Abstract
The first step of base excision repair utilizes glycosylase enzymes to find damage within a genome. A persistent question in the field of DNA repair is how glycosylases interact with DNA to specifically find and excise target damaged bases with high efficiency and specificity. Ensemble studies have indicated that glycosylase enzymes rely upon both sliding and distributive modes of search, but ensemble methods are limited in their ability to directly observe these modes. Here we review insights into glycosylase scanning behavior gathered through single-molecule fluorescence studies of enzyme interactions with DNA and provide a context for these results in relation to ensemble experiments.
Keywords: DNA glycosylases, base excision repair, single-molecule fluorescence microscopy, facilitated diffusion, DNA scanning
1. Introduction
1.1 Base Excision Repair
Endogenously produced DNA damages, including oxidative damages, are repaired by the base excision repair (BER) pathway (for reviews see [1–6]). Base excision repair is comprised of five enzymatic steps and is initiated by DNA glycosylases, enzymes which locate and excise a single damaged base leaving an abasic (AP) site in the DNA backbone. The next step in the pathway is cleavage of the DNA strand 5’ to the AP site by an AP endonuclease. Alternatively, DNA glycosylases that remove oxidized DNA bases contain a lyase activity that cleaves the DNA backbone leaving a sugar or a phosphate group attached to the 3’ end that needs to be removed by a phosphodiesterase or phosphatase activity. After these initial steps, downstream enzymes, including a DNA polymerase and a ligase, work to fill the single nucleotide gap. In mammalian cells this is called short patch repair.
Glycosylases are capable of specifically locating a single damaged DNA base among a sea of undamaged bases. The glycosylase search process involves non-covalent contacts between the enzyme and DNA and utilizes thermal energy to provide a driving force for movement along DNA. One lingering question is how glycosylase enzymes bind and move along DNA to enact an efficient search for damage.
1.2. Insights into the Glycosylase Search for Damage from Ensemble Studies
Early studies of the Lac repressor protein led to the suggestion that DNA-binding proteins may interact with DNA in a process known as “facilitated diffusion” [7–9]. The existence of facilitated diffusion has been supported by the observation that certain transcription factors and other DNA binding proteins are able to find binding sites at a rate that is faster than would be predicted from a simple three-dimensional diffusive search. A reasonable explanation for the enhanced binding rates is that the DNA is “facilitating” the search by serving as a conduit along which a protein molecule can travel between sites. The simplest interpretation of facilitated diffusion would be sliding along the DNA backbone. Facilitated diffusion is typically observed at low salt concentrations that enhance the electrostatic interactions between proteins and DNA, and these conditions are unlikely to be biologically relevant. However, facilitated diffusion by the Lac repressor has recently been reported in vivo on bacterial chromosomal DNA [10]. Thus, the experimental evidence indicates that facilitated diffusion plays some role in how DNA-search proteins interact with DNA. A precise search mechanism has yet to be resolved, and the relationship between facilitated diffusion and random diffusion has served as an active area of research for over 30 years.
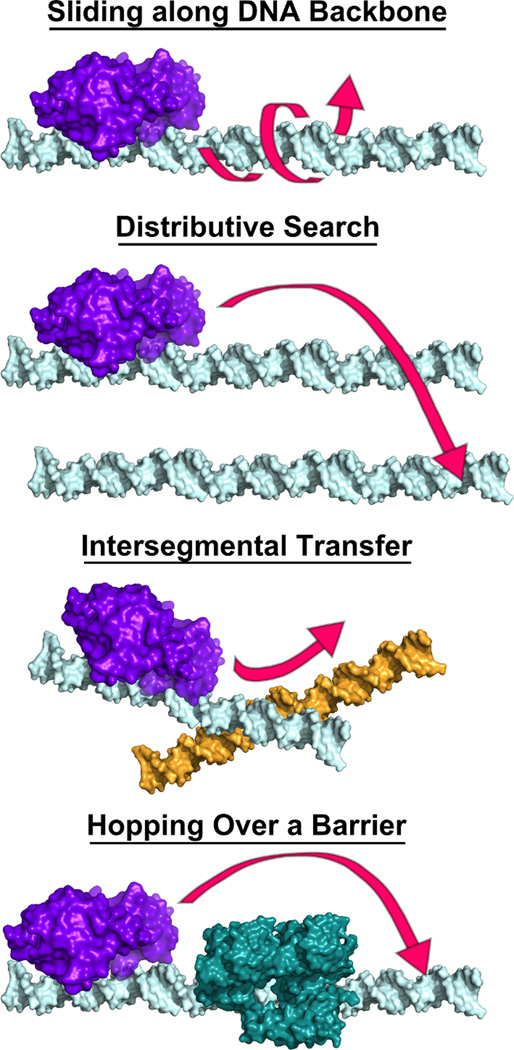
The mechanism by which a glycosylase scans DNA to find damage is likely complicated. From a theoretical standpoint, a glycosylase search mechanism that utilizes only sliding along the backbone is difficult to justify. A particle that moves randomly in one dimension is predicted to remain confined to a region close to its initial position [11, 12]. Thus, a sliding search of DNA would be too redundant for the efficient search of an entire genome. Similarly, a purely three-dimensional diffusive search of DNA would be inadequate [8]. A “distributive search” is often defined as dissociation from one DNA molecule followed by three-dimensional diffusion and binding to another molecule (Figure 1). In the distributive search model, in order for catalysis to take place, the enzyme would have to collide with the damaged substrate in an orientation that facilitates promotion to a reaction transition state. While it has been shown to be theoretically feasible for a glycosylase to rely upon a distributive search to find the small number of damage sites in the nucleus within a reasonable time frame, the likelihood that the enzyme could selectively find them in the presence of a vast excess of undamaged competitor DNA is low [13]. A combination of sliding and distributive interactions seems likely, and facilitated diffusion may employ other search modes. One such mode, known as “hopping,” involves dissociation and rebinding to a nearby location on the same DNA molecule (Figure 1). Hopping can be considered a hybrid between a sliding and a distributive interaction because the enzyme binds to a close location within the same DNA strand [8]. Another possible method of glycosylase travel is “intersegmental transfer,” wherein DNA secondary structure facilitates glycosylase relocation to a very distant location on the same strand or to new DNA strand. It has been suggested that glycosylases may use all of these various types of interactions with DNA to find lesions (discussed in [8] and reviewed in [12]).
Figure 1. Some possible mechanisms for glycosylase diffusion on DNA.
Early ensemble experiments to test the mechanism of glycosylase search on DNA revealed evidence that glycosylase enzymes employ facilitated diffusion. These experiments investigated T4 endonuclease V repair of UV-induced pyrimidine dimers on plasmid DNA. Lloyd and coworkers created varying numbers of damage sites in plasmid DNA and monitored the accumulation of linear DNA products after incubation with T4 endonuclase V [14]. When limited numbers of enzymes interacted with plasmids that contained several damage sites, the rate of cleavage within a single plasmid exceeded the rate of cleavage among different plasmids. Therefore, the cleavage of sites within a single plasmid was “correlated.” These results led to the suggestion that the encounter and enzymatic cleavage of sites was processive, which would imply facilitated diffusion between damage sites. Similar to the Lac repressor experiments, these T4 endonuclease V correlated cleavage experiments were carried out at very low salt, which would enhance glycosylase interactions with DNA. Interestingly, the observation of processive cleavage in T4 endonuclease V preceded confirmation that it was a bifunctional enzyme capable of both glycosylase and lyase activities!
Correlated cleavage experiments also provided evidence for a distributive search mode. When the processive mechanism of T4 endonuclease V was tested using the ensemble plasmid assay at increasing salt conditions, it was found that processivity was highly salt dependent [15, 16]. At salt concentrations below 50 mM, correlated cleavage was detected. When the NaCl concentration was above 50 mM, each glycosylase was able to repair several plasmid molecules, indicating a switch to a distributive search. This left the question of whether facilitated diffusion was physiologically relevant for glycosylase enzymes. In order to determine whether a processive mechanism could occur in vivo, T4 endonuclease V activity was examined in irradiated Escherichia coli cells [17, 18]. The first study confirmed that correlated cleavage could occur in the in vivo salt and crowding environment of the bacterium by monitoring T4 endonuclease V activity on plasmids within a repair-deficient strain of E. coli [17]. The second study introduced enzymatically active, scanning-deficient T4 endonuclease V variants into the repair-deficient strain of E. coli. Later structural studies showed that one of the mutated residues (Arg26) is in the catalytic site, while the other residue (Lys33) is within Van der Waals radius of the backbone (PDB:1VAS; PDB: 2FCC) [19, 20]. These variants had lower processivity in vitro, and were unable to effectively protect against UV-damage in vivo [18]. These studies showed that correlated cleavage could occur within the cell and indicated that a processive search was necessary for cell survival.
