Abstract
Posttraumatic stress disorder (PTSD) is associated with smaller volumes of the hippocampus, as has been demonstrated by meta-analyses. Proposed mechanistic relationships are reviewed briefly, including the hypothesis that sleep disturbances mediate the effects of PTSD on hippocampal volume. Evidence for this includes findings that insomnia and restricted sleep are associated with changes in hippocampal cell regulation and impairments in cognition. We present results of a new study of 187 subjects in whom neither PTSD nor poor sleep was associated with lower hippocampal volume. We outline a broad research agenda centered on the hypothesis that sleep changes mediate the relationship between PTSD and hippocampal volume.
Keywords: Neurogenesis, Neuroimaging, Dementia, Veterans, Sleep, PTSD
1. Introduction
Multiple studies have identified smaller hippocampal volumes in subjects with posttraumatic stress disorder (PTSD) [5,12,13,42,79,85]. Not all studies have found smaller hippocampal volumes [17,39,67,68], and various groups have suggested that hippocampal volume differences in PTSD are attributable to comorbid conditions such as alcoholism [84] or depression [14]. Still, in most studies of hippocampal volume in PTSD investigators have controlled for major psychiatric comorbidities, including substance abuse, and meta-analyses reveal that the correlation between PTSD and smaller hippocampal volume is widely replicated [1,10,21,79]. Studies by our group suggest that hippocampal size in PTSD may change in subjects who either go into remission or have worsening of symptoms over time [5,12,13,20,42,79,85]. In addition, the severity of the PTSD has been inversely correlated with hippocampal volume [13,17,35,39,67,68,76].
Decreased hippocampal size is not specific for PTSD, because it is present in such varied psychiatric conditions as depression [16,84], schizophrenia [14,55], and alcoholism [1,2,10,21,79,84]. Sleep deficits are present in each of the aforementioned disorders [23,59,70]. As recently reviewed by Germain [34], PTSD is highly correlated with sleep deficits, including increased stage 1 sleep, decreased slow-wave sleep, and increased density of rapid eye movement sleep. In fact, some authors (in particular, Ross et al. [66]) have proposed that PTSD is a problem, primarily, of sleep. This raises the possibility that differences in hippocampal size are mediated by sleep pathology. (A similar mediating relationship has been found by our group, for example, in the association between PTSD and decreased parietal-occipital gamma-aminobutyric acid concentration [52].)
The relationship between sleep disturbance and hippocampal volume has been investigated with mixed results. Rieman et al. [64] measured hippocampal volumes in eight patients with chronic primary insomnia (PI) and found them to be significantly smaller than those of matched controls, although the statistical significance did not survive correction for multiple comparisons. Winkelman et al. [82] followed this with a study of 20 patients with PI and 15 good-sleeping control patients in which they used manually defined hippocampal traces and found no difference in hippocampal volumes between the 2 groups. Noh et al. [58] found that, in 20 individuals with PI, hippocampal volume was negatively correlated with duration of insomnia symptoms. In this study, manually measured hippocampal volumes did not differ statistically from matched controls, although subjects classified as having PI did not differ significantly in total sleep time or sleep efficiency. Similarly, Winkelman et al. [83] found that hippocampal volumes did not differ between subjects with PI and normal controls, although their primary region of interest was the rostral anterior cingulate cortex. However, hippocampal volume was negatively correlated with wake after sleep onset and positively correlated with sleep efficiency.
Several voxel-based morphometry studies in which the authors used whole-brain instead of a priori regions of interest analyses found no differences in gray matter (GM) in the area of the hippocampus between good and poor sleepers [3,41,69,71]. Our group found that worse insomnia was correlated with smaller volumes of the CA3 and dentate areas of the hippocampus [57]. Recently, in a region-of-interest study, we found no significant difference between self-assessments of sleep and hippocampal volume, although there was an inverse correlation with total cortical and frontal GM volume at 1.5 Tesla [19]. Few studies of hippocampal volume aside from these have controlled for sleep quality or quantity. Overall, numerous studies have demonstrated hippocampal size differences inversely correlated with insomnia severity or severity of sleep deprivation. Still, the evidence is mixed, with most (but not all) whole-brain analyses (rather than region-of-interest approaches) tending to show no hippocampal differences (Table 1). This may be because whole-brain approaches have less power to detect differences in any one region when results are subjected to stringent controls for multiple comparisons.
Table 1.
Presentation of studies of brain gray matter in sleep disorders discussed in the text
| Study | Method | Samples | Hippocampal findings |
|---|---|---|---|
| Chao et al. [19] | ROI | 144 Gulf War veterans | No significant difference in hippocampal size based on self-rated sleep quality |
| Winkelman et al. [83] | ROI including hippocampus | 20 patients with PI and 20 matched controls—same cohort as [82] | Hippocampal volumes did not differ between groups; left hippocampal volume correlated with wake after sleep onset (negative correlation) and sleep efficiency (positive correlation) measured objectively |
| Spiegelhalder et al. [69] | VBM with DARTEL, ROI | 28 patients with PI and 38 matched controls | No difference in hippocampal size between groups |
| Joo et al. [41] | VBM with DARTEL | 27 patients with PI and 27 matched controls | No apparent differences in region of hippocampus between groups |
| Killgore, Schwab, Kipman, DelDonno, and Weber, 2012 | VBM with DARTEL | 36 healthy subjects | No significant difference in hippocampal size based on self-rated daytime sleepiness based on whole-brain a priori results |
| Taki et al. [72] | VBM with DARTEL | 290 children with various degrees of sleep decrease on school nights | Hippocampus smaller in children sleeping less (finding survived correction for multiple comparisons) |
| Stoffers et al. [71] | VBM with DARTEL using ROI based on the peak voxel from whole-brain analysis | 65 healthy subjects | Hippocampal differences not discussed but hippocampus not identified as within the defined ROI |
| Noh et al. [58] | Manual morphometry | 20 patients with PI and 20 matched controls | Hippocampal volume negatively correlated with duration of insomnia symptoms; hippocampal volumes did not differ statistically from matched controls, although subjects classified as having PI did not differ significantly in total sleep time or sleep efficiency |
| Winkelman et al. [82] | Manual morphometry | 20 patients with PI and 15 matched controls | No difference in hippocampal size between groups |
| Neylan et al. [57] | Manual morphometry | Subjective sleep assessment of 17 patients with PTSD and 19 matched controls | Worse insomnia correlated with smaller volumes of the CA3 and dentate areas of the hippocampus |
| Altena et al. [3] | VBM | 24 patients with PI and 13 matched controls | No gray matter differences in the area of the hippocampus between groups |
| Riemann et al. [64] | Manual morphometry | 8 patients with PI and 8 matched controls | Hippocampus smaller in insomnia but finding did not survive correction for multiple comparisons |
Abbreviations: DARTEL, diffeomorphic anatomical registration using exponentiated lie algebra; PI, primary insomnia; PTSD, posttraumatic stress disorder; ROI, region of interest; VBM, voxel-based morphometry.
