Abstract
Thorough analysis of translational endophenotypes is needed to improve therapeutic development in schizophrenia. Abnormal sensory gating, one such endophenotype, is associated with reduced expression of the α7 nicotinic receptor. However, typical gating measures such as the P50 evoked response are often low-pass filtered, and it is unclear how α7 expression affects gating at higher frequencies. Therefore, this study used time-frequency analysis to compare sensory gating at the beta and gamma frequencies between human patients and healthy controls as well as between α7 heterozygote mutant mice and wild-type. Gating of total beta (15–26 Hz) and gamma (30–50 Hz) power during paired clicks was assessed from mouse in vivo hippocampal CA3 recordings. Gating was also assessed in schizophrenia patients and healthy controls using electroencephalography. Relative to wild-type, α7 heterozygote mice showed impaired gating of total beta and gamma power. Similarly, relative to controls, patients showed impaired gating of total beta and gamma power. Poor beta gating was associated with negative symptoms. These results demonstrate that schizophrenia patients and α7 heterozygote mice show similar deficits in gating high frequency power. Time-frequency analysis of beta and gamma gating may thus be a translational method of assessing the genetic basis of gating deficits in schizophrenia.
Keywords: Gamma, Beta, Nicotinic, Schizophrenia, Gating
1. Introduction
Patients with schizophrenia often report being unable to filter out distracting background noise, leading to hypervigilance and problems in focusing attention (McGhie and Chapman, 1961). These filtering deficits are hypothesized to reflect general inhibitory dysfunction in cortical feedback circuitry in the illness (Miwa et al., 2011), and can be physiologically examined by measuring neural response to auditory stimuli. An advantage of this approach is that similar methods can be used to analyze response in human patients and animal models of schizophrenia.
On a translational level, the sensory flooding phenotype has been most thoroughly investigated using the P50 auditory paired-click paradigm (Turetsky et al., 2007). The P50 response is an early (50 ms post-stimulus) event related potential (ERP) that occurs in response to an auditory stimulus. In this experimental paradigm, two identical click sounds are played at an easily heard volume; the clicks are typically separated by 500 ms. In healthy subjects, the electrophysiological response to the second stimulus in the pair is reduced in comparison to the first. However, in schizophrenia and in animal models of the illness, the response to the second stimulus is not diminished, indicating a deficit in the ability of the brain to process neuronal impulses from the first click that affects the physiological responses that follow (Adler et al., 1982; Olincy et al., 2010; Turetsky et al., 2007). This is proposed to be a mechanism by which patients are less able to ignore irrelevant, unimportant sounds in the environment (e.g. the clicking of a clock distracts from balancing a checkbook) (Adler et al., 1998).
The neurobiological mechanisms underlying P50 gating and its pathological disruption are an active area of investigation. Neuroanatomically, suppression of the P50 electrophysiological response is localized to the hippocampus in rodent models (Stevens et al., 1996) and in the hippocampus, medial frontal gyrus, insula, and auditory cortex in healthy human subjects (Bak et al., 2011; Mathiak et al., 2011). On a microscopic scale, P50 gating deficits may be linked to reduced expression of presynaptic and postsynaptic α7 nicotinic acetylcholine receptors (nAChRs) on inhibitory interneurons (Adams et al., 2001; Adams et al., 2008; Freedman et al., 2000). This reduction in turn may reduce the ability of these cells to release gamma-aminobutyric acid (GABA) and inhibit brain response to repeated stimulation (Miwa et al., 2011). In support of this hypothesis, mice with reduced α7 nAChR expression (e.g. α7 heterozygotes) show impaired P50 sensory gating (Adams et al., 2008). In addition, single nucleotide polymorphisms in the promoter region for the α7 receptor gene are associated with gating deficits in humans and risk for schizophrenia (Leonard et al., 2002).
A limitation of P50 ERP studies is that they often do not reveal how neural oscillatory activity is modulated by sounds at higher frequency ranges, as EEG researchers typically low-pass filter out the higher frequency ranges in P50 recordings due to historical biases in attributing these signals to noise. It is important to fill this knowledge gap, however, as the most important frequencies for information transfer may be at higher frequencies (as with human speech) (Pena and Melloni, 2012; Shahin et al., 2009). On this last point, speech, which is also dominated by low frequency content, can easily be made unintelligible to the human ear by low-pass filtering what at first glance appears to be noise at higher frequencies (French and Steinberg, 1947; Pollack, 1948). A more complete understanding of how sensory gating deficits are related to circuit dynamics and the neural pathology of schizophrenia therefore requires an examination of activity across a broad range of frequencies. Indeed, in an early study of gamma-band contributions to sensory gating deficits in schizophrenia, Clementz et al. (1997) reported that gamma-band responses overlapping temporally with the P50 response separated schizophrenia and control subjects better than the P50 response.
