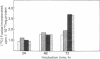Abstract
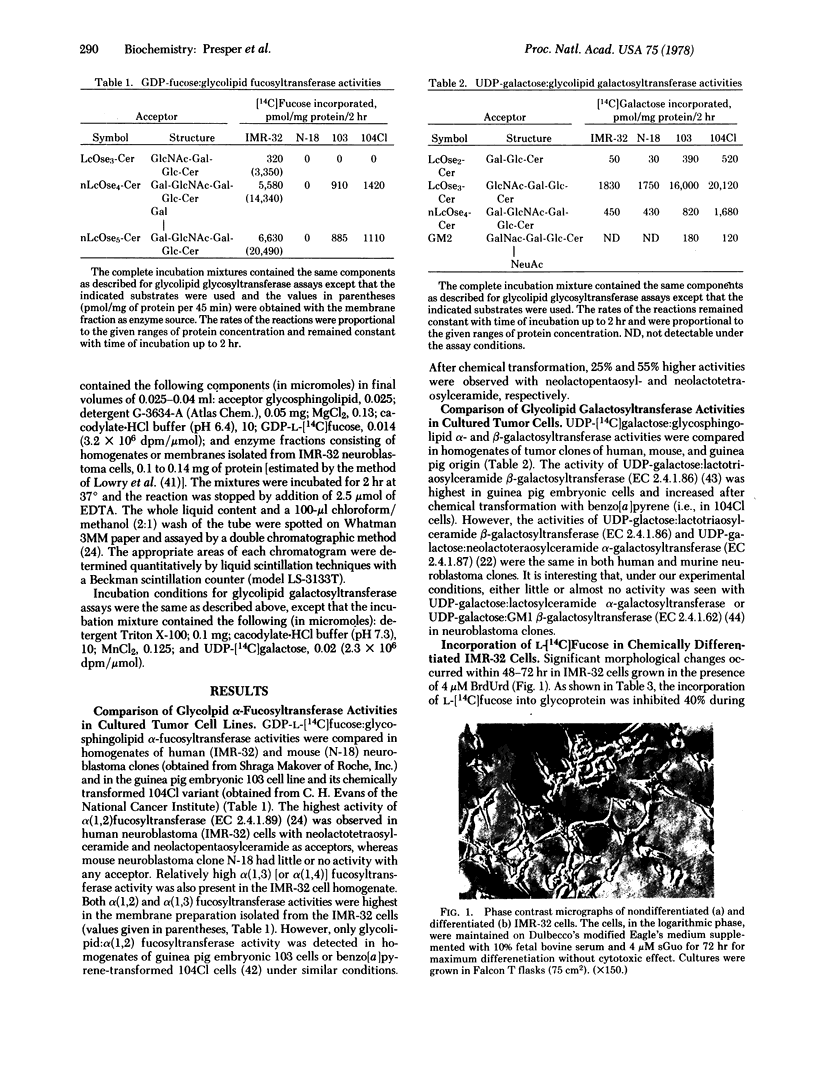
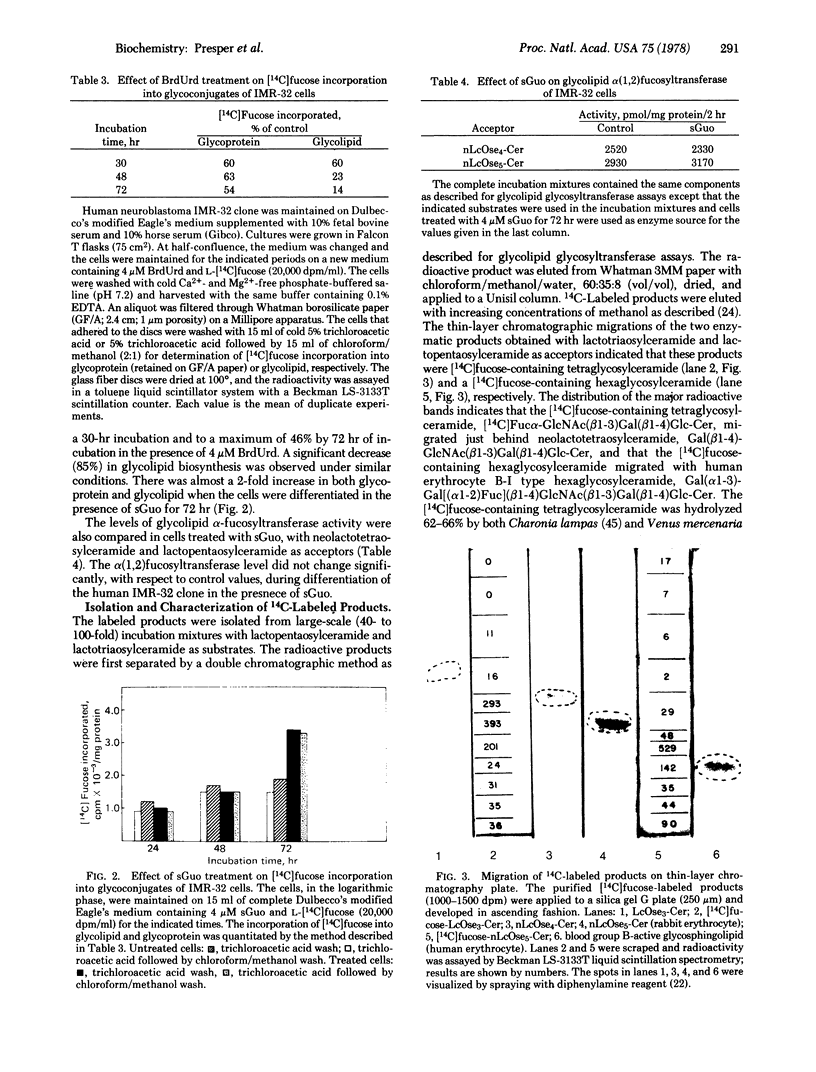
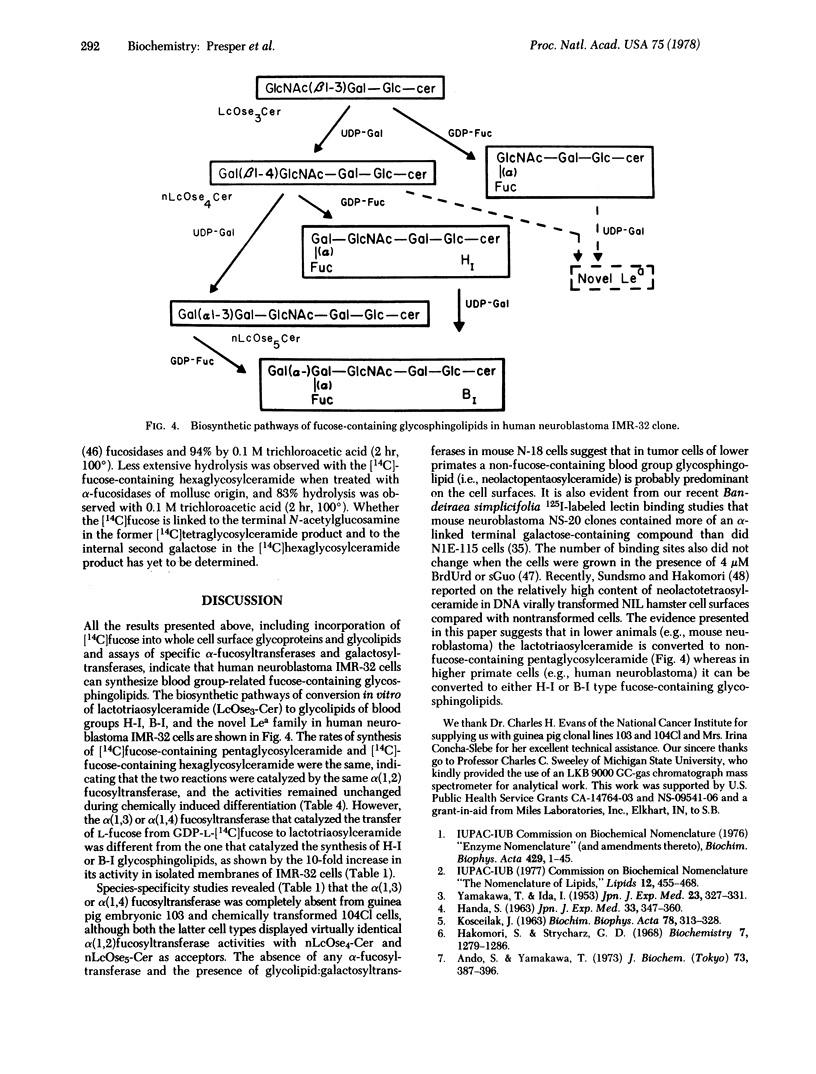
Two different glycolipid:fucosyltransferase activities involved in the biosynthesis in vitro of blood group-related glycosphingolipids have been detected in a membrane preparation isolated from a human neuroblastoma-derived clonal cell line, IMR-32. The membrane preparation contains an alpha (1,2)-fucosyltransferase (EC 2.4.1.89) that catalyzed the transfer of vucose from GDP--[14C]fucose to neolactotetraosylceramide or neolactopentaosylceramide to form types H-I and B-I glycolipids, respectively. The second fucosyltransferase catalyzes the transfer of fucose to lactotriaosylceramide [GlcNAc(beta1-3)Gal(beta1-4)Glc-Cer] to form a tetraglycosylceramide intermediate of the novel Lea-type glycolipid. UDP-galactose:lactotriaosylceramide beta-galactosyltransferase (EC 2.4.1.86) had 4 times the activity of UDP-galactose:alpha-galactosyltransferase (EC 2.4.1.87) when tested under similar conditions. alpha-Fucosyltransferase activities and the incorporation of [14C]fucose into glycoproteins and glycolipids were also compared in cells differentiated in the presence of 4 micron BrdUrd and 6-mercaptoguanosine.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando S., Yamakawa T. Separation of polar glycolipids from human red blood cells with special reference to blood group-A activity. J Biochem. 1973 Feb;73(2):387–396. [PubMed] [Google Scholar]
