Abstract
Aging phenotypes are dictated by myriad cellular changes including telomere shortening. In most tissues, telomere shortening is accelerated during replication if unrepaired oxidative damage to telomere sequences is present. However, the effect of reactive oxygen species exposure on skeletal muscle telomeres is unknown. We sought to determine if oxidative stress shortens telomeres in isolated adult rodent skeletal muscle fibers. Flexor digitorum brevis muscles were dissected from male mice (C57BL/6, long telomere and CAST/Ei, wild-derived, short telomere) and dissociated into single fibers. Fibers were cultured at an oxygen tension of 2%–5% for 5 days in control, hydrogen peroxide (oxidant), or a combination of N-acetylcysteine (antioxidant) and oxidant containing media. Telomere length, telomerase enzyme activity, and protein content of TRF1 and TRF2 were subsequently measured. In both strains, oxidative stress resulted in significant telomere shortening in isolated skeletal muscle fibers, likely by different mechanisms. Telomerase activity was not altered by oxidative stress treatment but was significantly different between strains, with greater telomerase activity in long-telomere–bearing C57BL/6 mice. These results provide important insights into mechanisms by which oxidative stress could shorten skeletal muscle telomeres.
Key Words: Telomere length, Telomere-binding proteins, Skeletal muscle fibers, Low oxygen tissue culture, Oxidative stress.
Telomeres are repetitive DNA sequences (5′-TTAGGGN-3′) at the ends of linear chromosomes (1). In most tissues, telomeres shorten over time due to a combination of incomplete replication, chromosome end processing, and unrepaired telomere DNA damage (2–4). In addition to being important in the regenerative potential of cells, adequate telomere length is important in maintaining genome stability and regulating gene expression via telomere position effects and chromosome looping (5–7). The enzyme telomerase can maintain and lengthen telomeres in germline, stem, and other highly proliferative cells (8–11). Telomere shortening has thus been studied as a biomarker of aging and is implicated in cancers and other age-related diseases such as cardiovascular disease (12,13). A variety of environmental factors important to age-related disease have been studied as modifiers of age-related telomere shortening. Among these, chronic exercise has emerged as a protective stimulus for a variety of cell types, attenuating age-related telomere shortening in heart, vascular tissue, and lymphocytes, though the response of skeletal muscle to exercise training is less clear (14–17).
Contrary to most tissues, telomere length does not change with age in skeletal muscle, likely stemming from its postmitotic status and low cell turnover (18–23). Results from our laboratory have shown that skeletal muscle telomere length is shorter from long-term exercise–trained animals compared with young and age-matched sedentary animals, indicating that skeletal muscle telomeres shorten in response to chronic physiological stress (20). In contrast, a recent publication indicates that the rate of telomere attrition is similar across all somatic tissues including skeletal muscle (2); however, this is a controversial finding and requires clarification. This study was cross-sectional in nature, and future longitudinal studies are required to validate these findings, as they are inconsistent with what this group and others have observed concerning telomere length with age in skeletal muscle (2,20,21,24). Literature about the mechanism(s) of how skeletal muscle telomeres can shorten despite the relatively low cellular turnover in adult muscles is not well established. In addition, a thorough understanding of skeletal muscle fiber telomeres (not satellite cell or myoblast telomeres) cultured at physiological oxygen tension (~5% O2) is lacking.
One well-studied and recognized factor associated with aging skeletal muscle is an increase in exposure of muscle fibers to reactive oxygen species (ROS) (25,26). The rate of telomere shortening in other tissues is accelerated in response to ROS exposure (27), yet how skeletal muscle telomeres respond to ROS is unknown. Skeletal muscle is extremely sensitive to oxidative DNA damage because of low antioxidant capacity compared with other tissues and deficiency in DNA repair enzymes (28). Telomeres, particularly the 5′-GGG triplet, are extremely sensitive to oxidative DNA damage (27,29) and are also refractory to repair (30). Thus, with aging, disease, or long-term physiological stress (ie, regular vigorous endurance exercise training), it could be possible that exposure to ROS at telomere DNA could induce DNA damage that could result in telomere shortening in skeletal muscle. Telomerase, which plays a role in maintaining telomere length, and the telomere repeat–binding factors 1 and 2 (TRF1 and TRF2) are critical in the response to DNA-damaging agents (ie, oxidative stress) and may play a role in telomere length regulation in skeletal muscle following physiological stress (31–33). However, the response of skeletal muscle telomeres and telomere-regulatory components to oxidative stress per se is not known. To clarify the skeletal muscle telomere length contradiction, we investigated telomeres dynamics in an in vitro model of adult skeletal muscle using two strains of mice: one with short (CAST/Ei) and one with long (C57BL/6) telomeres. We hypothesized that oxidative stress would decrease skeletal muscle telomere length, increase telomerase activity, and alter the protein content of telomere-maintaining proteins. Our results indicate that oxidative stress results in shorter telomeres in adult skeletal muscle in both short- and long-telomere–bearing mice, and that this was not associated with changes in telomerase activity.
Methods
Animals
All animal experiments were approved by the University of Maryland Institutional Animal Care and Use Committee and conformed to the National Institutes of Health’s Guide for the Use and Care of Laboratory Animals (NIH Pub. No. 85-23, revised 1996). Twenty male CAST/Ei (Mus m usculus c astaneus, 7–9 weeks old) and 15 male C57BL/6 (Mus m usculus, 8–10 weeks old) mice were purchased from Jackson Laboratory (Bar Harbor, ME). Animals were acclimated to the animal facility and randomly assigned to treatment groups. The animals were housed at 25°C on a 12-hour light–dark cycle. Animals were fed ad libitum laboratory mouse chow (Prolab RMH 3000, 5P00; LabDiet, Nestle Purina, Vevey, Switzerland) and given free access to water. The animals were anesthetized (3% isoflurane) and euthanized by exsanguination.
Single Muscle Fiber Isolation Procedure
Flexor digitorum brevis (FDB) skeletal muscles were dissected with a stereoscope (Trinocular Stereo 3.5X-90X model SM-2TZ; AmScope, Irvine, CA) in a sterile phosphate-buffered saline solution (pH 7.4, room temperature). Following dissection and removal of excess connective tissue, muscles were placed in dissociation media containing 10% fetal bovine serum (Gibco, Carlsbad, CA), 1% Pennstrep (PS; Invitrogen, Carlsbad, CA), with 5,000 units penicillin and 5,000 µg streptomycin in minimal essential medium (MEM; Gibco) with Liberase TM (13 Wunsch units/mL; Roche, Indianapolis, IN), a blend of type I and II collagenases. One FDB was placed in 2 mL of dissociation media in a 35-mm culture dish and then rested in a cell culture incubator for 90 minutes in ambient air (37°C, 5% CO2). After the 90-minute incubation, FDB muscles were rinsed of excess collagenase by a series of three 1-minute washes in 35-mm dishes containing prewarmed MEM supplemented with 1% PS. The muscle was then placed into a single well of a six-well culture plate (Cell Star; Greiner Bio-One, Frickenhausen, Germany) with 2mL of MEM supplemented with 1% PS and gently titrated until the muscle was visibly dissociated. To reduce the incidence of muscle fiber dedifferentiation and myoblast and/or fibroblast proliferation, fetal bovine serum was not used in the final culture media, as described previously (34).