We conducted a voxel-based morphometry analysis comparing GM volume with the severity of PTSD severity and with self-reported sleep quality by using standardized measures. Our a priori hypothesis was that sleep changes would mediate hippocampal volume changes associated with PTSD. We used a data set composed of veterans with PTSD, without PTSD but exposed to trauma, with recovered PTSD, and without any history of PTSD or trauma exposure. We designed a study in which we would test for differences in hippocampal size associated with severity of PTSD symptoms and then investigate whether those differences were mediated by differences in self-reported insomnia severity. In case no hippocampal size differences were associated with PTSD severity, we planned to test whether, in the same data set, hippocampal size differences were associated independently with self-reported insomnia severity.
2. Methods
Our study included 136 participants recruited via a list of Gulf War veterans provided by the Department of Defense and by advertisements placed in the community. Details of the recruitment methods have been reported elsewhere [18]. There was no effort to specifically recruit participants with PTSD. However, because it was recognized that PTSD, depression, and alcohol abuse/dependence might affect cognition, clinical symptoms, brain structure, and metabolism at the onset of the study, quantitative measurements of these variables were obtained. Inclusion criterion was being a US veteran of the First Persian Gulf War; exclusion criteria were severe physical impairment or medical illness, current or lifetime history of psychosis or of suicidal or homicidal ideation, history of neurologic or systemic illness affecting central nervous system functioning, a history of head injury with loss of consciousness for 10 minutes or longer, severe claustrophobia, and/or ferromagnetic objects in the body.
All subjects included in study completed, before undergoing the scanning, a Clinician-Administered PTSD Scale (CAPS) questionnaire and a Pittsburgh Sleep Quality Index (PSQI) questionnaire. The CAPS is a 30-item clinician-administered standardized interview assessing for PTSD based on criteria from the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition [6,80]. Greater scores indicate more severe PTSD. The PSQI is a self-rated 19-item questionnaire used to evaluate qualitative and quantitative aspects of sleep during the previous 1-month period. Greater PSQI scores indicate worse sleep [15]. A sleep-disordered breathing (SDB) score was assigned on the basis of the PSQI responses to questions related to not being able to breathe comfortably and coughing or snoring loudly and assessments by bed partners of snoring and pauses between breaths. Because obesity is associated with SDB [30], we separately controlled for body mass index (BMI) when employing our SDB score.
Each subject had undergone brain magnetic resonance imaging with a 4-Tesla (Bruker/Siemens, Malvern, PA) system with a birdcage transmit and eight-channel receive coil. T1-weighted scans were obtained with a 3-dimensional volumetric magnetization prepared rapid gradient echo sequence, TR/TE/TI = 2300/3/950 ms timing; 71-degree flip angle; isotropic 1.0 mm3 resolution; 157 contiguous sagittal slices. Scans were processed using SPM8 (Welcome Department of Cognitive Neurology, Institute of Neurology, University College London, UK; http://www.fil.ion.ucl.ac.uk/spm). The initial processing steps were drawn from this program’s voxel-based morphometry toolboxes as described by Taki et al. [72]. Scans were aligned, segmented into gray and white matter and cerebrospinal fluid, affine-registered to a standard Montreal Neurological Institute [29] brain template, and smoothed using an 8 mm smoothing kernel.
All results were analyzed in SPM8 using multivariate analysis of covariance. In our initial planned studies, we defined as the independent and dependent variables CAPS score and GM volume, respectively, and then PSQI score and GM volume. Results were corrected for multiple comparisons using the method of topological false-discovery rate at a threshold of T = 3 [22] and a minimum voxel size of 10. In subsequent post-hoc analyses, results were analyzed at P < .01 without separate correction for multiple comparisons. Results were analyzed using two-dimensional “glass brains” native to SPM8. In cases in which analysis revealed differences in GM between groups, areas of difference were projected onto sections of the “Colin27” normal brain in the same space from the Montreal Neurological Institute (http://www.nil.wustl.edu/labs/kevin/man/answers/mnispace.html) to facilitate identification of anatomical regions.
Initial tests for association of hippocampal size with PTSD and insomnia severity were conducted using the following as covariates: total intracranial volume, age, and sex. In case of findings using these covariates, further analyses included these additional covariates: SDB score, BMI, tobacco smoking status, maximum alcohol use in the past week, Beck’s Depression Inventory score, presence or absence of Gulf War illness (GWI) and history of exposure to sarin toxin. GWI is a chronic multisystem syndrome affecting military personnel and civilians who were in Iraq in 1991 [32]. Sarin is a neurotoxin implicated in the etiology of GWI. We previously found exposure to sarin to be associated with certain cognitive deficits and overall lower gray and white matter volumes in a study sample with significant overlap with the one described here [18]. GWI was previously identified in a fraction of study participants considered here, whereas exposure to sarin was found to be associated with lower GM volume in an overlapping data set [18]. Methods of determining GWI and sarin exposure are described in Fukuda et al. [32] and Chao et al. [18]; respectively. To summarize in brief, GWI was determined using criteria defined by the Centers for Disease Control and Prevention [32] and information on sarin exposure was provided by the US Deputy Assistant Secretary of Defense for Force Health Protection and Readiness [18].
Finally, data were processed using an explicit, inclusive mask over the regions of the hippocampus and parahippocampal regions to confirm that areas of differences in GM were located in a predefined region that included the bilateral hippocampus. The mask was obtained by combining the right and left regions labeled “hippocampus” and “parahippocampal” from an atlas used in the automated anatomical labeling procedure described by Tzourio-Mazoyer et al. [73]. We included parahippocampal areas in our mask to decrease the chance of type II statistical error in this study.
