Abstract
Prenatal cigarette smoke exposure (PCSE) has been linked to problems in behavioral inhibition and attention deficit hyperactivity disorder in children in several epidemiological studies. We used event-related potentials (ERPs) to examine the effects of PCSE on neural correlates of inhibitory control of behavior. In a prospective longitudinal study on child development in the Canadian Arctic, we assessed 186 Inuit children (mean age = 11.3 years) on a visual Go/No-go response inhibition paradigm. PCSE was assessed through maternal recall. Potential confounders were documented from a maternal interview, and exposure to neurotoxic environmental contaminants was assessed from umbilical cord and child blood samples. PCSE was not related to behavioral performance on this simple response inhibition task. Nevertheless, this exposure was associated with smaller amplitudes of the N2 and P3 components elicited by No-go stimuli, suggesting an impairment in the neural processes underlying response inhibition. Amplitude of the No-go P3 component was also inversely associated with behavioral measures of externalizing problems and hyperactivity/impulsivity in the classroom. This study is the first to report neurophysiological evidence of impaired response inhibition in school-aged children exposed to tobacco smoke in utero. Effects were found on ERP components associated with conflict processing and inhibition of a prepotent response, indicating neurophysiological deficits that may play a critical role in the attention and behavior problems observed in children with PCSE.
Keywords: Cigarette, Event-related potentials, Go/No-go, Inhibition, Nicotine, Pregnancy
1. Introduction
Maternal cigarette smoking during pregnancy has been associated with behavior problems among the exposed offspring in several epidemiological studies (Bastra et al., 2003; Braun et al., 2008; Brook et al., 2006; Kotimaa et al., 2003; Obel et al., 2009; Robinson et al., 2010; Rückinger et al., 2010; Wakschloag et al., 2010). These children are at increased risk for externalizing behavioral problems, conduct disorder, and attention deficit hyperactivity disorder (ADHD), all of which are characterized by impulsive behavior. Cognitive assessments in these children have revealed impairments in executive function, notably in inhibitory control (Cornelius et al., 2011; Huijbregts et al., 2008; Julvez et al., 2007). The mechanisms of action for these adverse neurobehavioral effects have not been determined, but they have been hypothesized to reflect dysfunction in the monoamine and cholinergic neurotransmitter systems (Baler et al., 2008; Blood-Siegfried and Rende, 2010; Gold et al., 2009; Muneoka et al., 1997; Slikker et al., 2005; Xu et al., 2001).
Most studies investigating the relation of prenatal cigarette smoke exposure (PCSE) to behavior problems and cognitive impairments have relied on standard behavioral and neuropsychological assessments. Neurophysiological measures, such as event-related potentials (ERPs), could provide additional information on how PCSE impairs the brain processes underlying executive control of behavior and help elucidate the mechanisms of action responsible for its adverse neurobehavioral effects. We recently reported an association of PCSE with increased externalizing behavior problems and ADHD-related behaviors, as measured by behavioral questionnaires completed by the classroom teacher, in school-aged Inuit children from the Canadian Arctic, where tobacco smoking is a major public health issue (Desrosiers et al., 2013). This population is also exposed to neurotoxic environmental contaminants from their traditional diet based on hunting and fishing, including lead (Pb), methylmercury, and polychlorinated biphenyls (PCBs) (Muckle et al., 2001), which have been related to behavioral problems and cognitive impairments similar to those associated with PCSE (Boucher et al., 2012a, 2012b). The present study we used ERPs to examine the neurophysiological correlates of PCSE-related impairments in inhibitory control in our prospective birth-cohort of Inuit children, after statistical adjustment for other neurotoxic exposures.
2. Methods
2.1 Participants
The study participants were 204 school-aged Inuit children from Nunavik, a region of Québec located north of the 55th parallel, about 1,500 km from Montreal. These children were originally recruited in the Cord Blood Monitoring Program (1993–1998), which was designed to document levels of environmental contaminants and nutrients in newborns in Arctic Québec (Dallaire et al., 2003). Six of these children also participated in the Environmental Contaminants and Child Development Study (ECCDS; 1996–2000) (Boucher et al., 2014; Jacobson et al., 2008), which was initiated during the latter part of the Cord Blood Monitoring Program, and one child was recruited only for the ECCDS.
Assessments were conducted between September 2005 and April 2007 in the three largest Nunavik villages when the children averaged 11.3 years of age. Participants who resided in other communities were transported by plane to one of the larger villages for testing. A maternal interview was conducted during the child assessment to document demographic background, smoking, alcohol and drug use during pregnancy as well as other maternal characteristics. Inclusion criteria were age between 9.0 and 13.0 years, birth weight ≥ 2.5 kg, gestation duration ≥ 35 weeks, no known neurological or clinically significant developmental disorder, and no medication for attention problems. Two participants with a history of epilepsy, two with a history of head trauma associated with loss of consciousness and/or requiring hospitalisation, one with multiple sclerosis and one with a history of meningitis were excluded after data collection. Written informed consent was obtained from a parent of each participant; oral assent, from each child. The research was approved by the Laval University and Wayne State University ethics committees.
2.2 Go/No-go protocol
Each participant was seated 57 cm from a 43-cm flat panel monitor on which letters were displayed centrally within a 7 x 7 cm space. The child held a button box in his/her hand and was instructed to press the button as quickly and accurately as possible with his/her index finger to all individually presented letters (the “Go” trials) except the target “X” (the “No-go” trials). The stimuli were presented for 500 ms, with random inter-stimulus intervals ranging between 1200–1400 ms. The first block consisted of 40 Go trials and served to prime Go responses within the second. This second block consisted of 126 Go trials (70%) randomly intermixed with 54 No-go trials (30%). Correct and incorrect responses were tabulated. Mean reaction time (RT; time between stimulus onset and button press) and response accuracy (% Correct) for Go and No-go trials during the second block of trials were computed. Data from the initial block of 40 Go trials were not analyzed (Burden et al., 2011).
Response inhibition in the Go/No-go task employed in this study has been shown to be associated with increased activity in the prefrontal cortex (PFC; Casey et al., 1997). The stimulus-locked ERPs elicited during this task include two late-latency components associated with the task conditions, the N2 and the P3 (Boucher et al., 2012a; Davis et al., 2003). The N2, which is maximal > 300 ms post-stimulus over frontal electrode locations, has been attributed by some investigators to detection of a conflict regarding whether to inhibit a response (Donkers and van Boxtel, 2004; Falkenstein, 2006); by others, to conflict between competing Go and No-go decisions without regard to inhibition (Randall and Smith et al., 2011). The P3, maximal ≈ 500 ms post-stimulus at centro-parietal electrodes, shows enhanced activity for No-go stimuli and is generally thought to reflect the amount of resources needed for task processing and/or efficiently inhibiting a motor response (Davis et al., 2003; Smith et al., 2007).
In addition, response-locked ERPs are obtained following erroneous motor responses (i.e., false alarms), offering a window on brain processes underlying error monitoring. These ERPs show two successive components: (1) the “error-related negativity” (ERN), maximal over fronto-central electrodes ≈ 80 ms after an incorrect response, which is thought to reflect the dynamics of response selection and conflict; and (2) the error positivity (Pe), peaking 200–400 ms after an incorrect response over central electrodes, which is thought to reflect the conscious recognition, or motivational significance, of an error (Hughes and Yeung, 2011; Overbeek et al., 2005).