Using time-frequency analysis techniques, recent work has begun to examine the contribution of other frequencies of neural oscillations during sensory processing. Gamma (30–80 Hz and higher) oscillations are associated with local sensory binding, i.e. the ability of the brain to link parts of stimuli to form a scene or object (Eckhorn et al., 1988). Beta (13–29 Hz) oscillations are associated with encoding and consolidating sensory information over long distances in the cortex, and may be correlated with stimulus salience (Bibbig et al., 2001; Haenschel et al., 2000; Kopell et al., 2000). Based on the proposed role(s) of activity in these frequency ranges for stimulus processing, it is possible to speculate that sensory flooding is associated with the inability to reduce beta and gamma oscillatory power in order to reduce stimulus salience following repetitive stimulation. This modulation likely involves complex interactions between inhibitory, excitatory, and cholinergic neurotransmitter systems. Indeed, nicotine modulates high frequency oscillatory power following white noise bursts (Cao et al., 2012). The relationship between gamma and beta oscillations during auditory sensory gating and nicotinic receptors, however, has yet to be investigated.
To investigate the relationship between gamma and beta frequencies and the α7 nAChR during sensory gating, we carried out a parallel study of gating in humans and mice. Time-frequency analysis was used to compare gating of beta and gamma power in α7 nAChR heterozygote mice to wild-type. In addition, identical analysis techniques were used to compare gating of beta and gamma power in patients to healthy controls. We hypothesized that gating of gamma and beta frequency power would be reduced in both heterozygous mice and patients with schizophrenia, indicating deficits in post-stimulus processing.
2. Materials and methods
2.1. Mice
Breeder C3H mice carrying the null mutation for the α7 nicotinic receptor were obtained from the Institute for Behavioral Genetics breeding colony at the University of Colorado, Boulder, CO, and bred at the University of Colorado Denver, Anschutz Medical Campus rodent colony. The C3H α7 heterozygote mice were derived by mating α7 mixed background (129 × C57BL/6) mice with C3H heterozygote mice (both sexes used). The progeny that result from this crossing are +/+ and 9+/− at the α7 locus. Heterozygote offspring were then bred back to the C3H line for a minimum of 10 generations. Food (Purina Rodent Chow) and water were available ad libitum and lighting was cycled at 12-h intervals (lights on at 6 AM). “Principles of Laboratory Animal Care” (NIH Publication No. 85–23, revised 1985) were followed. The Institutional Animal Care and Use Committee of the Denver Veterans Affairs Medical Center and/or the University of Colorado Anschutz Medical Campus approved the experimental protocols.
2.2. Mouse recordings
Adult C3H wild type (n = 9) and C3H α7 heterozygote (n = 11) mice (20–25 g) were anesthetized with chloral hydrate (400 mg/kg ip) and pyrazole (400 mg/kg ip). Mice were then placed in a stereotaxic apparatus, and hollow ear bars with attached earphones were positioned adjacent to the mouse′s ears. Body temperature was maintained at 37 °C with a heating pad. A teflon-coated stainless-steel recording electrode was inserted into the pyramidal layer of hippocampal area CA3 at 1.8 mm posterior to bregma, 2.7 mm lateral to the midline and 1.5–1.7 mm below the surface of the dura. Final location was identified by the presence of complex action potentials typical of hippocampal pyramidal neurons (Miller and Freedman, 1995). A similar reference electrode was placed on the dura, contralateral to the recording electrode, just anterior to bregma. Tones (300 Hz, 10 ms, 70 dB SPL) were presented in pairs separated by 500 ms with 10 s between tone pairs. A baseline period of 50 ms preceded each tone pairing. Recordings were segmented from −50 to 350 ms after stimulus onset. Five sets (10 m duration/set) of responses to 16 tone-pairs were recorded per animal. Activity was recorded at a sampling rate of 20 kHz and subsequently downsampled to 1 kHz to match the sampling rate of human data recordings. Custom software (SciWorks, DataWave, Loveland, CO) was used for all recordings. P50 data from these mice have been published previously (Adams et al., 2008).
2.3. Human subjects
Participants included 51 outpatients who met DSM-IV criteria for schizophrenia (as determined by the Structured Clinical Interview for DSM-IV) and 46 healthy comparison subjects recruited from the local community (see Table 1 for demographic information). Patients were recruited by referral from a clinician involved in the study (A.O.) as well as by other mental health professionals. Exclusion criteria included a current diagnosis of substance abuse, neurological disorders, or head trauma. Scores on the Scale for the Assessment of Negative Symptoms (SANS; five items) and the Scale for the Assessment of Positive Symptoms (SAPS; four items) scores were determined by a clinician (A.O.) (see Table 2). All patients were stable and were not hospitalized at the time of scoring. Medication and smoking status were not significantly associated with any measure of interest in the present study. Healthy comparison subjects were excluded for Axis I disorders including schizophrenia, bipolar disorder, depression, anxiety, and lifetime substance dependence as well as a first-degree family history of psychosis. Subjects were compensated for participation and provided written informed consent. The study was approved by the Colorado Multiple Institutional Review Board.
Table 1.
Demographic data of participants. ( ) indicate the S.D.
| Group | Healthy controls | Schizophrenia |
|---|---|---|
| Number | 46 | 51 |
| Age (years) | 31 (9) | 37 (12) |
| Gender (male:female) | 14:32 | 38:13 |
| Medication | n/a | 46 |
| Atypical antipsychotics | n/a | 44 |
| Typical antipsychotics | n/a | 2 |
| Chlorpromazine eq daily dose (mg) | n/a | 566 (484) |
| Dose range (mg) | n/a | 100–2550 |
| Time on current medication (months) | n/a | 30 (37) |
| Duration of illness (years) | n/a | 13 (11) |
| Smoking status (smoker:nonsmoker) | 7:39 | 14:37 |
Table 2.