3. Results
Mean subject age was 50.2 years (SD 7.7), mean education level was 15.5 years (SD 2.3), and 13 subjects (7%) were female. Eight (6%) subjects reported that they smoked cigarettes regularly at the time of scanning, whereas 42 (31%) subjects reported drinking alcohol in the past week. Of those who reported drinking alcohol in the past week, the average number of drinks consumed was 6.4 (range 1–28). The average BMI was 29 (SD 4.0). The average Beck’s Depression Inventory score was 7.4 (SD 8.3). The average score on the PSQI for the total sample was eight (SD 4.3). Of 52 (38%) non-zero CAPS scores, the average was 22.3, with nine (7%) subjects categorized as having current PTSD. Fourteen (10%) subjects had been categorized as having GWI, and 60 (44%) had been determined to have been exposed to sarin.
In our initial planned analysis of hippocampal size and CAPS score, we found no differences in GM volume in any brain region at the predefined threshold of T = 3 when we controlled for total intracranial volume, age, and sex. In subsequent post-hoc analyses, no differences emerged at a threshold of P <.01 when we used a minimum voxel size 0.
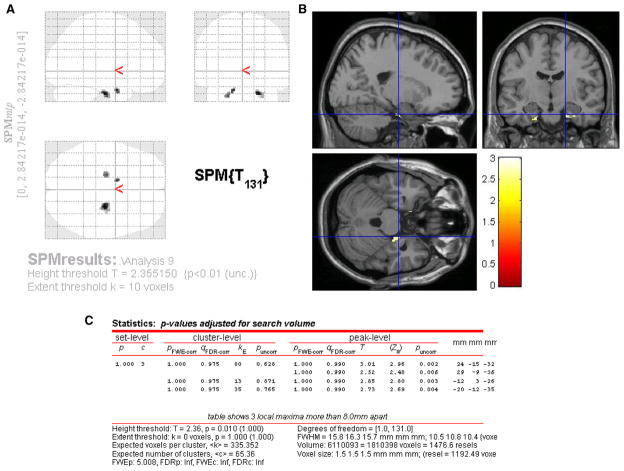
In our initial planned analysis of hippocampal size and PSQI score, we found no differences in GM volume in any brain region at the predefined threshold of T = 3 when we controlled for total intracranial volume, age, and sex. In subsequent post-hoc analyses, using threshold of P < .01 and controlling for intracranial volume, age, and sex, we found PSQI score to be negatively associated with GM in the area of the left hippocampus and entorhinal cortex bilaterally. The findings in the area of the hippocampus were absent at a minimum voxel size setting of 20 voxels, whereas all findings at P < .01 remained present at a minimum voxel size threshold of 10 voxels. Repeating our post-hoc analysis using our inclusive mask of the hippocampal region provided independent confirmation of anatomical location within the area of the hippocampus. These results are presented in Figure 1.
Fig. 1.
Results of voxel-based morphometry study of gray matter correlations with self-assessed sleep quality. Images are presented in radiologic format, with subject’s left to reader’s left. Areas of reduced gray matter volume are superimposed on “glass brain” (A) and standardized brain template Colin27 (B) from the Montreal Neurological Institute (http://www.nil.wustl.edu/labs/kevin/man/answers/mnispace.html). Statistics for each cluster are presented in (C).
4. Discussion
Our results indicate that, in our sample, neither PTSD nor insomnia severity predicted hippocampal size differences when we corrected data for multiple comparisons using the method of false-discovery rate. Using less-stringent statistical controls, we found that post-hoc analyses likewise demonstrated no association between the severity of PTSD and hippocampal size but did reveal an association between the severity of insomnia and hippocampal size, in particular on the left side.
Overall, caps scores were relatively low for this sample among those with PTSD. It could be that lower hippocampal size is an effect only seen in patients with severe PTSD [61]. For instance, Gilbertson et al. [35] found differences in hippocampal size only in subjects with CAPS scores greater than 65. A meta-analysis [42] of studies of hippocampal volume in PTSD found this to be the case. The CAPS scores of subjects in this study were relatively low.
It is widely recognized that sleep facilitates learning and memory in rodents and humans [77]. Inversely, sleep impairment leads predictably to numerous cognitive deficits. In humans, these include prolonged reaction times, decreased resistance to cognitive fatigue, impaired decision-making, increased impulsivity and risk taking, decreased self-monitoring of error (decreased error-monitoring), altered moral judgment, diminished appreciation for humor, and reduced ability to discriminate odors [8,9,24,31,40,50,65,74,75,78]. Thus, in humans, the known cognitive changes secondary to sleep deficits, although not all readily attributable to limbic network changes, would seem to evidence neural pathology on a very broad scale. In rodents, sleep loss leads to decreased hippocampal neural plasticity [25], α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor phosphorylation [38], decreased hippocampal cell proliferation [36], and decreased hippocampal N-methyl-D-aspartate receptor number [37]. Although the exact mechanisms responsible remain speculative, it is generally accepted that sleep effects cognition in humans in part via such structural and functional neuronal changes [63]. This finding is further supported by evidence that sleep problems predict and precede dementia [60].
Most of the studies to date that have investigated structural brain differences in chronic insomnia [3,64,69,82,83] have relied on criteria for the diagnosis of PI from the Diagnostic and Statistical Manual for Mental Disorders, IV edition [6], and 2 [41,58] have used criteria from the International Classification of Sleep Disorders or relied on a model of acutely reduced sleep quantity [72]. PI and environmental sleep restrictions may each have limitations as a model for the sleep patterns observed in PTSD. Criteria for PI include nonrestorative sleep [6], a feature not necessary for the diagnosis of PTSD. Subjective sleep complaints in PTSD vary but likely include altered perception of total sleep time compared with healthy patients [45]. The sleep features observed in PTSD include greater fraction of stage 1 sleep relative to slow-wave sleep and greater rapid eye movement sleep density [44], which are features that have not been consistently found in PI.