2.3 EEG recording and analyses
The electroencephalogram (EEG) was recorded with 30 Ag-AgCl electrodes placed according to the international 10–20 system (Jasper, 1958), referenced online to vertex (Cz) electrode, with forehead ground. The electro-oculogram (EOG) was recorded from bipolar miniature electrodes placed vertically above and below the right eye. Impedance was kept < 10 kΩ. EOG and EEG gain were amplified with gains of 5,000 and 50,000 respectively. The bandpass filter was 0.1–30 Hz, and a 60-Hz notch filter was engaged. The digitization rate was 200 Hz.
ERPs were derived and analysed using Brain Vision Analyzer 2.0 (Brain Products, Munich, Germany) software. EEG channels were re-referenced offline to linked earlobes. EOG correction (Gratton and Coles, 1983), artifact rejection (± 100 µV) and baseline correction (100 ms) were applied. All responses occurring 200–1600 ms post-stimulus onset were considered valid. ERPs were averaged for “Correct Go”, “Correct No-go” and “Incorrect No-go” responses separately. The “stimulus-locked” segments, which are measured in relation to when the stimulus first appeared on the screen, were segmented 100 ms before and 1,000 ms after stimulus onset; the “response-locked” segments, which are measured in relation to when the child pressed the button, were segmented 300 ms before and 500 ms after button press. Each ERP component was examined at the electrode site showing the maximum (P3, Pe) or minimum (N2, ERN) voltage1. Peak amplitude (µV) and latency to peak (ms) of the stimulus-locked N2 (Fz; 250–500 ms) component were identified using automatic detection. Mean amplitude values were computed for the stimulus-locked P3 component (Pz; 400–700). Mean amplitude values were also computed for both response-locked components: ERN (FCz; 0–125 ms) and Pe (Cz; 100–500 ms).
Twelve participants were excluded after data analysis for the following reasons: technical problems during recording (n = 4); too much noise in the EEG signal to produce a reliable ERP waveform (n = 3); random responding on the task (n = 2); and insufficient number (< 12 trials) of acceptable trials in their stimulus-locked ERP average (n = 3). These excluded participants did not differ from the remaining children in terms of PCSE (χ2(1) = 0.766, p = 0.381). An additional 28 children were excluded from the analyses of the response-locked components because of an insufficient number of incorrect No-go trials in their averaged ERP waveform (< 8 trials). Among the remaining participants, the mean number of epochs retained in the averaged ERPs was 102.7 (SD: 20.7, range: 37 – 126) for correct Go stimulus-locked components, 31.2 (SD: 9.0, range: 12 – 51) for correct No-go stimulus-locked components, and 17 (SD: 6.4, range: 8 – 35) for incorrect No-go response-locked components2.
2.4 Exposure to tobacco smoke
Maternal recall of smoking during pregnancy was assessed at 1-month postpartum for children originally involved in the ECCDS (n = 7), at 5 years for children who participated to a 5-year assessment (n = 77), and during the 11-year interview for the remaining participants. Comparisons of maternal reports at 11 years with those obtained at 1 -month postpartum and at 5 years confirm the validity of the maternal reports of smoking during pregnancy (yes/no) provided a full decade after delivery. All 7 mothers for whom 1 -month postpartum data were available reported smoking while pregnant with their child at both interviews. Among participants with maternal reports at 5 years, 88.7 % of the mothers who reported smoking during pregnancy, and 80.0% of those who reported having abstained from smoking, provided the same information at the 11-year assessment. However, retrospective quantification of smoking during pregnancy appeared less accurate, as only one of four (25.0 %) mothers who reported light smoking (< 10 cigarettes per day) at the 1-month postpartum interview reported consistent information at the 11-year assessment (the others all recalled heavy smoking, i.e. ≥ 10 cigarettes per day). By contrast, all three mothers who reported heavy smoking at 1 month postpartum also reported heaving smoking at the 11-year assessment. Retrospective quantification of PCSE was missing for five participants. For these reasons, a dichotomous (yes/no) variable was used as the primary variable of exposure in the statistical analyses. Postnatal environmental exposure (yes/no) was estimated at the 11-year assessment by asking the mother if at least one person smoked tobacco inside the house.
2.5 Environmental contaminant analyses
A blood sample (30 mL) obtained from the umbilical cord was used to determine prenatal exposure to Pb, methylmercury, and PCBs, and a venous blood sample (20 mL) obtained from each child was used to document the body burden of these contaminants at the time of testing. Umbilical cord and child whole blood samples were analysed for concentrations of Pb and total mercury (Hg), whereas concentrations of PCBs were determined in corresponding plasma samples. Contaminant analyses were performed at the Laboratoire de Toxicologie, Institut National de Santé Publique du Québec (Québec, Canada). Details regarding quantification of contaminants in biological samples have been described elsewhere (Dallaire et al., in preparation).
2.6 Additional variables
The following control variables were assessed: age and gender of child; whether or not the child was adopted; birth weight; children’s IQ [Wechsler, 2003; see Boucher et al. (2014) for a detailed description of IQ assessment], maternal age at delivery, marital status (living with a partner or not) and education (years); socioeconomic status (SES) of the primary caregiver (Hollingshead, 2011); maternal non-verbal reasoning abilities (Raven Progressive Matrices; Raven et al., 1992); breast-feeding status; binge drinking (at least one episode of ≥ 5 standard alcohol drinks; yes/no) and marijuana use (yes/no) during pregnancy; and contaminant [Pb, Hg, and PCB congener 153 (PCB-153)] concentrations in cord and child blood samples. Child behavior was assessed using the Teacher Report Form (TRF) from the Child Behavior Checklist (Achenbach, 1991), and the Disruptive Behavior Disorders Rating Scale (DBD; Pelham et al., 1992) completed by the child’s classroom teacher. Details regarding these questionnaires and their associations with PCSE have been provided in a previous paper (Desrosiers et al., 2013).
2.7 Statistical analyses
Normality of distribution was inspected visually for each variable and checked for skewness (normality range: −2.0 to 2.0). Each of the contaminant variables was log transformed since they exhibited log-normal distributions. The following variables with extreme values (>3 standard deviations from the mean) were recoded to one point greater than the highest observed non-outlying value: education of the primary caregiver (n = 8), gestation duration (n = 1), maternal age at delivery (n = 1), and maternal Raven score (n = 1) (Winer, 1971).
The effects of the PCSE (yes/no) on the stimulus-locked ERP parameters were examined in repeated measures multivariate analysis of variance (RM-MANOVA) with condition (Go vs. No-go) as the within-subjects factor and exposure group (exposed vs. non-exposed) as the between-subjects factor. The relation of PCSE to behavioral performance and to the response-locked ERPs was examined using analysis of covariance (ANCOVA). In both sets of analyses, child age at testing, child gender, and all other control variables associated (at p < 0.15) with PCSE status were included as covariates. In addition, because of their previously-documented effects on the ERP protocol used, current Pb and current PCB-153 concentrations were included as obligatory covariates for the stimulus-locked ERPs and response-locked ERPs, respectively, and both were included in the models for behavioral performance (Boucher et al., 2012a). Because the data on maternal alcohol and marijuana use during pregnancy were missing for 29 and 28 cases, respectively, these variables were included only in additional statistical analyses to determine if their inclusion altered the results. For each significant difference between the prenatal cigarette exposed vs. unexposed, analyses were re-run including postnatal environmental tobacco smoke exposure as a covariate in order to see its contribution to the effects attributed to PCSE. Finally, all significant results were explored further by replacing the dichotomous PCSE variable by a three-group variable taking into account the estimated quantification of smoking during pregnancy [1 = no exposure (n = 35); 2 = low exposure (< 10 cigarettes per day; n = 81), and 3 = high exposure (≥ 10 cigarettes per day; n = 65)].