- Basu M., Basu S. Enzymatic synthesis of a blood group B-related pentaglycosylceramide by an alpha-galactosyltransferase from rabbit bone marrow. J Biol Chem. 1973 Mar 10;248(5):1700–1706. [PubMed] [Google Scholar]
- Basu M., Basu S. Enzymatic synthesis of a tetraglycosylceramide by a galactosyltransferase from rabbit bone marrow. J Biol Chem. 1972 Mar 10;247(5):1489–1495. [PubMed] [Google Scholar]
- Basu M., Basu S., Shanabruch W. G., Moskal J. R., Evans C. H. Lectin and cholera toxin binding to guinea pig tumor (104C1) cell surfaces before and after glycosphingolipid incorporation. Biochem Biophys Res Commun. 1976 Jul 12;71(1):385–392. doi: 10.1016/0006-291x(76)90294-1. [DOI] [PubMed] [Google Scholar]
- Basu M., Moskal J. R., Gardener D. A., Basu S. Biosynthesis in vitro and the pattern of lectin binding receptors in monkey kidney cell surfaces. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1380–1388. doi: 10.1016/0006-291x(75)90512-4. [DOI] [PubMed] [Google Scholar]
- Basu S., Basu M., Chien J. L. Enzymatic synthesis of a blood group H-related glycosphingolipid by an alpha-fucosyltransferase from bovine spleen. J Biol Chem. 1975 Apr 25;250(8):2956–2962. [PubMed] [Google Scholar]
- Basu S., Kaufman B., Roseman S. Conversion of Tay-Sachs ganglioside to monosialoganglioside by brain uridine diphosphate D-galactose: glycolipid galactosyltransferase. J Biol Chem. 1965 Oct;240(10):4115–4117. [PubMed] [Google Scholar]