Cell Culture and Single Muscle Fiber Treatments
After fibers from all animals were completely dissociated, six-well plates were placed into an airtight cell culture chamber. In an attempt to culture skeletal muscle fibers at or near physiological conditions (PO2 in resting skeletal muscle is ~6–7 Torr or ~1%), we reduced the oxygen levels to 2%–5% in an airtight cell culture chamber (35–37). To achieve low oxygen conditions, the chamber was flushed with a gas mixture for 5 minutes (2% oxygen, 5% carbon dioxide, and 93% nitrogen) designed to replace the ambient oxygen level (21%) (~2%–5% as previously described (37)) and allowed to rest overnight in the 37°C cell culture incubator. The following day, the fibers were inspected and treated with randomly assigned culture conditions (control = MEM, 1% PS + water as a vehicle; H2O2 = 1 µM, in MEM + 1% PS; H2O2 + N-acetylcysteine [NAC] = 1 µM H2O2 plus 100 µM NAC in MEM + 1% PS) for 5 days. For the C57BL/6 mice experiments, five animals were used for each treatment group, and for the CAST/Ei mice experiments, seven animals were used in the control group and six animals each in the H2O2 and combination of H2O2 and NAC treatment groups. Drug treatment conditions were determined during preliminary experiments, and 1 µM H2O2 (Sigma, St Louis, MO) found to be the maximum dose tolerable for the duration of treatment (38). Treatments were replaced and the chamber re-flushed with low oxygen gas mixture every 24 hours. Treatment with 1 µM H2O2 in cell culture media has been shown to induce intracellular concentrations of approximately 0.1 µM in skeletal muscle fibers, which is similar to the amount of ROS produced by FDB fibers (38). Because previous observations (39,40) indicated that telomeric responses may accumulate over time (ie, repeat exposure effect) and lead to telomere shortening, we treated our fibers for 5 days rather than with a single dose.
The two FDBs from each animal were placed into separate wells of a six-well plate and treated identically, and each treatment group consisted of fibers from at least five animals (exact sample sizes are reported in the Results section). After 5 days of treatment, approximately 800 fibers per animal were removed via inspection using a stereoscope and 200-µL pipette and placed into four individual microcentrifuge tubes (~200 fibers/tube) for processing. Data were collected for each assay from approximately 200 fibers per treatment group. Skeletal muscle fibers from C57BL/6 or CAST/Ei mice were pelleted (17,000g, 25°C for 5 minutes). Following centrifugation, the supernatant was removed, and lysis buffers for extraction of proteins, DNA, or RNA were added to the pelleted fibers and frozen at −80°C until further analysis (details below).
Fiber Width
Fiber width was quantified by measurement with a calibrated (pixels per millimeter) line tool (41). At minimum, nine fibers were counted per treatment group. Three measurements of fiber width were made along the length of the fiber and then averaged to create the fiber width measurement for each fiber. The width measures of each fiber were averaged to generate the treatment group averages.
Telomere Length
DNA was isolated from approximately 200 fibers and quantified as previously described (15,42). Telomere length was measured using a quantitative PCR method based on the ratio of telomere repeat copy number (T PCR) to single-copy gene copy number (42). Between 12.5 and 20ng of total genomic DNA was added to a reaction mixture as previously described (15). All samples were run in triplicate, with a standard curve and reference samples run on the T and S PCR plates to ensure linearity. Technical outliers between replicates were identified by a difference in critical threshold values greater than 3. The reference samples were from a previous study of CAST/Ei mice that had telomere length determined via telomere restriction fragment analysis (TRF Southern blotting technique) to ensure accuracy of the assay (20,42). The intra-assay coefficient of variation for the telomere repeat copy assay was 2.5%, and the single-copy gene copy assay was 2.3%.
Telomerase
Isolated fibers were thawed on ice at 4°C and briefly centrifuged in a bench-top centrifuge. Samples were lysed in 200 µL of quantitative telomerase lysis buffer (Quantitative Telomerase Detection Kit; US Biomax, Rockville, MD) on ice with intermittent vortex mixing (every 15 minutes) for 1 hour followed by centrifugation (12,000g, 4°C for 30 minutes). Following centrifugation, supernatants were removed and assayed for protein content using a bicinchoninic acid protein assay (Pierce, Rockford, IL). In addition, cells from a telomerase-positive human cancer cell line were treated as above and included as a positive control (HeLa, ATCC CCL-2; ATCC, Manassas, VA). For unknowns and HeLa control samples, 1 µg of extracted protein was added to the reaction mixture according to the recommendations of the manufacturer and as previously performed in our lab (20). Telomerase activity was determined using a commercially available kit utilizing the telomere repeat amplification protocol (Quantitative Telomerase Detection Kit). Beyond the kit-provided standards, heat-treated samples were assayed as negative controls. Heat-treated samples were concluded to be telomerase negative if the mean of the critical threshold (Ct) for the heat-treated sample duplicates was 3 SDs above that of the telomerase-positive sample (15,20).
Immunoblotting
Prior to freezing, fibers were lysed in 300 µL of lysis buffer (100 mmol/L Tris [pH 6.8], 4% sodium dodecyl sulfate, 20% glycerol, and protease inhibitor cocktail; Complete mini EDTA-free; Roche). Lysates were thawed on ice for 1 hour, and total protein content was determined as above. Fifty- (TRF2 and GAPDH) and 90-µg (TRF1 and GAPDH) samples were prepared for immunoblotting. Proteins were resolved on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels, transferred to polyvinylidene fluoride membranes and blocked for 30 minutes in 5% nonfat dry milk, and exposed to primary antibodies overnight at 4°C as follows: TRF1 (C-19, SC 1977, 1:200; Santa Cruz Biotechnologies, Santa Cruz, CA), TRF2 (H-300, SC 9143, 1:200; Santa Cruz Biotechnologies), and GAPDH (14C10 Rabbit mAb #2118 1:1,000; Cell signaling, Beverly, MA). All products were visualized with horseradish peroxidase-linked secondary antibodies using high sensitivity enhanced chemiluminescence (SuperSignal West Dura Chemiluminescent Substrate; Pierce) on the Gene Gnome (Syngene Bio Imaging, Fredrick, MD). Band intensities were analyzed by densitometry using ImageJ software (41).