Because of the size difference between the PCSE groups, type III sum of squares were used in all multivariate analyses, since this method is considered as the most conservative when dealing with unequal sample sizes (Tabachnick and Fidell, 2007). Furthermore, Box’s M test for homogeneity of covariance matrices were tested for all MANOVA models, and none reached statistical significance (all p’s ≥ 0.35), supporting the assumption of homogeneity of variance-covariance matrices. All data analyses were conducted with SPSS 12.0.1 (SPSS, Chicago, IL).
3. Results
3.1 Descriptive statistics
Sample characteristics are summarized in Table 1. Smoking during pregnancy was common within our sample; a large majority of the children (81.2%) were prenatally exposed to cigarette smoke. Cigarette smoking during pregnancy was associated with lower birth weight, as expected, and with poorer maternal non-verbal reasoning abilities, higher cord blood Hg and Pb concentrations, and higher prevalence of maternal marijuana use during pregnancy. Mothers who smoked while pregnant also tended to be more likely to engage in binge drinking during this period.
Table 1.
Descriptive characteristics for the study participants by prenatal cigarette smoking exposure
| Not exposed |
Exposed |
||||||
|---|---|---|---|---|---|---|---|
| N | Mean ± SD | % | n | Mean (SD) | % | p-value | |
| Child characteristics | |||||||
| Age (years) | 35 | 11.3 ± 0.6 | 151 | 11.3 ± 0.6 | 0.60 | ||
| Sex (% girls) | 35 | 51.4 | 151 | 56.3 | 0.60 | ||
| Adopted (% yes) | 35 | 8.6 | 151 | 15.2 | 0.31 | ||
| Birth weight (g) | 35 | 3656 ± 558 | 150 | 3446 ± 432 | 0.02 | ||
| Gestation duration (weeks) | 35 | 39.5 ± 1.1 | 151 | 39.1 ± 1.5 | 0.19 | ||
| Estimated full-scale IQ | 35 | 95.9 ± 11.8 | 151 | 92.8 ± 12.4 | 0.18 | ||
| Principal caregiver/ family | |||||||
| Age at delivery | 35 | 23.5 ± 5.7 | 151 | 23.7 ± 5.6 | 0.87 | ||
| Marital status (% single) | 35 | 28.6 | 151 | 24.0 | 0.57 | ||
| Education (years) | 35 | 9.1 ± 2.3 | 151 | 8.2 ± 2.3 | 0.05 | ||
| SESa | 35 | 31.3 ± 14.2 | 151 | 28.4 ± 12.1 | 0.21 | ||
| Non-verbal reasoning skillsb | 35 | 39.3 ± 9.5 | 146 | 33.9 ± 10.2 | 0.01 | ||
| Breastfeeding (% breastfed) | 34 | 73.5 | 147 | 68.0 | 0.53 | ||
| Maternal use or consumption during pregnancy |
|||||||
| Binge drinking (% yes) | 32 | 18.8 | 125 | 36.8 | 0.05 | ||
| Marijuana (% yes) | 32 | 9.4 | 126 | 27.0 | 0.04 | ||
| 11-year exposure to environmental tobacco smoke (% yes) |
34 | 23.5 | 149 | 35.6 | 0.18 | ||
| Contaminants | |||||||
| Cord Pb (µg/dL) | 35 | 3.7 ± 2.4 | 149 | 4.9 ± 3.2 | 0.02 | ||
| Current Pb (µg/dL) | 35 | 2.7 ± 2.8 | 148 | 2.6 ± 2.0 | 0.49 | ||
| Cord Hg (µg/L) | 35 | 17.0 ± 17.9 | 149 | 21.9 ± 17.4 | 0.03 | ||
| Current Hg (µg/L) | 35 | 3.2 ± 2.2 | 148 | 4.9 ± 5.5 | 0.34 | ||
| Cord PCB-153 (µg/kg fat) | 35 | 97.4 ± 64.2 | 146 | 122.4 ± 97.6 | 0.22 | ||
| Current PCB-153 (µg/kg fat) | 35 | 58.2 ± 55.2 | 147 | 75.1 ± 73.4 | 0.18 | ||
Assessed with the Hollingshead index, which is computed from predefined scores given for parental occupation status and education (Hollingshead, 1971).
Based on the Raven Progressive Matrices (Raven et al., 1992).
3.2 Behavioral performance
Behavioral performance on the Go/No-go task is summarized in Table 2. As can been seen, PCSE was not associated with any of the behavioral outcomes. The same results were obtained when using the three-group PCSE variable as the index of exposure (data not shown).
Table 2.
Adjusted mean (± SD) behavioral performance during the Go/No-go task by prenatal cigarette smoking exposure
| Not exposed (n = 35) | Exposed (n = 139) | F-ratio for smoking | |
|---|---|---|---|
| Mean hit RT (msec) | 471.0 ± 65.1 | 475.7 ± 74.6 | 0.11 (n.s.) |
| Correct Go trials (%) | 91.1 ± 6.9 | 89.7 ± 10.4 | 0.50 (n.s.) |
| Correct No-go trials (%) | 60.5 ± 13.7 | 64.6 ± 14.5 | 2.08 (n.s.) |
F-ratio for ANCOVAs adjusting for child age and sex, current Pb and PCB-153 concentrations, birth weight, education of the primary caregiver, maternal non-verbal reasoning abilities, cord Pb, and cord Hg concentrations. n.s.: non significant (p ≥ 0.15).