Previous investigators have found negative correlations between hippocampal size and sleep quality or quantity, although this has also been a finding that has not been completely consistent in previous studies. In this study, we did find a negative correlation in post-hoc analyses between hippocampal size and sleep quality, although at lower statistical thresholds than those used in our initially planned analyses. This is a relatively large sample size that used a subjective measure of sleep quality. Most previous studies have depended on the diagnosis of PI, rather than PSQI scores, and have included approximately 20 subjects. To date, the largest study of hippocampal size and sleep quality (290 subjects) found smaller hippocampal sizes in subjects sleeping fewer hours and did not depend on the diagnosis of PI. It is possible that the PSQI scores in our data set were too high or were not variable enough to reveal differences in hippocampal size using more stringent statistical thresholds. Overall, however, the larger studies thus far, including this one, have demonstrated a trend toward smaller hippocampal sizes in subjects with poorer sleep.
The mechanism by which PTSD confers increased risk of dementia has not been identified, although proposed hypotheses have included reduced cognitive reserve and glucocorticoid-mediated or inflammatory cell damage [62,86]. More research is needed in the area of PTSD and cognitive changes. It is vitally important to establish the mechanism by which PTSD precipitates cognitive changes as well as the process by which the disorder increases the risk of dementia later in life. The hippocampus is vitally important to the formation of new memories [27,54]. The cognitive changes associated with PTSD in conjunction with findings of changes in the hippocampus raise the possibility that PTSD confers significant risk for progressive cognitive decline by a mechanism related to hippocampal functional changes.
The rationale for structural imaging of the hippocampus in PTSD has centered on the hypothesis that volumetric differences may correlate with critical neurophysiology and neuronal function. It is well established at this point that human neurons are capable of structural changes throughout life [43] and that neurogenesis remains possible in the adult [28]. Furthermore, imaging studies from humans indicate that hippocampal size increases in the setting of treatment [11,48] and learning [26,49]. Studies in animals reveal that learning leads to hippocampal neurogenesis, increased dendritic branching, changes in the number of dendritic synapses [47], and increases in long-term potentiation [81]. In rodents, stress results in decreased hippocampal neurogenesis as well as decreased overall hippocampal volume [51].
The diagnosis of PTSD carries with it certain implications of limbic dysfunction. The disorder is characterized by emotional activation from minimal contextual cues. This process bypasses the contextual contributions to memory that depend on the hippocampus [1]. Investigators have thus searched for hippocampal structural differences in this disorder characterized by hippocampal dysfunction at the phenotype level.
Available data indicate a range of cognitive abnormalities associated with PTSD. These include lower general cognitive ability, impairments in attention, visuospatial deficits, more negative appraisals, decrements on verbal and autobiographical memory, source monitoring difficulties, and attentional biases [46,53]. Studies by Yaffe et al. [86] and by Qureshi et al. [62] indicate that PTSD poses significant added risk for dementia later in life.
Figures 2–4 illustrate explanatory models linking poor sleep, PTSD, and hippocampal changes. Figure 2 illustrates 2 predominant models in the current literature. In one model relating hippocampal volume and PTSD (Fig. 2A), a subject might develop PTSD and its attendant sleep and cognitive changes, which then impair limbic circuitry, as evidenced by hippocampal volume changes. Alternatively, in Figure 2B, differences in hippocampal volume might confer risk for PTSD, which then precipitates numerous behavioral changes, including sleep impairment. Poor sleep is generally considered a consequence of PTSD (as represented by its placement to the right of PTSD in Figs. 2A and 2B), whereas hippocampal volume has been considered separately to either result from PTSD or increase the risk of the disorder.
Fig. 2.
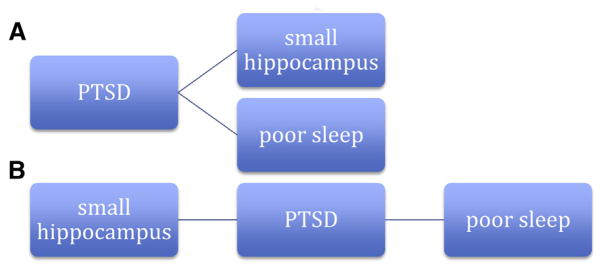
Two separate current working models (A and B) relating posttraumatic stress disorder to hippocampal size. Potential causal pathways proceed left to right.
Fig. 4.
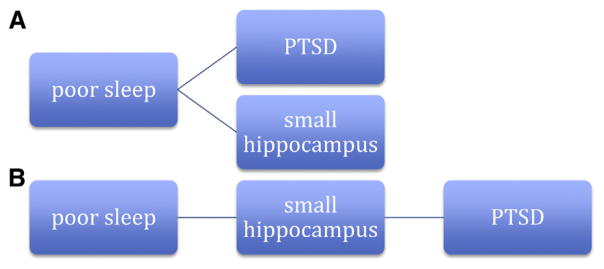
Alternate models (A and B) including sleep in association of posttraumatic stress disorder and hippocampal volume differences.
Figure 3 presents the aforementioned explanatory models identified but includes sleep changes as a mediating variable. If hippocampal volumes in subjects with PTSD are smaller because of changes in volume on the onset of the disorder (Fig. 2A), then this may be mediated by changes in sleep (Fig. 3A). The cognitive deficits associated with impaired sleep might be accompanied by changes in hippocampal volume. Given that PTSD is also accompanied by various cognitive changes, it could be that sleep deficits mediate both cognitive deficits and hippocampal volume changes. Although studies have shown that poor sleep is associated with smaller hippocampal volumes, only one study to date has considered the relative contributions of PTSD and sleep to hippocampal volume.
Fig. 3.
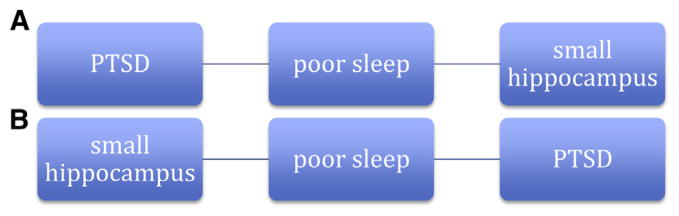
Two models (A and B) illustrating poor sleep as a mediating variable in association between posttraumatic stress disorder and hippocampal size.