Statistical Analysis
All values, unless otherwise stated, are presented as means ± standard error of the mean. Generalized linear models were used to analyze the data with SAS version 9.2. We collapsed all treatment groups and compared absolute values of each strain to assess strain differences. Treatment effects for each strain were determined with one-way analysis of variance. Tukey's honestly significant difference (HSD) was used to correct for post hoc comparisons. Differences were considered significant at p < .05.
Results
Single Muscle Fiber Morphology Is Not Altered by ROS Treatment
Because oxygen levels of cultured murine cells are related to increased DNA damage, reducing the exposure of the skeletal muscle fibers to near physiological oxygen tensions allowed us to more accurately assess the effects of our oxidant exposure (43). No noticeable differences in single muscle fiber morphology between strains or among treatment groups were observed in the cultures (Figure 1). We quantified the width of the skeletal muscle fibers and found no differences between groups (C57BL/6: control = 0.02±0.006 mm, H2O2 = 0.02±0.004 mm, H2O2 + NAC = 0.03±0.017 mm, p = .9; CAST/Ei: control = 0.03±0.006 mm, H2O2 = 0.02±0.003 mm, H2O2 + NAC = 0.03±0.005mm ± SD, p = .9). In addition, we did not observe any fiber dedifferentiation (ie, nuclei blebbing) or myoblast and/or fibroblast integration into single muscle fibers in any strain or treatment group.
Figure 1.

Morphology of skeletal muscle fibers cultured at physiologic oxygen and treated with H2O2 with and without NAC for 5 days. Skeletal muscle fibers from both strains of mice were cultured in low oxygen conditions and treated for 5 days with H2O2 (1 µM), H2O2 (1 µM) plus N-acetylcysteine (H2O2 + NAC, 10 µM), or control (vehicle, water) treated. Treatments were replaced every 24 hours. Micrographs of C57BL/6 and CAST/Ei skeletal muscle fibers cultured over 5 days at low oxygen conditions. No visible differences were observed among treatment groups. Images were taken at 40× magnification from an inverted light microscope. Representative images are presented from each treatment group. CONT = control; H2O2 = hydrogen peroxide treated; H2O2 + NAC = hydrogen peroxide and NAC treated. Scale bar = 25 µm.
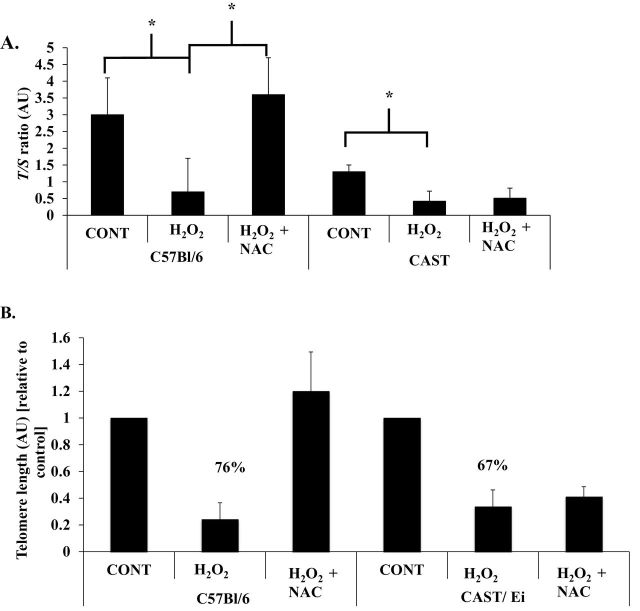
Telomere Length Is Reduced in H2O2-Treated Adult Single Muscle Fibers
Telomeres were shortened (76%) in H2O2-treated fibers from C57BL/6 mice compared with control-treated fibers (p = .05). Telomere shortening was significantly attenuated by treatment with the antioxidant NAC (H2O2 vs H2O2 + NAC, p = .05; Figure 2), indicating a role of oxidative stress in telomere shortening. In CAST/Ei mice, H2O2 shortened telomeres (67%) compared with control (p = .04; Figure 2); however, antioxidant treatment did not result in attenuation of telomere shortening (H2O2 vs H2O2 + NAC; Figure 2).
Figure 2.
Oxidative stress shortens skeletal muscle fiber telomere length. Skeletal muscle fibers from both strains of mice were cultured in low oxygen conditions and treated for 5 days with H2O2 (1 µM; C57BL/6 n = 5 mice, CAST/Ei n = 6 mice), H2O2 plus N-acetylcysteine (H2O2 + NAC, 10 µM and 1 µM H2O2; C57BL/6 n = 5 mice, CAST/Ei n = 6 mice), or control (vehicle, water; C57BL/6 n = 5 mice, CAST/Ei n = 7 mice). (A) Telomere length as measured by T/S ratio. (B) Telomere length expressed relative to each strain’s control condition to show the relative change in telomere length. *Significantly different (p ≤ .05). Data are presented as means ± standard error of the mean. CONT = control; H2O2 = hydrogen peroxide treated; H2O2 + NAC = hydrogen peroxide and NAC treated. T/S ratio = telomere repeat copy PCR to single-copy gene PCR ratio. AU = arbitrary units. Analysis of variance with Tukey’s HSD post hoc tests were performed to determine statistical significance for each strain of mice. Approximately 200 fibers from each animal were analyzed (thus ~1,000 fibers [~200 fibers times the number of animals per treatment] per treatment were analyzed). Number of animals per treatment was used to determine degrees of freedom for each statistical test. HSD, honestly significant difference.
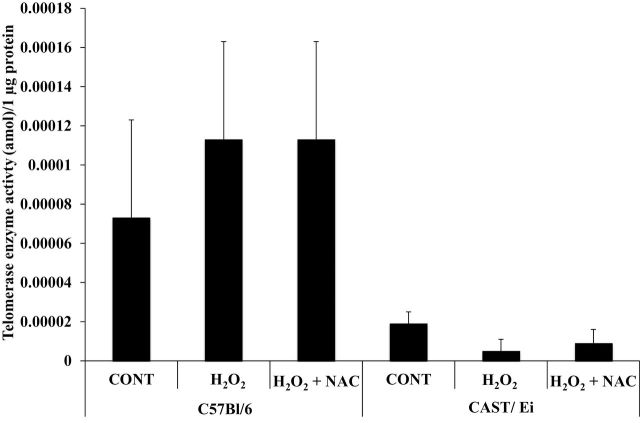
Telomerase Enzyme Activity Was Not Altered by Oxidant Treatment in Muscle Fibers
As shown in Figure 3, H2O2 treatment did not result in any significant change in telomerase enzyme activity in either strain of mice, nor did the addition of NAC impact telomerase activity in either strain.
Figure 3.