3.3 ERP results
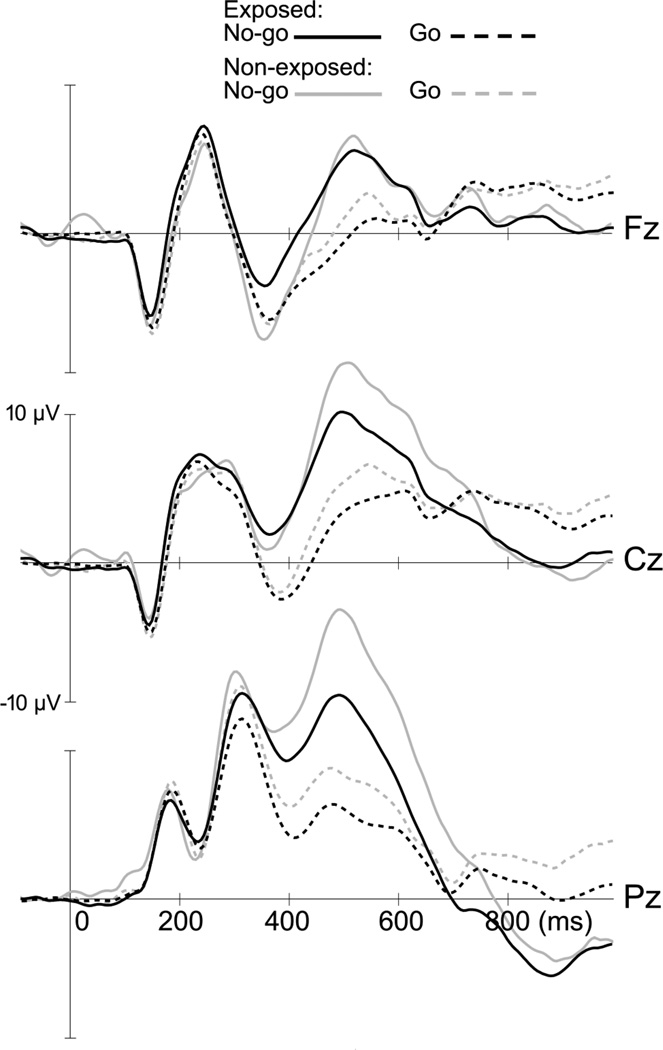
Results from the RM-MANOVAs examining the effects of PCSE on the stimulus-locked ERP parameters are presented in Table 3. PCSE is associated with reductions in N2 and P3 amplitudes (see Fig. 1). However, there were significant interaction effects between PCSE and task condition for both N2 and P3 amplitudes. These interaction effects were explored further by running separate ANCOVAs for the Go and No-go conditions, using the same predictor and covariates as in the RM-MANOVA models. There was a significant effect of smoking on N2 amplitude in the No-go condition (F(1,165) = 11.74, p = 0.001), but not in the Go condition (F(1,165) = 0.01, p = 0.931). Non-exposed children showed a significant Go/No-go N2 effect (i.e., more negative amplitude for No-go vs. Go trials; unadjusted F(1,33) = 7.64; p = 0.009), whereas the exposed children did not (F(1,151) = 0.56; p = 0.455). There was also a strong effect of PCSE status on P3 amplitude in the No-go condition (F(1,165) = 10.63, p = 0.001), whereas in the Go condition its effect fell short of statistical significance (F(1,165) = 2.50, p = 0.116).
Table 3.
Adjusted mean (± SD) stimulus-locked ERP parameters by prenatal cigarette smoking exposure
| Not exposed (n = 35) |
Exposed (n = 140) |
F-ratio |
||||
|---|---|---|---|---|---|---|
| Go | No-go | Go | No-go | Smoking | Smoking x condition | |
| N2 latency (msec) | 376.9 ± 34.8 | 376.5 ± 50.1 | 377.0 ± 43.6 | 368.5 ± 54.1 | 0.31 (n.s.) | 0.38 (n.s.) |
| N2 amplitude (µV) | −7.4 ± 5.6 | −11.7 ±9.0 | −7.3 ± 5.0 | −6.5 ± 7.3 | 5.88* | 13.41** |
| P3 amplitude (µV) | 6.5 ± 4.4 | 13.9 ± 6.7 | 4.5 ± 5.1 | 9.2 ± 7.2 | 8.97** | 5.30* |
F-ratio for RM_MANOVAs adjusting for child age and sex, current Pb concentrations, birth weight, education of the primary caregiver, maternal non-verbal reasoning abilities, cord Pb, and cord Hg concentrations.
p < 0.05
p < 0.01.
n.s.: non significant (p > 0.15).
Figure 1.
Grand average for stimulus-locked ERPs at midline electrode s comparing children with PCSE (n = 151) to unexposed children (n = 35). The N2 is a negative component (≈375 ms) more pronounced at the frontal lead (Fz). The P3 peaks ≈500 ms post-stimulus and is larger at the parietal lead (Pz).
Additional ANCOVA analyses examining the effect of the three-group PCSE variable on N2 and P3 amplitudes in the No-go condition also revealed significant differences between groups (N2: F(2,159) = 5.99, p = 0.003; P3: F(2,159) = 5.56, p = 0.005). Pairwise comparisons with Bonferroni correction revealed that the non-exposed children have significantly larger No-go N2 (estimated mean = −11.64 µV) and P3 (estimated mean = 13.91 µV) amplitudes than children with low exposure (N2: estimated mean = −6.05 µV, p = 0.002; P3: estimated mean = 8.87 µV, p = 0.005) and with high exposure (N2: estimated mean = −7.35 µV; p = 0.038; P3: estimated mean = 9.22 µV, p = 0.015). Children with low and high exposure did not differ on N2 (p = 0.994) or P3 (p = 1.000) amplitude.
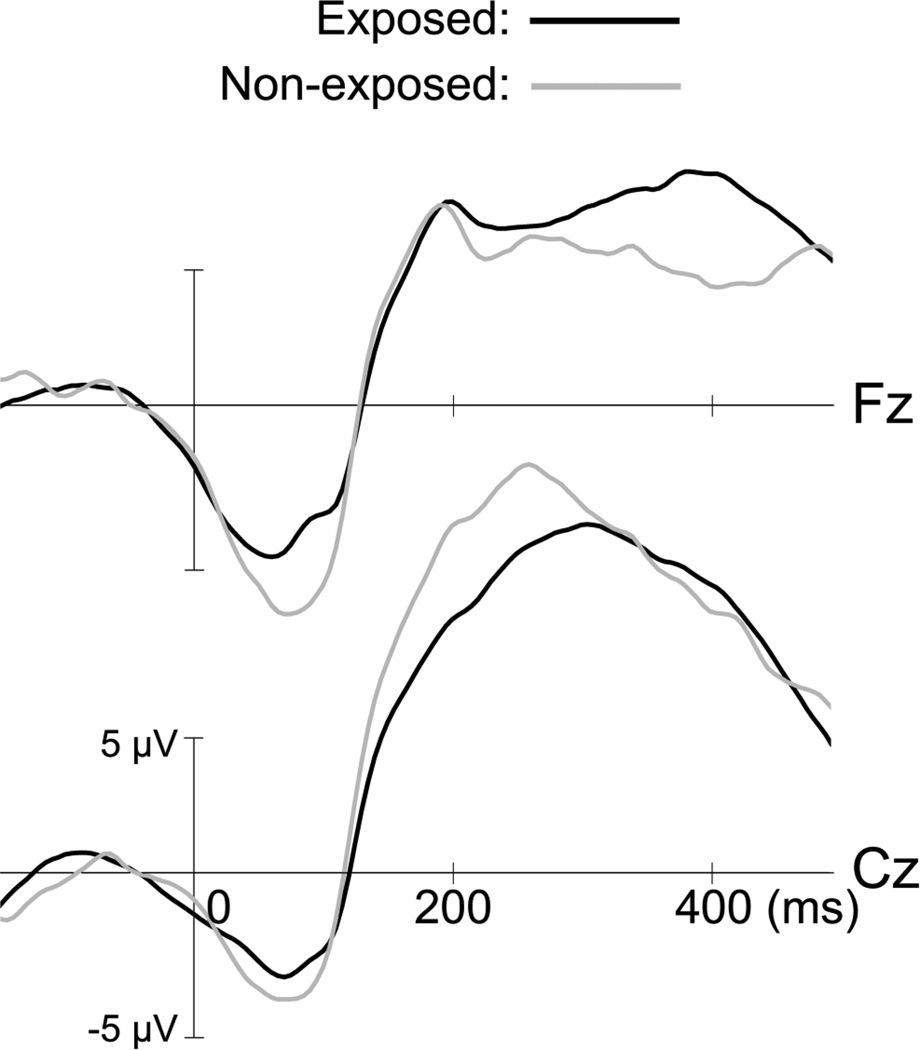
Results for the response-locked ERPs according to prenatal exposure to tobacco smoke are presented on Table 4. There is no significant effect of PCSE on these ERPs (ps > 0.20; see Fig. 2). No additional effect was observed when replacing the PCSE variable by the three-group variable (data not shown).