- Bauer C., Köttgen E., Reutter W. Elevated activities of alpha-2-and alpha-3-fucosyltransferases in human serum as a new indicator of malignancy. Biochem Biophys Res Commun. 1976 May 23;76(2):488–494. doi: 10.1016/0006-291x(77)90751-3. [DOI] [PubMed] [Google Scholar]
- Bella A., Jr, Kim Y. S. Biosynthesis of intestinal glycoprotein: a study of an (1 lead to 2) fucosyltransferase in rat small intestinal mucosa. Arch Biochem Biophys. 1971 Dec;147(2):753–761. doi: 10.1016/0003-9861(71)90435-8. [DOI] [PubMed] [Google Scholar]
- Chou T. H., Murphy C., Kessel D. Selective inhibition of a plasma fucosyltransferase by N-ethylmaleimide. Biochem Biophys Res Commun. 1977 Feb 7;74(3):1001–1006. doi: 10.1016/0006-291x(77)91617-5. [DOI] [PubMed] [Google Scholar]
- Evans C. H., DiPaolo J. A. Neoplastic transformation of guinea pig fetal cells in culture induced by chemical carcinogens. Cancer Res. 1975 Apr;35(4):1035–1044. [PubMed] [Google Scholar]
- HANDA S. BLOOD GROUP ACTIVE GLYCOLIPID FROM HUMAN ERYTHROCYTES. Jpn J Exp Med. 1963 Dec;33:347–360. [PubMed] [Google Scholar]
- Hakomori S., Strycharz G. D. Investigations on cellular blood-group substances. I. Isolation and chemical composition of blood-group ABH and Le-b isoantigens of sphingoglycolipid nature. Biochemistry. 1968 Apr;7(4):1279–1286. doi: 10.1021/bi00844a005. [DOI] [PubMed] [Google Scholar]
- Hanfland P. Characterization of B and H blood-group active glycosphingolopids from human B erythrocyte membranes. Chem Phys Lipids. 1975 Nov;15(2):105–124. doi: 10.1016/0009-3084(75)90035-3. [DOI] [PubMed] [Google Scholar]