Oxidative stress does not alter telomerase enzyme activity in skeletal muscle fibers. Overall comparison between strains revealed greater telomerase enzyme activity in C57BL/6 compared with CAST/Ei animals (p < .01). HeLa control telomerase activity was 0.018±0.0045amol per microgram of protein. Data are presented as means ± standard error of the mean. CONT = control; H2O2 = hydrogen peroxide treated; H2O2 + NAC = hydrogen peroxide and N-acetylcysteine treated. Amol = attomoles. Analysis of variance with Tukey’s HSD post hoc tests were performed to determine statistical significance for each strain of mice. Approximately 200 fibers from each animal were analyzed (thus ~1,000 fibers [~200 fibers times the number of animals per treatment] per treatment were analyzed). Number of animals per treatment was used to determine degrees of freedom for each statistical test. HSD = honestly significant difference.
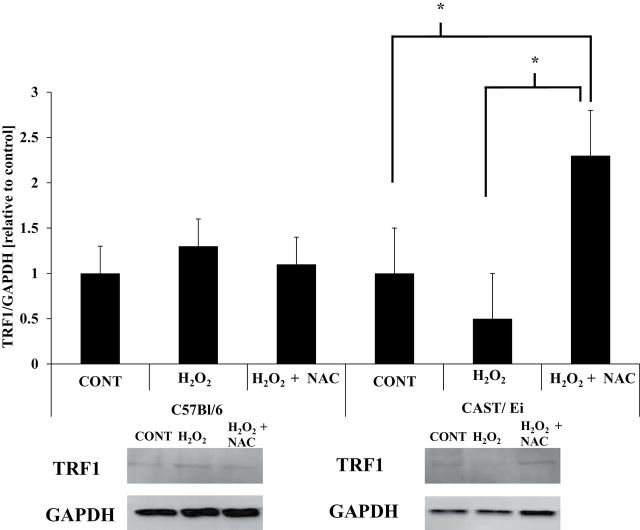
Oxidative Stress Treatment Resulted in Altered Protein Content of TRF1 and TRF2
Shelterin is a six-protein complex that binds telomere DNA and functions to regulate telomere length (31–33), protects chromosome ends from being recognized as broken DNA (32), and regulates telomerase action at the telomere (44–46). From in vitro gain-of-function experiments, TRF1 has been shown to act as both a positive (lengthening) and negative (shortening) regulator of telomere length and is important in cell cycle progression (47). TRF2 is critical in prevention of a telomere-specific DNA damage response, telomere length regulation, and prevention of end-to-end fusions (32). Similar to TRF1, TRF2 in vitro and in vivo (rodent model) gain-of-function experiments have shown TRF2 to be a negative regulator of telomere length (48,49).
TRF1.—
In C57BL/6 fibers, no differences in protein content of TRF1 between H2O2 or H2O2 + NAC treatments were observed (Figure 4). In the CAST/Ei fibers, no differences in TRF1 protein content were observed between control and H2O2 (p = .5; Figure 4); however, CAST/Ei fibers treated with a combination of NAC and H2O2 had significantly greater TRF1 protein content than both control (p = .04) and H2O2-treated fibers (p = .01; Figure 4).
Figure 4.
TRF1 protein content is not altered by oxidative stress treatment. Densitometric analysis and representative immunoblot images of TRF1 and GAPDH (a loading control) are shown. Data are presented as means ± standard error of the mean. All presented images are derived from the same immunoblot for each strain of mice. CONT = control; H2O2 = hydrogen peroxide treated; H2O2 + NAC = hydrogen peroxide and N-acetylcysteine treated. AU = arbitrary units. *Significantly different (p ≤ .05). Analysis of variance with Tukey’s HSD post hoc tests were performed to determine statistical significance for each strain of mice. Approximately 200 fibers from each animal were analyzed (thus ~1,000 fibers [~200 fibers times the number of animals per treatment] per treatment were analyzed). Number of animals per treatment was used to determine degrees of freedom for each statistical test. HSD = honestly significant difference.
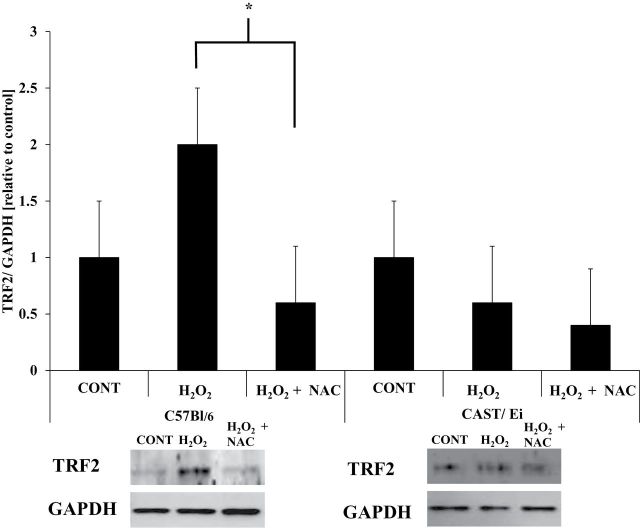
TRF2.—
In C57BL/6 fibers, H2O2 treatment apparently (p = .1) resulted in increased protein content of TRF2 compared with control (Figure 5) that was attenuated in H2O2 + NAC–treated fibers (p = .05; Figure 5), indicating a role of oxidative stress in the TRF2 response. In CAST/Ei fibers, no significant alterations in TRF2 protein content were observed in either treatment group (Figure 5).
Figure 5.
TRF2 protein content is increased in response to H2O2 in C57BL/6 muscle fibers. Densitometric analysis and representative immunoblot images of TRF2 and GAPDH are shown. GAPDH was a loading control. All presented images are derived from the same immunoblot for each strain of mice. Data are presented as means ± standard error of the mean. CONT = control; H2O2 = hydrogen peroxide treated; H2O2 + NAC = hydrogen peroxide and N-acetylcysteine treated. AU = arbitrary units. *Significantly different (p ≤ .05). Analysis of variance with Tukey’s HSD post hoc tests were performed to determine statistical significance for each strain of mice. Approximately 200 fibers from each animal were analyzed (thus ~1,000 [~200 fibers times the number of animals per treatment] fibers per treatment were analyzed). Number of animals per treatment was used to determine degrees of freedom for each statistical test. HSD = honestly significant difference.
Strain Comparisons Reveal Differences in Telomere Length and Telomerase Activity but Not in Shelterin Protein Content
To determine strain differences in the telomere dynamics, we compared C57BL/6 and CAST/Ei mice regardless of treatment. As shown in Table 1, typical laboratory mice (C57BL/6) had longer telomeres compared with wild-derived CAST/Ei mice, confirming earlier reports (p < .01; Figure 2 and Table 1 (50)). C57BL/6 mice had significantly greater telomerase enzyme activity compared with CAST/Ei (p < .01; Figure 3 and Table 1), similar to previous findings (51). For both TRF1 and TRF2 protein content, no differences were observed between strains (TRF1, p = .4; TRF2, p = .7; Figures 4 and 5, respectively, and Table 1).