Alternatively, if (as in Fig. 2B), smaller hippocampal volume constitutes a risk factor for PTSD, then poor sleep might mediate this relationship (Fig. 3B). In this case, individuals with small hippocampal volumes would be less capable of achieving high-quality sleep and thus are less resilient in the setting of acute stressors to developing PTSD. The hippocampus is vitally important to contextual memory encoding during sleep and likely relies on hippocampal-cortical connectivity during slow-wave sleep [4]. Slow-wave sleep is generally reduced in PTSD [44]. There is no evidence that small hippocampal volumes constitute a risk factor for poor sleep and it has not been determined whether individuals with poor sleep who develop PTSD initially have smaller hippocampal volumes to begin with. However, evidence does indicate that poor sleep is a risk factor for PTSD [33,56].
As illustrated in Figure 4, poor sleep could represent a risk factor for decreased hippocampal volume. In Figure 4A, poor sleep is a confounding variable in the relationship between hippocampal volume and PTSD status—poor sleep leads independently to both. Given the aforementioned evidence, it is highly unlikely that poor sleep is entirely unrelated to hippocampal volume in individuals with PTSD. The scenario outlined in Figure 4B has less evidence to support it. Although some evidence suggests that smaller hippocampal volumes constitute a risk factor for PTSD, it has not been established that poor sleep leads to smaller hippocampal volumes in these patients.
5. Future directions
Structural imaging studies have thus far been conducted in an arena in which information about brain physiology has been elusive, especially as it relates to psychiatric disease. The limitations of structural imaging to augment our knowledge of psychiatric physiology should be considered in light of our restricted access to the brain of living humans. Autopsy investigations, usually considered the “gold standard” in neuropathologic inquiries, carry the obvious shortcomings of not being able to interrogate living tissue or provide for longitudinal investigations. Thus, there remains an important role for studies considering the hypothesis that hippocampal size is smaller in PTSD. Future studies should ideally have increased power or new methods of investigation that may yield increased internal validity.
We posit that sleep changes may mediate differences in hippocampal size in subjects with PTSD. Few studies have considered sleep quality or quantity as a confounding variable in testing the hypothesis that PTSD is associated with smaller hippocampus sizes. To date, such a hypothesis has been considered in only one study [57]. Attempts to replicate these results would help to further our understanding of the relationship between stress, sleep and cognition.
If poor sleep were found to mediate changes in hippocampal volume, it would be easier to test other hypotheses regarding PTSD and hippocampal changes. Ultimately, it would be important to determine the relevance of hippocampal changes. This would best be accomplished by investigating what cellular and molecular changes gave rise to gross anatomical volume differences. The discovery of a mediating variable in structural imaging studies of PTSD would provide future studies with additional statistical power. In light of recent findings that PTSD confers an increased risk of dementia, it has become especially important to determine the mechanism of cognitive changes in PTSD and whether any effective treatment might ameliorate this risk. In particular, it would be valuable to know whether differences in sleep that are associated with PTSD contribute to the elevated risk of dementia associated with the disorder, as these sleep disturbances may be amenable to treatment.
References
- 1.Acheson DT, Gresack JE, Risbrough VB. Hippocampal dysfunction effects on context memory: possible etiology for posttraumatic stress disorder. Neuropharmacology. 2012;62:674–85. doi: 10.1016/j.neuropharm.2011.04.029. http://dx.doi.org/10.1016/j.neuropharm.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW. Hippocampal volume in patients with alcohol dependence. Arch General Psychiatry. 1999;56:356–63. doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- 3.Altena E, Vrenken H, Van Der Werf YD, van den Heuvel OA, Van Someren EJ. Reduced orbitofrontal and parietal gray matter in chronic insomnia: a voxel-based morphometric study. Biol Psychiatry. 2010;67:182–5. doi: 10.1016/j.biopsych.2009.08.003. http://dx.doi.org/10.1016/j.biopsych.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Andrade KC, Spoormaker VI, Dresler M, Wehrle R, Holsboer F, Samann PG, et al. Sleep spindles and hippocampal functional connectivity in human NREM sleep. J Neurosci. 2011;31:10331–9. doi: 10.1523/JNEUROSCI.5660-10.2011. http://dx.doi.org/10.1523/JNEUROSCI.5660-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Apfel BA, Ross J, Hlavin J, Meyerhoff DJ, Metzler TJ, Marmar CR, et al. Hippocampal volume differences in Gulf War veterans with current versus lifetime posttraumatic stress disorder symptoms. Biol Psychiatry. 2011;69:541–8. doi: 10.1016/j.biopsych.2010.09.044. http://dx.doi.org/10.1016/j.biopsych.2010.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Vol. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 7.Babson K, Feldner M, Badour C, Trainor C, Blumenthal H, Sachs-Ericsson N, et al. Posttraumatic stress and sleep: differential relations across types of symptoms and sleep problems. J Anxiety Disord. 2011;25:706–13. doi: 10.1016/j.janxdis.2011.03.007. http://dx.doi.org/10.1016/j.janxdis.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balkin TJ. Performance deficits during sleep loss: Effects of time awake, time of day, and time on task. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Elsevier Health Sciences; 2010. [Google Scholar]