Ensemble correlated cleavage studies utilizing linear DNA oligodeoxyribonucleotides (oligos) that contain engineered damages have allowed for the study of glycosylase enzymes that recognize oxidative lesions, methylated sites, and DNA mismatches. These experiments measure the rate at which a glycosylase cleaves consecutive damage sites within a single DNA strand of a duplex. The processivity of E. coli uracil DNA glycosylase (Udg) was examined using both plasmid [21] and linear DNA substrates [22–25]. Similar to what was observed for T4 endonuclease V, the processivity of Udg appears to be highly dependent on salt conditions for both substrates. However, Udg also appears to switch between a distributive and processive mechanism depending on the identity of the linear substrate [21–24, 26]. As evidence for a combination of sliding and distributive search modes grew, linear substrates were designed with precisely-spaced damage sites to allow for a determination of how far the glycosylase slides before dissociating from the DNA strand. At low salt concentrations where processivity is favored, MutY glycosylase and formamidopyrimidine DNA glycosylase (Fpg) appear to slide along 175 bp and 275 bp before dissociation, respectively [27]. Similarly, Udg was shown to remove consecutive uracils within a span of 200–225 bases [23]. A later study of Udg suggested a shorter sliding length of ~20 bp [24], and the processive behavior of Udg decreased as the distance between uracils increased. These results further supported the existence of a finite average sliding distance and a search mechanism that relied on a combination of sliding and distributive search processes.
The ability to pass over barriers is important for function in an in vivo environment that includes nucleosomes and transcription machinery. Correlated cleavage studies have used linear DNA with blockades or breaks to determine whether a glycosylase can “hop” over the impedence. The first study looked at the enzymatic turnover of human alkyladenine DNA glycosylase (AAG) as it interacted with two lesions separated by an EcoRI binding site [28]. The presence of the EcoRI enzyme bound to the site between the lesions decreased the processivity of AAG by only 50%, indicating that some of the glycosylases were able to hop over the restriction enzyme to process the second lesion. In other studies, Udg was shown to be able to bypass both nicks and single-stranded gaps, albeit with reduced rates of correlated cleavage of uracils on the opposite side of the gap [25, 26]. In order to detect intersegmental transfer, Hedglin et al. designed substrates consisting of two damaged oligos connected by a flexible PEG tether, increasing the local concentration of the two oligos while preventing sliding between them. They found that AAG performed correlated cleavage of the two sites, indicating that AAG was able to dissociate and transfer from one oligo to another [29].
Even though the correlated cleavage experiments suggest that the glycosylase search process likely involves a combination of sliding with distributive dissociative and associative steps, correlated cleavage is an indirect measurement of facilitated diffusion. No ensemble experiment is able to directly observe the combination of these two mechanisms during a glycosylase search on DNA. One of the biggest limitations of ensemble experiments is their inability to characterize interactions between glycosylases with undamaged DNA. The movement of glycosylases along an undamaged DNA strand cannot be controlled or synchronized. Binding, scanning, and releasing are continuous stochastic processes under almost all physiological conditions. Thus, ensemble characterization of the kinetics of glycosylase sliding along undamaged DNA is nearly impossible due to extreme heterogeneity in rates and relative position for individual enzymes. Similarly, the measurement of a simple affinity constant for a glycosylase binding to an undamaged substrate is exceedingly difficult using traditional gel-based assays. Given the relatively low occurrence of damage in the genome, the vast majority of glycosylase encounters involve undamaged bases. Thus, full characterization of the search mechanism must also include the characterization of glycosylase behavior on undamaged DNA. It has been possible to use undamaged λ-DNA as a substrate for single-molecule (SM) fluorescence microsocopy of glycosylases, and for this reason, SM experiments have been an ideal way to gain new and exciting insights into the glycosylase search process. SM experiments hold much promise not only for observing individual glycosylases as they interact with specific damages on DNA but also for characterizing the entire BER pathway.
2. Single-Molecule Studies of the Glycosylase Search
2.1 What are the tools for examining the glycosylase search?
The SM studies discussed in this review were carried out using total-internal reflection microscopy (TIRFM) to observe a single glycosylase as it moves along a single DNA molecule. SM TIRFM is a well-established method [30, 31]; however, the SM sample conditions have been specially developed for study of glycosylase diffusion. The experiments require monitoring the glycosylase for time frames from seconds to minutes, and the enzyme must be labeled with long-lived fluorophores. This labeling has been accomplished through covalent attachment of organic dyes [32, 33], or an antibody-mediated attachment of a quantum dot (Qdot) [34, 35] without altering the enzymatic activity of the glycosylase. These fluorophores are stabilized to photobleaching and blinking by the addition of thiol reducing agents.
One of the biggest challenges for SM microscopy of DNA-scanning proteins is the immobilization and stretching of long pieces of DNA which serve as substrates for the long-distance scanning events. The first SM experiments with glycosylases were performed using λ-DNA that was tethered to a polyethylene glycol passivated surface through a single biotin linkage [32]. The DNA was stretched under continuous flow of buffer during the experiment. Later studies [34] used an alternative strategy to immobilize and stretch λ-DNA that was based on the same approach used for the study of UvrA and UvrB nucleotide excision repair proteins [36]. The technique utilized silica beads as a platform for the creation of “DNA tightropes” that remained extended in the absence of flow (Figures 2A and 2B). The silica beads elevated the DNA molecules above the surface, allowing for imaging with reduced surface background effects. The detection of DNA and glycosylase molecules that are bound to tightropes at 3–5 µm above the surface required modified TIRFM. The excitation beam was adjusted to a sub-critical oblique angle using a lens that defocuses the beam at the back aperture of the objective to create a far-TIRF excitation field.
Figure 2. Sample configuration for SM DNA tightrope experiments.
(A) λ-DNA is extended across 5 µm beads using hydrodynamic flow. (B) Image of Qdot labeled glycosylase bound to DNA stretched between two beads. (C) Conjugation scheme for attachment of Qdot to glycosylase. A streptavidin-modified PEG coated QD is attached through biotin to an antibody that is specific for the 6-histidine tag on the C-terminus of the glycosylase (not to scale).
Movies of glycosylases scanning are collected at up to 100 frames per second, and show random 1D scanning of the extended DNA (Movie 1). The spatial resolution of TIRFM is limited to approximately 10 nm under ideal conditions, which is too low to directly distinguish rotational from linear diffusion. The time-dependent position of a glycosylase may be analyzed using a tracking program (DiaTrack [32, 33] or Spot Tracker for ImageJ [34, 35]), and mean squared displacement (MSD) analysis may then be used to determine an apparent 1D diffusion constant for glycosylase sliding of each individual molecule [33]. The mean binding lifetime of the glycosylase may be calculated through exponential fits to binding lifetime distributions.
2.2 hOGG1 Diffusion on Undamaged DNA
The first SM experiments with glycosylases on DNA, reported by Blainey, et al., looked at a human oxoguanine DNA glycosylase (hOGG1) while it interacted with undamaged λ-DNA [33]. In these experiments, the glycosylase was labeled using dyes covalently attached at an engineered cysteine, which gave unencumbered movement along DNA. This initial study observed hundreds of wild-type and variant glycosylases as they interacted with an undamaged substrate. These experiments revealed several basic aspects of glycosylase DNA scanning behavior that were consistent with predictions from ensemble experiments. The movement of hOGG1 along DNA was bidirectional, random, and involved sliding distances that were kilobases long and could be detected with ~10 nm spatial resolution and 10 ms frame rates. Although the experiments were performed under continuous buffer flow, the force of flow did not influence the directionality of glycosylase motion. However, it was unclear in these studies how flow might have affected the binding lifetime of the glycosylase on the DNA substrate. Nevertheless, these experiments gave the first direct, and exciting observations of glycosylases as they scanned along DNA.