Table 1.
Animal Characteristics and Strain Comparisons
| Strain | Age (wk) | Body Mass (g) | Telomere Length (T/S ratio) | Telomerase Activity (amol/µg) | TRF1 (AU) | TRF2 (AU) |
|---|---|---|---|---|---|---|
| C57BL/6 | 9.5±0.1 | 23.8±0.3 | 2.6±0.5 | 1 × 10–4 ± 2 × 10–5 | 0.43±0.2 | 0.05±0.01 |
| CAST/Ei | 7.7±0.1* | 13.5±0.2* | 0.75±0.5* | 1 × 10–5 ± 2 × 10–5* | 0.76±0.2 | 0.04±0.01 |
Notes: Amol = attomoles; AU = arbitrary units; T/S ratio = telomere copy PCR to single-copy gene PCR ratio. Data are presented as mean ± standard error of the mean. Age was significantly different between strains but not within strain between treatment groups (data not shown). C57BL/6 data were collected on a total of 15 male animals and ~200 fibers per assay per animal, thus each assay contains data from ~3,000 fibers. CAST/Ei data were collected on a total of 20 male animals and ~200 fibers per assay per animal, thus each assay contains data from ~4,000 fibers. TRF1 and TRF2 protein content was normalized to GAPDH. The arbitrary units for TRF1 and TRF2 were calculated as the ratio of TRF protein to GAPDH protein for a specific series of immunoblots, and thus the quantity of arbitrary units cannot be compared between the two proteins. Student’s paired t tests were used to compare mouse strains with animal number used to calculate degrees of freedom. Statistical significance was accepted at *p < .05
Discussion
We demonstrate for the first time in isolated adult skeletal muscle fibers of mice that oxidative stress exposure results in telomere shortening. The oxidant-induced telomere shortening was significantly prevented by antioxidant treatment in the long-telomere–bearing C57BL/6 strain, but not in the short-telomere–bearing CAST/Ei mice, indicating potentially different mechanisms of oxidant-induced telomere shortening between long- and short-telomere–bearing mice. Because CAST/Ei mice have telomere length more similar to human telomeres, their response may more accurately depict what the response of telomeres following oxidative insult in human muscle fibers. Telomerase activity was not impacted by the treatments, but we describe differences in enzyme activity between typical laboratory (long telomere) and wild-derived (short telomere) strains of mice that impact interpretation of the data. Moreover, both TRF1 and TRF2 showed differential, strain-specific responses to oxidant and antioxidant treatment indicating strain-specific telomere dynamics in skeletal muscle. These results provide insights into the complex regulation of telomeres in postmitotic tissues and begin to clarify how age-associated increases in ROS may result in shortened telomeres in skeletal muscle and other differentiated tissues.
Current dogma states that telomeres shorten with replication and exposure to DNA-damaging agents (eg, ROS) coupled with replication that results in accelerated shortening (27). Telomere shortening caused by either mechanism may result in telomeres reaching a critical length and causing telomere dysfunction and potentially cell and tissue dysfunction (52). Although this dogma holds true for aging of most human somatic cells, skeletal muscle cells most likely do not follow this pattern of age-related telomere attrition. Our results and those of others have shown little to no age-related telomere shortening in skeletal muscle of healthy older mammals, both human and rodent (19,20). However, a recent report has indicated that the rate of telomere shortening in human skeletal muscle is similar to that of other tissues, such as lymphocytes (2). Recent reports and data from our lab have shown skeletal muscle telomere shortening in skeletal muscle homogenates from mice and humans that have undergone chronic exercise training (20,53). In addition, we have recently observed that a single bout of treadmill exercise produced a stress response that resulted in Trf1 downregulation in skeletal muscle (40). These data indicate that skeletal muscle telomeres are responsive (ie, shorten) to physiological stressors. The current data support the notion that the cellular environment induced by aging (ie, increased ROS production) may also contribute to changes in skeletal muscle telomere phenotype.
Telomere Length Shortens in Skeletal Muscle Under Oxidative Stress
In vivo, it is thought that telomere length in skeletal muscle only shortens when the satellite cells are induced to divide and incorporate into existing fibers (54), such that nuclei with shorter telomeres accumulate in the tissue over time. This type of telomere shortening is only observed in skeletal muscles from diseases associated with increase degeneration–regeneration cycles, such as Duchenne muscular dystrophy (18,22,54,55). Instead, we show for the first time, similar to other tissues (56,57), that oxidative stress can cause telomere shortening in skeletal muscle fibers. Interestingly, treatment with the antioxidant NAC attenuated telomere shortening in skeletal muscle fibers of C57BL/6 mice but not in CAST/Ei fibers. Intrinsic differences in telomere length and telomere dynamics between the strains could underlie this differential response. Further, because CAST/Ei mice have telomere lengths similar to human tissues (50), the fibers from these animals may be responding to oxidative stress in a similar fashion to how human skeletal muscle telomeres may respond to ROS. The differential results between the CAST/Ei and C57BL/6 strains could be due to genotype or differences in telomere length (3,58). Mammalian species with short telomeres tend to have an enhanced capacity to resist and/or to repair oxidative damage, whereas long-telomere–bearing species tend to lose telomere length more readily (3). However, because both strains had similar telomere shortening responses (76% and 67%) to oxidative insult, it is difficult to determine if this particular wild-derived rodent strain has a higher resistance or propensity to repair oxidative damage compared with the long-telomere–bearing C57BL/6 strain. These data could indicate that rodents, regardless of telomere length, tend to lose telomere length more readily compared with other mammalian species (3). However, because the antioxidant treatment rescued the telomere shortening observed in the long-telomere–bearing mice but not in the short-telomere–bearing mice, this likely indicates that the telomeres shortened via different mechanisms between the mouse strains. The long-telomere–bearing mice likely lost telomere length due to oxidant-induced DNA damage to the telomeres, whereas a different mechanism likely caused the telomere shortening in the short-telomere–bearing mice, such as cellular proliferation of fiber-associated satellite cells in response to both cell culture treatments. However, future experiments will be needed to test this hypothesis because proliferation was not directly assessed in the current study. The relatively recent inbreeding of the CAST/Ei strain compared with the long-term inbreeding of the C57BL/6 strain could represent evolutionary divergence within the species (3,50,59) may also explain the differential results. Overall, we report that ROS is indeed a possible mechanism for telomere shortening in adult skeletal muscle fibers of both typical and wild-derived strains of mice, though the dynamics of this shortening are strain specific.