Clinical data.
| SAPS | Mean (S.D.) | SANS | Mean (S.D.) |
|---|---|---|---|
| Hallucinations | 1.68 (1.72) | Affective flattening | 1.27 (1.37) |
| Delusions | 2.52 (2.00) | Alogia | 0.78 (1.29) |
| Bizarre behavior | 0.71 (1.51) | Avolition | 1.29 (1.80) |
| Thought disorder | 1.19 (1.80) | Anhedonia | 1.38 (2.15) |
| Total | 6.10 (5.22) | Attention | 0.68 (1.14) |
| Total (not including attention) | 4.71 (5.13) |
2.4. Human EEG recordings
Pairs of click stimuli were presented as described previously for measurement of P50 sensory gating (Adler et al., 1982). Briefly, EEG activity was recorded from a gold disk electrode affixed to the vertex, referenced to linked ears. The electroculogram was simultaneously recorded between the right superior orbit and lateral canthus. Impedances were less than 10 kΩ. EEG recording and analysis was performed using LEA2003 software (Rave Wave Systems, Inc.).
During recording, subjects sat semi-recumbent in a comfortable chair. They were instructed to remain awake and to fix their eyes on a cross on the wall 2 m across from the chair. A 0.04-ms duration square wave pulse was amplified in the 20–12,000 Hz bandwidth and delivered through headphones to produce 2.5-ms click stimuli. Clicks were presented binaurally with headphones (Sony MDR V-600) and LEA2003 hardware (Rave Wave Systems, Inc). The click stimulus intensity was set to 50 dB above the subject's hearing threshold to optimize brain response while minimizing startle. Threshold was defined as the minimum volume at which a set of paired clicks could be discerned by the subject. The clicks were presented in pairs separated by 500 ms, with 10 s between click pairs. Click pairs were presented in five blocks of 16–30 conditioning-test trials. Total recording time was approximately 30 min. Subjects were given no special instructions concerning the clicks they were hearing.
EEG data were first filtered from 10 to 100 Hz and segmented from −100 to 350 ms relative to stimulus onset using a second order Butterworth filter. Data was recorded at a sampling rate of 1 kHz. Trials (evoked response pairs) were rejected during recording if they contained muscle startle artifact or eye blinks as indicated by an EEG or electro-oculographic voltage of ± 35 µV at 50 ms after stimulus or if there was evidence of drowsiness in the EEG, as indicated by three or more delta waves greater than 20 µV in amplitude. P50 data from these subjects have been published previously (Olincy et al., 2010; Smucny et al., 2013).
2.5. Time-frequency data generation
Time-frequency plots and extracted data were generated in Matlab utilizing EEGLAB v.10 scripts (http://sccn.ucsd.edu/eeglab/). As a default, plots and data generated from these scripts are masked to remove all data susceptible to edge artifacts (Roach and Mathalon, 2008). Time-varying spectral total power was computed by convolving the average of individual trials with a complex-valued Morlet′s wavelet. Cycle length varied from three to six over the 15- to 60-Hz frequency range. So that equivalent Morlet wavelet lengths could be used for both human and mouse time-frequency analyses, 50 ms of zero-padding were added to the beginning of each epoch of the mouse data. Power was derived from the squared amplitude coefficient of the wavelet transform. Beta was defined as the average power from 15 to 26 Hz between 30 and 150 ms post-stimulus with 22 frequency bins. Gamma was defined as the average power from 30 to 50 Hz between 30 and 150 ms post-stimulus with 40 frequency bins.
2.6. Data analysis
For all analyses, the primary measure of interest (gating of beta or gamma power) refers to the net difference in power between the test stimulus (stimulus two, or S2) and the conditioning stimulus (stimulus one, or S1), calculated as the value S2-S1. Larger S2-S1 values thus imply worse sensory gating. The S2-S1 calculation inherently subtracts baseline power (i.e. power before S1). Between-group differences of S1 and S2 were also analyzed to explore the relative contribution of each stimulus to the effect, although a limitation of this analysis is that the calculated S1 and S2 power includes baseline signal which may potentially confound any observed effects. This signal could not be subtracted, as it was relatively short (100 ms) and is therefore susceptible to edge artifacts during wavelet analysis (Roach and Mathalon, 2008). Power was analyzed 30–150 ms post-stimulus, as healthy subjects gate beta power in this time window (Hong et al., 2008). In addition, this epoch captures the peak of the auditory stimulus-associated transient gamma band response (Tiitinen et al., 1993). The beta range was defined as 15–26 Hz, and the gamma range was defined as 30–50 Hz.
The dependent measures (S1 power, S2 power, S2-S1 power) were compared between groups with repeated measures analysis of variance (ANOVA), with frequency band (beta, gamma) as a within-subjects factor and genotype (wildtype vs. α7 heterozygote for mice, control vs. patient for human) as a between-subjects factor. Significant main effects were followed up by post-hoc (Fisher′s Least Significant Difference (LSD)) testing of simple effects between groups at each frequency band. All values used in human data analysis were adjusted for the effects of age and gender, as groups were not age- and gender-matched.