The authors were also able to compare binding lifetimes and diffusion constants for the wild-type, the glycosylase deficient K249Q variant and a pH-insensitive H270A variant of hOGG1 at various pH and salt conditions. Faster 1D diffusion with increasing salt concentration has been suggested to be indicative of hopping among DNA sites [33, 37]. No change was observed in the hOGG1 diffusion constant under various salt conditions and the authors concluded that hOGG1 does not hop on DNA. The binding lifetime of hOGG1 was on the order of 0.2 s and highly salt dependent; in the presence of increasing salt, the binding lifetime decreased sharply. Blainey and coworkers further characterized the diffusive behavior of hOGG1. They reported a mean diffusion constant of 5×106 bp2/s for hOGG1 sliding along DNA, which is too fast to allow for extrusion of each base in the chain. Instead, they propose that glycosylases find a DNA lesion through a process that is under kinetic control. In this scheme, glycosylases would search a small region of the DNA with high redundancy, and the lesion is selected because it is extruded at a higher rate than undamaged bases.
A second study of hOGG1 diffusion sought to characterize the nature of glycosylase motion along DNA, specifically testing whether the protein tracks rotationally along the DNA molecule [32]. The diffusion constant of a particle in motion is dependent on the radius of the particle, and the mathematical relationship between the apparent 1D diffusion constant and particle size scales differently for linear versus rotation diffusion. Whereas the sliding diffusion constant is dependent on 1/R, rotational diffusion will scale on the order of 1/R3. In this study, the size of the protein (R) and the distance between the protein center and DNA (ROC) were varied by tethering a large inert protein molecule (streptavidin) to hOGG1. The diffusion constants of the two forms of hOGG1 were measured using the SM assay, and it was found that the diffusion constant for hOGG1 sliding depended on R and ROC with a magnitude consistent with rotation-coupled sliding. This study also compared the diffusive behavior of several DNA scanning proteins of varying R and Roc, which included enzymes from very different structural families and having very different biological functions. For all of these proteins the diffusion constant scaled at close to 1/R3, suggesting that rotational diffusion is a universal feature of the DNA scanning mechanism. In the case of the glycosylase enzymes, the authors suggested that rotation may keep the glycosylase properly positioned for identification of lesions in the DNA strand.
2.2 The Role of Structure in the Scanning Behavior of the E. coli Glycosylases
Glycosylases that recognize oxidized DNA bases can be separated into two different structural families (for reviews see [38–40]). The first contains a helix-hairpin-helix motif, a Gly/Pro loop, and a catalytic aspartate, and is known as the helix-hairpin-helix (HhH) superfamily [41, 42]. The founding member of the HhH superfamily is E. coli endonuclease III (Nth), which recognizes oxidized pyrimidines [43–46]. The second family is known as the Fpg/Nei family and is named after formamidopyrimidine DNA glycosylase (Fpg, also known as MutM) [47] and endonuclease VIII (Nei) [48, 49]. These proteins contain a helix two turns helix motif and a zinc finger motif [50–53]. Despite having a similar structure to Fpg, Nei has a substrate specificity closer to that of Nth and recognizes oxidized pyrimidines [48, 54], while Fpg recognizes oxidized purines such as 8-oxoguanine [55, 56]. Although hOGG1 also recognizes the oxidized purine, 8-oxoguanine [57–59], it is actually a member of the HhH structural family and not structurally similar to Fpg [60].
In order to establish whether structural features or substrate specificity dictate aspects of search behavior, the three E. coli glycosylases Nth, Nei and Fpg were studied using SM microscopy [34]. As was observed for hOGG1, the E. coli enzymes, Fpg, Nei, and Nth, showed sliding behavior along undamaged DNA that was bidirectional, random, and highly redundant. Using MSD analysis, the apparent 1D diffusion constants and binding lifetimes were measured for these enzymes under various salt conditions. Similar to what was observed for hOGG1, the binding lifetimes of the E. coli enzymes were highly dependent on salt (Figure 3); however, in contrast to hOGG1, the binding lifetimes of the E. coli enzymes were consistently longer possibly due to the differences in flow conditions of the experiments. The E. coli enzymes had a broad distribution of 1D diffusion constants that ranged from 0.001 to 0.1 µm2/s, and had diffusion constants that are consistent with rotational diffusion along the DNA backbone [34]. The diffusion constants for the E. coli enzymes are slower than that of hOGG1, which is attributable to the Stokes drag from the Qdot label. The diffusion constant was not dependent on salt for any of the E. coli enzymes, confirming that like hOGG1, the E. coli enzymes do not hop while scanning DNA. The conclusion from these studies is that scanning behavior is consistent for glycosylase enzymes, regardless of structural family or substrate specificity.
Figure 3. Salt dependence of the binding lifetime of human and E. coli glycosylases.
Mean binding lifetimes were obtained from single exponential fits to binding lifetime histograms.
2.3 The Role of the Wedge Residue in Glycosylase Scanning
Structural studies of Fpg have been useful in characterizing the steps of damage recognition (for a review see [40]). The first crystal structure of an Fpg from Thermus thermophilus was determined in the absence of DNA (PDB:1EE8) [50]. The protein has two domains surrounding a positively charged binding cleft that makes contacts with the DNA. Within this cleft are residues that are essential for enzymatic activity, including an N-terminal proline that acts as a nucleophile. The cleft also contains zinc finger and helix-two-turns-helix motifs essential for binding to DNA. Nei has been shown to have a similar fold and the two enzymes make up the Fpg/Nei structural family [50–53].
A series of structural papers were able to capture snapshots of base interrogation by Bacillus stearothermophilus Fpg [61–65], which suggested a possible mechanism for lesion search (Figure 4). The first structure captured a catalytically inactive E3Q variant of Fpg with an extruded 8-oxoG lesion inserted into the protein active site (PDB:1R2Y) [61]. This structure showed three amino acid side chains (Met77, Arg112, and Phe114) inserted into the void left by the everted base. A follow-up structure utilized sulfur chemistry to crosslink wild-type (WT) B. stearothermophilus Fpg to undamaged DNA (PDB:2F5O). Although all of the DNA bases remained intrahelical in this structure, the undamaged target base pair was buckled due to insertion of the sterically repulsive residue, Phe114, directly into the stack [62]. Another structure was able to chemically crosslink a catalytically inactive Fpg variant in close proximity to the lesion site (but with the damage site remaining within the helix) (PDB:3GPP) [63]. This structure once again showed Phe114 inserted directly into the stack and inducing base pair disruption, puckering, and backbone strain. These structural studies suggest that Phe114 may act as a “wedge” residue able to disrupt base pairing without forcing extrusion of the interrogated base.
Figure 4. Crystal structure of Fpg WT Phe114 wedge residue and Fpg F114A variant interrogating damaged and undamaged guanosine DNA bases.
Residue 114 is colored red, while the interrogated base is colored purple. (A) Phe114 interaction with guanosine-containing DNA (2F5O). (B) Phe114 interaction with 8-oxoG containing DNA (1R2Y). (C) Ala114 interaction with guanosine-containing DNA (4G4Q). (D) Ala114 interaction with 8-oxoG-containing DNA (4G4R).
In order to directly investigate the effect of the wedge residue on Fpg scanning behavior, Dunn and coworkers utilized the SM assay to measure diffusion constants for an E. coli Fpg F111A wedge variant on undamaged λ-DNA [34]. Fpg F111A and WT Fpg had similar binding lifetimes (Figure 3), suggesting that the variant is capable of maintaining the same long-distance contacts with undamaged DNA. However, the variant showed scanning behavior that is significantly different from that of the WT protein. The mean diffusion constant of Fpg F111A calculated using the typical MSD analysis was (0.0286 ± 0.005 µm2/s), which is an order of magnitude faster than the WT Fpg diffusion constant of (0.00323 ± 0.0009 µm2/s). The displacement plots of glycosylase sliding along λ-DNA show several types of motion, including fast sliding, slow sliding, and pauses. Thus, a sliding-window MSD analysis was developed to extract diffusion constants describing the heterogeneous phases within an individual trace [35]. This in-depth analysis shows that the WT Fpg has a broadly distributed range of diffusion constants while the Fpg F111A variant has a narrower distribution that lacks periods of slow diffusion corresponding to the wedge-driven search. To explore if intrahelical base interrogation is used by E. coli Nei and Nth, the corresponding wedge variants Nei Y72A and Nth L81A were generated and their sliding behavior observed on undamaged λ-DNA. Similar to what was observed for Fpg F111A, Nei Y27A and Nth L81A scan faster with mean diffusion constants significantly greater than their WT counterparts. Furthermore, the Nei and Nth wedge variants lack the slow component of diffusion, suggesting that for the E. coli glycosylases, the wedge is responsible for a slow intrahelical mode of scanning along DNA to find lesions [35].