- 9.Belenky G, Wesensten NJ, Thorne DR, Thomas ML, Sing HC, Redmond DP, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 10.Bonne O, Brandes D, Gilboa A, Gomori JM, Shenton ME, Pitman RK, et al. Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. Am J Psychiatry. 2001;158:1248–51. doi: 10.1176/appi.ajp.158.8.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bremner JD, Vermetten E. Neuroanatomical changes associated with pharmacotherapy in posttraumatic stress disorder. Ann N Y Acad Sci. 2004;1032:154–7. doi: 10.1196/annals.1314.012. http://dx.doi.org/10.1196/annals.1314.012. [DOI] [PubMed] [Google Scholar]
- 12.Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:973–81. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry. 2003;160:924–32. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- 14.Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68:748–66. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- 15.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 16.Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- 17.Cardenas VA, Samuelson K, Lenoci M, Studholme C, Neylan TC, Marmar CR, et al. Changes in brain anatomy during the course of posttraumatic stress disorder. Psychiatry Res. 2011;193:93–100. doi: 10.1016/j.pscychresns.2011.01.013. http://dx.doi.org/10.1016/j.pscychresns.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chao LL, Abadjian L, Hlavin J, Meyerhoff DJ, Weiner MW. Effects of low-level sarin and cyclosarin exposure and Gulf War Illness on brain structure and function: a study at 4T. Neurotoxicology. 2011;32:814–22. doi: 10.1016/j.neuro.2011.06.006. http://dx.doi.org/10.1016/j.neuro.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Chao LL, Mohlenhoff BS, Weiner MW, Neylan TC. Associations between subjective sleep quality and brain volume in Gulf War Veterans. Sleep. 2014;37:445–52. doi: 10.5665/sleep.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chao L, Weiner M, Neylan T. Regional cerebral volumes in veterans with current versus remitted posttraumatic stress disorder. Psychiatry Res. 2013;213:193–201. doi: 10.1016/j.pscychresns.2013.03.002. http://dx.doi.org/10.1016/j.pscychresns.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Childress JE, McDowell EJ, Dalai VV, Bogale SR, Ramamurthy C, Jawaid A, et al. Hippocampal volumes in patients with chronic combat-related posttraumatic stress disorder: a systematic review. J Neuropsychiatry Clin Neurosci. 2013;25:12–25. doi: 10.1176/appi.neuropsych.12010003. http://dx.doi.org/10.1176/appi.neuropsych.12010003. [DOI] [PubMed] [Google Scholar]
- 22.Chumbley J, Worsley K, Flandin G, Friston K. Topological FDR for neuroimaging. NeuroImage. 2010;49:3057–64. doi: 10.1016/j.neuroimage.2009.10.090. http://dx.doi.org/10.1016/j.neuroimage.2009.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohrs S. Sleep disturbances in patients with schizophrenia. CNS Drugs. 2008;22:939–62. doi: 10.2165/00023210-200822110-00004. [DOI] [PubMed] [Google Scholar]
- 24.Davidson LM, Fleming R, Baum A. Chronic stress, catecholamines, and sleep disturbance at three mile island. J Human Stress. 1987;13:75–83. doi: 10.1080/0097840X.1987.9936798. http://dx.doi.org/10.1080/0097840X.1987.9936798. [DOI] [PubMed] [Google Scholar]
- 25.Davis CJ, Meighan PC, Taishi P, Krueger JM, Harding JW, Wright JW. REM sleep deprivation attenuates actin-binding protein cortactin: a link between sleep and hippocampal plasticity. Neurosci Lett. 2006;400:191–6. doi: 10.1016/j.neulet.2006.02.046. http://dx.doi.org/10.1016/j.neulet.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 26.Draganski B. Temporal and spatial dynamics of brain structure changes during extensive learning. J Neurosci. 2006;26:6314–7. doi: 10.1523/JNEUROSCI.4628-05.2006. http://dx.doi.org/10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eichenbaum H. What HM taught us. J Cogn Neurosci. 2013;25:14–21. doi: 10.1162/jocn_a_00285. [DOI] [PubMed] [Google Scholar]
- 28.Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–7. doi: 10.1038/3305. http://dx.doi.org/10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 29.Evans AC, Collins DL, Mills SR, Brown ED, Kelly RL, Peters TM. 3D statistical neuroanatomical models from 305 MRI volumes. 1993 IEEE Conference Record; Nuclear Science Symposium and Medical Imaging Conference; San Francisco. October 31 to November 6, 1993; 1993. pp. 1813–7. [Google Scholar]
- 30.Flemons WW. Clinical practice. Obstructive sleep apnea. N Engl J Med. 2002;347:498–504. doi: 10.1056/NEJMcp012849. http://dx.doi.org/10.1056/NEJMcp012849. [DOI] [PubMed] [Google Scholar]
- 31.Fortier-Brochu É, Beaulieu-Bonneau S, Ivers H, Morin CM. Insomnia and daytime cognitive performance: a meta-analysis. Sleep Med Rev. 2012;16:83–94. doi: 10.1016/j.smrv.2011.03.008. http://dx.doi.org/10.1016/j.smrv.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Fukuda K, Nisenbaum R, Stewart G, Thompson WW, Robin L, Washko RM, et al. Chronic multisymptom illness affecting Air Force veterans of the Gulf War. JAMA. 1998;280:981–8. doi: 10.1001/jama.280.11.981. [DOI] [PubMed] [Google Scholar]
- 33.Gehrman P, Seelig AD, Jacobson IG, Boyko EJ, Hooper TI, Gackstetter GD, et al. Predeployment sleep duration and insomnia symptoms as risk factors for new-onset mental health disorders following military deployment. Sleep. 2013;36:1009–18. doi: 10.5665/sleep.2798. http://dx.doi.org/10.5665/sleep.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatry. 2013;170:372–82. doi: 10.1176/appi.ajp.2012.12040432. http://dx.doi.org/10.1176/appi.ajp.2012.12040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–7. doi: 10.1038/nn958. http://dx.doi.org/10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guzman-Marin R, Bashir T, Suntsova N, Szymusiak R, McGinty D. Hippocampal neurogenesis is reduced by sleep fragmentation in the adult rat. Neuroscience. 2007;148:325–33. doi: 10.1016/j.neuroscience.2007.05.030. http://dx.doi.org/10.1016/j.neuroscience.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guzman-Marin R, Suntsova N, Methippara M, Greiffenstein R, Szymusiak R, McGinty D. Sleep deprivation suppresses neurogenesis in the adult hippocampus of rats. Eur J Neurosci. 2005;22:2111–6. doi: 10.1111/j.1460-9568.2005.04376.x. http://dx.doi.org/10.1111/j.1460-9568.2005.04376.x. [DOI] [PubMed] [Google Scholar]
