(96)00425-3. [DOI] [PubMed] [Google Scholar]
- 93.Balfour DJ, Fagerstrom KO. Pharmacology of nicotine and its therapeutic use in smoking cessation and neurodegenerative disorders. Pharmacol Ther. 1996;72:51–81. doi: 10.1016/s0163-7258(96)00099-x. [DOI] [PubMed] [Google Scholar]
- 94.Summers KL, Giacobini E. Effects of local and repeated systemic administration of (−)nicotine on extracellular levels of acetylcholine, norepinephrine, dopamine, and serotonin in rat cortex. Neurochem Res. 1995;20:753–759. doi: 10.1007/BF01705545. [DOI] [PubMed] [Google Scholar]
- 95.Linner L, Endersz H, Ohman D, Bengtsson F, Schalling M, Svensson TH. Reboxetine modulates the firing pattern of dopamine cells in the ventral tegmental area and selectively increases dopamine availability in the prefrontal cortex. J Pharmacol Exp Ther. 2001;297:540–546. [PubMed] [Google Scholar]
- 96.Winzer-Serhan UH, Leslie FM. Codistribution of nicotinic acetylcholine receptor subunit alpha3 and beta4 mRNAs during rat brain development. J Comp Neurol. 1997;386:540–554. doi: 10.1002/(sici)1096-9861(19971006)386:4<540::aid-cne2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 97.Lena C, de Kerchove D'Exaerde A, Cordero-Erausquin M, Le Novere N, del Mar Arroyo-Jimenez M, Changeux JP. Diversity and distribution of nicotinic acetylcholine receptors in the locus ceruleus neurons. Proc Natl Acad Sci U S A. 1999;96:12126–12131. doi: 10.1073/pnas.96.21.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vincler MA, Eisenach JC. Immunocytochemical localization of the alpha3, alpha4, alpha5, alpha7, beta2, beta3 and beta4 nicotinic acetylcholine receptor subunits in the locus coeruleus of the rat. Brain Res. 2003;974:25–36. doi: 10.1016/s0006-8993(03)02546-0. [DOI] [PubMed] [Google Scholar]
- 99.Luo S, Kulak JM, Cartier GE, Jacobsen RB, Yoshikami D, Olivera BM, et al. alpha-conotoxin AuIB selectively blocks alpha3 beta4 nicotinic acetylcholine receptors and nicotine-evoked norepinephrine release. J Neurosci. 1998;18:8571–859. doi: 10.1523/JNEUROSCI.18-21-08571.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Azam L, McIntosh JM. Characterization of nicotinic acetylcholine receptors that modulate nicotine-evoked [3H]norepinephrine release from mouse hippocampal synaptosomes. Mol Pharmacol. 2006;70:967–976. doi: 10.1124/mol.106.024513. [DOI] [PubMed] [Google Scholar]
- 101.Kulak JM, Nguyen TA, Olivera BM, McIntosh JM. Alpha-conotoxin MII blocks nicotine-stimulated dopamine release in rat striatal synaptosomes. J Neurosci. 1997;17:5263–5270. doi: 10.1523/JNEUROSCI.17-14-05263.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McCallum SE, Collins AC, Paylor R, Marks MJ. Deletion of the beta 2 nicotinic acetylcholine receptor subunit alters development of tolerance to nicotine and eliminates receptor upregulation. Psychopharmacology. 2006;184:314–327. doi: 10.1007/s00213-005-0076-6. [DOI] [PubMed] [Google Scholar]
- 103.Exley R, Clements MA, Hartung H, McIntosh JM, Cragg SJ. alpha6-Containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens. Neuropsychopharmacology. 2008;33:2158–2166. doi: 10.1038/sj.npp.1301617. [DOI] [PubMed] [Google Scholar]
- 104.Exley R, Cragg SJ. Presynaptic nicotinic receptors: a dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. Br J Pharmacol. 2008;153(Suppl 1):S283–S297. doi: 10.1038/sj.bjp.0707510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gaimarri A, Moretti M, Riganti L, Zanardi A, Clementi F, Gotti C. Regulation of neuronal nicotinic receptor traffic and expression. Brain Res Rev. 2007;55:134–143. doi: 10.1016/j.brainresrev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 106.Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans-Borgmeyer I, Heinemann SF, et al. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci. 1992;12:2765–2784. doi: 10.1523/JNEUROSCI.12-07-02765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mao D, Perry DC, Yasuda RP, Wolfe BB, Kellar KJ. The alpha4beta2alpha5 nicotinic cholinergic receptor in rat brain is resistant to up-regulation by nicotine in vivo. J Neurochem. 2008;104:446–456. doi: 10.1111/j.1471-4159.2007.05011.x. [DOI] [PubMed] [Google Scholar]
- 108.Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, et al. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- 109.Nashmi R, Xiao C, Deshpande P, McKinney S, Grady SR, Whiteaker P, et al. Chronic nicotine cell specifically upregulates functional alpha 4* nicotinic receptors: basis for both tolerance in midbrain and enhanced long-term potentiation in perforant path. J Neurosci. 2007;27:8202–8218. doi: 10.1523/JNEUROSCI.2199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Parker SL, Fu Y, McAllen K, Luo J, McIntosh JM, Lindstrom JM, et al. Up-regulation of brain nicotinic acetylcholine receptors in the rat during long-term self-administration of nicotine: disproportionate increase of the alpha6 subunit. Mol Pharmacol. 2004;65:611–622. doi: 10.1124/mol.65.3.611. [DOI] [PubMed] [Google Scholar]