The SM studies of glycosylase wedge variants, together with kinetic and crystallographic studies of Fpg lesion recognition and repair, paint a consistent picture of the role of the wedge residue. Fpg scans DNA so quickly that forced extrusion of every DNA base during interrogation by the wedge residue would be inefficient for probing an entire genome, and spontaneous capture of extruded bases would occur too infrequently for effective repair [32–34]. Therefore, glycosylases likely sense damage through other DNA structural changes that precede eversion, such as backbone sugar pucker, base pairing strength, or steric bulk of the lesion. Recent structures of the B. stearothermophilus Fpg F114A variant bound to undamaged (PDB 4G4Q) and damaged (PDB 4G4R) DNA confirm the importance of Phe114 in lesion search; in both complexes, the target bases remain intrahelical and stacked [65] (Figure 4). Taken together, the crystallographic data show that the positively charged binding cleft of Fpg serves to bind the DNA while the enzyme scans, and the Phe wedge residue is able to probe individual base pairs to find the lesion without eversion of undamaged bases. Support for this mechanism has come from stopped flow experiments that examined changes in fluorescence during repair by E. coli Fpg [66–69]. By correlating DNA conformational states with changes in protein conformation, the authors were able to assign the two significant changes in substrate structure to a) Fpg recognition of the damage site before eversion and b) insertion of the three void-filling residues after base eversion [66, 67]. Because insertion of the void-filling residues occurred after eversion of the base, the authors suggest that Fpg does not use the wedge residue to “push” the damaged base into the enzyme active site. The SM traces show that fast 1D glycosylase sliding is punctuated by periods of slow wedge-mediated intrahelical interrogation to identify lesions. These studies demonstrate that the wedge residue plays an important role during sliding along undamaged DNA to find lesions, where eversion of an 8-oxoG lesion would be rare.
2.4 Glycosylase Scanning Behavior on Damaged DNA
The SM studies of glycosylases on undamaged λ-DNA have revealed new information about scanning behavior, but this is only part of the picture. What happens when a glycosylase encounters damage? Some specific questions include whether the enzyme pauses at a damage site, if the glycosylase continues scanning after encountering damage, and whether the presence of damage has any effect on scanning rates or binding lifetimes.
The scanning behavior of E. coli glycoslyases has been studied with the SM method using λ-DNA substrates that contain randomly distributed damage sites [35]. Fpg is specific for oxidized purines, and 8-oxoG was generated using methylene blue and visible light [70–72]. Nei and Nth, however, are specific for oxidized pyrimidines, and thymine glycol (Tg) sites were generated using osmium tetroxide and heat [46, 73, 74]. For both types of damage, the average number of damages per λ-DNA molecule was varied to give high dose and low dose conditions. The binding lifetimes of all three enzymes increased in the presence of specifically recognized damage. Furthermore, the amount of time in contact with DNA increased as the amount of damage increased, suggesting that the presence of damage increases the binding lifetime regardless of the glycosylase structure or substrate specificity.
When the diffusive behavior of Fpg on damaged λ-DNA was analyzed using a sliding window analysis to identify different scanning modes, the distribution of diffusive modes was broad with both fast and slow diffusive behavior [35]. As the amount of damage increased, the slow mode of diffusion made a greater contribution to the overall distribution of diffusive modes, suggesting pauses at the damage sites. The pauses, presumably to remove the lesion, were a little over a second in reasonable agreement with the turnover time of the enzymes [75]. A model for Fpg scanning behavior based on SM data proposes that the glycosylase scans along the backbone of undamaged DNA at the rotational limit with a diffusion constant of 0.050 µm2/s (Figure 5). This sliding state forms an equilibrium with various immobilized states, including probing an undamaged site, probing a damaged site, and probing a non-specific damage site. The probing state on an undamaged site has a shorter lifetime than the probing state on a damage site. Probing in the presence of damage can lead to a long lived eversion and excision complex. This excision complex is then able to return to the scanning state directly; in fact, approximately 36% of glycosylase pause events are followed by the glycosylase continuing to scan the same DNA molecule, while 64% of events are followed by dissociation [35]. Stopped flow kinetic studies of protein tryptophan fluorescence have identified rates for protein conformational steps during Fpg binding and repair with various lesions [66, 68, 69]. Using these parameters and the models described therein, the distribution of diffusion constants is well fitted, thus confirming that glycosylases stop at a specific damage before continuing a processive scan along DNA.
Figure 5. Kinetic scheme for glycosylase scanning in the presence of damage.
The glycosylase scans non-specific DNA with a 1D diffusion constant of 0.05 µm2/s. This scanning process is in equilibrium with several short-lived paused states, including interrogation of undamaged bases, damaged bases that are not specific substrates for the glycosyase, and specifically recognized damaged bases. From the specific damage recognition complex, the glycosylase may equilibrate with a long-lived eversion and excision complex before returning to a 1D scanning state.
3. Outlook and Perspective
3.1 The Future of SM Experiments with Glycosylases
Even though SM experiments have indirectly answered several questions about glycosylase behavior on damaged DNA, it would be exciting to watch a glycosylase as it interacted with a specific damage site. In order to directly observe scanning around the lesion, the SM experiment would utilize a DNA molecule with a visually identifiable damage site. From these experiments, we might expect to learn how long a glycosylase pauses at the damage site, whether the glycosylase will recognize the damage when traveling from either direction, what direction the glycosylase continues to scan after encountering the damage, and if the damage decreases the apparent 1D diffusion constant around the lesion. λ-DNA substrates that contain oxidative damages in a specific position are yet to be developed, but recent SM studies that have examined recognition of DNA mismatches by mismatch repair proteins demonstrate the possibilities for similar experiments with glycosylases [76].
Despite the fact that significant progress has been made in understanding glycosylase scanning behavior, it must be pointed out that ensemble and SM experiments are nowhere near emulating the in vivo environment experienced by a glycosylase as it repairs damage in a cell. It is difficult to predict the local solution environment around cellular DNA, which includes molecular crowding and microenvironments of pH and charge. Furthermore, both oligos and linearly-extended λ-DNA are oversimplified substrates. Glycosylase activity assays that utilize short (12–40) bp oligos underemphasize the role of sliding, and lesion recognition and processing in these experiments may primarily rely upon collisions. Thus, glycosylase reactions on short oligos have faster kinetics during the early and late stages of the reaction [69]. Another consideration is that eukaryotic genomic DNA has significant secondary structure, associated proteins, and chromatin packing that will affect how glycosylases are able to access regions for repair. Glycosylase activity is attenuated in the presence of nucleosomes [77–81], and the rates of base cleavage by hNTH1, NEIL1, and UDG depend upon whether the lesion is facing outward toward the solution or buried against the histone surface [78, 81]. These studies suggest that exposure of lesions during transient breathing of nucleosomes plays a large role in the efficiency of glycosylase repair [82]. Although there are a few reports of the use of λ-DNA containing nucleosomes in SM studies [83–85], these substrates have yet to be used in SM studies of glycosylase activity.
The SM studies to date have not explored the scanning behavior of other members of the BER/short-patch repair pathway. For several glycosylases, including E. coli MutY, murine MYH, hNTH1, and hOGG1, product release is a rate-limiting step in the glycosylase reaction [57, 86–89]. It has been shown in several studies that downstream members of the repair pathway, specifically apurinic-apyrimidinic endonucleases, are capable of enhancing the rate of product release by these glycosylases through competitive binding to the product site [90–95]. This interaction between glycosylases and downstream repair proteins undoubtedly influences the sliding length on damaged DNA substrates, and may influence processivity of scanning after lesion repair. Furthermore, it has been suggested that eukaryotic glycosylase enzymes are able to form complexes with other proteins involved in repair. For example, hMYH, a human glycosylase whose function is important for prevention of colon cancer, has been shown to interact in vitro with AP endonuclease (APE1), proliferating cell nuclear antigen (PCNA), and replication protein A (RPA) [94]. The functional importance of the interaction between MYH and PCNA in vivo has been shown for the yeast homologs of these proteins [96]. Similarly, Y-box binding protein (YB-1) and XPG endonuclease have been shown to modulate hNTH glycosylase activity [88, 97, 98], and the Nei-like proteins NEIL1 and NEIL2 interact with replication-associated proteins [99–102] and transcriptional machinery [103], respectively. It is likely that a glycosylase in complex with other “repairosome” factors would have modulated scanning behavior when compared with a glycosylase alone, especially with regard to binding lifetimes, processivity, and rate of diffusion.
