Abstract
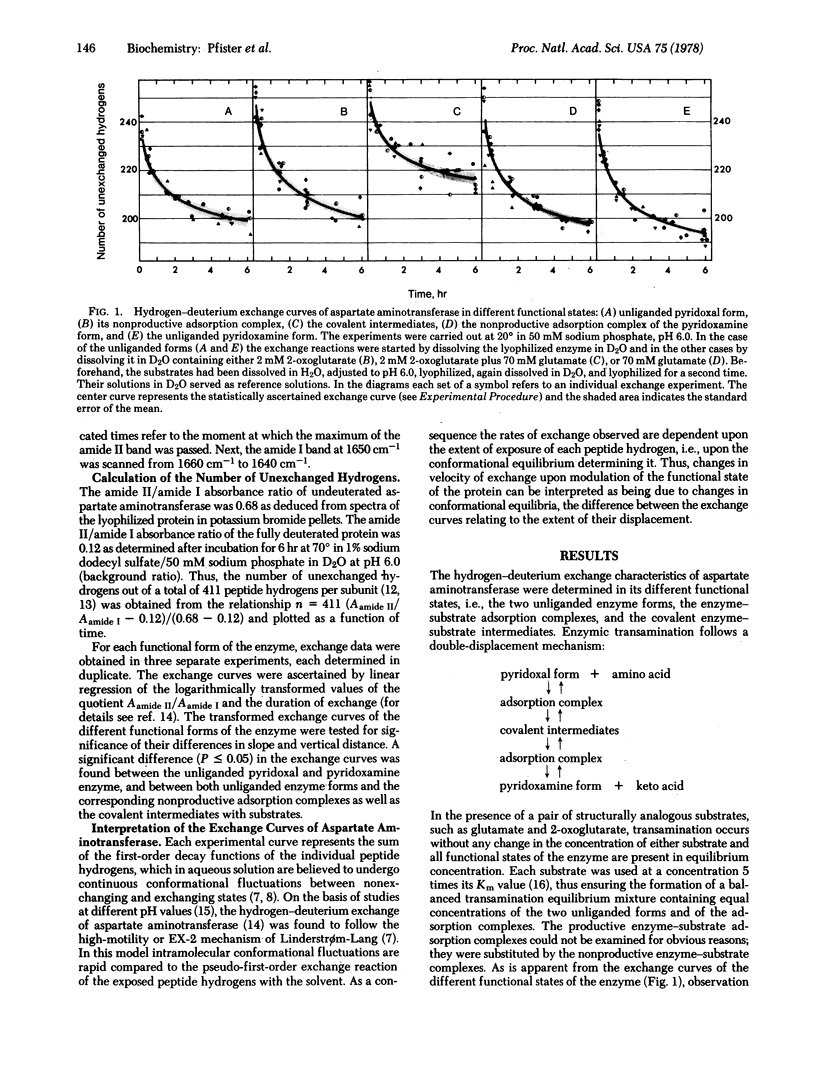
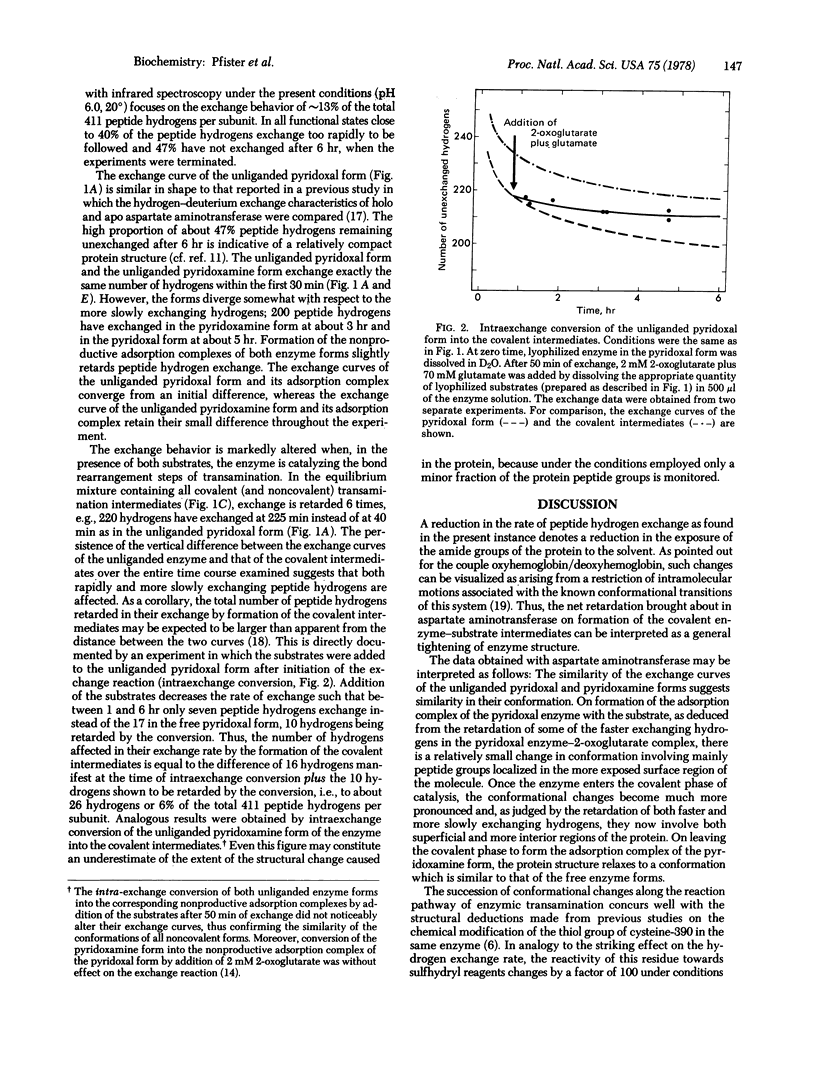
Catalysis-linked conformational transitions of aspartate aminotransferase (cytosolic isoenzyme from pig heart; L-aspartate:2-oxoglutarate aminotransferase, EC 2.6.1.1) have been probed by infrared spectrophotometric measurement of hydrogen-deuterium exchange. In the unliganded pyridoxal form of the enzyme at pH 6.0 and 20 degrees, 43% of the total 411 peptide hydrogens per subunit exchange within the first 10 min. An additional 9% exchange slowly in the following time period to 360 min. A quite similar exchange curve is obtained with the pyridoxamine form of the enzyme, indicating close correspondence in conformation of both unliganded forms of the enzyme. Formation of a nonproductive adsorption complex of the pyridoxal enzyme with 2-oxoglutarate or of the pyridoxamine enzyme with glutamate alters the exchange characteristics only slightly. In contrast, the formation of an equilibrium mixture of the covalent transamination intermediates, which occurs in the silultaneous presence of the amino acid and the keto acid substrate, results in a marked retardation of hydrogen exchange, reflecting a substantial tightening of the structure of the enzyme. The exchange reactions of at least 26 peptide hydrogens per subunit (6% of the total) are retarded by a factor of 6 on the average. The occurrence of such syncatalytic conformational changes reflects energetic coupling of the covalency changes at the active site with conformational changes of the macromolecular protein matrix that may contribute to optimizing the free energy profile of enzymic transamination.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAUNSTEIN A. E. BINDING AND REACTIONS OF THE VITAMIN B6 COENZYME IN THE CATALYTIC CENTER OF ASPARTATE TRANSAMINASE. Vitam Horm. 1964;22:451–484. doi: 10.1016/s0083-6729(08)60348-9. [DOI] [PubMed] [Google Scholar]