Table 4.
Adjusted mean (± SD) response-locked ERP parameters by prenatal cigarette smoking exposure
| Not exposed (n = 32) | Exposed (n = 117) | F-ratio for smoking | |
|---|---|---|---|
| ERN amplitude (µV) | −4.3 ± 6.1 | −4.2 ± 6.0 | 0.01 (n.s.) |
| Pe amplitude (µV) | 8.8 ± 6.7 | 7.6 ± 8.1 | 0.56 (n.s.) |
F-ratio for ANCOVAs adjusting for child age and sex, current PCB-153 concentrations, birth weight, education of the primary caregiver, maternal non-verbal reasoning abilities, cord Pb, and cord Hg concentrations. n.s.: non significant (p > 0.15).
Figure 2.
Grand average for response-locked ERPs comparing participants with PCSE (n = 126) to non-exposed participants (n = 32) for incorrect No-go trials. Erroneous responses elicit an early frontal component of negative voltage (ERN) followed by a large positive wave maximal at the vertex (Pe).
All analyses were re-run with maternal binge alcohol drinking and marijuana consumption during pregnancy added to the list of covariates. The results remained essentially unchanged, with the exception of the main effect of PCSE on N2 amplitude in the RM-MANOVA models, which fell short of statistical significance (p = 0.10). Nevertheless, the interaction effect between PCSE and task condition on N2 amplitude, and the effect of PCSE on No-go N2 amplitude in the ANCOVA model both remained statistically significant, further confirming the link between PCSE and the N2 amplitude response to task condition. Results were also unchanged when adding TRF symptom scores or DBD-based diagnoses as additional covariates (not shown), suggesting that the group differences in ERP components are not attributable to differences in behavioral problems or psychiatric disorders. Analyses were also re-run adding postnatal environmental exposure to tobacco smoke variable as a covariate (not shown). Again, the results remained virtually unchanged, suggesting that the observed differences between groups were not attributable to 11-year environmental exposure to tobacco smoke.
3.4 Correlations between ERPs and behavioral indicators
Pearson correlations between ERP parameters associated with PCSE, and child behavior, assessed from behavioral performance on the Go/No-go task and with questionnaires completed by the classroom teacher, were also examined (Table 5). Smaller P3 amplitudes, especially when elicited by No-go trials, are associated with poorer recognition of Go trials, and with a higher prevalence of classroom behaviors associated with ADHD–Hyperactive/impulsive type. There was only one significant correlation with N2 amplitude, which indicated that larger amplitude in the Go condition is associated with higher response accuracy on No-go trials.
Table 5.
Pearson correlations between Go/No-go ERP amplitudes and behavioral indicators
| N2 amplitude |
P3 amplitude |
||||
|---|---|---|---|---|---|
| Behavioral outcomes | n | Go | No-go | Go | No-go |
| Go/No-go performance | |||||
| Mean hit RT | 185 | 0.01 | 0.10 | −0.15* | −0.12 |
| Correct Go trials | 185 | −0.04 | −0.10 | 0.20** | 0.28** |
| Correct No-go trials | 185 | −0.16* | −0.10 | 0.12 | 0.01 |
| TRF Symptom score (log) | |||||
| Internalizing problems | 181 | 0.08 | 0.07 | −0.01 | −0.07 |
| Externalizing problems | 181 | −0.02 | −0.04 | −0.11 | −0.18* |
| Attention problems | 181 | 0.12 | 0.09 | −0.11 | −0.20** |
| DBD-based diagnoses | |||||
| ADHD – Inattention type | 183 | 0.08 | 0.04 | −0.07 | −0.03 |
| ADHD – Hyperactive/impulsive type | 183 | 0.05 | 0.03 | −0.24** | −0.27** |
Note. Negative (−) correlations with N2 amplitude indicate greater (more negative) amplitude.
p < 0.05
p < 0.01.
4. Discussion
This study examined the effects of PCSE on the neurophysiological correlates of response inhibition using ERPs recorded during a visual Go/No-go task. The study participants were school-aged children in whom PCSE has recently been linked to increased behavior problems, as assessed by their classroom teachers (Desrosiers et al., 2013). PCSE was associated with an amplitude reduction in both the N2 and the P3 components elicited by the No-go trials, even though it had no significant effect on behavioral performance parameters. The absence of group differences in behavioral performance on this task provides support for the finding that the smaller N2 and P3 amplitudes in children with PCSE were not attributable to reduced motivation or capacity to perform the task. Differences in markers of brain activity, but not in behavioral performance, have also been reported in other studies with other populations and/or using other functional brain imaging techniques (Burden et al., 2009; Ma et al., 2012; Wiersema et al., 2009), and suggest that children with PCSE were able to compensate for their response inhibition deficits in this simple task. However, it seems likely that impaired behavioral inhibition would have been observed in the context of a more challenging task. There were no group differences in the post-response error-related ERPs, suggesting that PCSE is more specifically associated with an impairment in response inhibition, rather than with a cognitive deficit that generalizes across multiple domains.
ERPs elicited in the No-go condition, in comparison to the Go condition, were more sensitive to the effects of PCSE, and this was especially marked for the N2 component. By contrast to the non-exposed participants, the exposed children did not exhibit the expected increase in N2 amplitude following No-go compared to Go trials. A similar pattern of results has previously been observed in adult ADHD patients assessed on an auditory Go/No-go task (Fisher et al., 2011). Reduced No-go N2 and P3 amplitudes have also been reported in children with ADHD (Burden et al., 2010; Johnstone et al., 2009; Overtoom et al., 1998). Furthermore, we found an inverse correlation between P3 amplitude elicited by No-go trials, and behavioral indicators of externalizing problems and hyperactivity/impulsivity at school. The present ERP findings thus appear to provide a key to understanding the mechanisms underlying the relation between PCSE and ADHD. However, that the No-go ERP components, in comparison to the Go ERP components, were especially sensitive to the effects of PCSE does not imply that these effects are specific to PCSE. As we have shown in a previous paper (Boucher et al., 2012a), other factors also associated with ADHD and externalized behaviors may interfere similarly and independently with these ERP components.
Previous structural imaging studies have found developmental anomalies in anterior regions of the cerebral cortex, such as the PFC, in children and adolescents exposed to cigarette smoke during the prenatal period (El Marroun et al., 2014; Jacobsen et al., 2007; Liu et al., 2011; Toro et al., 2008). Enhanced vulnerability of the PFC to the effects of PCSE would be consistent with findings of aggressive and impulsive behavior, hyperactivity, inattention, and impairments in executive function in children with PCSE (Batstra et al., 2003; Braun et al., 2008; Brook et al., 2006; Cornelius et al., 2011, 2012; Piper and Corbett, 2012) and with the specific effect of nicotine exposure on nicotinic cholinergic receptor expression in this region of the brain (Chen et al., 2005). The No-go N2 component has been proposed to reflect conflict processes taking place in or near the anterior cingulate cortex (Bekker et al., 2005; Donkers and van Boxtel., 2004; Jonkman et al., 2007; Nieuwenhuis et al., 2003). The No-go P3 component indexes later stages of information processing, occurring when attentional resources are allocated for efficiently inhibiting a response. Although the generators of the P3 signal in the brain are less well characterized (and probably more broadly distributed) than those of the N2 component, they are also thought to involve regions of the PFC (Davis et al., 2003; Smith et al., 2013). Thus, it seems plausible that altered PFC development might account for the findings obtained in our study, although functional neuroimaging methods with better spatial resolution would be necessary to verify this hypothesis. To our knowledge, only one study to date used functional magnetic resonance imaging to study the brain activity during response inhibition in adolescents prenatally exposed to tobacco (Bennett et al., 2009). Compared to 11 unexposed controls, the 7 exposed children in this study showed greater activation in widespread regions of the brain, including the left frontal, but also right occipital and bilateral temporal and parietal regions. However, this study was limited by the very small size of its sample.