- 38.Hagewoud R, Havekes R, Novati A, Kaijser JN, Van der Zee EA, Meerlo P. Sleep deprivation impairs spatial working memory and reduces hippocampal AMPA receptor phosphorylation. J Sleep Res. 2010;19:280–8. doi: 10.1111/j.1365-2869.2009.00799.x. http://dx.doi.org/10.1111/j.1365-2869.2009.00799.x. [DOI] [PubMed] [Google Scholar]
- 39.Herringa R, Phillips M, Almeida J, Insana S, Germain A. Posttraumatic stress symptoms correlate with smaller subgenual cingulate, caudate, and insula volumes in unmedicated combat veterans. Psychiatry Res. 2012;203:139–45. doi: 10.1016/j.pscychresns.2012.02.005. http://dx.doi.org/10.1016/j.pscychresns.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hursh SR, Van Dongen HP. Fatigue and performance modeling. In: Kryger MH, editor. Principles and practice of sleep medicine. Elsevier Health Sciences; 2010. [Google Scholar]
- 41.Joo EY, Noh HJ, Kim JS, Koo DL, Kim D, Hwang KJ, et al. Brain gray matter deficits in patients with chronic primary insomnia. Sleep. 2013;36:999–1007. doi: 10.5665/sleep.2796. http://dx.doi.org/10.5665/sleep.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karl A, Schaefer M, Malta LS, Dörfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehavioral Rev. 2006;30:1004–31. doi: 10.1016/j.neubiorev.2006.03.004. http://dx.doi.org/10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 43.Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 2010;33:121–9. doi: 10.1016/j.tins.2010.01.001. http://dx.doi.org/10.1016/j.tins.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi I, Boarts JM, Delahanty DL. Polysomnographically measured sleep abnormalities in PTSD: a meta-analytic review. Psychophysiology. 2007;44:660–9. doi: 10.1111/j.1469-8986.2007.537.x. http://dx.doi.org/10.1111/j.1469-8986.2007.537.x. [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi I, Huntley E, Lavela J, Mellman TA. Subjectively and objectively measured sleep with and without posttraumatic stress disorder and trauma exposure. Sleep. 2012;35:957–65. doi: 10.5665/sleep.1960. http://dx.doi.org/10.5665/-sleep.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kremen WS, Koenen KC, Afari N, Lyons MJ. Twin studies of posttraumatic stress disorder: differentiating vulnerability factors from sequelae. Neuropharmacology. 2012;62:647–53. doi: 10.1016/j.neuropharm.2011.03.012. http://dx.doi.org/10.1016/j.neuropharm.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leuner B, Gould E. Structural plasticity and hippocampal function. Ann Rev Psychol. 2010;61:111–40. doi: 10.1146/annurev.psych.093008.100359. http://dx.doi.org/10.1146/annurev.psych.093008.100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levy-Gigi E, Szabó C, Kelemen O, Kéri S. Association among clinical response, hippocampal volume, and FKBP5 gene expression in individuals with posttraumatic stress disorder receiving cognitive behavioral therapy. Biol Pyschiatry. 2013;74:793–800. doi: 10.1016/j.biopsych.2013.05.017. http://dx.doi.org/10.1016/j.biopsych.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 49.Maguire EA, Woollett K, Spiers HJ. London taxi drivers and bus drivers: a structural MRI and neuropsychological analysis. Hippocampus. 2006;16:1091–101. doi: 10.1002/hipo.20233. http://dx.doi.org/10.1002/hipo.20233. [DOI] [PubMed] [Google Scholar]
- 50.Mauss IB, Troy AS, LeBourgeois MK. Poorer sleep quality is associated with lower emotion-regulation ability in a laboratory paradigm. Cogn Emot. 2013;27:567–76. doi: 10.1080/02699931.2012.727783. http://dx.doi.org/10.1080/02699931.2012.727783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McEwen BS, Magarinos AM. Stress effects on morphology and function of the hippocampus. Ann N Y Acad Sci. 1997;821:271–84. doi: 10.1111/j.1749-6632.1997.tb48286.x. [DOI] [PubMed] [Google Scholar]
- 52.Meyerhoff DJ, Mon A, Metzler TJ, Neylan TC. Cortical GABA and glutamate in Posttraumatic Stress Disorder and their relationships to self-reported sleep quality. Sleep. 2014;37:893–900. doi: 10.5665/sleep.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moore SA. Cognitive abnormalities in posttraumatic stress disorder. Curr Op Psychiatry. 2009;22:19–24. doi: 10.1097/YCO.0b013e328314e3bb. http://dx.doi.org/10.1097/YCO.0-b013e328314e3bb. [DOI] [PubMed] [Google Scholar]
- 54.Nadel L, Hupbach A, Gomez R, Newman-Smith K. Memory formation, consolidation and transformation. Neurosci Biobehav Rev. 2012;36:1640–5. doi: 10.1016/j.neubiorev.2012.03.001. http://dx.doi.org/10.1016/j.neubiorev.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 55.Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch General Psychiatry. 1998;55:433–40. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- 56.Neylan TC. Sleep and psychological vulnerability to traumatic stress. Sleep. 2013;36:967–8. doi: 10.5665/sleep.2782. http://dx.doi.org/10.5665/sleep.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neylan TC, Mueller SG, Wang Z, Metzler TJ, Lenoci M, Truran D, et al. Insomnia severity is associated with a decreased volume of the CA3/dentate gyrus hippocampal subfield. Biol Psychiatry. 2010;68:494–6. doi: 10.1016/j.biopsych.2010.04.035. http://dx.doi.org/10.1016/j.biopsych.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Noh HJ, Joo EY, Kim ST, Yoon SM, Koo DL, Kim D, et al. The Relationship between hippocampal volume and cognition in patients with chronic primary insomnia. J Clin Neurol. 2012;8:130–8. doi: 10.3988/jcn.2012.8.2.130. http://dx.doi.org/10.3988/jcn.2012.8.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nutt D, Wilson S, Paterson L. Sleep disorders as core symptoms of depression. Dialogues Clin Neurosci. 2008;10:329. doi: 10.31887/DCNS.2008.10.3/dnutt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Osorio RS, Pirraglia E, Aguera-Ortiz LF, During EH, Sacks H, Ayappa I, et al. Greater risk of Alzheimer’s disease in older adults with insomnia. J Am Geriatr Soc. 2011;59:559–62. doi: 10.1111/j.1532-5415.2010.03288.x. http://dx.doi.org/10.1111/j.1532-5415.2010.03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13:769–87. doi: 10.1038/nrn3339. http://dx.doi.org/10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qureshi SU, Kimbrell T, Pyne JM, Magruder KM, Hudson TJ, Petersen NJ, et al. Greater prevalence and incidence of dementia in older veterans with posttraumatic stress disorder. J Am Geriatr Soc. 2010;58:1627–33. doi: 10.1111/j.1532-5415.2010.02977.x. http://dx.doi.org/10.1111/j.1532-5415.2010.02977.x. [DOI] [PubMed] [Google Scholar]