- Banks B. E., Doonan S., Lawrence A. J., Vernon C. A. The molecular weight and other properties of aspartate aminotransferase from pig heart muscle. Eur J Biochem. 1968 Sep 24;5(4):528–539. doi: 10.1111/j.1432-1033.1968.tb00402.x. [DOI] [PubMed] [Google Scholar]
- Birchmeier W., Wilson K. J., Christen P. Cytoplasmic aspartate aminotransferase: syncatalytic sulfhydryl group modification. J Biol Chem. 1973 Mar 10;248(5):1751–1759. [PubMed] [Google Scholar]
- Englander S. W. Measurement of structural and free energy changes in hemoglobin by hydrogen exchange methods. Ann N Y Acad Sci. 1975 Apr 15;244:10–27. doi: 10.1111/j.1749-6632.1975.tb41518.x. [DOI] [PubMed] [Google Scholar]
- Gehring H., Christen P. Syncatalytic sulfhydryl group modification in mitochondrial aspartate aminotransferase from chicken and pig heart. Biochem Biophys Res Commun. 1975 Mar 17;63(2):441–447. doi: 10.1016/0006-291x(75)90707-x. [DOI] [PubMed] [Google Scholar]
- HAMMES G. G. MECHANISM OF ENZYME CATALYSIS. Nature. 1964 Oct 24;204:342–343. doi: 10.1038/204342a0. [DOI] [PubMed] [Google Scholar]
- Hvidt A., Nielsen S. O. Hydrogen exchange in proteins. Adv Protein Chem. 1966;21:287–386. doi: 10.1016/s0065-3233(08)60129-1. [DOI] [PubMed] [Google Scholar]
- JENKINS W. T. GLUTAMIC-ASPARTIC TRANSAMINASE. VII. EQUILIBRIUM KINETICS WITH ERYTHRO-BETA-HYDROXYASPARTIC ACID. J Biol Chem. 1964 Jun;239:1742–1747. [PubMed] [Google Scholar]
- Jencks W. P. Binding energy, specificity, and enzymic catalysis: the circe effect. Adv Enzymol Relat Areas Mol Biol. 1975;43:219–410. doi: 10.1002/9780470122884.ch4. [DOI] [PubMed] [Google Scholar]
- Jenkins W. T., D'Ari L. Glutamic-aspartic transaminase. IX. Equilibria with glutamate and alpha-ketoglutarate. J Biol Chem. 1966 Jun 25;241(12):2845–2854. [PubMed] [Google Scholar]