- 111.Lai A, Parameswaran N, Khwaja M, Whiteaker P, Lindstrom JM, Fan H, et al. Long-term nicotine treatment decreases striatal alpha 6* nicotinic acetylcholine receptor sites and function in mice. Mol Pharmacol. 2005;67:1639–1647. doi: 10.1124/mol.104.006429. [DOI] [PubMed] [Google Scholar]
- 112.Perry DC, Mao D, Gold AB, McIntosh JM, Pezzullo JC, Kellar KJ. Chronic nicotine differentially regulates alpha6- and beta3-containing nicotinic cholinergic receptors in rat brain. J Pharmacol Exp Ther. 2007;322:306–315. doi: 10.1124/jpet.107.121228. [DOI] [PubMed] [Google Scholar]
- 113.McCallum SE, Parameswaran N, Bordia T, Fan H, McIntosh JM, Quik M. Differential regulation of mesolimbic alpha 3/alpha 6 beta 2 and alpha 4 beta 2 nicotinic acetylcholine receptor sites and function after long-term oral nicotine to monkeys. J Pharmacol Exp Ther. 2006;318:381–388. doi: 10.1124/jpet.106.104414. [DOI] [PubMed] [Google Scholar]
- 114.Drenan RM, Grady SR, Whiteaker P, McClure-Begley T, McKinney S, Miwa JM, et al. In vivo activation of midbrain dopamine neurons via sensitized, high-affinity alpha 6 nicotinic acetylcholine receptors. Neuron. 2008;60:123–136. doi: 10.1016/j.neuron.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Briggs CA, Gubbins EJ, Marks MJ, Putman CB, Thimmapaya R, Meyer MD, et al. Untranslated region-dependent exclusive expression of high-sensitivity subforms of alpha4beta2 and alpha3beta2 nicotinic acetylcholine receptors. Mol Pharmacol. 2006;70:227–240. doi: 10.1124/mol.105.020198. [DOI] [PubMed] [Google Scholar]
- 116.Moroni M, Zwart R, Sher E, Cassels BK, Bermudez I. alpha4beta2 nicotinic receptors with high and low acetylcholine sensitivity: pharmacology, stoichiometry, and sensitivity to long-term exposure to nicotine. Mol Pharmacol. 2006;70:755–768. doi: 10.1124/mol.106.023044. [DOI] [PubMed] [Google Scholar]
- 117.Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J. Alternate stoichiometries of alpha4beta2 nicotinic acetylcholine receptors. Mol Pharmacol. 2003;63:332–341. doi: 10.1124/mol.63.2.332. [DOI] [PubMed] [Google Scholar]
- 118.Tapia L, Kuryatov A, Lindstrom J. Ca2+ permeability of the (alpha4)3(beta2)2 stoichiometry greatly exceeds that of (alpha4)2(beta2)3 human acetylcholine receptors. Mol Pharmacol. 2007;71:769–776. doi: 10.1124/mol.106.030445. [DOI] [PubMed] [Google Scholar]
- 119.Vallejo YF, Buisson B, Bertrand D, Green WN. Chronic nicotine exposure upregulates nicotinic receptors by a novel mechanism. J Neurosci. 2005;25:5563–5572. doi: 10.1523/JNEUROSCI.5240-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gotti C, Moretti M, Meinerz NM, Clementi F, Gaimarri A, Collins AC, et al. Partial deletion of the nicotinic cholinergic receptor alpha 4 or beta 2 subunit genes changes the acetylcholine sensitivity of receptor-mediated 86Rb+ efflux in cortex and thalamus and alters relative expression of alpha 4 and beta 2 subunits. Mol Pharmacol. 2008;73:1796–1807. doi: 10.1124/mol.108.045203. [DOI] [PubMed] [Google Scholar]
- 121.Balfour DJ. Neural mechanisms underlying nicotine dependence. Addiction. 1994;89:1419–1423. doi: 10.1111/j.1360-0443.1994.tb03738.x. [DOI] [PubMed] [Google Scholar]
- 122.Dani JA, De Biasi M. Cellular mechanisms of nicotine addiction. Pharmacol Biochem Behav. 2001;70:439–446. doi: 10.1016/s0091-3057(01)00652-9. [DOI] [PubMed] [Google Scholar]
- 123.Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not "either/or": activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–342. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Galzi JL, Changeux JP. Neuronal nicotinic receptors: molecular organization and regulations. Neuropharmacology. 1995;34:563–582. doi: 10.1016/0028-3908(95)00034-4. [DOI] [PubMed] [Google Scholar]
- 125.Miyazawa A, Fujiyoshi Y, Unwin N. Structure and gating mechanism of the acetylcholine receptor pore. Nature. 2003;423:949–955. doi: 10.1038/nature01748. [DOI] [PubMed] [Google Scholar]
- 126.Wang H, Sun X. Desensitized nicotinic receptors in brain. Brain Res. 2005;48:420–437. doi: 10.1016/j.brainresrev.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 127.Quick MW, Lester RA. Desensitization of neuronal nicotinic receptors. J Neurobiol. 2002;53:457–478. doi: 10.1002/neu.10109. [DOI] [PubMed] [Google Scholar]
