Abstract
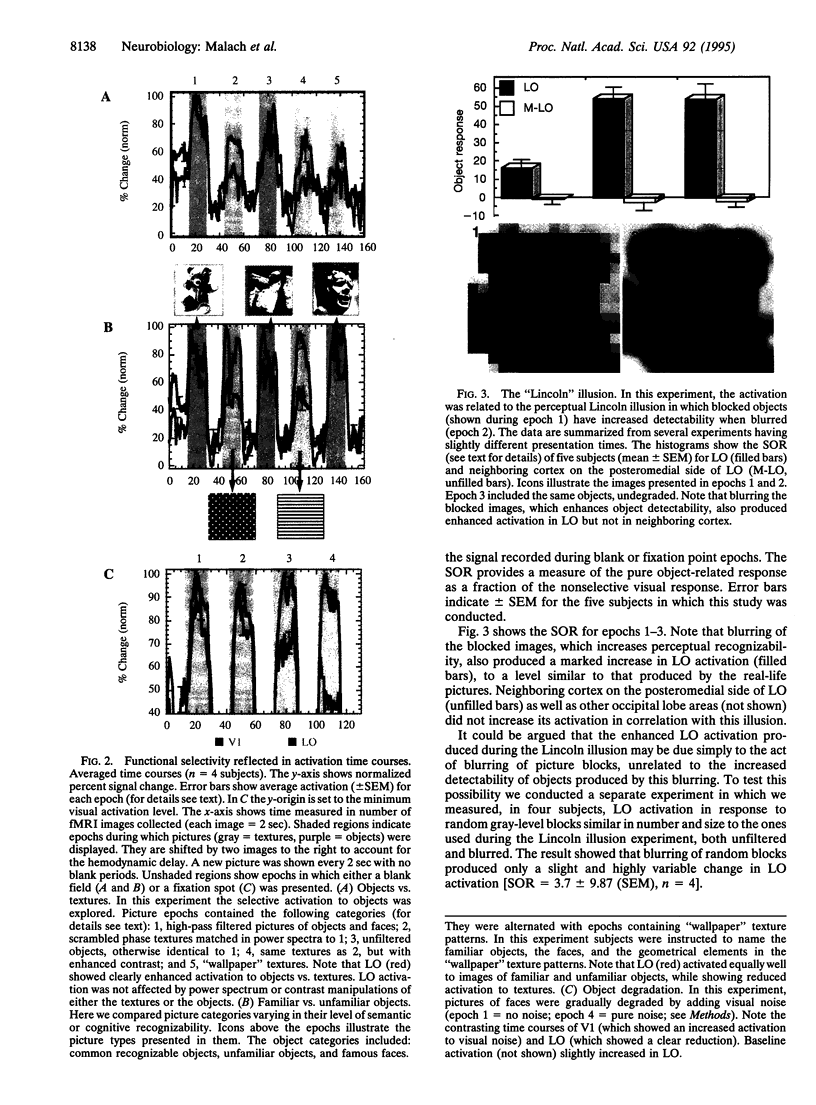
The stages of integration leading from local feature analysis to object recognition were explored in human visual cortex by using the technique of functional magnetic resonance imaging. Here we report evidence for object-related activation. Such activation was located at the lateral-posterior aspect of the occipital lobe, just abutting the posterior aspect of the motion-sensitive area MT/V5, in a region termed the lateral occipital complex (LO). LO showed preferential activation to images of objects, compared to a wide range of texture patterns. This activation was not caused by a global difference in the Fourier spatial frequency content of objects versus texture images, since object images produced enhanced LO activation compared to textures matched in power spectra but randomized in phase. The preferential activation to objects also could not be explained by different patterns of eye movements: similar levels of activation were observed when subjects fixated on the objects and when they scanned the objects with their eyes. Additional manipulations such as spatial frequency filtering and a 4-fold change in visual size did not affect LO activation. These results suggest that the enhanced responses to objects were not a manifestation of low-level visual processing. A striking demonstration that activity in LO is uniquely correlated to object detectability was produced by the "Lincoln" illusion, in which blurring of objects digitized into large blocks paradoxically increases their recognizability. Such blurring led to significant enhancement of LO activation. Despite the preferential activation to objects, LO did not seem to be involved in the final, "semantic," stages of the recognition process. Thus, objects varying widely in their recognizability (e.g., famous faces, common objects, and unfamiliar three-dimensional abstract sculptures) activated it to a similar degree. These results are thus evidence for an intermediate link in the chain of processing stages leading to object recognition in human visual cortex.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison T., Ginter H., McCarthy G., Nobre A. C., Puce A., Luby M., Spencer D. D. Face recognition in human extrastriate cortex. J Neurophysiol. 1994 Feb;71(2):821–825. doi: 10.1152/jn.1994.71.2.821. [DOI] [PubMed] [Google Scholar]
- Bandettini P. A., Wong E. C., Hinks R. S., Tikofsky R. S., Hyde J. S. Time course EPI of human brain function during task activation. Magn Reson Med. 1992 Jun;25(2):390–397. doi: 10.1002/mrm.1910250220. [DOI] [PubMed] [Google Scholar]