- Kägi J. H., Ulmer D. D. Hydrogen-deuterium exchange of cytochrome c. II. Effect of pH. Biochemistry. 1968 Aug;7(8):2718–2723. doi: 10.1021/bi00848a004. [DOI] [PubMed] [Google Scholar]
- Lumry R. Conformational mechanisms for free energy transduction in protein systems: old ideas and new facts. Ann N Y Acad Sci. 1974 Feb 18;227:46–73. doi: 10.1111/j.1749-6632.1974.tb14373.x. [DOI] [PubMed] [Google Scholar]
- Ottesen M. Methods for measurement of hydrogen isotope exchange in globular proteins. Methods Biochem Anal. 1971;20:135–168. doi: 10.1002/9780470110393.ch5. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Egorov C. A., Aldanova N. A., Feigina M. Y., Lipkin V. M., Abdulaev N. G., Grishin E. V., Kiselev A. P., Modyanov N. N., Braunstein A. E. The complete amino acid sequence of cytoplasmic aspartate aminotransferase from pig heart. FEBS Lett. 1973 Jan 1;29(1):31–34. doi: 10.1016/0014-5793(73)80008-0. [DOI] [PubMed] [Google Scholar]
- Schlegel H., Christen P. Cytosolic aspartate aminotransferase. Studies on subunit interactions with the apo/holo hybrid dimer. Biochim Biophys Acta. 1978 Jan 25;532(1):6–16. doi: 10.1016/0005-2795(78)90442-7. [DOI] [PubMed] [Google Scholar]
- Schlegel H., Zaoralek P. E., Christen P. Aspartate aminotransferase. Determination of the active site occupancy pattern indicates independent transamination of the two subunits. J Biol Chem. 1977 Aug 25;252(16):5835–5838. [PubMed] [Google Scholar]
- Ulmer D. D., Kägi J. H. Hydrogen-deuterium exchange of cytochrome c. I. Effect of oxidation state. Biochemistry. 1968 Aug;7(8):2710–2717. doi: 10.1021/bi00848a003. [DOI] [PubMed] [Google Scholar]
- VELICK S. F., VAVRA J. A kinetic and equilibrium analysis of the glutamic oxaloacetate transaminase mechanism. J Biol Chem. 1962 Jul;237:2109–2122. [PubMed] [Google Scholar]
- Willumsen L. Hydrogen-deuterium exchange in heavy meromyosin. Biochim Biophys Acta. 1966 Oct 10;126(2):382–388. doi: 10.1016/0926-6585(66)90075-6. [DOI] [PubMed] [Google Scholar]