- 128.Giniatullin R, Nistri A, Yakel JL. Desensitization of nicotinic ACh receptors: shaping cholinergic signaling. Trends Neurosci. 2005;28:371–378. doi: 10.1016/j.tins.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 129.Matsubayashi H, Inoue A, Amano T, Seki T, Nakata Y, Sasa M, et al. Involvement of alpha7- and alpha4beta2-type postsynaptic nicotinic acetylcholine receptors in nicotine-induced excitation of dopaminergic neurons in the substantia nigra: a patch clamp and single-cell PCR study using acutely dissociated nigral neurons. Brain Res Mol Brain Res. 2004;129:1–7. doi: 10.1016/j.molbrainres.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 130.Yang K, Hu J, Lucero L, Liu Q, Zheng C, Zhen X, et al. Distinctive nicotinic acetylcholine receptor functional phenotypes of rat ventral tegmental area dopaminergic neurons. J Physiol. 2009;587:345–361. doi: 10.1113/jphysiol.2008.162743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bohler S, Gay S, Bertrand S, Corringer PJ, Edelstein SJ, Changeux JP, et al. Desensitization of neuronal nicotinic acetylcholine receptors conferred by N-terminal segments of the beta 2 subunit. Biochemistry. 2001;40:2066–2074. doi: 10.1021/bi0020022. [DOI] [PubMed] [Google Scholar]
- 132.Sudweeks SN, Yakel JL. Functional and molecular characterization of neuronal nicotinic ACh receptors in rat CA1 hippocampal neurons. J Physiol. 2000;527(Pt 3):515–528. doi: 10.1111/j.1469-7793.2000.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nelson ME, Lindstrom J. Single channel properties of human alpha3 AChRs: impact of beta2, beta4 and alpha5 subunits. J Physiol. 1999;516(Pt 3):657–678. doi: 10.1111/j.1469-7793.1999.0657u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fenster CP, Rains MF, Noerager B, Quick MW, Lester RA. Influence of subunit composition on desensitization of neuronal acetylcholine receptors at low concentrations of nicotine. J Neurosci. 1997;17:5747–5759. doi: 10.1523/JNEUROSCI.17-15-05747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dani JA, Heinemann S. Molecular and cellular aspects of nicotine abuse. Neuron. 1996;16:905–908. doi: 10.1016/s0896-6273(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 136.Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D, et al. Cigarette smoking saturates brain alpha 4 beta 2 nicotinic acetylcholine receptors. Arch Gen Psychiatry. 2006;63:907–915. doi: 10.1001/archpsyc.63.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shiffman S, Ferguson SG. The effect of a nicotine patch on cigarette craving over the course of the day: results from two randomized clinical trials. Curr Med Res Opin. 2008;24:2795–2804. doi: 10.1185/03007990802380341. [DOI] [PubMed] [Google Scholar]
- 138.Ferguson SG, Shiffman S, Gwaltney CJ. Does reducing withdrawal severity mediate nicotine patch efficacy? A randomized clinical trial. J Consult Clin Psychol. 2006;74:1153–1161. doi: 10.1037/0022-006X.74.6.1153. [DOI] [PubMed] [Google Scholar]
- 139.Buccafusco JJ, Beach JW, Terry AV., Jr Desensitization of nicotinic acetylcholine receptors as a strategy for drug development. J Pharmacol Exp Ther. 2009;328:364–370. doi: 10.1124/jpet.108.145292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Garwood CL, Potts LA. Emerging pharmacotherapies for smoking cessation. Am J Health Syst Pharm. 2007;64:1693–1698. doi: 10.2146/ajhp060427. [DOI] [PubMed] [Google Scholar]
- 141.Hurt RD, Krook JE, Croghan IT, Loprinzi CL, Sloan JA, Novotny PJ, et al. Nicotine patch therapy based on smoking rate followed by bupropion for prevention of relapse to smoking. J Clin Oncol. 2003;21:914–920. doi: 10.1200/JCO.2003.08.160. [DOI] [PubMed] [Google Scholar]
- 142.Wileyto P, Patterson F, Niaura R, Epstein L, Brown R, Audrain-McGovern J, et al. Do small lapses predict relapse to smoking behavior under bupropion treatment? Nicotine Tob Res. 2004;6:357–366. doi: 10.1080/1462220042000202463. [DOI] [PubMed] [Google Scholar]
- 143.Hughes JR, Higgins ST, Bickel WK. Nicotine withdrawal versus other drug withdrawal syndromes: similarities and dissimilarities. Addiction. 1994;89:1461–1470. doi: 10.1111/j.1360-0443.1994.tb03744.x. [DOI] [PubMed] [Google Scholar]
- 144.Rollema H, Coe JW, Chambers LK, Hurst RS, Stahl SM, Williams KE. Rationale, pharmacology and clinical efficacy of partial agonists of alpha4beta2 nACh receptors for smoking cessation. Trends Pharmacol Sci. 2007;28:316–325. doi: 10.1016/j.tips.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 145.Hays JT, Ebbert JO, Sood A. Efficacy and safety of varenicline for smoking cessation. Am J Med. 2008;121:S32–S42. doi: 10.1016/j.amjmed.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 146.McColl SL, Burstein AH, Reeves KR, Billing CB, Jr, Stolar M, Sellers EM. Human abuse liability of the smoking cessation drug varenicline in smokers and nonsmokers. Clin Pharmacol Ther. 2008;83:607–614. doi: 10.1038/sj.clpt.6100510. [DOI] [PubMed] [Google Scholar]