Clinical correlations were analyzed at a Bonferroni-corrected threshold of p < 0.01 for SANS (five-point) subscales and p < 0.0125 for SAPS (four-point) subscales. Total SANS and SAPS scores were analyzed at a threshold of p < 0.05 (uncorrected). All correlations were adjusted for the effects of age and gender. All data analyses were completed using SPSS version 20 (IBM, New York).
3. Results
3.1. Mouse data
Time-frequency plots of S2 (test stimulus)-S1 (conditioning stimulus) total power are shown in Fig. 1. Repeated measures ANOVA revealed a significant main effect of group on S2-S1 power (i.e. gating) (F(1,18) = 10.8, p < 0.01), with α7 heterozygote mice showing larger S2-S1 power (and therefore worse gating). Posthoc testing showed the groups differed significantly in beta (p = 0.002) and gamma (p = 0.011) gating (Fig. 1; Table 3). The effect was driven by a relative increase in S2 power relative to S1 in heterozygote mice, but little to no change in S2 power relative to S1 in wild-type mice.
Fig. 1.
Left: Time-frequency plots of S2-S1 power (i.e. gating) from α7 heterozygote and wild-type mouse. Right: Bar graphs showing higher S2-S1 power (i.e. gating deficits) in the beta and gamma frequency ranges in heterozygote mice compared to wild-type. Average beta and gamma power was calculated between 30 and 150 ms post-stimulus. Error bars represent the standard error. *p < 0.05.
Table 3.
Power values. Human data are adjusted for the effect of age and gender.± values represent the standard error.
| Total power (mV2) | Wild-type (mouse) | Alpha7 Het (mouse) | p Het vs. wild-type | Control | Patient | p patient vs. control |
|---|---|---|---|---|---|---|
| Beta S1:30–150 ms | 4.16 ± 0.62 | 4.56 ± 0.56 | 0.63 | 52.15 ± 2.57 | 54.74 ± 2.42 | 0.53 |
| Beta S2:30–150 ms | 4.03 ± 0.75 | 6.62 ± 0.68 | 0.020* | 45.62 ± 2.62 | 52.15 ± 2.47 | 0.10 |
| S2-S1 | −0.13 ± 0.46 | 2.16 ± 0.42 | 0.002* | −6.54 ± 0.78 | −2.59 ± 0.74 | 0.001* |
| Gamma S1:30–150 ms | 2.85 ± 0.48 | 3.61 ± 0.43 | 0.26 | 19.65 ± 1.08 | 20.70 ± 1.02 | 0.56 |
| Gamma S2:30–150 ms | 2.95 ± 0.58 | 4.93 ± 0.53 | 0.022* | 18.32 ± 1.06 | 20.28 ± 1.00 | 0.24 |
| S2-S1 | 0.10 ± 0.32 | 1.32 ± 0.29 | 0.011* | −1.34 ± 0.29 | −0.42 ± 0.27 | 0.035* |
p < 0.05.
Comparing S1 and S2 power between groups, no significant main effect of group was observed for S1 power (F(1,18) = 0.62, p = 0.44). A significant main effect of group was observed for S2 power (F(1,18) = 6.53, p = 0.020). Post-hoc testing showed the groups differed significantly in S2 power for the beta (p = 0.020) and gamma (p = 0.022) bands (Table 3).
3.2. Human data
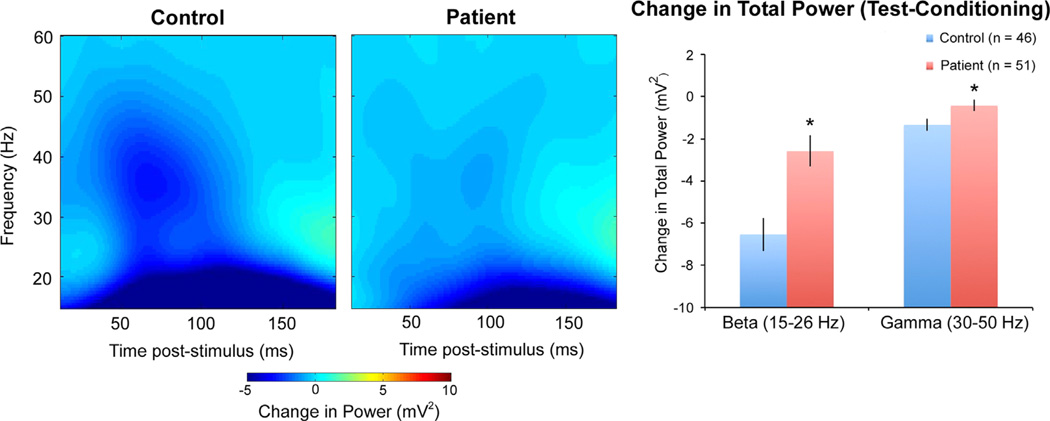
Time-frequency plots of human S2-S1 total power are shown in Fig. 2. Repeated measures ANOVA revealed a significant main effect of group on S2-S1 power (i.e. gating) (F(1,93) = 12.0, p < 0.001), with patients showing larger S2-S1 power (and therefore worse gating). Post-hoc testing showed the groups differed significantly in beta (p < 0.001) and gamma (p = 0.035) gating (Fig. 2; Table 3). Although both groups showed reduced beta and gamma power to the second click relative to the first, patients showed less of a reduction.
