Abstract
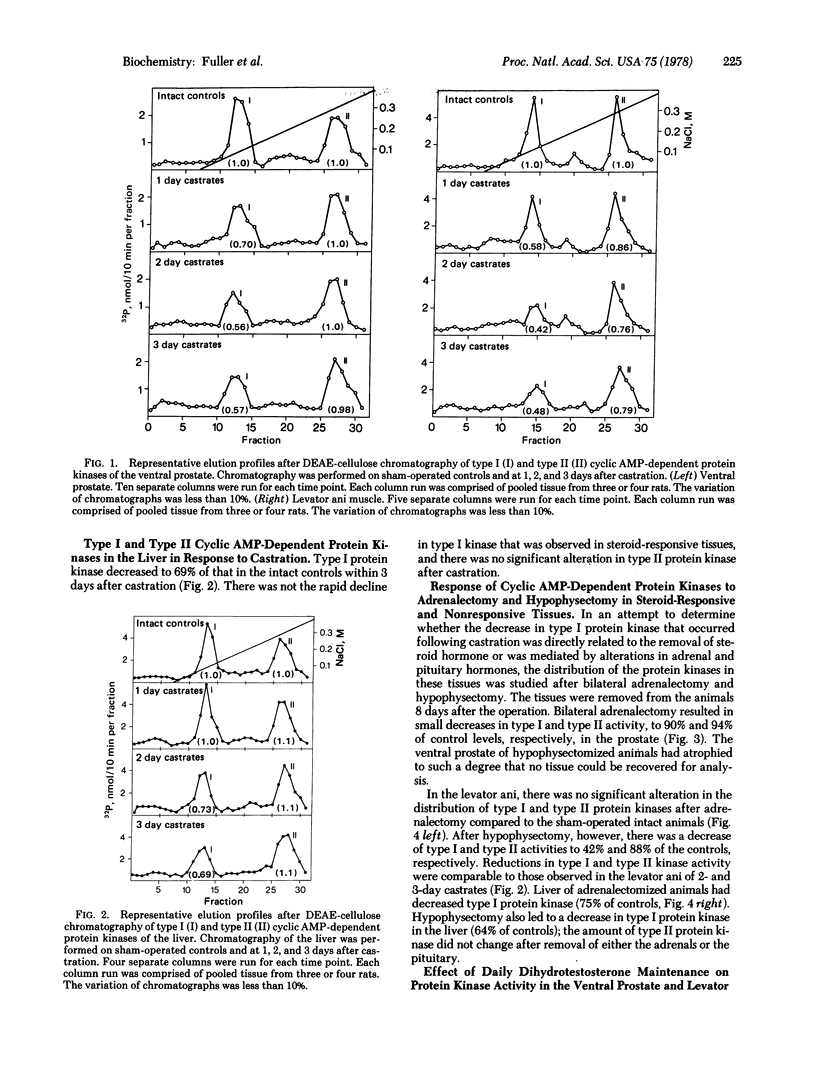
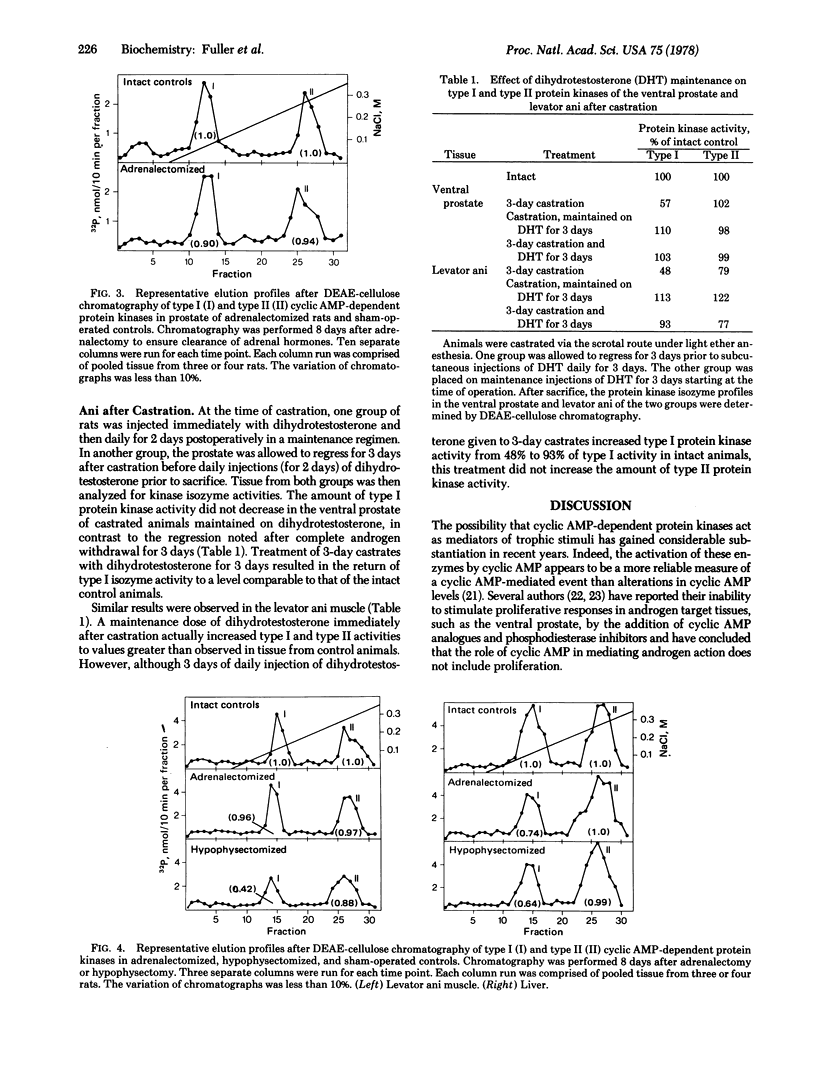
The total amounts of type I and type II cytoplasmic cyclic AMP-dependent protein kinase activities were measured in various tissues of intact rats and rats subjected to castration, hypophysectomy, or adrenalectomy. After castration, the total amount of type I activity decreased rapidly in classifically steroid-responsive tissues such as the ventral prostate and levator ani muscle and less rapidly in the liver. After hypophysectomy and adrenalectomy, type I activity in the liver decreased to the same extent as after castration. Type I activity could be maintained in the ventral prostate and levator ani muscle at control levels by the daily injection of dihydrotestosterone. Furthermore, after post-castration regression of the prostate for 3 days, three daily subcutaneous injections of dihydrotestosterone resulted in a complete restoration of type I activity toe the intact level. The amount of type II activity was not altered by any of the experimental ablations. This study provides evidence linking steroid action to the ability of steroid-responsive tissues to maintain a substantial activity of type I cyclic AMP-dependent protein kinase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Byus C. V., Chubb J. M., Huxtable R. J., Russell D. H. Increase in type I adenosine 3',5'-monophosphate-dependent protein kinase during isoproterenol-induced cardiac hypertrophy. Biochem Biophys Res Commun. 1976 Dec 6;73(3):694–702. doi: 10.1016/0006-291x(76)90866-4. [DOI] [PubMed] [Google Scholar]
- Byus C. V., Klimpel G. R., Lucas D. O., Russell D. H. Type I and type II cyclic AMP-dependent protein kinase as opposite effectors of lymphocyte mitogenesis. Nature. 1977 Jul 7;268(5615):63–64. doi: 10.1038/268063a0. [DOI] [PubMed] [Google Scholar]
- Colby H. D., Kitay J. I. Effects of gonadal hormones on adrenocortical secretion of 5 -reduced metabolites of corticosterone in the rat. Endocrinology. 1972 Dec;91(6):1523–1527. doi: 10.1210/endo-91-6-1523. [DOI] [PubMed] [Google Scholar]