- 147.Schuh LM, Schuh KJ, Henningfield JE. Pharmacologic determinants of tobacco dependence. Am J Ther. 1996;3:335–341. doi: 10.1097/00045391-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 148.Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, et al. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- 149.Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, et al. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007b;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 150.Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- 151.Faessel HM, Gibbs MA, Clark DJ, Rohrbacher K, Stolar M, Burstein AH. Multiple-dose pharmacokinetics of the selective nicotinic receptor partial agonist, varenicline, in healthy smokers. J Clin Pharmacol. 2006;46:1439–1448. doi: 10.1177/0091270006292624. [DOI] [PubMed] [Google Scholar]
- 152.Obach RS, Reed-Hagen AE, Krueger SS, Obach BJ, O'Connell TN, Zandi KS, et al. Metabolism and disposition of varenicline, a selective alpha4beta2 acetylcholine receptor partial agonist, in vivo and in vitro. Drug Metab Dispos. 2006;34:121–130. doi: 10.1124/dmd.105.006767. [DOI] [PubMed] [Google Scholar]
- 153.Fagerstrom K, Hughes J. Varenicline in the treatment of tobacco dependence. Neuropsychiatr Dis Treat. 2008;4:353–363. doi: 10.2147/ndt.s927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cahill K, Stead L, Lancaster T. A preliminary benefit-risk assessment of varenicline in smoking cessation. Drug Saf. 2009;32:119–135. doi: 10.2165/00002018-200932020-00005. [DOI] [PubMed] [Google Scholar]
- 155.Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 156.Food and Drug Administration. FDA issues public health advisory on Chantix. [[Accessed 2009 May 18]];2008 [online] Available from URL: http://www.fda.gov/CDER/Drug/early_comm/varenicline.htm.
- 157.Papke RL, Heinemann SF. Partial agonist properties of cytisine on neuronal nicotinic receptors containing the beta 2 subunit. Mol Pharmacol. 1994;45:142–149. [PubMed] [Google Scholar]
- 158.Grady SR, Murphy KL, Cao J, Marks MJ, McIntosh JM, Collins AC. Characterization of nicotinic agonist-induced [(3)H]dopamine release from synaptosomes prepared from four mouse brain regions. J Pharmacol Exp Ther. 2002;301:651–660. doi: 10.1124/jpet.301.2.651. [DOI] [PubMed] [Google Scholar]
- 159.Abin-Carriquiry JA, Voutilainen MH, Barik J, Cassels BK, Iturriaga-Vasquez P, Bermudez I, et al. C3-halogenation of cytisine generates potent and efficacious nicotinic receptor agonists. Eur J Pharmacol. 2006;536:1–11. doi: 10.1016/j.ejphar.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 160.Marks MJ, Whiteaker P, Collins AC. Deletion of the alpha7, beta2, or beta4 nicotinic receptor subunit genes identifies highly expressed subtypes with relatively low affinity for [3H]epibatidine. Mol Pharmacol. 2006;70:947–959. doi: 10.1124/mol.106.025338. [DOI] [PubMed] [Google Scholar]
- 161.Salminen O, Whiteaker P, Grady SR, Collins AC, McIntosh JM, Marks MJ. The subunit composition and pharmacology of alpha-Conotoxin MII-binding nicotinic acetylcholine receptors studied by a novel membrane-binding assay. Neuropharmacology. 2005;48:696–705. doi: 10.1016/j.neuropharm.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 162.Etter JF, Lukas RJ, Benowitz NL, West R, Dresler CM. Cytisine for smoking cessation: a research agenda. Drug Alcohol Depend. 2008;92:3–8. doi: 10.1016/j.drugalcdep.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 163.Stolerman IP, Garcha HS, Mirza NR. Dissociations between the locomotor stimulant and depressant effects of nicotinic agonists in rats. Psychopharmacology. 1995;117:430–437. doi: 10.1007/BF02246215. [DOI] [PubMed] [Google Scholar]
- 164.Chandler CJ, Stolerman IP. Discriminative stimulus properties of the nicotinic agonist cytisine. Psychopharmacology. 1997;129:257–264. doi: 10.1007/s002130050188. [DOI] [PubMed] [Google Scholar]
- 165.Rasmussen T, Swedberg MD. Reinforcing effects of nicotinic compounds: intravenous self-administration in drug-naive mice. Pharmacol Biochem Behav. 1998;60:567–573. doi: 10.1016/s0091-3057(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 166.Museo E, Wise RA. Place preference conditioning with ventral tegmental injections of cytisine. Life Sci. 1994;55:1179–1186. doi: 10.1016/0024-3205(94)00656-3. [DOI] [PubMed] [Google Scholar]