Fig. 2.
Left: Time-frequency plots of S2-S1 power (i.e. gating) for human schizophrenia patients and healthy controls. Right: Bar graphs showing higher S2-S1 power (i.e. gating deficits) in the beta and gamma frequency ranges for patients compared to controls. Average beta and gamma power was calculated between 30 and 150 ms post-stimulus. Error bars represent the standard error. *p < 0.05.
Comparing S1 and S2 power between groups, no significant main effect of group was observed for S1 power (F(1,93) = 0.52, p = 0.47). A trend towards a main effect of group was observed for S2 power (F(1,93) = 2.81, p = 0.097). Exploratory post-hoc testing showed a trend towards higher S2 power in patients for the beta (p = 0.093) band (Table 3).
3.3. Clinical correlates
Poor beta gating was associated with a higher total (sum of all subscores, not including attention) SANS score (R = 0.43, F(3,41) = 3.12, p = 0.036). In addition, poor beta gating was associated with a higher SANS attention subscore (R = 0.46, F(3,41) = 3.60, p = 0.021) and SANS alogia subscore (R = 0.46, F(3,41) = 3.64, p = 0.020), although the latter correlations did not survive a Bonferroni correction (see Methods). No correlations with SAPS scores were observed.
4. Discussion
In this study, we examined neuronal oscillations associated with auditory sensory gating in a mouse model with decreased expression of the α7 nAChR and in patients with schizophrenia. We found that gating of total beta and gamma frequency power was decreased in α7 heterozygotic mice compared to wild type C3H mice. Similarly, we found that gating of total beta and gamma frequency power was decreased in patients with schizophrenia compared to healthy controls. These results are in agreement with previous work demonstrating that sensory gating abnormalities in schizophrenia extend to neural oscillations in the gamma and beta frequency ranges (Hall et al., 2011; Hong et al., 2004). Additionally, these results suggest that the P50 gating deficits observed in α7 heterzygous mice (Adams et al., 2008) and schizophrenia patients (Adler et al., 1982) are also shared at high frequency ranges.
Although both α7 heterozygotic mice and patients with schizophrenia showed deficits in gating relative to their comparison controls, the effects differed in that the mutant mice showed increased S2 relative to S1 (compared to no change for wild-type), whereas patients showed reduced inhibition of S2 power relative to S1 (compared to controls). The translational applicability of the described approach must therefore be further evaluated in future studies. For example, non-nicotinic mediated mechanisms (e.g. loss of GABAergic interneurons (Zhang and Reynolds, 2002)) may contribute to the observed effects in human patients to a greater extent than in α7 mutant mice. Future analysis of the contribution of α7 receptor-related genotypes (Araud et al., 2011; Stephens et al., 2009) to the observed effect in patients should prove useful in evaluating this possibility.
Time frequency analysis of neural oscillations provides insight into the functioning of circuits within the brain that is not possible with ERP analyses, as it is not restricted to signals that are dominated by low frequency activity. The results of the present study extend previous findings that have demonstrated a role for α7 nAChRs in gating of P50 ERPs by showing that deficits extend to beta and gamma frequency ranges. In the adult hippocampus, nicotinic α7 nAChRs are primarily expressed on inhibitory interneurons; upon stimulation, these receptors depolarize these interneurons and induce GABA release (Miwa et al., 2011). A deficit in α7 receptor expression would thus reduce GABAergic tone and promote hyperexcitability. On a network level, this change in excitability may be associated with an increase in total oscillatory power, as demonstrated in a computational model of interneuronal dysfunction (Spencer, 2009), as well as in a study using optogenetics to increase excitatory tone in the medial prefrontal cortex (Yizhar et al., 2011). Increased beta and gamma power during S2 may therefore reflect a general increase in cortical excitability conveyed through loss of α7 receptors on inhibitory interneurons. Interestingly, α7 receptor activation improves prepulse inhibition in transgenic mouse models of schizophrenia (Kucinski et al., 2012) and reduces hippocampal hyperactivity during a sensorimotor gating task in patients (Tregellas et al., 2010). These findings suggest that nicotinic modulation can modulate inhibitory neurotransmitter systems to attenuate baseline response to stimuli.
The fact that beta and gamma gating is impaired in heterozygote mice as well as schizophrenia patients has important implications for how sensory processing may be abnormal in the illness. Gamma oscillations during sensory tasks are thought to be related to local processing and encode the stimuli as a discrete object with recognizable features (Eckhorn et al., 1988; Tallon-Baudry and Bertrand, 1999). Beta oscillations may be related to long-distance processing and the salience of the stimulus (Haenschel et al., 2000; Kopell et al., 2000). Reduced gamma and beta power after the second click may therefore be indicative of a reduction in stimulus salience and encoding to help prevent repeated stimuli from being consciously processed—perhaps indicative of the “unconscious” ability to ignore a ticking clock. Although we cannot rule out the possibility that beta and gamma gating deficits in patients are conferred through non-α7-mediated mechanisms, the demonstrated roles for this receptor in P50 gating, cortical hyperexcitability, and risk for schizophrenia suggest that it is important for modulating network dynamics across a range of frequencies.