Telomerase Enzyme Activity Is Not Increased in Single Muscle Fibers Exposed to Oxidative Stress
Previous studies have reported ambiguous results regarding telomerase enzyme activity following oxidative stress treatments across a range of tissues (60–63). Interestingly, we report no effect of oxidant challenge on telomerase activity, but that telomerase activity is greater in muscle fibers of C57BL/6 compared with short-telomere–bearing CAST/Ei mice, supporting previous findings (51). Our results confirm those of Cattan and coworkers (64) who showed that telomerase enzyme activity was not increased in skeletal muscle of CAST/Ei animals exposed to oxidative stress (long-term glutathione depletion). The low levels of telomerase enzyme activity detected in skeletal muscle likely is originating from the satellite cells associated with the isolated fibers and is tightly regulated in a cell cycle–dependent fashion (21). This could indicate that telomerase activity in satellite cells of skeletal muscle of these mice is detectable, but extremely low compared with other tissues and, therefore, plays a minimal role in skeletal muscle fiber telomere dynamics.
TRF Protein Content Is Altered in Response to Oxidative Challenge but Differs by Strain
Several recent studies have observed altered expression of TRF1 and TRF2 induced by multiple stimuli (16,17,65,66). We have previously shown that Trf1 gene expression was reduced with acute exercise in skeletal muscle (40). In chronically exercised animals, exercise prevented an age-associated increase in TRF1 protein content and an age-associated decrease in TRF2 gene expression and protein content in skeletal muscle (20). These results indicated the physiological stressors can induce changes in the expression telomere-binding proteins.
In the present study, we show that TRF1 protein content is not influenced by oxidative stress treatment in skeletal muscle fibers of C57BL/6 mice. In the CAST/Ei fibers, oxidative challenge alone did not change TRF1 protein content, but the combination of antioxidant and oxidative stress increased TRF1 protein content; however, this result is difficult to interpret without an antioxidant-only group. A study of skeletal muscle from Duchenne muscular dystrophy patients (a condition associated with increased skeletal muscle ROS) compared with control skeletal muscle showed that TRF1 protein was increased along with shortened telomeres (67). We hypothesize that short-telomere–bearing mammals (humans and CAST/Ei mice) may preferentially upregulate TRF1 under stress, whereas long-telomere–bearing mammals (C57BL/6) do not upregulate TRF1 to stabilize telomere length in skeletal muscle. Future antioxidant dose–dependent experiments are needed to clarify these findings.
TRF2 protein content was increased in H2O2-treated skeletal muscle fibers of C57BL/6 animals compared with combination of oxidant- and antioxidant-treated fibers. TRF2 content was not altered in CAST/Ei control fibers in any treatment group. These data suggest that TRF2 may be associated with the response of ROS-induced telomere shortening in C57BL/6 skeletal muscle. TRF2 was not induced in short-telomere–bearing mice, possibly related to underlying differences between strains in either telomere biology and/or antioxidant capacity. Together, these data indicate that oxidative stress–related signaling may be important for the regulation of TRF1, TRF2, and telomere length in rodents.
Several limitations of the current study should be pointed out. We did not directly assess proliferation of muscle-associated satellite cells or other doses of antioxidant treatment that would significantly improve the interpretation of this study’s data. Further, we cannot rule out the possibility that the oxidant treatment induced cellular senescence of the fiber-associated satellite cells. We also cannot address whether telomere length shortened due to proliferation or DNA damage–induced mechanisms, both of which are exciting topics of future investigation. That being said, we have devised a novel tissue culture model system to study the effects of oxidative stress on telomere length between short- and long-telomere–bearing strains of mice. These initial studies provide the basis for future mechanistic studies of skeletal muscle fiber and satellite cell telomere dynamics.
Thus, aging and increased ROS production in skeletal muscle by any number of mechanisms including disease and muscle contraction may result in telomere shortening, such as that observed in our current investigation. In summary, we describe that ROS results in telomere shortening in skeletal muscle fibers of mice, but with strain-specific responses. These data indicate important strain differences in the response of telomeres and telomere-related proteins that may be driven by intrinsic differences related to telomere length and/or antioxidant enzymes or other unknown factors. We suggest a potential mechanism by which increased oxidative stress could induce telomere shortening in skeletal muscles.
Funding
UTSWMC National Cancer Institute Cell Biology Department Training Grant (CA12433404 to A.T.L, 2012–2013). This work was also supported by the National Institutes of Health (T32 AG000268 to A.T.L, 2010).
References
- 1. Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med. 2006;12:1133–1138. 10.1038/nm1006-1133 [DOI] [PubMed] [Google Scholar]
- 2. Daniali L, Benetos A, Susser E, et al. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun. 2013;4:1597. 10.1038/ncomms2602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gomes NM, Ryder OA, Houck ML, et al. Comparative biology of mammalian telomeres: hypotheses on ancestral states and the roles of telomeres in longevity determination. Aging Cell. 2011;10:761–768. 10.1111/j.1474-9726.2011.00718.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shay JW, Wright WE. Senescence and immortalization: role of telomeres and telomerase. Carcinogenesis. 2005;26:867–874. 10.1093/carcin/bgh296 [DOI] [PubMed] [Google Scholar]
- 5. Martinez P, Thanasoula M, Carlos AR, et al. Mammalian Rap1 controls telomere function and gene expression through binding to telomeric and extratelomeric sites. Nat Cell Biol. 2010;12:768–780. 10.1038/ncb2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Perrod S, Gasser SM. Long-range silencing and position effects at telomeres and centromeres: parallels and differences. Cell Mol Life Sci. 2003;60:2303–2318. 10.1007/s00018-003-3246-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stadler G, Rahimov F, King OD, et al. Telomere position effect regulates DUX4 in human facioscapulohumeral muscular dystrophy. Nat Struct Mol Biol. 2013;20:671–678. 10.1038/nsmb.2571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Greider CW. Telomeres, telomerase and senescence. Bioessays. 1990;12:363–369. 10.1002/bies.950120803 [DOI] [PubMed] [Google Scholar]