- 167.Zatonski W, Cedzynska M, Tutka P, West R. An uncontrolled trial of cytisine (Tabex) for smoking cessation. Tob Control. 2006;15:481–484. doi: 10.1136/tc.2006.016097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cohen C, Bergis OE, Galli F, Lochead AW, Jegham S, Biton B, et al. SSR591813, a novel selective and partial alpha4beta2 nicotinic receptor agonist with potential as an aid to smoking cessation. J Pharmacol Exp Ther. 2003;306:407–420. doi: 10.1124/jpet.103.049262. [DOI] [PubMed] [Google Scholar]
- 169.Fagerstrom K, Balfour DJ. Neuropharmacology and potential efficacy of new treatments for tobacco dependence. Expert Opin Investig Drugs. 2006;15:107–116. doi: 10.1517/13543784.15.2.107. [DOI] [PubMed] [Google Scholar]
- 170.Sanfi Aventis Press Release. [February 18, 2007];:14. http://www.info-financiere.fr/upload/FCECO005544_20080212.pdf. [Google Scholar]
- 171.Xiao Y, Fan H, Musachio JL, Wei ZL, Chellappan SK, Kozikowski AP, et al. Sazetidine-A, a novel ligand that desensitizes alpha4beta2 nicotinic acetylcholine receptors without activating them. Mol Pharmacol. 2006;70:1454–1460. doi: 10.1124/mol.106.027318. [DOI] [PubMed] [Google Scholar]
- 172.Zwart R, Carbone AL, Moroni M, Bermudez I, Mogg AJ, Folly EA, et al. Sazetidine-A is a potent and selective agonist at native and recombinant alpha 4 beta 2 nicotinic acetylcholine receptors. Mol Pharmacol. 2008;73:1838–1843. doi: 10.1124/mol.108.045104. [DOI] [PubMed] [Google Scholar]
- 173.Xiao Y, Woolverton WL, Sahibzada N, Yasuda RP, Kellar KJ. Pharmacological properties of sazetidine-A, a desensitizer of alpha4beta2 nicotinic acetylcholine receptors. Soc Neurosci Abstr. 2007;33:574.6. [Google Scholar]
- 174.Zwart R, Vijverberg HP. Four pharmacologically distinct subtypes of alpha4beta2 nicotinic acetylcholine receptor expressed in Xenopus laevis oocytes. Mol Pharmacol. 1998;54:1124–1131. [PubMed] [Google Scholar]
- 175.Dwoskin LP, Rauhut AS, King-Pospisil KA, Bardo MT. Review of the pharmacology and clinical profile of bupropion, an antidepressant and tobacco use cessation agent. CNS Drug Rev. 2006;12:178–207. doi: 10.1111/j.1527-3458.2006.00178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zislis G, Desai TV, Prado M, Shah HP, Bruijnzeel AW. Effects of the CRF receptor antagonist D-Phe CRF(12–41) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats. Neuropharmacology. 2007;53:958–966. doi: 10.1016/j.neuropharm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Le Foll B, Goldberg SR. Control of the reinforcing effects of nicotine by associated environmental stimuli in animals and humans. Trends Pharmacol Sci. 2005;26:287–293. doi: 10.1016/j.tips.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 178.Perkins KA, Sanders M, Fonte C, Wilson AS, White W, Stiller R, et al. Effects of central and peripheral nicotinic blockade on human nicotine discrimination. Psychopharmacology. 1999;142:158–164. doi: 10.1007/s002130050875. [DOI] [PubMed] [Google Scholar]
- 179.Rose JE, Behm FM, Westman EC, Kukovich P. Precessation treatment with nicotine skin patch facilitates smoking cessation. Nicotine Tob Res. 2006;8:89–101. doi: 10.1080/14622200500431866. [DOI] [PubMed] [Google Scholar]
- 108.Rose JE, Behm FM, Westman EC, Levin ED, Stein RM, Ripka GV. Mecamylamine combined with nicotine skin patch facilitates smoking cessation beyond nicotine patch treatment alone. Clin Pharmacol Ther. 1994;56:86–99. doi: 10.1038/clpt.1994.105. [DOI] [PubMed] [Google Scholar]
- 181.DeNoble VJ, Mele PC. Intravenous nicotine self-administration in rats: effects of mecamylamine, hexamethonium and naloxone. Psychopharmacology. 2006;184:266–272. doi: 10.1007/s00213-005-0054-z. [DOI] [PubMed] [Google Scholar]
- 182.Glick SD, Visker KE, Maisonneuve IM. An oral self-administration model of nicotine preference in rats: effects of mecamylamine. Psychopharmacology. 1996;128:426–431. doi: 10.1007/s002130050153. [DOI] [PubMed] [Google Scholar]
- 183.Miller DK, Wilkins LH, Bardo MT, Crooks PA, Dwoskin LP. Once weekly administration of nicotine produces long-lasting locomotor sensitization in rats via a nicotinic receptor-mediated mechanism. Psychopharmacology. 2001;156:469–476. doi: 10.1007/s002130100747. [DOI] [PubMed] [Google Scholar]
- 184.Nakahara D. Influence of nicotine on brain reward systems: study of intracranial self-stimulation. Ann NY Acad Sci. 2004;1025:489–490. doi: 10.1196/annals.1316.060. [DOI] [PubMed] [Google Scholar]