3.2 Conclusions
SM studies of glycosylases on λ-DNA have revealed sliding behavior which is consistent with what has been observed in correlated cleavage studies; that is, glycosylases show a combination of 1D sliding and 3D diffusion with sliding lengths that are comparable to those measured in the correlated cleavage studies [33–35]. The binding lifetimes are highly salt dependent, supporting the salt sensitivity of the correlated cleavage studies. This sliding behavior is influenced by individual residues involved in either contact with the DNA backbone [33], or interrogation for damage [34, 35]. Glycosylases pause in the presence of damage sites, and this pausing likely reflects interrogation by the wedge residue followed by removal of the damage [35].
The use of SM methods to study BER/short patch repair is still in its infancy, leaving plenty of room for new ideas and discoveries. The continual innovation in SM substrates, instrumentation, and analysis will allow for many questions about glycosylase interaction with DNA to be answered in the near future. It is also possible that we will soon be able to observe the glycosylase repair process as it occurs in vivo [10, 104]. This is an exciting time in the field of SM DNA repair!
Supplementary Material
The Nei protein is colored red, while the extended DNA tightrope is colored green. Movie was collected at 15 fps.
Highlights.
We review single-molecule fluorescence studies of the glycosylase search for damage
These studies confirm that glycosylases use sliding and distributive search modes
Search behavior is consistent for hOGG1, Nth, Fpg, and Nei glycosylases
E. coli DNA glycosylases utilize a wedge residue to search DNA for oxidative damage
Single-molecule studies are discussed in the context of the ensemble literature
Acknowledgements
The authors would like to thank National Institutes of Health Grant PO1 CA 098993 awarded by the National Cancer Institute for supporting the work in our laboratory. The Authors are also grateful to Dr. Andrew Dunn for initiating the SM studies in our laboratory, Dr. Shane Nelson for computational simulation of the data, and Dr. Aishwarya Prakash for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors declare no conflict of interest.
References
- 1.Krokan HE, Nilsen H, Skorpen F, Otterlei M, Slupphaug G. Base excision repair of DNA in mammalian cells. FEBS letters. 2000;476:73–77. doi: 10.1016/s0014-5793(00)01674-4. [DOI] [PubMed] [Google Scholar]
- 2.Mitra S, Izumi T, Boldogh I, Bhakat KK, Hill JW, Hazra TK. Choreography of oxidative damage repair in mammalian genomes. Free radical biology & medicine. 2002;33:15–28. doi: 10.1016/s0891-5849(02)00819-5. [DOI] [PubMed] [Google Scholar]
- 3.Izumi T, Wiederhold LR, Roy G, Roy R, Jaiswal A, Bhakat KK, Mitra S, Hazra TK. Mammalian DNA base excision repair proteins: their interactions and role in repair of oxidative DNA damage. Toxicology. 2003;193:43–65. doi: 10.1016/s0300-483x(03)00289-0. [DOI] [PubMed] [Google Scholar]
- 4.Barnes DE, Lindahl T. Repair and genetic consequences of endogenous DNA base damage in mammalian cells. Annual review of genetics. 2004;38:445–476. doi: 10.1146/annurev.genet.38.072902.092448. [DOI] [PubMed] [Google Scholar]
- 5.Fromme JC, Banerjee A, Verdine GL. DNA glycosylase recognition and catalysis. Current opinion in structural biology. 2004;14:43–49. doi: 10.1016/j.sbi.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Duclos S, Doublié S, Wallace SS. Consequences and repair of oxidative DNA damage. In: Greim H, Albertini RJ, editors. The Cellular Response to the Genotoxic Insult: The Question of Threshold for Genotoxic Carcinogens. Cambridge, United Kingdom: The Royal Society of Chemistry; 2012. pp. 109–153. [Google Scholar]
- 7.Riggs AD, Bourgeois S, Cohn M. The lac repressor-operator interaction. 3. Kinetic studies. Journal of molecular biology. 1970;53:401–417. doi: 10.1016/0022-2836(70)90074-4. [DOI] [PubMed] [Google Scholar]
- 8.Berg OG, Winter RB, Vonhippel PH. Diffusion-Driven Mechanisms of Protein Translocation on Nucleic-Acids .1. Models and Theory. Biochemistry-Us. 1981;20:6929–6948. doi: 10.1021/bi00527a028. [DOI] [PubMed] [Google Scholar]
- 9.Winter RB, Berg OG, Vonhippel PH. Diffusion-Driven Mechanisms of Protein Translocation on Nucleic-Acids .3. The Escherichia-Coli-Lac Repressor-Operator Interaction - Kinetic Measurements and Conclusions. Biochemistry-Us. 1981;20:6961–6977. doi: 10.1021/bi00527a030. [DOI] [PubMed] [Google Scholar]
- 10.Hammar P, Leroy P, Mahmutovic A, Marklund EG, Berg OG, Elf J. The lac repressor displays facilitated diffusion in living cells. Science. 2012;336:1595–1598. doi: 10.1126/science.1221648. [DOI] [PubMed] [Google Scholar]
- 11.Berg HC Expanded ed. Random walks in biology. Princeton, N.J.: Princeton University Press; 1993. [Google Scholar]
- 12.Zharkov DO, Grollman AP. The DNA trackwalkers: principles of lesion search and recognition by DNA glycosylases. Mutation research. 2005;577:24–54. doi: 10.1016/j.mrfmmm.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Friedman JI, Stivers JT. Detection of damaged DNA bases by DNA glycosylase enzymes. Biochemistry-Us. 2010;49:4957–4967. doi: 10.1021/bi100593a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lloyd RS, Hanawalt PC, Dodson ML. Processive action of T4 endonuclease V on ultraviolet-irradiated DNA. Nucleic Acids Res. 1980;8:5113–5127. doi: 10.1093/nar/8.21.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganesan AK, Seawell PC, Lewis RJ, Hanawalt PC. Processivity of T4 endonuclease V is sensitive to NaCl concentration. Biochemistry-Us. 1986;25:5751–5755. doi: 10.1021/bi00367a060. [DOI] [PubMed] [Google Scholar]
- 16.Gruskin EA, Lloyd RS. The DNA scanning mechanism of T4 endonuclease V. Effect of NaCl concentration on processive nicking activity. J Biol Chem. 1986;261:9607–9613. [PubMed] [Google Scholar]
- 17.Gruskin EA, Lloyd RS. Molecular analysis of plasmid DNA repair within ultraviolet-irradiated Escherichia coli. I. T4 endonuclease V-initiated excision repair. J Biol Chem. 1988;263:12728–12737. [PubMed] [Google Scholar]
- 18.Dowd DR, Lloyd RS. Biological significance of facilitated diffusion in protein-DNA interactions. Applications to T4 endonuclease V-initiated DNA repair. J Biol Chem. 1990;265:3424–3431. [PubMed] [Google Scholar]
- 19.Vassylyev DG, Kashiwagi T, Mikami Y, Ariyoshi M, Iwai S, Ohtsuka E, Morikawa K. Atomic model of a pyrimidine dimer excision repair enzyme complexed with a DNA substrate: structural basis for damaged DNA recognition. Cell. 1995;83:773–782. doi: 10.1016/0092-8674(95)90190-6. [DOI] [PubMed] [Google Scholar]
- 20.Golan G, Zharkov DO, Grollman AP, Dodson ML, McCullough AK, Lloyd RS, Shoham G. Structure of T4 pyrimidine dimer glycosylase in a reduced imine covalent complex with abasic site-containing DNA. Journal of molecular biology. 2006;362:241–258. doi: 10.1016/j.jmb.2006.06.059. [DOI] [PubMed] [Google Scholar]
- 21.Higley M, Lloyd RS. Processivity of uracil DNA glycosylase. Mutation research. 1993;294:109–116. doi: 10.1016/0921-8777(93)90019-d. [DOI] [PubMed] [Google Scholar]
- 22.Purmal AA, Lampman GW, Pourmal EI, Melamede RJ, Wallace SS, Kow YW. Uracil DNA N-glycosylase distributively interacts with duplex polynucleotides containing repeating units of either TGGCCAAGCU or TGGCCAAGCTTGGCCAAGCU. J Biol Chem. 1994;269:22046–22053. [PubMed] [Google Scholar]