- 63.Ribeiro S. Sleep and plasticity. Pflügers Arch. 2011;463:111–20. doi: 10.1007/s00424-011-1031-5. http://dx.doi.org/10.1007/s00424-011-1031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Riemann D, Voderholzer U, Spiegelhalder K, Hornyak M, Buysse DJ, Nissen C, et al. Chronic insomnia and MRI-measured hippocampal volumes: a pilot study. Sleep. 2007;30:955–8. doi: 10.1093/sleep/30.8.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rosekind MR, Gander PH, Miller DL, Gregory KB, Smith RM, Weldon KJ, et al. Fatigue in operational settings: examples from the aviation environment. Human Factors. 1994;36:327–38. doi: 10.1177/001872089403600212. http://dx.doi.org/10.1177/001872089403600212. [DOI] [PubMed] [Google Scholar]
- 66.Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989;146:697–707. doi: 10.1176/ajp.146.6.697. [DOI] [PubMed] [Google Scholar]
- 67.Schuff N, Neylan TC, Fox-Bosetti S, Lenoci M, Samuelson KW, Studholme C, et al. Abnormal N-acetylaspartate in hippocampus and anterior cingulate in posttraumatic stress disorder. Psychiatry Res. 2008;162:147–57. doi: 10.1016/j.pscychresns.2007.04.011. http://dx.doi.org/10.1016/j.pscychresns.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schuff N, Neylan TC, Lenoci MA, Du AT, Weiss DS, Marmar CR, et al. Decreased hippocampal N-acetylaspartate in the absence of atrophy in posttraumatic stress disorder. Biol Psychiatry. 2001;50:952–9. doi: 10.1016/s0006-3223(01)01245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spiegelhalder K, Regen W, Baglioni C, Klöppel S, Abdulkadir A, Hennig J, et al. Insomnia does not appear to be associated with substantial structural brain changes. Sleep. 2013;36:731–7. doi: 10.5665/sleep.2638. http://dx.doi.org/10.5665/sleep.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stein MD, Friedmann PD. Disturbed sleep and its relationship to alcohol use. Substance Abuse. 2005;26:1–13. doi: 10.1300/j465v26n01_01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stoffers D, Moens S, Benjamins J, van Tol MJ, Penninx BW, Veltman DJ, et al. Orbitofrontal gray matter relates to early morning awakening: a neural correlate of insomnia complaints? Front Neurol. 2012;3:105. doi: 10.3389/fneur.2012.00105. http://dx.doi.org/10.3389/fneur.2012.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Taki Y, Hashizume H, Thyreau B, Sassa Y, Takeuchi H, Wu K, et al. Sleep duration during weekdays affects hippocampal gray matter volume in healthy children. NeuroImage. 2012;60:471–5. doi: 10.1016/j.neuroimage.2011.11.072. http://dx.doi.org/10.1016/j.neuroimage.2011.11.072. [DOI] [PubMed] [Google Scholar]
- 73.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. http://dx.doi.org/10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 74.Van Dongen HP, Hursh SR. Fatigue, performance, errors, and accidents. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Elsevier Health Sciences; 2010. [Google Scholar]
- 75.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–29. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 76.Villarreal G, Hamilton DA, Petropoulos H, Driscoll I, Rowland LM, Griego JA, et al. Reduced hippocampal volume and total white matter volume in posttraumatic stress disorder. Biol Psychiatry. 2002;52:119–25. doi: 10.1016/s0006-3223(02)01359-8. [DOI] [PubMed] [Google Scholar]
- 77.Walker MP. Sleep, memory and emotion. Prog Brain Res. 2010;185:49–68. doi: 10.1016/B978-0-444-53702-7.00004-X. http://dx.doi.org/10.1016/B978-0-444-53702-7.00004-X. [DOI] [PubMed] [Google Scholar]
- 78.Walker MP, Van der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychol Bull. 2009;135:731–48. doi: 10.1037/a0016570. http://dx.doi.org/10.1037/a0016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Z, Neylan TC, Mueller SG, Lenoci M, Truran D, Marmar CR, et al. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch General Psychiatry. 2010;67:296–303. doi: 10.1001/archgenpsychiatry.2009.205. http://dx.doi.org/10.1001/archgenpsychiatry.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: a review of the first ten years of research. Depression Anxiety. 2001;13:132–56. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- 81.Whitlock JR. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–7. doi: 10.1126/science.1128134. http://dx.doi.org/10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- 82.Winkelman JW, Benson KL, Buxton OM, Lyoo IK, Yoon S, O’Connor S, et al. Lack of hippocampal volume differences in primary insomnia and good sleeper controls: an MRI volumetric study at 3 Tesla. Sleep Med. 2010;11:576–82. doi: 10.1016/j.sleep.2010.03.009. http://dx.doi.org/10.1016/j.sleep.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 83.Winkelman JW, Plante DT, Schoerning L, Benson K, Buxton OM, O’Connor SP, et al. Increased rostral anterior cingulate cortex volume in chronic primary insomnia. Sleep. 2013;36:991–8. doi: 10.5665/sleep.2794. http://dx.doi.org/10.5665/sleep.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Woodward SH, Kaloupek DG, Streeter CC, Kimble MO, Reiss AL, Eliez S, et al. Hippocampal volume, PTSD, and alcoholism in combat veterans. Am J Psychiatry. 2006;163:674–81. doi: 10.1176/ajp.2006.163.4.674. http://dx.doi.org/10.1176/appi.ajp.163.4.674. [DOI] [PubMed] [Google Scholar]
- 85.Woon FL, Sood S, Hedges DW. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1181–8. doi: 10.1016/j.pnpbp.2010.06.016. http://dx.doi.org/10.1016/j.pnpbp.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 86.Yaffe K, Vittinghoff E, Lindquist K, Barnes D, Covinsky KE, Neylan T, et al. Posttraumatic stress disorder and risk of dementia among US Veterans PTSD and dementia in US veterans. Archives of General Psychiatry. 2010;67:608–13. doi: 10.1001/archgenpsychiatry.2010.61. http://dx.doi.org/10.1001/archgenpsychiatry.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]