- 185.Palmatier MI, Liu X, Caggiula AR, Donny EC, Sved AF. The role of nicotinic acetylcholine receptors in the primary reinforcing and reinforcement-enhancing effects of nicotine. Neuropsychopharmacology. 2007;32:1098–1108. doi: 10.1038/sj.npp.1301228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Risner ME, Goldberg SR. A comparison of nicotine and cocaine self-administration in the dog: fixed-ratio and progressive-ratio schedules of intravenous drug infusion. J Pharmacol Exp Ther. 1983;224:319–326. [PubMed] [Google Scholar]
- 187.Shoaib M, Schindler CW, Goldberg SR. Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology. 1997;129:35–43. doi: 10.1007/s002130050159. [DOI] [PubMed] [Google Scholar]
- 188.Spealman RD, Goldberg SR. Maintenance of schedule-controlled behavior by intravenous injections of nicotine in squirrel monkeys. J Pharmacol Exp Ther. 1982;223:402–408. [PubMed] [Google Scholar]
- 189.Stolerman IP, Naylor C, Elmer GI, Goldberg SR. Discrimination and self-administration of nicotine by inbred strains of mice. Psychopharmacology. 1999;141:297–306. doi: 10.1007/s002130050837. [DOI] [PubMed] [Google Scholar]
- 190.Mansbach RS, Chambers LK, Rovetti CC. Effects of the competitive nicotinic antagonist erysodine on behavior occasioned or maintained by nicotine: comparison with mecamylamine. Psychopharmacology. 2000;148:234–242. doi: 10.1007/s002130050047. [DOI] [PubMed] [Google Scholar]
- 191.Liu X, Caggiula AR, Yee SK, Nobuta H, Poland RE, Pechnick RN. Reinstatement of nicotine-seeking behavior by drug-associated stimuli after extinction in rats. Psychopharmacology. 2006;184:417–425. doi: 10.1007/s00213-005-0134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Liu X, Caggiula AR, Yee SK, Nobuta H, Sved AF, Pechnick RN, et al. Mecamylamine attenuates cue-induced reinstatement of nicotine-seeking behavior in rats. Neuropsychopharmacology. 2007;32:710–718. doi: 10.1038/sj.npp.1301129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Schnoll RA, Lerman C. Current and emerging pharmacotherapies for treating tobacco dependence. Expert Opin Emerg Drugs. 2006;11:429–444. doi: 10.1517/14728214.11.3.429. [DOI] [PubMed] [Google Scholar]
- 194.Lundahl LH, Henningfield JE, Lukas SE. Mecamylamine blockade of both positive and negative effects of IV nicotine in human volunteers. Pharmacol Biochem Behav. 2000;66:637–643. doi: 10.1016/s0091-3057(00)00252-5. [DOI] [PubMed] [Google Scholar]
- 195.Rose JE, Behm FM, Westman EC. Nicotine-mecamylamine treatment for smoking cessation: the role of pre-cessation therapy. Exp Clini Psychopharmacol. 1998;6:331–343. doi: 10.1037//1064-1297.6.3.331. [DOI] [PubMed] [Google Scholar]
- 196.Rose J. New findings on nicotine addiction and treatment. In: Bevins RA, Caggiula AR, editors. The motivational impact of nicotine and its role in tobacco use. Vol. 55. New York: Springer; 2009. pp. 131–141. [Google Scholar]
- 197.Cousins MS, Stamat HM, de Wit H. Acute doses of d-amphetamine and bupropion increase cigarette smoking. Psychopharmacology. 2001;157:243–253. doi: 10.1007/s002130100802. [DOI] [PubMed] [Google Scholar]
- 198.Dalsgareth OJ, Hansen NC, Soes-Petersen U, Evald T, Hoegholm A, Barber J, et al. A multicenter, randomized, double-blind, placebo-controlled, 6-month trial of bupropion hydrochloride sustained-release tablets as an aid to smoking cessation in hospital employees. Nicotine Tob Res. 2004;6:55–61. doi: 10.1080/14622200310001656867. [DOI] [PubMed] [Google Scholar]
- 199.Jorenby D. Clinical efficacy of bupropion in the management of smoking cessation. Drugs. 2002;62(Suppl 2):25–35. doi: 10.2165/00003495-200262002-00003. [DOI] [PubMed] [Google Scholar]
- 200.Shiffman S, Johnston JA, Khayrallah M, Elash CA, Gwaltney CJ, Paty JA, et al. The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology. 2000;148:33–40. doi: 10.1007/s002130050022. [DOI] [PubMed] [Google Scholar]
- 201.Sidhpura N, Redfern P, Rowley H, Heal D, Wonnacott S. Comparison of the effects of bupropion and nicotine on locomotor activation and dopamine release in vivo. Biochem Pharmacol. 2007;74:1292–1298. doi: 10.1016/j.bcp.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 202.Holm KJ, Spencer CM. Bupropion: a review of its use in the management of smoking cessation. Drugs. 2000;59:1007–1024. doi: 10.2165/00003495-200059040-00019. [DOI] [PubMed] [Google Scholar]
- 203.Berzewski H, Van Moffaert M, Gagiano CA. Efficacy and tolerability of reboxetine compared with imipramine in a double-blind study in patients suffering from major depressive offsodes. Eur Neuropsychopharmacol. 1997;7(Suppl 1):S37–S47. doi: 10.1016/s0924-977x(97)00418-5. discussion S71-3. [DOI] [PubMed] [Google Scholar]