- 23.Bennett SE, Sanderson RJ, Mosbaugh DW. Processivity of Escherichia coli and rat liver mitochondrial uracil-DNA glycosylase is affected by NaCl concentration. Biochemistry-Us. 1995;34:6109–6119. doi: 10.1021/bi00018a014. [DOI] [PubMed] [Google Scholar]
- 24.Porecha RH, Stivers JT. Uracil DNA glycosylase uses DNA hopping and short-range sliding to trap extrahelical uracils. Proc Natl Acad Sci U S A. 2008;105:10791–10796. doi: 10.1073/pnas.0801612105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mechetin GV, Zharkov DO. Mechanism of translocation of uracil-DNA glycosylase from Escherichia coli between distributed lesions. Biochem Biophys Res Commun. 2011;414:425–430. doi: 10.1016/j.bbrc.2011.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sidorenko VS, Mechetin GV, Nevinsky GA, Zharkov DO. Correlated cleavage of single- and double-stranded substrates by uracil-DNA glycosylase. FEBS letters. 2008;582:410–414. doi: 10.1016/j.febslet.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Francis AW, David SS. Escherichia coli MutY and Fpg utilize a processive mechanism for target location. Biochemistry-Us. 2003;42:801–810. doi: 10.1021/bi026375+. [DOI] [PubMed] [Google Scholar]
- 28.Hedglin M, O'Brien PJ. Hopping enables a DNA repair glycosylase to search both strands and bypass a bound protein. ACS chemical biology. 2010;5:427–436. doi: 10.1021/cb1000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hedglin M, Zhang Y, O'Brien PJ. Isolating contributions from intersegmental transfer to DNA searching by alkyladenine DNA glycosylase. J Biol Chem. 2013;288:24550–24559. doi: 10.1074/jbc.M113.477018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selvin PR, Ha T. Single-molecule techniques : a laboratory manual. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press; 2008. [Google Scholar]
- 31.Roy R, Hohng S, Ha T. A practical guide to single-molecule FRET. Nature methods. 2008;5:507–516. doi: 10.1038/nmeth.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blainey PC, Luo GB, Kou SC, Mangel WF, Verdine GL, Bagchi B, Xie XS. Nonspecifically bound proteins spin while diffusing along DNA. Nat Struct Mol Biol. 2009;16 doi: 10.1038/nsmb.1716. 1224-U1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blainey PC, van Oijent AM, Banerjee A, Verdine GL, Xie XS. A base-excision DNA-repair protein finds intrahelical lesion bases by fast sliding in contact with DNA. P Natl Acad Sci USA. 2006;103:5752–5757. doi: 10.1073/pnas.0509723103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunn AR, Kad NM, Nelson SR, Warshaw DM, Wallace SS. Single Qdot-labeled glycosylase molecules use a wedge amino acid to probe for lesions while scanning along DNA. Nucleic Acids Res. 2011;39:7487–7498. doi: 10.1093/nar/gkr459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson SR, Dunn AR, Kathe SD, Warshaw DM, Wallace SS. Two glycosylase families diffusively scan DNA using a wedge residue to probe for damages and stop upon encountering a damage. Proc Natl Acad Sci USA. 2014 May 20;111(20):E2091–E2099. doi: 10.1073/pnas.1400386111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kad NM, Wang H, Kennedy GG, Warshaw DM, Van Houten B. Collaborative Dynamic DNA Scanning by Nucleotide Excision Repair Proteins Investigated by Single-Molecule Imaging of Quantum-Dot-Labeled Proteins. Mol Cell. 2010;37:702–713. doi: 10.1016/j.molcel.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorman J, Greene EC. Visualizing one-dimensional diffusion of proteins along DNA. Nat Struct Mol Biol. 2008;15:768–774. doi: 10.1038/nsmb.1441. [DOI] [PubMed] [Google Scholar]
- 38.McCullough AK, Dodson ML, Lloyd RS. Initiation of base excision repair: glycosylase mechanisms and structures. Annual review of biochemistry. 1999;68:255–285. doi: 10.1146/annurev.biochem.68.1.255. [DOI] [PubMed] [Google Scholar]
- 39.Huffman JL, Sundheim O, Tainer JA. DNA base damage recognition and removal: new twists and grooves. Mutation research. 2005;577:55–76. doi: 10.1016/j.mrfmmm.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 40.Prakash A, Doublie S, Wallace SS. The Fpg/Nei family of DNA glycosylases: substrates, structures, and search for damage. Progress in molecular biology and translational science. 2012;110:71–91. doi: 10.1016/B978-0-12-387665-2.00004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuo CF, McRee DE, Fisher CL, O'Handley SF, Cunningham RP, Tainer JA. Atomic structure of the DNA repair [4Fe-4S] enzyme endonuclease III. Science. 1992;258:434–440. doi: 10.1126/science.1411536. [DOI] [PubMed] [Google Scholar]
- 42.Thayer MM, Ahern H, Xing D, Cunningham RP, Tainer JA. Novel DNA binding motifs in the DNA repair enzyme endonuclease III crystal structure. The EMBO journal. 1995;14:4108–4120. doi: 10.1002/j.1460-2075.1995.tb00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strniste GF, Wallace SS. Endonucleolytic incision of x-irradiated deoxyribonucleic acid by extracts of Escherichia coli. Proc Natl Acad Sci U S A. 1975;72:1997–2001. doi: 10.1073/pnas.72.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Armel PR, Strniste GF, Wallace SS. Studies on Escherichia coli x-ray endonuclease specificity. Roles of hydroxyl and reducing radicals in the production of DNA lesions. Radiation research. 1977;69:328–338. [PubMed] [Google Scholar]
- 45.Gates FT, Linn S. Endonuclease from Escherichia coli that acts specifically upon duplex DNA damaged by ultraviolet light, osmium tetroxide, acid, or x-rays. J Biol Chem. 1977;252:2802–2807. [PubMed] [Google Scholar]
- 46.Katcher HL, Wallace SS. Characterization of the Escherichia coli X-ray endonuclease, endonuclease III. Biochemistry-Us. 1983;22:4071–4081. doi: 10.1021/bi00286a013. [DOI] [PubMed] [Google Scholar]
- 47.Chetsanga CJ, Lindahl T. Release of 7-methylguanine residues whose imidazole rings have been opened from damaged DNA by a DNA glycosylase from Escherichia coli. Nucleic Acids Res. 1979;6:3673–3684. doi: 10.1093/nar/6.11.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Melamede RJ, Hatahet Z, Kow YW, Ide H, Wallace SS. Isolation and characterization of endonuclease VIII from Escherichia coli. Biochemistry-Us. 1994;33:1255–1264. doi: 10.1021/bi00171a028. [DOI] [PubMed] [Google Scholar]
- 49.Jiang D, Hatahet Z, Blaisdell JO, Melamede RJ, Wallace SS. Escherichia coli endonuclease VIII: cloning, sequencing, and overexpression of the nei structural gene and characterization of nei and nei nth mutants. Journal of bacteriology. 1997;179:3773–3782. doi: 10.1128/jb.179.11.3773-3782.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sugahara M, Mikawa T, Kumasaka T, Yamamoto M, Kato R, Fukuyama K, Inoue Y, Kuramitsu S. Crystal structure of a repair enzyme of oxidatively damaged DNA, MutM (Fpg), from an extreme thermophile, Thermus thermophilus HB8. The EMBO journal. 2000;19:3857–3869. doi: 10.1093/emboj/19.15.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gilboa R, Zharkov DO, Golan G, Fernandes AS, Gerchman SE, Matz E, Kycia JH, Grollman AP, Shoham G. Structure of formamidopyrimidine-DNA glycosylase covalently complexed to DNA. J Biol Chem. 2002;277:19811–19816. doi: 10.1074/jbc.M202058200. [DOI] [PubMed] [Google Scholar]