Among the strengths of this study are the novel and innovative approach for studying the effects of PCSE on response inhibition, i.e. ERPs, the large number of children for which we conducted neurophysiological assessments, as well the statistical control for several potentially confounding factors, including maternal alcohol and drug use during pregnancy and exposure to neurotoxic environmental chemicals. The observed effects were robust as they remained significant after the inclusion of these covariables in the statistical models. Among the limitations is the absence of information on parental psychopathology. Since cigarette smoking is more prevalent in individuals with conduct disorder and/or ADHD and since these pathologies are highly heritable, we cannot exclude the possibility that the observed effects are at least partly attributable to genetic factors (Agrawal et al., 2010; Kollins et al., 2005; Langley et al., 2012). Another limitation is the small proportion of non-exposed children within the total sample. Although recall of smoking vs. non-smoking during pregnancy was excellent in general, the reliability data indicate that a certain proportion of the cases may have been misclassified. When the exposed children were divided into low- and high-exposed, no difference between these groups were observed on the ERP variables. This surprising finding may be related to the poorer reliability of retrospective quantification of smoking during pregnancy when provided a full decade after delivery and thus provides further support for the use of the yes/no variable as a valid index of exposure in this case. Future studies documenting PCSE prospectively are, therefore, warranted to permit assessment of the dose-response relationship between maternal cigarette use during pregnancy and ERP components recorded during response-inhibition paradigms.
5. Conclusion
This study is, to our knowledge, the first to provide neurophysiological evidence of impaired response inhibition in school-age children exposed to cigarette smoking in utero. Children with PCSE exhibited amplitude reductions in ERP N2 and P3 components elicited by No-go stimuli when compared to non-exposed children, suggesting impairments in conflict processing and allocation of attentional resources for inhibiting a prepotent response. Impairments in these processes, which are thought to involve the PFC, may mediate the previously reported increased risks for externalizing behavior problems and ADHD among these children. This study suggests that ERPs constitute a promising and sensitive measure in the assessment of the neurotoxic effects of PCSE, and that future prospective studies on PCSE toxicity should consider integrating such objective neurophysiological measures in their assessment protocol.
Supplementary Material
Highlights.
We used ERPs to study the effects of prenatal cigarette smoke exposure (PCSE).
Children performed a Go/No-go task to elicit the N2, P3, ERN, and Pe components.
PCSE was associated with decreased No-go N2 and No-go P3 amplitude.
Effects remained statistically significant after control for confounders.
No effect was found on the error-related ERN and Pe components.
Acknowledgements
We thank the Nunavik children and parents for their participation; Charles Nelson, Ph.D., and Alissa Westerlund of Children’s Hospital Boston, for their contributions to the development and implementation of the ERP task; the professional staff from the health centers and nursing stations of Puvirnituq, Kuujjuaq, and Inukjuak for their assistance; the Nunavik Nutrition and Health Committee and Municipal Councils for their support of this research; and Renee Sun, Line Roy, Brenda Tuttle, Alacie Pov, Johanne Varin, Jocelyne Gagnon, and Neil Dodge for their contributions to data collection and analysis.
Sources of funding: NIH/NIEHS R01-ES007902 (JLJ); Northern Contaminants Program, Indian and Northern Affairs Canada (GM); NIH/NIAAA F32-AA14730 (MB); the Joseph Young, Sr., Fund from the State of Michigan (SWJ), and postdoctoral grants from the Canadian Institutes for Health Research (OB).
List of abbreviations
- ADHD
Attention deficit hyperactivity disorder
- DBD
Disruptive Behavior Disorders Rating Scales
- ECCDS
Environmental Contaminants and Child Development Study
- ERN
Error-related negativity
- Hg
Mercury
- Pb
Lead
- PCB
Polychlorinated biphenyls
- Pe
Error positivity
- PCSE
Prenatal cigarette smoke exposure
- PFC
Prefrontal cortex
- TRF
Teacher Report Form
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: The authors declare that there are no conflicts of interest.
Examination of the topographic distribution of the Go/No-go response across the six midline electrode sites (Fz, FCz, Cz, Pz, POz, and Oz) among the unexposed children showed that the interaction effect between the Go and No-go conditions on ERP amplitude was maximal at Fz for N2, and at Pz for P3.
Relatively low numbers of epochs (≥ 12 for stimulus-locked components, and ≥ 8 for response-locked components) were required to limit the exclusion of subjects. To make sure that this liberal criterion did account for our findings, all significant results were re-examined using a stricter and more conventional criterion of ≥ 20 trials. Twenty-five additional participants had to be excluded. Despite the change in sample size, all results remained statistically significant in these new analyses involving 161 participants (31 unexposed, and 130 exposed; results not shown).