- 9. Greider CW. Telomerase RNA levels limit the telomere length equilibrium. Cold Spring Harb Symp Quant Biol. 2006;71:225–229. 10.1101/sqb.2006.71.063 [DOI] [PubMed] [Google Scholar]
- 10. Lee HW, Blasco MA, Gottlieb GJ, Horner JW, 2nd, Greider CW, DePinho RA. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–574. 10.1038/33345 [DOI] [PubMed] [Google Scholar]
- 11. Levy MZ, Allsopp RC, Futcher AB, Greider CW, Harley CB. Telomere end-replication problem and cell aging. J Mol Biol. 1992;225:951–960. 10.1016/0022-2836(92)90096-3 [DOI] [PubMed] [Google Scholar]
- 12. Ludlow AT, Roth SM. Physical activity and telomere biology: exploring the link with aging-related disease prevention. J Aging Res. 2011;2011:790378. 10.4061/2011/790378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van der Harst P, van der Steege G, de Boer RA, et al. ; MERIT-HF Study Group. Telomere length of circulating leukocytes is decreased in patients with chronic heart failure. J Am Coll Cardiol. 2007;49:1459–1464. 10.1016/j.jacc.2007.01.027 [DOI] [PubMed] [Google Scholar]
- 14. Cherkas LF, Hunkin JL, Kato BS, et al. The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med. 2008;168:154–158. 10.1001/archinternmed.2007.39 [DOI] [PubMed] [Google Scholar]
- 15. Ludlow AT, Zimmerman JB, Witkowski S, Hearn JW, Hatfield BD, Roth SM. Relationship between physical activity level, telomere length, and telomerase activity. Med Sci Sports Exerc. 2008;40:1764–1771. 10.1249/MSS.0b013e31817c92aa [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Werner C, Fürster T, Widmann T, et al. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation. 2009;120:2438–2447. 10.1161/CIRCULATIONAHA.109.861005 [DOI] [PubMed] [Google Scholar]
- 17. Werner C, Hanhoun M, Widmann T, et al. Effects of physical exercise on myocardial telomere-regulating proteins, survival pathways, and apoptosis. J Am Coll Cardiol. 2008;52:470–482. 10.1016/j.jacc.2008.04.034 [DOI] [PubMed] [Google Scholar]
- 18. Decary S, Hamida CB, Mouly V, Barbet JP, Hentati F, Butler-Browne GS. Shorter telomeres in dystrophic muscle consistent with extensive regeneration in young children. Neuromuscul Disord. 2000;10:113–120. 10.1016/S0960-8966(99)00093-0 [DOI] [PubMed] [Google Scholar]
- 19. Decary S, Mouly V, Hamida CB, Sautet A, Barbet JP, Butler-Browne GS. Replicative potential and telomere length in human skeletal muscle: implications for satellite cell-mediated gene therapy. Hum Gene Ther. 1997;8:1429–1438. 10.1089/hum.1997.8.12-1429 [DOI] [PubMed] [Google Scholar]
- 20. Ludlow AT, Witkowski S, Marshall MR, et al. Chronic exercise modifies age-related telomere dynamics in a tissue-specific fashion. J Gerontol A Biol Sci Med Sci. 2012;67:911–926. 10.1093/gerona/gls002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mouly V, Aamiri A, Bigot A, et al. The mitotic clock in skeletal muscle regeneration, disease and cell mediated gene therapy. Acta Physiol Scand. 2005;184:3–15. 10.1111/j.1365-201X.2005.01417.x [DOI] [PubMed] [Google Scholar]
- 22. Renault V, Thornell LE, Butler-Browne G, Mouly V. Human skeletal muscle satellite cells: aging, oxidative stress and the mitotic clock. Exp Gerontol. 2002;37:1229–1236. S0531556502001298 [pii] [DOI] [PubMed] [Google Scholar]
- 23. Renault V, Thornell LE, Eriksson PO, Butler-Browne G, Mouly V, Thorne LE. Regenerative potential of human skeletal muscle during aging. Aging Cell. 2002;1:132–139 [DOI] [PubMed] [Google Scholar]
- 24. Benetos A, Kimura M, Labat C, et al. A model of canine leukocyte telomere dynamics. Aging Cell. 2011;10:991–995. 10.1111/j.1474-9726.2011.00744.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fanò G, Mecocci P, Vecchiet J, et al. Age and sex influence on oxidative damage and functional status in human skeletal muscle. J Muscle Res Cell Motil. 2001;22:345–351. 10.1023/A1013122805060 [DOI] [PubMed] [Google Scholar]
- 26. Fulle S, Di Donna S, Puglielli C, et al. Age-dependent imbalance of the antioxidative system in human satellite cells. Exp Gerontol. 2005;40:189–197. 10.1016/j.exger.2004.11.006 [DOI] [PubMed] [Google Scholar]
- 27. Kawanishi S, Oikawa S. Mechanism of telomere shortening by oxidative stress. Ann N Y Acad Sci. 2004;1019:278–284. 10.1196/annals.1297.047 [DOI] [PubMed] [Google Scholar]
- 28. Narciso L, Fortini P, Pajalunga D, et al. Terminally differentiated muscle cells are defective in base excision DNA repair and hypersensitive to oxygen injury. Proc Natl Acad Sci USA. 2007;104:17010–17015. 10.1073/pnas.0701743104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oikawa S, Tada-Oikawa S, Kawanishi S. Site-specific DNA damage at the GGG sequence by UVA involves acceleration of telomere shortening. Biochemistry. 2001;40:4763–4768. 10.1021/bi002721g [DOI] [PubMed] [Google Scholar]
- 30. Rochette PJ, Brash DE. Human telomeres are hypersensitive to UV-induced DNA Damage and refractory to repair. PLoS Genet. 2010;6:e1000926. 10.1371/journal.pgen.1000926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bilaud T, Brun C, Ancelin K, Koering CE, Laroche T, Gilson E. Telomeric localization of TRF2, a novel human telobox protein. Nat Genet. 1997;17:236–239. 10.1038/ng1097-236 [DOI] [PubMed] [Google Scholar]
- 32. de Lange T. How shelterin solves the telomere end-protection problem. Cold Spring Harb Symp Quant Biol. 2010;75:167–177. 10.1101/sqb.2010.75.017 [DOI] [PubMed] [Google Scholar]
- 33. de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. 10.1101/gad.1346005 [DOI] [PubMed] [Google Scholar]
- 34. Brown LD, Schneider MF. Delayed dedifferentiation and retention of properties in dissociated adult skeletal muscle fibers in vitro. In Vitro Cell Dev Biol Anim. 2002;38:411–422. 10.1290/1071-2690(2002)038<0411DDAROP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 35. Chakravarthy MV, Spangenburg EE, Booth FW. Culture in low levels of oxygen enhances in vitro proliferation potential of satellite cells from old skeletal muscles. Cell Mol Life Sci. 2001;58:1150–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Richardson RS, Noyszewski EA, Leigh JS, Wagner PD. Lactate efflux from exercising human skeletal muscle: role of intracellular PO2 . J Appl Physiol. 1998;85:627–634. 10.1113/jphysiol.2005.102327 [DOI] [PubMed] [Google Scholar]
- 37. Wright WE, Shay JW. Inexpensive low-oxygen incubators. Nat Protoc. 2006;1:2088–2090. 10.1038/nprot.2006.374 [DOI] [PubMed] [Google Scholar]