- 52.Serre L, Pereira de Jesus K, Boiteux S, Zelwer C, Castaing B. Crystal structure of the Lactococcus lactis formamidopyrimidine-DNA glycosylase bound to an abasic site analogue-containing DNA. The EMBO journal. 2002;21:2854–2865. doi: 10.1093/emboj/cdf304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zharkov DO, Golan G, Gilboa R, Fernandes AS, Gerchman SE, Kycia JH, Rieger RA, Grollman AP, Shoham G. Structural analysis of an Escherichia coli endonuclease VIII covalent reaction intermediate. The EMBO journal. 2002;21:789–800. doi: 10.1093/emboj/21.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang D, Hatahet Z, Melamede RJ, Kow YW, Wallace SS. Characterization of Escherichia coli endonuclease VIII. J Biol Chem. 1997;272:32230–32239. doi: 10.1074/jbc.272.51.32230. [DOI] [PubMed] [Google Scholar]
- 55.Chung MH, Kasai H, Jones DS, Inoue H, Ishikawa H, Ohtsuka E, Nishimura S. An endonuclease activity of Escherichia coli that specifically removes 8-hydroxyguanine residues from DNA. Mutation research. 1991;254:1–12. doi: 10.1016/0921-8777(91)90035-n. [DOI] [PubMed] [Google Scholar]
- 56.Tchou J, Kasai H, Shibutani S, Chung MH, Laval J, Grollman AP, Nishimura S. 8-oxoguanine (8-hydroxyguanine) DNA glycosylase and its substrate specificity. Proc Natl Acad Sci U S A. 1991;88:4690–4694. doi: 10.1073/pnas.88.11.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zharkov DO, Rosenquist TA, Gerchman SE, Grollman AP. Substrate specificity and reaction mechanism of murine 8-oxoguanine-DNA glycosylase. J Biol Chem. 2000;275:28607–28617. doi: 10.1074/jbc.M002441200. [DOI] [PubMed] [Google Scholar]
- 58.Asagoshi K, Yamada T, Okada Y, Terato H, Ohyama Y, Seki S, Ide H. Recognition of formamidopyrimidine by Escherichia coli and mammalian thymine glycol glycosylases. Distinctive paired base effects and biological and mechanistic implications. J Biol Chem. 2000;275:24781–24786. doi: 10.1074/jbc.M000576200. [DOI] [PubMed] [Google Scholar]
- 59.Asagoshi K, Yamada T, Terato H, Ohyama Y, Monden Y, Arai T, Nishimura S, Aburatani H, Lindahl T, Ide H. Distinct repair activities of human 7,8-dihydro-8-oxoguanine DNA glycosylase and formamidopyrimidine DNA glycosylase for formamidopyrimidine and 7,8-dihydro-8-oxoguanine. J Biol Chem. 2000;275:4956–4964. doi: 10.1074/jbc.275.7.4956. [DOI] [PubMed] [Google Scholar]
- 60.Bruner SD, Norman DP, Verdine GL. Structural basis for recognition and repair of the endogenous mutagen 8-oxoguanine in DNA. Nature. 2000;403:859–866. doi: 10.1038/35002510. [DOI] [PubMed] [Google Scholar]
- 61.Fromme JC, Verdine GL. DNA lesion recognition by the bacterial repair enzyme MutM. J Biol Chem. 2003;278:51543–51548. doi: 10.1074/jbc.M307768200. [DOI] [PubMed] [Google Scholar]
- 62.Banerjee A, Santos WL, Verdine GL. Structure of a DNA glycosylase searching for lesions. Science. 2006;311:1153–1157. doi: 10.1126/science.1120288. [DOI] [PubMed] [Google Scholar]
- 63.Qi Y, Spong MC, Nam K, Banerjee A, Jiralerspong S, Karplus M, Verdine GL. Encounter and extrusion of an intrahelical lesion by a DNA repair enzyme. Nature. 2009;462:762–766. doi: 10.1038/nature08561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qi Y, Spong MC, Nam K, Karplus M, Verdine GL. Entrapment and structure of an extrahelical guanine attempting to enter the active site of a bacterial DNA glycosylase, MutM. J Biol Chem. 2010;285:1468–1478. doi: 10.1074/jbc.M109.069799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sung RJ, Zhang M, Qi Y, Verdine GL. Structural and Biochemical Analysis of DNA Helix Invasion by the Bacterial 8-Oxoguanine DNA Glycosylase MutM. J Biol Chem. 2013;288:10012–10023. doi: 10.1074/jbc.M112.415612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuznetsov NA, Koval VV, Zharkov DO, Vorobjev YN, Nevinsky GA, Douglas KT, Fedorova OS. Pre-steady-state kinetic study of substrate specificity of Escherichia coli formamidopyrimidine-DNA glycosylase. Biochemistry-Us. 2007;46:424–435. doi: 10.1021/bi060787r. [DOI] [PubMed] [Google Scholar]
- 67.Kuznetsov NA, Vorobjev YN, Krasnoperov LN, Fedorova OS. Thermodynamics of the multi-stage DNA lesion recognition and repair by formamidopyrimidine-DNA glycosylase using pyrrolocytosine fluorescence--stopped-flow pre-steady-state kinetics. Nucleic Acids Res. 2012;40:7384–7392. doi: 10.1093/nar/gks423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fedorova OS, Nevinsky GA, Koval VV, Ishchenko AA, Vasilenko NL, Douglas KT. Stopped-flow kinetic studies of the interaction between Escherichia coli Fpg protein and DNA substrates. Biochemistry-Us. 2002;41:1520–1528. doi: 10.1021/bi011524u. [DOI] [PubMed] [Google Scholar]
- 69.Koval VV, Kuznetsov NA, Zharkov DO, Ishchenko AA, Douglas KT, Nevinsky GA, Fedorova OS. Pre-steady-state kinetics shows differences in processing of various DNA lesions by Escherichia coli formamidopyrimidine-DNA glycosylase. Nucleic Acids Res. 2004;32:926–935. doi: 10.1093/nar/gkh237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boiteux S, Gajewski E, Laval J, Dizdaroglu M. Substrate specificity of the Escherichia coli Fpg protein (formamidopyrimidine-DNA glycosylase): excision of purine lesions in DNA produced by ionizing radiation or photosensitization. Biochemistry-Us. 1992;31:106–110. doi: 10.1021/bi00116a016. [DOI] [PubMed] [Google Scholar]
- 71.Czeczot H, Tudek B, Lambert B, Laval J, Boiteux S. Escherichia coli Fpg protein and UvrABC endonuclease repair DNA damage induced by methylene blue plus visible light in vivo and in vitro. Journal of bacteriology. 1991;173:3419–3424. doi: 10.1128/jb.173.11.3419-3424.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Muller E, Boiteux S, Cunningham RP, Epe B. Enzymatic recognition of DNA modifications induced by singlet oxygen and photosensitizers. Nucleic Acids Res. 1990;18:5969–5973. doi: 10.1093/nar/18.20.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ide H, Kow YW, Wallace SS. Thymine glycols and urea residues in M13 DNA constitute replicative blocks in vitro. Nucleic Acids Res. 1985;13:8035–8052. doi: 10.1093/nar/13.22.8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Laspia MF, Wallace SS. Excision repair of thymine glycols, urea residues, and apurinic sites in Escherichia coli. Journal of bacteriology. 1988;170:3359–3366. doi: 10.1128/jb.170.8.3359-3366.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guo Y, Bandaru V, Jaruga P, Zhao X, Burrows CJ, Iwai S, Dizdaroglu M, Bond JP, Wallace SS. The oxidative DNA glycosylases of Mycobacterium tuberculosis exhibit different substrate preferences from their Escherichia coli counterparts. DNA repair. 2010;9:177–190. doi: 10.1016/j.dnarep.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gorman J, Wang F, Redding S, Plys AJ, Fazio T, Wind S, Alani EE, Greene EC. Single-molecule imaging reveals target-search mechanisms during DNA mismatch repair. Proc Natl Acad Sci U S A. 2012;109:E3074–E3083. doi: 10.1073/pnas.1211364109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nilsen H, Lindahl T, Verreault A. DNA base excision repair of uracil residues in reconstituted nucleosome core particles. The EMBO journal. 2002;21:5943–5952. doi: 10.1093/emboj/cdf581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beard BC, Wilson SH, Smerdon MJ. Suppressed catalytic activity of base excision repair enzymes on rotationally positioned uracil in nucleosomes. Proc Natl Acad Sci U S A. 2003;100:7465–7470. doi: 10.1073/pnas.1330328100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Menoni H, Gasparutto D, Hamiche A, Cadet J, Dimitrov S, Bouvet P, Angelov D. ATP-dependent chromatin remodeling is required for base excision repair in conventional but not in variant H2A.Bbd nucleosomes. Molecular and cellular biology. 