- 204.Sacchetti G, Bernini M, Bianchetti A, Parini S, Invernizzi RW, Samanin R. Studies on the acute and chronic effects of reboxetine on extracellular noradrenaline and other monoamines in the rat brain. Br J Pharmacol. 1999;128:1332–1338. doi: 10.1038/sj.bjp.0702926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wong EH, Sonders MS, Amara SG, Tinholt PM, Piercey MF, Hoffmann WP, et al. Reboxetine: a pharmacologically potent, selective, and specific norepinephrine reuptake inhibitor. Biol Psychiatry. 2000;47:818–829. doi: 10.1016/s0006-3223(99)00291-7. [DOI] [PubMed] [Google Scholar]
- 206.Miller DK, Wong EH, Chesnut MD, Dwoskin LP. Reboxetine: functional inhibition of monoamine transporters and nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 2002;302:687–695. doi: 10.1124/jpet.302.2.687. [DOI] [PubMed] [Google Scholar]
- 207.Ascher JA, Cole JO, Colin JN, Feighner JP, Ferris RM, Fibiger HC, et al. Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry. 1995;56:395–401. [PubMed] [Google Scholar]
- 208.Ferris R, Cooper B. Mechanism of antidepressant activity of bupropion. J Clin Psychiatry. 1993;11:2–14. [PubMed] [Google Scholar]
- 209.Lecca D, Cacciapaglia F, Valentini V, Gronli J, Spiga S, Di Chiara G. Preferential increase of extracellular dopamine in the rat nucleus accumbens shell as compared to that in the core during acquisition and maintenance of intravenous nicotine self-administration. Psychopharmacology. 2006;184:435–446. doi: 10.1007/s00213-005-0280-4. [DOI] [PubMed] [Google Scholar]
- 210.Rau KS, Birdsall E, Hanson JE, Johnson-Davis KL, Carroll FI, Wilkins DG, et al. Bupropion increases striatal vesicular monoamine transport. Neuropharmacology. 2005;49:820–830. doi: 10.1016/j.neuropharm.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 211.Fryer JD, Lukas RJ. Noncompetitive functional inhibition at diverse, human nicotinic acetylcholine receptor subtypes by bupropion, phencyclidine, and ibogaine. J Pharmacol Exp Ther. 1999;288:88–92. [PubMed] [Google Scholar]
- 212.Slemmer JE, Martin BR, Damaj MI. Bupropion is a nicotinic antagonist. J Pharmacol Exp Ther. 2000;295:321–327. [PubMed] [Google Scholar]
- 213.Decker MW, Anderson DJ, Brioni JD, Donnelly-Roberts DL, Kang CH, O'Neill AB, et al. Erysodine, a competitive antagonist at neuronal nicotinic acetylcholine receptors. Eur J Pharmacol. 1995;280:79–89. doi: 10.1016/0014-2999(95)00191-m. [DOI] [PubMed] [Google Scholar]
- 214.Lukas RJ, Fryer JD, Eaton J, Gentry CL. Some methods for studies of nicotinic acetylcholine receptor pharmacology. In: Levin ED, editor. Nicotinic Receptors and the Nervous System. Boca Raton: CRC Press; 2002. pp. 3–27. [Google Scholar]
- 215.Marks MJ, Bullock AE, Collins AC. Sodium channel blockers partially inhibit nicotine-stimulated 86Rb+ efflux from mouse brain synaptosomes. J Pharmacol Exp Ther. 1995;274:833–841. [PubMed] [Google Scholar]
- 216.Marks MJ, Farnham DA, Grady SR, Collins AC. Nicotinic receptor function determined by stimulation of rubidium efflux from mouse brain synaptosomes. J Pharmacol Exp Ther. 1993;264:542–552. [PubMed] [Google Scholar]
- 217.Puttfarcken PS, Manelli AM, Arneric SP, Donnelly-Roberts DL. Evidence for nicotinic receptors potentially modulating nociceptive transmission at the level of the primary sensory neuron: studies with F11 cells. J Neurochem. 1997;69:930–938. doi: 10.1046/j.1471-4159.1997.69030930.x. [DOI] [PubMed] [Google Scholar]
- 218.Xiao Y, Meyer EL, Thompson JM, Surin A, Wroblewski J, Kellar KJ. Rat alpha3/beta4 subtype of neuronal nicotinic acetylcholine receptor stably expressed in a transfected cell line: pharmacology of ligand binding and function. Mol Pharmacol. 1998;54:322–333. doi: 10.1124/mol.54.2.322. [DOI] [PubMed] [Google Scholar]
- 219.Damaj MI, Carroll FI, Eaton JB, Navarro HA, Blough BE, Mirza S, et al. Enantioselective effects of hydroxy metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors. Mol Pharmacol. 2004;66:675–682. doi: 10.1124/mol.104.001313. [DOI] [PubMed] [Google Scholar]
- 220.Miller DK, Sumithran SP, Dwoskin LP. Bupropion inhibits nicotine-evoked [3H]overflow from rat striatal slices preloaded with [3H]dopamine and from rat hippocampal slices preloaded with [3H]norepinephrine. J Pharmacol Exp Ther. 2002;302:1113–1122. doi: 10.1124/jpet.102.033852. [DOI] [PubMed] [Google Scholar]
- 221.Yoshimura RF, Hogenkamp DJ, Li WY, Tran MB, Belluzzi JD, Whittemore ER, et al. Negative allosteric modulation of nicotinic acetylcholine receptors blocks nicotine self-administration in rats. J Pharmacol Exp Ther. 2007;323:907–915. doi: 10.1124/jpet.107.128751. [DOI] [PubMed] [Google Scholar]