- Corbin J. D., Keely S. L., Park C. R. The distribution and dissociation of cyclic adenosine 3':5'-monophosphate-dependent protein kinases in adipose, cardiac, and other tissues. J Biol Chem. 1975 Jan 10;250(1):218–225. [PubMed] [Google Scholar]
- Corbin J. D., Keely S. L., Soderling T. R., Park C. R. Hormonal regulation of adenosine 3',5'-monophosphate-dependent protein kinase. Adv Cyclic Nucleotide Res. 1975;5:265–279. [PubMed] [Google Scholar]
- Costa M., Gerner E. W., Russell D. H. Cell cycle-specific activity of type I and type II cyclic adenosine 3':5'-monophosphate-dependent protein kinases in Chinese hamster ovary cells. J Biol Chem. 1976 Jun 10;251(11):3313–3319. [PubMed] [Google Scholar]
- Costa M., Manen C. A., Russell D. H. In vivo activation of cAMP-dependent protein kinase by aminophylline and 1-methyl, 3-isobutylxanthine. Biochem Biophys Res Commun. 1975 Jul 8;65(1):75–81. doi: 10.1016/s0006-291x(75)80063-5. [DOI] [PubMed] [Google Scholar]
- Coyne M. D., Kitay J. I. Effect of orchiectomy on pituitary secretion of ACTH. Endocrinology. 1971 Oct;89(4):1024–1028. doi: 10.1210/endo-89-4-1024. [DOI] [PubMed] [Google Scholar]
- Craven S., Lesser B., Bruchovsky N. Evidence that adenosine 3', 5'-cyclic monophosphate is not involved in the growth response of prostate to androgens. Endocrinology. 1974 Oct;95(4):1177–1180. doi: 10.1210/endo-95-4-1177. [DOI] [PubMed] [Google Scholar]
- Gharrett A. J., Malkinson A. M., Sheppard J. R. Cyclic AMP-dependent protein kinases from normal and SV40-transformed 3T3 cells. Nature. 1976 Dec 16;264(5587):673–675. doi: 10.1038/264673a0. [DOI] [PubMed] [Google Scholar]
- Jungmann R. A., Russell D. H. Cyclic AMP, cyclic AMP-dependent protein kinase, and the regulation of gene expression. Life Sci. 1977 Jun 1;20(11):1787–1797. doi: 10.1016/0024-3205(77)90213-2. [DOI] [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. IV. Widespread occurrence of adenosine 3',5'-monophosphate-dependent protein kinase in various tissues and phyla of the animal kingdom. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1349–1355. doi: 10.1073/pnas.64.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P. C., Radloff D., Schweppe J. S., Jungmann R. A. Testicular protein kinases. Characterization of multiple forms and ontogeny. J Biol Chem. 1976 Feb 25;251(4):914–921. [PubMed] [Google Scholar]
- Liao S., Fang S. Receptor-proteims for androgens and the mode of action of androgens on gene transcription in ventral prostate. Vitam Horm. 1969;27:17–90. doi: 10.1016/s0083-6729(08)61124-3. [DOI] [PubMed] [Google Scholar]
- Mangan F. R., Pegg A. E., Mainwaring I. P. A reappraisal of the effects of adenosine 3':5'-cyclic monophosphate on the function and morphology of the rat prostate gland. Biochem J. 1973 May;134(1):129–142. doi: 10.1042/bj1340129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill J. P., Schröder C. H., Riddle J. C., Hsie A. W. The cell cycle specificity of the morphological conversion of chinese hamster ovary cells by N6, O2'-dibutyryl cyclic adenosine 3',5'-phosphate. Exp Cell Res. 1976 Jan;97:213–217. doi: 10.1016/0014-4827(76)90670-4. [DOI] [PubMed] [Google Scholar]
- Porter K. R., Puck T. T., Hsie A. W., Kelley D. An electron microscopy study of the effects on dibutyryl cyclic AMP on Chinese hamster ovary cells. Cell. 1974 Jul;2(3):145–162. doi: 10.1016/0092-8674(74)90089-0. [DOI] [PubMed] [Google Scholar]
- Rosen O. M., Erlichman J., Rubin C. S. Molecular structure and characterization of bovine heart protein kinase. Adv Cyclic Nucleotide Res. 1975;5:253–263. [PubMed] [Google Scholar]
- Russell D. H., Byus C. V., Manen C. A. Proposed model of major sequential biochemical events of a trophic response. Life Sci. 1976 Nov 1;19(9):1297–1305. [PubMed] [Google Scholar]
- Wilson M. J., Ahmed K. Enzymic characteristics and effects of testosterone treatment on nucleolar and chromatin-associated histone phosphokinase activity of rat ventral prostate. Exp Cell Res. 1977 Apr;106(1):151–157. doi: 10.1016/0014-4827(77)90251-8. [DOI] [PubMed] [Google Scholar]