Exploratory between-group analyses of S1 and S2 power in mice revealed higher S2 power in heterozygote mice, but no difference in S1 power; in humans, no differences between groups for either S1 or S2 were observed. Although these results are preliminary and must be interpreted with caution, they suggest that gamma and beta gating deficits may not be due to a failure to gate “in” the first stimulus. Rather, these results (particularly in the mutant mice) suggest that at higher frequency ranges the α7 receptor may play a role in S2 suppression but not S1 response. Previous findings using the α7 partial agonist DMXB-A found a similar pattern of effects on S1 and S2 P50 responses in patients with schizophrenia (Olincy et al., 2006) and in a mouse model that shows poor sensory gating (DBA/2) (Stevens et al., 2010). Nonetheless, given that S2 values did not differ between schizophrenia patients and controls, the interaction between S2 beta/gamma suppression, schizophrenia, and nicotinic receptor function should continue to be examined in future research.
A concern in the present experiments is that the neuronal mechanisms that underlie sensory gating as recorded by a scalp electrode may be different from those measured by an implanted hippocampal electrode. Typically, P50 gating studies in schizophrenia measure ERPs from an electrode on the top of the head, Cz, as this location elicits the most reliable gating differences between patients and controls (Clementz et al., 1998). In mouse models of the illness, sensory gating is measured using electrodes implanted within the CA3 region of the hippocampus, as it is relatively easy to locate electro-physiologically (Miller and Freedman, 1995) and normally shows robust auditory gating (Bickford-Wimer et al., 1990) that is disrupted in α7 receptor mutant mice (Stevens et al., 1996). In both cases, the deficit is thought to arise from a primary deficit in GABAergic (inhibitory neurotransmitter) tone, although the neural circuitry and anatomical localization may not be identical (e.g. interneuronal pathology in the reticular nucleus of the thalamus may contribute to gating deficits measured at the Cz electrode (Miwa et al., 2011)). Despite these concerns, both scalp EEG and hippocampal recordings suggest that P50 gating deficits are mediated in part by the α7 receptor. The present results suggest that the translational nature of this deficit may extend to higher frequency ranges despite the difference in recording locations. Nonetheless, future studies that incorporate additional electrode sources may prove useful in determining the whole-brain generalizability of high-frequency gating impairment. This approach would also allow for analysis of within-and between-network dynamics, i.e. how changes in high frequency power at a single source can influence neural activity at distal locations (as hypothesized for sensory binding (Eckhorn et al., 1988)).
Poor beta gating was associated with the severity of negative symptoms, as measured by total SANS score and (on a trend level) by SANS Alogia and Attention subscores. The majority of P50 gating studies have found no association between negative symptoms and gating ratio (Potter et al., 2006), although a significant correlation between M50 (the magnetoencephalographic equivalent of the EEG-measured P50) gating ratio and negative symptoms has been reported (Thoma et al., 2005). Previous studies using time-frequency analysis have not reported an association between gating at any frequency range and negative symptoms (Hall et al., 2011; Hong et al., 2008). SAPS and SANS scores were low (average of 1–2 per subscale) in the present subject population, and it is possible that floor effects influenced the observed clinical correlations. Additional studies are needed to clarify how sensory gating abnormalities at various frequencies relate to the clinical manifestation of the illness.
5. Conclusion
In summary, we measured beta and gamma frequency sensory gating in a genetic mouse model for schizophrenia and human patients with the illness. Both α7 heterozygote mice and patients showed reduced sensory gating in these frequency ranges, compared to wild-type mice and human comparison subjects, respectively. These results suggest the sensory gating deficit observed in schizophrenia is associated with abnormalities in neural oscillations that may be related to deficits in α7 nAChR expression.
Acknowledgments
The authors thank Robert Freedman, M.D., for assistance with manuscript preparation. This research was supported by the VA Biomedical Laboratory and Clinical Science Research and Development Service, the Brain and Behavior Foundation, and the Blowitz-Ridgeway Foundation.
