Abstract
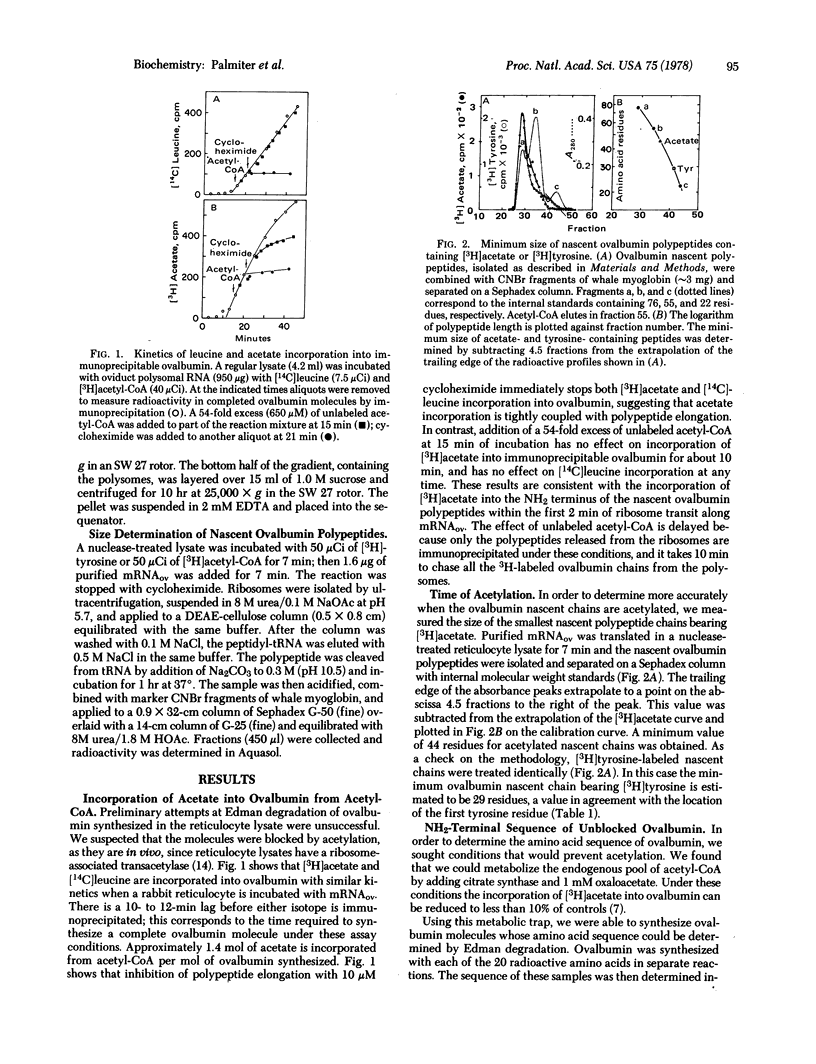
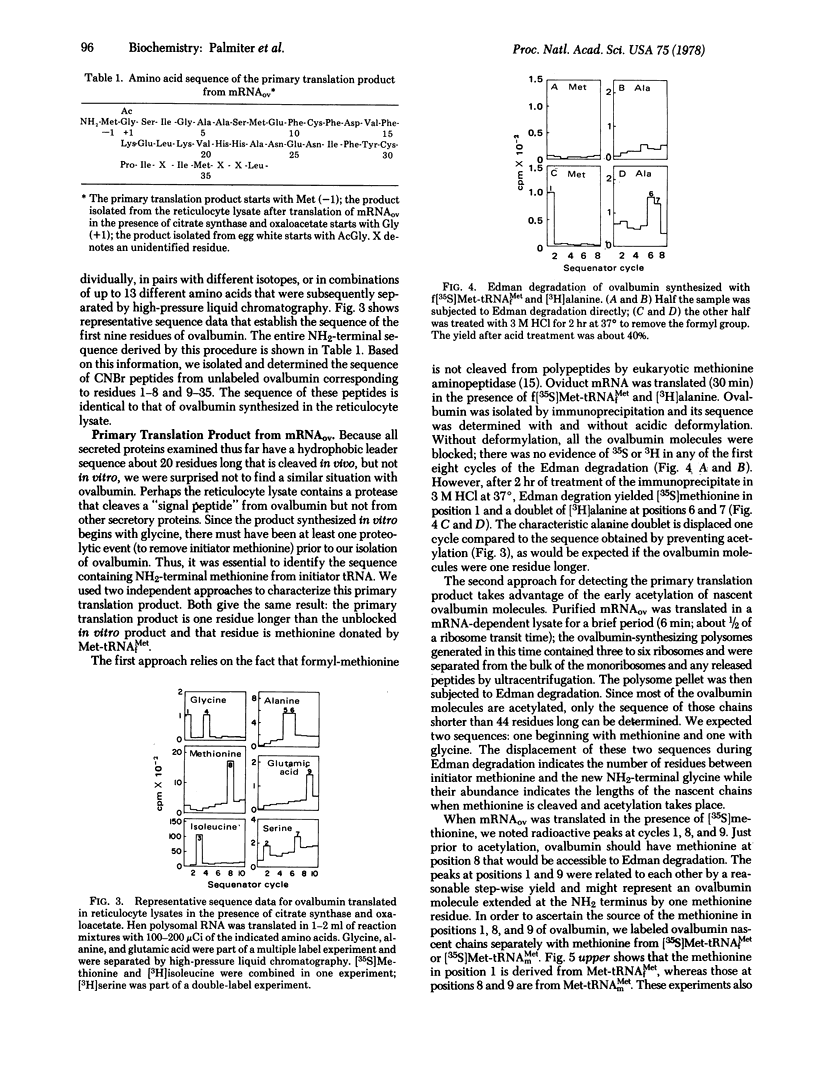
Ovalbumin mRNA was translated in a reticulocyte lysate. The primary translation product starts with methionine derived from Met-tRNAf. When the nascent polypeptide is about 20 residues long, this methionine is removed. The new NH2-terminal glycine is acetylated from acetyl-CoA when the polypeptide is 44 residues long. The sequence of 35 residues at the NH2 terminus of ovalbumin was determined by automated Edman degradation after a method was devised to prevent acetylation during protein synthesis in the reticulocyte lysate. This sequence is the same as that of secreted ovalbumin and does not resemble the transient "signal peptides" associated with most secretory proteins, including three other egg white proteins synthesized in the same cells as ovalbumin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson N. A., Brewer H. B., Anderson W. F. Hemoglobin switching in sheep and goats. 3. Cell-free initiation of sheep globin synthesis. J Biol Chem. 1974 Aug 25;249(16):5227–5235. [PubMed] [Google Scholar]
- Haines M. E., Carey N. H., Palmiter R. S. Purification and properties of ovalbumin messenger RNA. Eur J Biochem. 1974 Apr 16;43(3):549–560. doi: 10.1111/j.1432-1033.1974.tb03442.x. [DOI] [PubMed] [Google Scholar]
- Housman D., Jacobs-Lorena M., Rajbhandary U. L., Lodish H. F. Initiation of haemoglobin synthesis by methionyl-tRNA. Nature. 1970 Aug 29;227(5261):913–918. doi: 10.1038/227913a0. [DOI] [PubMed] [Google Scholar]
- Inouye S., Wang S., Sekizawa J., Halegoua S., Inouye M. Amino acid sequence for the peptide extension on the prolipoprotein of the Escherichia coli outer membrane. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1004–1008. doi: 10.1073/pnas.74.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R., Hunter T. Role of methionine in the initiation of haemoglobin synthesis. Nature. 1970 Aug 15;227(5259):672–676. doi: 10.1038/227672a0. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Gagnon J., Ericsson L. H., Walsh K. A. Precursor of egg white lysozyme. Amino acid sequence of an NH2-terminal extension. J Biol Chem. 1977 Sep 25;252(18):6386–6393. [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Ovalbumin messenger ribonucleic acid translation. Comparable rates of polypeptide initiation and elongation on ovalbumin and globin messenger ribonucleic acid in a rabbit reticulocyte lysate. J Biol Chem. 1973 Mar 25;248(6):2095–2106. [PubMed] [Google Scholar]