- 222.Johnstone TB, Hogenkamp DJ, Coyne L, Su J, Halliwell RF, Tran MB, et al. Modifying quinolone antibiotics yields new anxiolytics. Nature Med. 2004;10:31–32. doi: 10.1038/nm967. [DOI] [PubMed] [Google Scholar]
- 223.Wilkins LH, Jr, Grinevich VP, Ayers JT, Crooks PA, Dwoskin LP. N-n-alkylnicotinium analogs, a novel class of nicotinic receptor antagonists: interaction with alpha4beta2* and alpha7* neuronal nicotinic receptors. J Pharmacol Exp Ther. 2003;304:400–410. doi: 10.1124/jpet.102.043349. [DOI] [PubMed] [Google Scholar]
- 224.Wilkins LH, Jr, Haubner A, Ayers JT, Crooks PA, Dwoskin LP. N-n-alkylnicotinium analogs, a novel class of nicotinic receptor antagonist: inhibition of S(−)-nicotine-evoked [3H]dopamine overflow from superfused rat striatal slices. J Pharmacol Exp Ther. 2002;301:1088–1096. doi: 10.1124/jpet.301.3.1088. [DOI] [PubMed] [Google Scholar]
- 225.Grinevich VP, Crooks PA, Sumithran SP, Haubner AJ, Ayers JT, Dwoskin LP. N-n-alkylpyridinium analogs, a novel class of nicotinic receptor antagonists: selective inhibition of nicotine-evoked [3H] dopamine overflow from superfused rat striatal slices. J Pharmacol Exp Ther. 2003;306:1011–1020. doi: 10.1124/jpet.103.051789. [DOI] [PubMed] [Google Scholar]
- 226.Everett AJ, Lowe LA, Wilkinson S. Revision of the Structure of (+)-Tubocuarine Chloride and the (+) Chondrocuraine. Chem Commun Chem Soc London. 1970:1020–1021. [Google Scholar]
- 227.Koelle GB. Cholinergic and non-cholinergic pharmacology of the bronchioles. Postgrad Med J. 1975;51:63–68. [PubMed] [Google Scholar]
- 228.Michelson M, Zaimal E. Acetylcholine, an approach to the molecular mechanism of action. Oxford: Pergammon Press; 1973. [Google Scholar]
- 229.Ayers JT, Dwoskin LP, Deaciuc AG, Grinevich VP, Zhu J, Crooks PA. bis-Azaaromatic quaternary ammonium analogues: ligands for alpha4beta2* and alpha7* subtypes of neuronal nicotinic receptors. Bioorg Med Chem Lett. 2002;12:3067–3071. doi: 10.1016/s0960-894x(02)00687-x. [DOI] [PubMed] [Google Scholar]
- 230.Dwoskin LP, Sumithran SP, Zhu J, Deaciuc AG, Ayers JT, Crooks PA. Subtype-selective nicotinic receptor antagonists: potential as tobacco use cessation agents. Bioorg Med Chem Lett. 2004;14:1863–1867. doi: 10.1016/j.bmcl.2003.10.073. [DOI] [PubMed] [Google Scholar]
- 231.Dwoskin LP, Wooters TE, Sumithran SP, Siripurapu KB, Joyce BM, Lockman PR, et al. N,N'-Alkane-diyl-bis-3-picoliniums as Nicotinic Receptor Antagonists: Inhibition of Nicotine-induced Dopamine Release and Hyperactivity. J Pharmacol Exp Ther. 2008;326:563–576. doi: 10.1124/jpet.108.136630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Allen DD, Lockman PR, Roder KE, Dwoskin LP, Crooks PA. Active transport of high-affinity choline and nicotine analogs into the central nervous system by the blood-brain barrier choline transporter. J Pharmacol Exp Ther. 2003;304:1268–1274. doi: 10.1124/jpet.102.045856. [DOI] [PubMed] [Google Scholar]
- 233.Lockman PR, Manda VK, Geldenhuys WJ, Mittapalli RK, Thomas F, Albayati ZF, et al. Carrier-mediated transport of the quaternary ammonium neuronal nicotinic receptor antagonist N,N'-dodecyl-bis-picolinium dibromide at the blood-brain barrier. J Pharmacol Exp Ther. 2008;324:244–250. doi: 10.1124/jpet.107.130906. [DOI] [PubMed] [Google Scholar]
- 234.Rahman S, Neugebauer NM, Zhang Z, Crooks PA, Dwoskin LP, Bardo MT. The effects of a novel nicotinic receptor antagonist N,N-dodecane-1,12-diyl-bis-3-picolinium dibromide (bPiDDB) on acute and repeated nicotine-induced increases in extracellular dopamine in rat nucleus accumbens. Neuropharmacology. 2007;52:755–763. doi: 10.1016/j.neuropharm.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 235.Neugebauer NM, Zhang Z, Crooks PA, Dwoskin LP, Bardo MT. Effect of a novel nicotinic receptor antagonist, N,N'-dodecane-1,12-diyl-bis-3-picolinium dibromide, on nicotine self-administration and hyperactivity in rats. Psychopharmacology. 2006;184:426–434. doi: 10.1007/s00213-005-0163-8. [DOI] [PubMed] [Google Scholar]
- 236.Benwell ME, Balfour DJ. The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. Br J Pharmacol. 1992;105:849–856. doi: 10.1111/j.1476-5381.1992.tb09067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Cadoni C, Di Chiara G. Differential changes in accumbens shell and core dopamine in behavioral sensitization to nicotine. Eur J Pharmacol. 2000;387:R23–R25. doi: 10.1016/s0014-2999(99)00843-2. [DOI] [PubMed] [Google Scholar]
- 238.Albayati ZA, Dwoskin LP, Crooks PA. Pharmacokinetics of the novel nicotinic receptor antagonist N,N'-dodecane-1,12-diyl-bis-3-picolinium dibromide in the rat. Drug Metab Dispos. 2008;36:2024–2029. doi: 10.1124/dmd.108.020354. [DOI] [PMC free article] [PubMed] [Google Scholar]