- Belliveau J. W., Kennedy D. N., Jr, McKinstry R. C., Buchbinder B. R., Weisskoff R. M., Cohen M. S., Vevea J. M., Brady T. J., Rosen B. R. Functional mapping of the human visual cortex by magnetic resonance imaging. Science. 1991 Nov 1;254(5032):716–719. doi: 10.1126/science.1948051. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Miezin F. M., Dobmeyer S., Shulman G. L., Petersen S. E. Selective and divided attention during visual discriminations of shape, color, and speed: functional anatomy by positron emission tomography. J Neurosci. 1991 Aug;11(8):2383–2402. doi: 10.1523/JNEUROSCI.11-08-02383.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon L. D., Julesz B. Masking in visual recognition: effects of two-dimensional filtered noise. Science. 1973 Jun 15;180(4091):1194–1197. doi: 10.1126/science.180.4091.1194. [DOI] [PubMed] [Google Scholar]
- Haxby J. V., Grady C. L., Horwitz B., Ungerleider L. G., Mishkin M., Carson R. E., Herscovitch P., Schapiro M. B., Rapoport S. I. Dissociation of object and spatial visual processing pathways in human extrastriate cortex. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1621–1625. doi: 10.1073/pnas.88.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong K. K., Belliveau J. W., Chesler D. A., Goldberg I. E., Weisskoff R. M., Poncelet B. P., Kennedy D. N., Hoppel B. E., Cohen M. S., Turner R. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S., Tank D. W., Menon R., Ellermann J. M., Kim S. G., Merkle H., Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno M. I., Dale A. M., Reppas J. B., Kwong K. K., Belliveau J. W., Brady T. J., Rosen B. R., Tootell R. B. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995 May 12;268(5212):889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- Sergent J., Ohta S., MacDonald B. Functional neuroanatomy of face and object processing. A positron emission tomography study. Brain. 1992 Feb;115(Pt 1):15–36. doi: 10.1093/brain/115.1.15. [DOI] [PubMed] [Google Scholar]
- Tanaka K. Neuronal mechanisms of object recognition. Science. 1993 Oct 29;262(5134):685–688. doi: 10.1126/science.8235589. [DOI] [PubMed] [Google Scholar]
- Ungerleider L. G., Haxby J. V. 'What' and 'where' in the human brain. Curr Opin Neurobiol. 1994 Apr;4(2):157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Watson J. D., Myers R., Frackowiak R. S., Hajnal J. V., Woods R. P., Mazziotta J. C., Shipp S., Zeki S. Area V5 of the human brain: evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cereb Cortex. 1993 Mar-Apr;3(2):79–94. doi: 10.1093/cercor/3.2.79. [DOI] [PubMed] [Google Scholar]