References
- Adams CE, Stitzel JA, Collins AC, Freedman R. Alpha7-nicotinic receptor expression and the anatomical organization of hippocampal interneurons. Brain Research. 2001;922:180–190. doi: 10.1016/s0006-8993(01)03115-8. [DOI] [PubMed] [Google Scholar]
- Adams CE, Yonchek JC, Zheng L, Collins AC, Stevens KE. Altered hippocampal circuit function in C3H alpha7 null mutant heterozygous mice. Brain Research. 2008;1194:138–145. doi: 10.1016/j.brainres.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler L, Pachtman E, Franks R, Pecevich M, Waldo M, Freedman R. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biological Psychiatry. 1982;17:639–654. [PubMed] [Google Scholar]
- Adler LE, Olincy A, Waldo M, Harris JG, Griffith J, Stevens K, Flach K, Nagamoto H, Bickford P, Leonard S, Freedman R. Schizophrenia, sensory gating, and nicotinic receptors. Schizophrenia Bulletin. 1998;24:189–202. doi: 10.1093/oxfordjournals.schbul.a033320. [DOI] [PubMed] [Google Scholar]
- Araud T, Graw S, Berger R, Lee M, Neveu E, Bertrand D, Leonard S. The chimeric gene CHRFAM7A, a partial duplication of the CHRNA7 gene, is a dominant negative regulator of alpha7 nAChR function. Biochemical Pharmacology. 2011;82:904–914. doi: 10.1016/j.bcp.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak N, Glenthoj BY, Rostrup E, Larsson HB, Oranje B. Source localization of sensory gating: a combined EEG and fMRI study in healthy volunteers. Neuroimage. 2011;54:2711–2718. doi: 10.1016/j.neuroimage.2010.11.039. [DOI] [PubMed] [Google Scholar]
- Bibbig A, Faulkner HJ, Whittington MA, Traub RD. Self-organized synaptic plasticity contributes to the shaping of gamma and beta oscillations in vitro. The Journal of Neuroscience. 2001;21:9053–9067. doi: 10.1523/JNEUROSCI.21-22-09053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford-Wimer PC, Nagamoto H, Johnson R, Adler LE, Egan M, Rose GM, Freedman R. Auditory sensory gating in hippocampal neurons: a model system in the rat. Biological Psychiatry. 1990;27:183–192. doi: 10.1016/0006-3223(90)90648-l. [DOI] [PubMed] [Google Scholar]
- Cao YA, Featherstone RE, Gandal MJ, Liang Y, Jutzeler C, Saunders J, Tatard-Leitman V, Chen J, Weinberger DR, Lerman C, Siegel SJ. Nicotine normalizes event related potentials in COMT-Val-tg mice and increases gamma and theta spectral density. Behavioral Neuroscience. 2012;126:332–343. doi: 10.1037/a0027047. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Blumenfeld LD, Cobb S. The gamma band response may account for poor P50 suppression in schizophrenia. Neuroreport. 1997;8:3889–3893. doi: 10.1097/00001756-199712220-00010. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Geyer MA, Braff DL. Multiple site evaluation of P50 suppression among schizophrenia and normal comparison subjects. Schizophrenia Research. 1998;30:71–80. doi: 10.1016/s0920-9964(97)00122-9. [DOI] [PubMed] [Google Scholar]
- Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, Munk M, Reitboeck HJ. Coherent oscillations: a mechanism of feature linking in the visual cortex? Multiple electrode and correlation analyses in the cat. Biological Cybernetics. 1988;60:121–130. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- Freedman R, Adams CE, Leonard S. The alpha7-nicotinic acetylcholine receptor and the pathology of hippocampal interneurons in schizophrenia. Journal of Chemical Neuroanatomy. 2000;20:299–306. doi: 10.1016/s0891-0618(00)00109-5. [DOI] [PubMed] [Google Scholar]
- French NR, Steinberg JC. Factors governing the intelligibility of speech sounds. The Journal of the Acoustical Society of America. 1947;19:90–119. [Google Scholar]
- Haenschel C, Baldeweg T, Croft RJ, Whittington M, Gruzelier J. Gamma and beta frequency oscillations in response to novel auditory stimuli: a comparison of human electroencephalogram (EEG) data with in vitro models. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:7645–7650. doi: 10.1073/pnas.120162397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MH, Taylor G, Salisbury DF, Levy DL. Sensory gating event-related potentials and oscillations in schizophrenia patients and their unaffected relatives. Schizophrenia Bulletin. 2011;37:1187–1199. doi: 10.1093/schbul/sbq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Buchanan RW, Thaker GK, Shepard PD, Summerfelt A. Beta (~16 Hz) frequency neural oscillations mediate auditory sensory gating in humans. Psychophysiology. 2008;45:197–204. doi: 10.1111/j.1469-8986.2007.00624.x. [DOI] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, McMahon RP, Thaker GK, Buchanan RW. Gamma/beta oscillation and sensory gating deficit in schizophrenia. Neuroreport. 2004;15:155–159. doi: 10.1097/00001756-200401190-00030. [DOI] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, Mitchell BD, McMahon RP, Wonodi I, Buchanan RW, Thaker GK. Sensory gating endophenotype based on its neural oscillatory pattern and heritability estimate. Archives of General Psychiatry. 2008;65:1008–1016. doi: 10.1001/archpsyc.65.9.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucinski A, Syposs C, Wersinger S, Bencherif M, Stachowiak MK, Stachowiak EK. alpha7 neuronal nicotinic receptor agonist (TC-7020) reverses increased striatal dopamine release during acoustic PPI testing in a transgenic mouse model of schizophrenia. Schizophrenia Research. 2012;136:82–87. doi: 10.1016/j.schres.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Leonard S, Gault J, Hopkins J, Logel J, Vianzon R, Short M, Drebing C, Berger R, Venn D, Sirota P, Zerbe G, Olincy A, Ross RG, Adler LE, Freedman R. Association of promoter variants in the alpha7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Archives of General Psychiatry. 2002;59:1085–1096. doi: 10.1001/archpsyc.59.12.1085. [DOI] [PubMed] [Google Scholar]