- 38. Palomero J, Pye D, Kabayo T, Spiller DG, Jackson MJ. In situ detection and measurement of intracellular reactive oxygen species in single isolated mature skeletal muscle fibers by real time fluorescence microscopy. Antioxid Redox Signal. 2008;10:1463–1474. 10.1089/ars.2007.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brandl A, Meyer M, Bechmann V, Nerlich M, Angele P. Oxidative stress induces senescence in human mesenchymal stem cells. Exp Cell Res. 2011;317:1541–1547. 10.1016/j.yexcr.2011.02.015 [DOI] [PubMed] [Google Scholar]
- 40. Ludlow AT, Lima LC, Wang J, et al. Exercise alters mRNA expression of telomere-repeat binding factor 1 in skeletal muscle via p38 MAPK. J Appl Physiol. 2012;113:1737–1746. 10.1152/japplphysiol.00200.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rasband WS. ImageJ. U. S. National Institutes of Health 1997–2011. Retrieved from http://imagej.nih.gov/ij/
- 42. Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. 10.1093/nar/30.10.e47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol. 2003;5:741–747. 10.1038/ncb1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Greider CW. Telomeres do D-loop-T-loop. Cell. 1999;97:419–422. 10.1016/S0092-8674(00)80750-3 [DOI] [PubMed] [Google Scholar]
- 45. Griffith JD, Comeau L, Rosenfield S, et al. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503–514. 10.1016/S0092-8674(00)80760-6 [DOI] [PubMed] [Google Scholar]
- 46. Nandakumar J, Cech TR. Finding the end: recruitment of telomerase to telomeres. Nat Rev Mol Cell Biol. 2013;14:69–82. 10.1038/nrm3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McKerlie M, Zhu XD. Cyclin B-dependent kinase 1 regulates human TRF1 to modulate the resolution of sister telomeres. Nat Commun. 2011;2:371. 10.1038/ncomms1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Muñoz P, Blanco R, Flores JM, Blasco MA. XPF nuclease-dependent telomere loss and increased DNA damage in mice overexpressing TRF2 result in premature aging and cancer. Nat Genet. 2005;37:1063–1071. 10.1038/ng1633 [DOI] [PubMed] [Google Scholar]
- 49. Smogorzewska A, van Steensel B, Bianchi A, et al. Control of human telomere length by TRF1 and TRF2. Mol Cell Biol. 2000;20:1659–1668. 10.1128/MCB.20.5.1659-1668.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hemann MT, Greider CW. Wild-derived inbred mouse strains have short telomeres. Nucleic Acids Res. 2000;28:4474–4478. 10.1093/nar/28.22.4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Prowse KR, Greider CW. Developmental and tissue-specific regulation of mouse telomerase and telomere length. Proc Natl Acad Sci USA. 1995;92:4818–4822. 10.1073/pnas.92.11.4818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–528. 10.1038/nature08982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rae DE, Vignaud A, Butler-Browne GS, et al. Skeletal muscle telomere length in healthy, experienced, endurance runners. Eur J Appl Physiol. 2010;109:323–330. 10.1007/s00421-010-1353-6 [DOI] [PubMed] [Google Scholar]
- 54. Kadi F, Ponsot E. The biology of satellite cells and telomeres in human skeletal muscle: effects of aging and physical activity. Scand J Med Sci Sports. 2010;20:39–48. 10.1111/j.1600-0838.2009.00966.x [DOI] [PubMed] [Google Scholar]
- 55. Ponsot E, Echaniz-Laguna A, Delis AM, Kadi F. Telomere length and regulatory proteins in human skeletal muscle with and without ongoing regenerative cycles. Exp Physiol. 2012;97:774–784. 10.1113/expphysiol.2011.063818 [DOI] [PubMed] [Google Scholar]
- 56. Makpol S, Zainuddin A, Rahim NA, Yusof YA, Ngah WZ. Alpha-tocopherol modulates hydrogen peroxide-induced DNA damage and telomere shortening of human skin fibroblasts derived from differently aged individuals. Planta Med. 2010;76:869–875. 10.1055/s-0029-1240812 [DOI] [PubMed] [Google Scholar]
- 57. Richter T, von Zglinicki T. A continuous correlation between oxidative stress and telomere shortening in fibroblasts. Exp Gerontol. 2007;42:1039–1042. 10.1016/j.exger.2007.08.005 [DOI] [PubMed] [Google Scholar]
- 58. Mehrabian M, Allayee H, Wong J, et al. Identification of 5-lipoxygenase as a major gene contributing to atherosclerosis susceptibility in mice. Circ Res. 2002;91:120–126. 10.1161/01.RES.0000028008.99774.7F [DOI] [PubMed] [Google Scholar]
- 59. Keane TM, Goodstadt L, Danecek P, et al. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature. 2011;477:289–294. 10.1038/nature10413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Büchner N, Zschauer TC, Lukosz M, Altschmied J, Haendeler J. Downregulation of mitochondrial telomerase reverse transcriptase induced by H2O2 is Src kinase dependent. Exp Gerontol. 2010;45:558–562. 10.1016/j.exger.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 61. Haendeler J, Dröse S, Büchner N, et al. Mitochondrial telomerase reverse transcriptase binds to and protects mitochondrial DNA and function from damage. Arterioscler Thromb Vasc Biol. 2009;29:929–935. 10.1161/ATVBAHA.109.185546 [DOI] [PubMed] [Google Scholar]
- 62. Haendeler J, Hoffmann J, Diehl JF, et al. Antioxidants inhibit nuclear export of telomerase reverse transcriptase and delay replicative senescence of endothelial cells. Circ Res. 2004;94:768–775. 10.1161/01.RES.0000121104.05977.F3 [DOI] [PubMed] [Google Scholar]
- 63. Strong MA, Vidal-Cardenas SL, Karim B, Yu H, Guo N, Greider CW. Phenotypes in mTERT +/− and mTERT −/− mice are due to short telomeres, not telomere-independent functions of telomerase reverse transcriptase. Mol Cell Biol. 2011;31:2369–2379. 10.1128/MCB.05312-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cattan V, Mercier N, Gardner JP, et al. Chronic oxidative stress induces a tissue-specific reduction in telomere length in CAST/Ei mice. Free Radic Biol Med. 2008;44:1592–1598. 10.1016/j.freeradbiomed.2008.01.007 [DOI] [PubMed] [Google Scholar]
- 65. Spallarossa P, Altieri P, Aloi C, et al. Doxorubicin induces senescence or apoptosis in rat neonatal cardiomyocytes by regulating the expression levels of the telomere binding factors 1 and 2. Am J Physiol Heart Circ Physiol. 2009;297:H2169–H2181. 10.1152/ajpheart.00068.2009 [DOI] [PubMed] [Google Scholar]
- 66. Zhang Y, Cao EH, Qin JF. Up-regulation of telomere-binding TRF1, TRF2 related to reactive oxygen species induced by As(2)O(3) in MGC-803 cells. Eur J Pharmacol. 2005;516:1–9. 10.1016/j.ejphar.2005.04.022 [DOI] [PubMed] [Google Scholar]
- 67. Aguennouz M, Vita GL, Messina S, et al. Telomere shortening is associated to TRF1 and PARP1 overexpression in Duchenne muscular dystrophy. Neurobiol Aging. 2011;32:2190–2197. 10.1016/j.neurobiolaging.2010.01.008 [DOI] [PubMed] [Google Scholar]