- Palmiter R. D., Palacios R., Schimke R. T. Identification and isolation of ovalbumin-synthesizing polysomes. II. Quantification and immunoprecipitation of polysomes. J Biol Chem. 1972 May 25;247(10):3296–3304. [PubMed] [Google Scholar]
- Palmiter R. D. Quantitation of parameters that determine the rate of ovalbumin synthesis. Cell. 1975 Mar;4(3):189–189. doi: 10.1016/0092-8674(75)90167-1. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Prasad C., Peterkofsky A. Initiation of ovalbumin synthesis in chicken oviduct magnum. Transient labeling of the NH2-terminus by methionine. Arch Biochem Biophys. 1976 Aug;175(2):730–736. doi: 10.1016/0003-9861(76)90566-x. [DOI] [PubMed] [Google Scholar]
- Rhoads R. E., McKnight G. S., Schimke R. T. Synthesis of ovalbumin in a rabbit reticulocyte cell-free system programmed with hen oviduct ribonucleic acid. J Biol Chem. 1971 Dec 10;246(23):7407–7410. [PubMed] [Google Scholar]
- Shapiro D. J., Taylor J. M., McKnight G. S., Palacios R., Gonzalez C., Kiely M. L., Schimke R. T. Isolation of hen oviduct ovalbumin and rat live albumin polysomes by indirect immunoprecipitation. J Biol Chem. 1974 Jun 25;249(12):3665–3671. [PubMed] [Google Scholar]
- Stanley W. M., Jr Preparation and analysis of L-( 35 S)methionine labeled transfer ribonucleic acids from rabbit liver. Anal Biochem. 1972 Jul;48(1):202–216. doi: 10.1016/0003-2697(72)90183-2. [DOI] [PubMed] [Google Scholar]
- Struck D. K., Lennarz W. J. Evidence for the participation of saccharide-lipids in the synthesis of the oligosaccharide chain of ovalbumin. J Biol Chem. 1977 Feb 10;252(3):1007–1013. [PubMed] [Google Scholar]
- Traugh J. A., Sharp S. B. Protein modification enzymes associated with the protein-synthesizing complex from rabbit reticulocytes. Protein kinase, phosphoprotein phosphatase, and acetyltransferase. J Biol Chem. 1977 Jun 10;252(11):3738–3744. [PubMed] [Google Scholar]
- Yamamoto S., Lampen J. O. Membrane penicillinase of Bacillus licheniformis 749/C:sequence and possible repeated tetrapeptide structure of the phospholipopeptide region. Proc Natl Acad Sci U S A. 1976 May;73(5):1457–1461. doi: 10.1073/pnas.73.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]