References
- Achenbach TM. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. Burlington: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- Agrawal A, Scherrer JF, Grant JD, Sartor CE, Pergadia ML, Duncan AE, et al. The effects of maternal smoking during pregnancy on offspring outcomes. Prev Med. 2010;50:13–18. doi: 10.1016/j.ypmed.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baler RD, Volkow ND, Fowler JS, Benveniste H. Is fetal brain monoamine oxidase inhibition the missing link between maternal smoking and conduct disorders? J Psychiatry Neurosci. 2008;33:187–195. [PMC free article] [PubMed] [Google Scholar]
- Batstra L, Hadders-Algra M, Neeleman J. Effect of antenatal exposure to maternal smoking on behavioural problems and academic achievement in childhood: prospective evidence from a Dutch birth cohort. Early Hum Dev. 2003;75:21–33. doi: 10.1016/j.earlhumdev.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Bekker EM, Kenemans JL, Verbaten MN. Source analysis of the N2 in a cued Go/NoGo task. Brain Res Cogn Brain Res. 2005;22:221–231. doi: 10.1016/j.cogbrainres.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Bennett DS, Mohamed FB, Carmody DP, Bendersky M, Patel S, Khorrami M, et al. Response inhibition among early adolescents prenatally exposed to tobacco: an fMRI study. Neurotoxicol Teratol. 2009;31:283–290. doi: 10.1016/j.ntt.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood-Siegfried J, Rende EK. The long-term effects of prenatal nicotine exposure on neurologic development. J Midwifery Womens Health. 2010;55:143–152. doi: 10.1016/j.jmwh.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher O, Muckle G, Jacobson JL, Carter RC, Kaplan-Estrin M, Ayotte P, et al. Domain-specific effects of prenatal exposure to PCBs, mercury, and lead on infant cognition: results from the Environmental Contaminants and Child Development Study in Nunavik. Environ Health Perspect. 2014;122:310–316. doi: 10.1289/ehp.1206323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher O, Jacobson SW, Plusquellec P, Dewailly E, Ayotte P, Forget-Dubois N, et al. Prenatal methylmercury, postnatal lead exposure, and evidence of attention deficit/hyperactivity disorder among Inuit children in Arctic Québec. Environ Health Perspect. 2012b;120:1456–1461. doi: 10.1289/ehp.1204976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher O, Burden MJ, Muckle G, Saint-Amour D, Ayotte P, Dewailly E, et al. Response inhibition and error monitoring during a visual go/no-go task in Inuit children exposed to lead, polychlorinated biphenyls, and methylmercury. Environ Health Perspect. 2012a;120:608–615. doi: 10.1289/ehp.1103828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Froehlich TE, Daniels JL, Dietrich KN, Hornung R, et al. Association of environmental toxicants and conduct disorder in U.S. children: NHANES 2001–2004. Environ Health Perspect. 2008;116:956–962. doi: 10.1289/ehp.11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook DW, Zhang C, Rosenberg G, Brook JS. Maternal cigarette smoking during pregnancy and child aggressive behavior. Am J Addict. 2006;15:450–456. doi: 10.1080/10550490600998559. [DOI] [PubMed] [Google Scholar]
- Burden MJ, Andrew C, Saint-Amour D, Meintjes EM, Molteno CD, Hoyme HE, et al. The effects of fetal alcohol syndrome on response execution and inhibition: an event-related potential study. Alcohol Clin Exp Res. 2009;33:1994–2004. doi: 10.1111/j.1530-0277.2009.01038.x. [DOI] [PubMed] [Google Scholar]
- Burden MJ, Jacobson JL, Westerlund A, Lundahl LH, Morrison A, Dodge NC, et al. An event-related potential study of response inhibition in ADHD with and without prenatal alcohol exposure. Alcohol Clin Exp Res. 2010;34:617–627. doi: 10.1111/j.1530-0277.2009.01130.x. [DOI] [PubMed] [Google Scholar]
- Burden MJ, Westerlund A, Muckle G, Dodge N, Dewailly E, Nelson CA. The effects of maternal binge drinking during pregnancy on neural correlates of response inhibition and memory in childhood. Alcohol Clin Exp Res. 2011;35:69–82. doi: 10.1111/j.1530-0277.2010.01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, et al. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. J Cogn Neurosci. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Chen H, Parker SL, Matta SG, Sharp BM. Gestational nicotine exposure reduces nicotinic cholinergic receptor (nAChR) expression in dopaminergic brain regions of adolescent rats. Eur J Neurosci. 2005;22:380–388. doi: 10.1111/j.1460-9568.2005.04229.x. [DOI] [PubMed] [Google Scholar]
- Cornelius MD, De Genna NM, Leech SL, Willford JA, Goldschmidt L, Day NL. Effects of prenatal cigarette smoke exposure on neurobehavioral outcomes in 10-year-old children of adolescent mothers. Neurotoxicol Teratol. 2011;33:137–144. doi: 10.1016/j.ntt.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius MD, Goldschmidt L, De Genna LM, Larkby C. Long-term effects of prenatal cigarette smoke exposure on behavior dysregulation among 14-year-old offspring of teenage mothers. Matern Child Health J. 2012;16:694–705. doi: 10.1007/s10995-011-0766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallaire F, Dewailly E, Muckle G, Ayotte P. Time trends of persistent organic pollutants and heavy metals in umbilical cord blood of Inuit infants born in Nunavik (Québec, Canada) between 1994 and 2001. Environ Health Perspect. 2003;111:1660–1664. doi: 10.1289/ehp.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallaire R, Dewailly É, Ayotte P, Jacobson SW, Jacobson JL, Muckle G. Growth in Inuit children exposed to polychlorinated biphenyls and lead during fetal development and childhood. In preparation. doi: 10.1016/j.envres.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Bruce J, Snyder K, Nelson CA. The x-trials: neural correlates of an inhibitory control task in children and adults. J Cogn Neurosci. 2003;15:432–443. doi: 10.1162/089892903321593144. [DOI] [PubMed] [Google Scholar]
- Desrosiers C, Boucher O, Forget-Dubois N, Dewailly E, Ayotte P, Jacobson SW, et al. Associations between prenatal cigarette smoke exposure and externalized behaviors at school age among Inuit children exposed to environmental contaminants. Neurotoxico Teratol. 2013;39:84–90. doi: 10.1016/j.ntt.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkers FCL, van Boxtel GJM. The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain Cogn. 2004;56:165–176. doi: 10.1016/j.bandc.2004.04.005. 2004. [DOI] [PubMed] [Google Scholar]
- El Marroun H, Schmidt MN, Franken IH, Jaddoe VW, Hofman A, van der Lugt A, et al. Prenatal tobacco exposure and brain morphology: a prospective study in young children. Neuropsychopharmacology. 2014;39:792–800. doi: 10.1038/npp.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein M. Inhibition, conflict and the Nogo-N2. Clin Neurophysiol. 2006;117:1638–1640. doi: 10.1016/j.clinph.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Fisher T, Aharon-Peretz J, Pratt H. Dis-regulation of response inhibition in adult Attention Deficit Hyperactivity Disorder (ADHD): an ERP study. Clin Neurophysiol. 2011;112:2390–2399. doi: 10.1016/j.clinph.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Gold AB, Keller AB, Perry DC. Prenatal exposure of rats to nicotine causes persistent alterations of nicotinic cholinergic receptors. Brain Res. 2009;1250:88–100. doi: 10.1016/j.brainres.2008.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E. A new method for off-line removal of ocular artefact. Electroenceph Clin Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. Yale J Sociol. 2011;8:21–51. [Google Scholar]