2007;27:5949–5956. doi: 10.1128/MCB.00376-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Prasad A, Wallace SS, Pederson DS. Initiation of base excision repair of oxidative lesions in nucleosomes by the human, bifunctional DNA glycosylase NTH1. Molecular and cellular biology. 2007;27:8442–8453. doi: 10.1128/MCB.00791-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Odell ID, Newick K, Heintz NH, Wallace SS, Pederson DS. Non-specific DNA binding interferes with the efficient excision of oxidative lesions from chromatin by the human DNA glycosylase, NEIL1. DNA repair. 2010;9:134–143. doi: 10.1016/j.dnarep.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maher RL, Prasad A, Rizvanova O, Wallace SS, Pederson DS. Contribution of DNA unwrapping from histone octamers to the repair of oxidatively damaged DNA in nucleosomes. DNA repair. 2013;12:964–971. doi: 10.1016/j.dnarep.2013.08.010. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Visnapuu ML, Greene EC. Single-molecule imaging of DNA curtains reveals intrinsic energy landscapes for nucleosome deposition. Nat Struct Mol Biol. 2009;16:1056–1062. doi: 10.1038/nsmb.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Finkelstein IJ, Visnapuu ML, Greene EC. Single-molecule imaging reveals mechanisms of protein disruption by a DNA translocase. Nature. 2010;468:983–987. doi: 10.1038/nature09561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gorman J, Plys AJ, Visnapuu ML, Alani E, Greene EC. Visualizing one-dimensional diffusion of eukaryotic DNA repair factors along a chromatin lattice. Nat Struct Mol Biol. 2010;17:932–938. doi: 10.1038/nsmb.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Porello SL, Leyes AE, David SS. Single-turnover and pre-steady-state kinetics of the reaction of the adenine glycosylase MutY with mismatch-containing DNA substrates. Biochemistry-Us. 1998;37:14756–14764. doi: 10.1021/bi981594+. [DOI] [PubMed] [Google Scholar]
- 87.Pope MA, David SS. DNA damage recognition and repair by the murine MutY homologue. DNA repair. 2005;4:91–102. doi: 10.1016/j.dnarep.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 88.Marenstein DR, Ocampo MT, Chan MK, Altamirano A, Basu AK, Boorstein RJ, Cunningham RP, Teebor GW. Stimulation of human endonuclease III by Y box-binding protein 1 (DNA-binding protein B). Interaction between a base excision repair enzyme and a transcription factor. J Biol Chem. 2001;276:21242–21249. doi: 10.1074/jbc.M101594200. [DOI] [PubMed] [Google Scholar]
- 89.Bjoras M, Luna L, Johnsen B, Hoff E, Haug T, Rognes T, Seeberg E. Opposite base-dependent reactions of a human base excision repair enzyme on DNA containing 7,8-dihydro-8-oxoguanine and abasic sites. The EMBO journal. 1997;16:6314–6322. doi: 10.1093/emboj/16.20.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hill JW, Hazra TK, Izumi T, Mitra S. Stimulation of human 8-oxoguanine-DNA glycosylase by AP-endonuclease: potential coordination of the initial steps in base excision repair. Nucleic Acids Res. 2001;29:430–438. doi: 10.1093/nar/29.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marenstein DR, Chan MK, Altamirano A, Basu AK, Boorstein RJ, Cunningham RP, Teebor GW. Substrate specificity of human endonuclease III (hNTH1). Effect of human APE1 on hNTH1 activity. J Biol Chem. 2003;278:9005–9012. doi: 10.1074/jbc.M212168200. [DOI] [PubMed] [Google Scholar]
- 92.Vidal AE, Hickson ID, Boiteux S, Radicella JP. Mechanism of stimulation of the DNA glycosylase activity of hOGG1 by the major human AP endonuclease: bypass of the AP lyase activity step. Nucleic Acids Res. 2001;29:1285–1292. doi: 10.1093/nar/29.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang H, Clendenin WM, Wong D, Demple B, Slupska MM, Chiang JH, Miller JH. Enhanced activity of adenine-DNA glycosylase (Myh) by apurinic/apyrimidinic endonuclease (Ape1) in mammalian base excision repair of an A/GO mismatch. Nucleic Acids Res. 2001;29:743–752. doi: 10.1093/nar/29.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Parker A, Gu Y, Mahoney W, Lee SH, Singh KK, Lu AL. Human homolog of the MutY repair protein (hMYH) physically interacts with proteins involved in long patch DNA base excision repair. J Biol Chem. 2001;276:5547–5555. doi: 10.1074/jbc.M008463200. [DOI] [PubMed] [Google Scholar]
- 95.Pope MA, Porello SL, David SS. Escherichia coli apurinic-apyrimidinic endonucleases enhance the turnover of the adenine glycosylase MutY with G:A substrates. J Biol Chem. 2002;277:22605–22615. doi: 10.1074/jbc.M203037200. [DOI] [PubMed] [Google Scholar]
- 96.Chang DY, Lu AL. Functional interaction of MutY homolog with proliferating cell nuclear antigen in fission yeast, Schizosaccharomyces pombe. J Biol Chem. 2002;277:11853–11858. doi: 10.1074/jbc.M111739200. [DOI] [PubMed] [Google Scholar]
- 97.Bessho T. Nucleotide excision repair 3' endonuclease XPG stimulates the activity of base excision repairenzyme thymine glycol DNA glycosylase. Nucleic Acids Res. 1999;27:979–983. doi: 10.1093/nar/27.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Klungland A, Hoss M, Gunz D, Constantinou A, Clarkson SG, Doetsch PW, Bolton PH, Wood RD, Lindahl T. Base excision repair of oxidative DNA damage activated by XPG protein. Mol Cell. 1999;3:33–42. doi: 10.1016/s1097-2765(00)80172-0. [DOI] [PubMed] [Google Scholar]
- 99.Dou H, Theriot CA, Das A, Hegde ML, Matsumoto Y, Boldogh I, Hazra TK, Bhakat KK, Mitra S. Interaction of the human DNA glycosylase NEIL1 with proliferating cell nuclear antigen. The potential for replication-associated repair of oxidized bases in mammalian genomes. J Biol Chem. 2008;283:3130–3140. doi: 10.1074/jbc.M709186200. [DOI] [PubMed] [Google Scholar]
- 100.Hegde ML, Theriot CA, Das A, Hegde PM, Guo Z, Gary RK, Hazra TK, Shen B, Mitra S. Physical and functional interaction between human oxidized base-specific DNA glycosylase NEIL1 and flap endonuclease 1. J Biol Chem. 2008;283:27028–27037. doi: 10.1074/jbc.M802712200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Theriot CA, Hegde ML, Hazra TK, Mitra S. RPA physically interacts with the human DNA glycosylase NEIL1 to regulate excision of oxidative DNA base damage in primer-template structures. DNA repair. 2010;9:643–652. doi: 10.1016/j.dnarep.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hegde ML, Hegde PM, Bellot LJ, Mandal SM, Hazra TK, Li GM, Boldogh I, Tomkinson AE, Mitra S. Prereplicative repair of oxidized bases in the human genome is mediated by NEIL1 DNA glycosylase together with replication proteins. Proc Natl Acad Sci U S A. 2013;110:E3090–E3099. doi: 10.1073/pnas.1304231110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Banerjee D, Mandal SM, Das A, Hegde ML, Das S, Bhakat KK, Boldogh I, Sarkar PS, Mitra S, Hazra TK. Preferential repair of oxidized base damage in the transcribed genes of mammalian cells. J Biol Chem. 2011;286:6006–6016. doi: 10.1074/jbc.M110.198796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Uphoff S, Reyes-Lamothe R, Garza de Leon F, Sherratt DJ, Kapanidis AN. Single-molecule DNA repair in live bacteria. Proc Natl Acad Sci U S A. 2013;110:8063–8068. doi: 10.1073/pnas.1301804110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Nei protein is colored red, while the extended DNA tightrope is colored green. Movie was collected at 15 fps.