- Mathiak K, Ackermann H, Rapp A, Mathiak KA, Shergill S, Riecker A, Kircher TT. Neuromagnetic oscillations and hemodynamic correlates of P50 suppression in schizophrenia. Psychiatry Research: Neuroimaging. 2011;194:95–104. doi: 10.1016/j.pscychresns.2011.01.001. [DOI] [PubMed] [Google Scholar]
- McGhie A, Chapman J. Disorders of attention and perception in early schizophrenia. The British Journal of Medical Psychology. 1961;34:103–116. doi: 10.1111/j.2044-8341.1961.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Miller CL, Freedman R. The activity of hippocampal interneurons and pyramidal cells during the response of the hippocampus to repeated auditory stimuli. Neuroscience. 1995;69:371–381. doi: 10.1016/0306-4522(95)00249-i. [DOI] [PubMed] [Google Scholar]
- Miwa JM, Freedman R, Lester HA. Neural systems governed by nicotinic acetylcholine receptors: emerging hypotheses. Neuron. 2011;70:20–33. doi: 10.1016/j.neuron.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olincy A, Braff DL, Adler LE, Cadenhead KS, Calkins ME, Dobie DJ, Green MF, Greenwood TA, Gur RE, Gur RC, Light GA, Mintz J, Nuechterlein KH, Radant AD, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Wagner BD, Freedman R. Inhibition of the P50 cerebral evoked response to repeated auditory stimuli: results from the Consortium on Genetics of Schizophrenia. Schizophrenia Research. 2010;119:175–182. doi: 10.1016/j.schres.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olincy A, Harris JG, Johnson LL, Pender V, Kongs S, Allensworth D, Ellis J, Zerbe GO, Leonard S, Stevens KE, Stevens JO, Martin L, Adler LE, Soti F, Kem WR, Freedman R. Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Archives of General Psychiatry. 2006;63:630–638. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- Pena M, Melloni L. Brain oscillations during spoken sentence processing. Journal of Cognitive Neuroscience. 2012;24:1149–1164. doi: 10.1162/jocn_a_00144. [DOI] [PubMed] [Google Scholar]
- Pollack I. Effects of high pass and low pass filtering on the intelligibility of speech in noise. The Journal of the Acoustical Society of America. 1948;20:259–266. [Google Scholar]
- Potter D, Summerfelt A, Gold J, Buchanan RW. Review of clinical correlates of P50 sensory gating abnormalities in patients with schizophrenia. Schizophrenia Bulletin. 2006;32:692–700. doi: 10.1093/schbul/sbj050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach BJ, Mathalon DH. Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophrenia Bulletin. 2008;34:907–926. doi: 10.1093/schbul/sbn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahin AJ, Picton TW, Miller LM. Brain oscillations during semantic evaluation of speech. Brain and Cognition. 2009;70:259–266. doi: 10.1016/j.bandc.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucny J, Olincy A, Eichman LC, Lyons E, Tregellas JR. Early sensory processing deficits predict sensitivity to distraction in schizophrenia. Schizophrenia Research. 2013;147(1):196–200. doi: 10.1016/j.schres.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM. The functional consequences of cortical circuit abnormalities on gamma oscillations in schizophrenia: insights from computational modeling. Frontiers in Human Neuroscience. 2009;3:33. doi: 10.3389/neuro.09.033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens SH, Logel J, Barton A, Franks A, Schultz J, Short M, Dickenson J, James B, Fingerlin TE, Wagner B, Hodgkinson C, Graw S, Ross RG, Freedman R, Leonard S. Association of the 5′-upstream regulatory region of the alpha7 nicotinic acetylcholine receptor subunit gene (CHRNA7) with schizophrenia. Schizophrenia Research. 2009;109:102–112. doi: 10.1016/j.schres.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens KE, Cornejo B, Adams CE, Zheng L, Yonchek J, Hoffman KL, Christians U, Kem WR. Continuous administration of a selective alpha7 nicotinic partial agonist, DMXBA, improves sensory inhibition without causing tachyphylaxis or receptor upregulation in DBA/2 mice. Brain Research. 2010;1352:140–146. doi: 10.1016/j.brainres.2010.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens KE, Freedman R, Collins AC, Hall M, Leonard S, Marks MJ, Rose GM. Genetic correlation of inhibitory gating of hippocampal auditory evoked response and alpha-bungarotoxin-binding nicotinic cholinergic receptors in inbred mouse strains. Neuropsychopharmacology. 1996;15:152–162. doi: 10.1016/0893-133X(95)00178-G. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends in Cognitive Sciences. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- Thoma RJ, Hanlon FM, Moses SN, Ricker D, Huang M, Edgar C, Irwin J, Torres F, Weisend MP, Adler LE, Miller GA, Canive JM. M50 sensory gating predicts negative symptoms in schizophrenia. Schizophrenia Research. 2005;73:311–318. doi: 10.1016/j.schres.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Tiitinen H, Sinkkonen J, Reinikainen K, Alho K, Lavikainen J, Naatanen R. Selective attention enhances the auditory 40-Hz transient response in humans. Nature. 1993;364:59–60. doi: 10.1038/364059a0. [DOI] [PubMed] [Google Scholar]
- Tregellas JR, Olincy A, Johnson L, Tanabe J, Shatti S, Martin LF, Singel D, Du YP, Soti F, Kem WR, Freedman R. Functional magnetic resonance imaging of effects of a nicotinic agonist in schizophrenia. Neuropsychopharmacology. 2010;35:938–942. doi: 10.1038/npp.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophrenia Bulletin. 2007;33:69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O′Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZJ, Reynolds GP. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophrenia Research. 2002;55:1–10. doi: 10.1016/s0920-9964(01)00188-8. [DOI] [PubMed] [Google Scholar]