- Hughes G, Yeung N. Dissociable correlates of response conflict and error awareness in error-related brain activity. Neuropsychologia. 2011;49:405–415. doi: 10.1016/j.neuropsychologia.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbregts SC, Warren AJ, de Sonneville LM, Swaab-Barneveld H. Hot and cool forms of inhibitory control and externalizing behavior in children of mothers who smoked during pregnancy: an exploratory study. J Abnorm Child Psychol. 2008;36:323–333. doi: 10.1007/s10802-007-9180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Picciotto MR, Heath CJ, Frost SJ, Tsou KA, Dwan RA, et al. Prenatal and adolescent exposure to tobacco smoke modulates the development of white matter microstructure. J Neurosci. 2007;27:13491–13498. doi: 10.1523/JNEUROSCI.2402-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Muckle G, Kaplan-Estrin M, Ayotte P, Dewailly E. Beneficial effects of a polyunsaturated fatty acid on infant development : evidence from the inuit of arctic Quebec. J Pediatr. 2008;152:356–364. doi: 10.1016/j.jpeds.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Jasper HA. The ten-twenty system of the international federation. Electroencephalogr Clin Neurophysiol. 1958;10:371–375. [Google Scholar]
- Johnstone SJ, Barry RJ, Markovska V, Dimoska A, Clarke AR. Response inhibition and interference control in children with AD/HD: a visual ERP investigation. Int J Psychophysiol. 2009;72:145–153. doi: 10.1016/j.ijpsycho.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Jonkman LM, Sniedt FL, Kemner C. Source localization of the Nogo-N2: a developmental study. Clin Neurophysiol. 2007;118:1069–1077. doi: 10.1016/j.clinph.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Julvez J, Ribas-Fitó N, Torrent M, Forns M, Garcia-Esteban R, Sunyer J. Maternal smoking habits and cognitive development of children at age 4 years in a population-based birth cohort. Int J Epidemiol. 2007;36:825–832. doi: 10.1093/ije/dym107. [DOI] [PubMed] [Google Scholar]
- Kollins SH, McClernon FJ, Fuemmeler BF. Association between smoking and attention-deficit/hyperactivity disorder symptoms in a population-based sample of young adults. Arch Gen Psychiatry. 2005;62:1142–1147. doi: 10.1001/archpsyc.62.10.1142. [DOI] [PubMed] [Google Scholar]
- Kotimaa AJ, Moilanen I, Taanila A, Ebeling H, Smalley SL, McGough JJ, et al. Maternal smoking and hyperactivity in 8-year-old children. J Am Acad Child Adolesc Psychiatry. 2003;42:826–833. doi: 10.1097/01.CHI.0000046866.56865.A2. [DOI] [PubMed] [Google Scholar]
- Langley K, Heron J, Smith GD, Thapar A. Maternal and paternal smoking during pregnancy and risk of ADHD symptoms in offspring: testing for intrauterine effects. Am J Epidemiol. 2012;176:261–268. doi: 10.1093/aje/kwr510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Cohen RA, Gongvatana A, Sheinkopf SJ, Lester BM. Impact of prenatal exposure to cocaine and tobacco on diffusion tensor imaging and sensation seeking in adolescents. J Pediatr. 2011;159:771–775. doi: 10.1016/j.jpeds.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Lei D, Jin X, Du X, Jiang F, Li F, et al. Compensatory brian activation in children with attention deficit/hyperactivity disorder during a simplified Go/No-go task. J Neural Transm. 2012;119:613–619. doi: 10.1007/s00702-011-0744-0. [DOI] [PubMed] [Google Scholar]
- Muckle G, Ayotte P, Dewailly E, Jacobson SW, Jacobson JL. Prenatal exposure of the northern Québec Inuit infants to environmental contaminants. Environ Health Perspect. 2001;109:1291–1299. doi: 10.1289/ehp.011091291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muneoka K, Ogawa T, Kamei K, Muraoka S, Tomiyoshi R, Mimura Y, et al. Prenatal nicotine exposure affects the development of the central serotonergic system as well as the dopaminergic system in rat offspring: involvement of route of drug administrations. Brain Res Dev Brain Res. 1997;102:117–126. doi: 10.1016/s0165-3806(97)00092-8. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, van den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: effects of response conflict and trial type frequency. Cogn Affect Behav Neurosci. 2003;3:17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- Obel C, Linnet KM, Henriksen TB, Rodriguez A, Järvelin MR, Kotimaa A, et al. Smoking during pregnancy and hyperactivity-inattention in the offspring - comparing results from three Nordic cohorts. Int J Epidemiol. 2009;38:698–705. doi: 10.1093/ije/dym290. [DOI] [PubMed] [Google Scholar]
- Overbeek TJM, Nieuwenhuis S, Ridderinkhof DR. Dissociable components of error processing: on the functional significance of the Pe vis-à-vis the ERN/Ne. J Psychophysiol. 2005;19:319–329. [Google Scholar]
- Overtoom CC, Verbaten MN, Kemner C, Kenemans JL, van Engeland H, Buitelaar JK, et al. Associations between event-related potentials and measures of attention and inhibition in the Continuous Performance Task in children with ADHD and normal controls. J Am Acad Child Adolesc Psychiatry. 1998;73:977–985. doi: 10.1097/00004583-199809000-00018. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Jr, Gnagy EM, Greenslade KE, Milich R. Teacher ratings of DMS-III-R symptoms for the disruptive behavior disorders. J Am Acad Child Adolesc Psychiatry. 1992;31:210–218. doi: 10.1097/00004583-199203000-00006. [DOI] [PubMed] [Google Scholar]
- Piper BJ, Corbett SM. Executive function profile in the offspring of women that smoked during pregnancy. Nicotine Tob Res. 2012;14:191–199. doi: 10.1093/ntr/ntr181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall WM, Smith JL. Conflict and inhibition in the cued-Go/NoGo task. Clin Neurophysiol. 2011;122:2400–2407. doi: 10.1016/j.clinph.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Raven JC, Court JH, Raven J. Manual for Raven’s progressive matrices and vocabulary scales: standard progressive matrices. Oxford: Psychologists; 1992. [Google Scholar]
- Robinson M, McLean NJ, Oddy WH, Mattes E, Bulsara M, Li J, et al. Smoking cessation during pregnancy and the risk of child behavoural problems: a longitudinal prospective cohort study. J Epideimol Community Health. 2010;64:622–629. doi: 10.1136/jech.2009.088658. [DOI] [PubMed] [Google Scholar]
- Rückinger S, Rzehak P, Chen CM, Sausenthaler S, Koletzko S, Bauer CP, et al. Prenatal and postnatal tobacco exposure and behavioral problems in 10-year-old children: results from the GINI-plus prospective birth cohort study. Environ Health Perspect. 2010;118:150–154. doi: 10.1289/ehp.0901209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slikker W, Jr, Xu ZA, Levin ED, Slotkin TA. Mode of action: disruption of brain cell replication, second messenger, and neurotransmitter systems during development leading to cognitive dysfunction - developmental neurotoxicity of nicotine. Crit Rev Toxicol. 2005;35:703–711. doi: 10.1080/10408440591007421. [DOI] [PubMed] [Google Scholar]
- Smith JL, Jamadar S, Provost AL, Michie PT. Motor and non-motor inhibition in the Go/NoGo task: an ERP and fMRI study. Int J Psychophysiol. 2013;87:244–253. doi: 10.1016/j.ijpsycho.2012.07.185. [DOI] [PubMed] [Google Scholar]
- Smith JL, Johnstone SJ, Barry RJ. Response priming in the Go/NoGo task: the N2 reflects neither inhibition nor conflict. Clin Neurophysiol. 2007;118:343–355. doi: 10.1016/j.clinph.2006.09.027. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 5th ed. Boston: Pearson Education Inc; 2007. [Google Scholar]
- Toro R, Leonard G, Lerner JV, Lerner RM, Perron M, Pike GB, et al. Prenatal exposure to maternal cigarette smoking and the adolescent cerebral cortex. Neuropsychopharmacology. 2008;33:1019–1027. doi: 10.1038/sj.npp.1301484. [DOI] [PubMed] [Google Scholar]
- Wakschloag LS, Kistner EO, Pine DS, Biesecker G, Pickett KE, Skol AD, et al. Interaction of prenatal exposure to cigarettes and MAOA genotype in pathways to youth antisocial behavior. Mol Psychoatry. 2010;15:928–937. doi: 10.1038/mp.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scales for Children. 4th ed. San Antonio: Psychological Corporation; 2003. [Google Scholar]
- Wiersema JR, van der Meere JJ, Roeyers H. ERP correlates of error monitoring in adult ADHD. J Neural Transm. 2009;116:371–379. doi: 10.1007/s00702-008-0165-x. [DOI] [PubMed] [Google Scholar]
- Winer BJ. Statistical principles in experimental design. 2nd ed. New York: McGraw-Hill; 1971. [Google Scholar]
- Xu Z, Seidler FJ, Ali SF, Slikker W, Jr, Slotkin TA. Fetal and adolescent nicotine administration: effects on CNS serotonergic systems. Brain Res. 2001;914:166–178. doi: 10.1016/s0006-8993(01)02797-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.