- Iijima Y., Egami F. Purification of alpha-L-fucosidose from the liver of a marine gastropod, Chariona lampas. J Biochem. 1971 Jul;70(1):75–78. doi: 10.1093/oxfordjournals.jbchem.a129628. [DOI] [PubMed] [Google Scholar]
- Jabbal I., Schachter H. Pork liver guanosine diphosphate-L-fucose glycoprotein fucosyltransferases. J Biol Chem. 1971 Aug 25;246(16):5154–5161. [PubMed] [Google Scholar]
- KOSCIELAK J. BLOOD GROUP A SPECIFIC GLYCOLIPIDS FROM HUMAN ERYTHROCYTES. Biochim Biophys Acta. 1963 Oct 29;78:313–328. doi: 10.1016/0006-3002(63)91642-1. [DOI] [PubMed] [Google Scholar]
- Kościelak J., Miller-Podraza H., Krauze R., Piasek A. Isolation and characterization of poly(glycosyl)ceramides (megaloglycolipids) with A, H and I blood-group activities. Eur J Biochem. 1976 Dec;71(1):9–18. doi: 10.1111/j.1432-1033.1976.tb11083.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsumoto M., Taki T. Blood group H active glycolipid from rat ascites hepatoma AH 7974F. Biochem Biophys Res Commun. 1976 Jul 26;71(2):472–476. doi: 10.1016/0006-291x(76)90811-1. [DOI] [PubMed] [Google Scholar]
- McKibbin J. M., Smith E. L., Månsson J. E., Li Y. T. Characterization of dog small intestinal fucolipids with human blood group A activity. Differences in dog and human A-active fucolipids. Biochemistry. 1977 Mar 22;16(6):1223–1228. doi: 10.1021/bi00625a030. [DOI] [PubMed] [Google Scholar]
- Pacuszka T., Kościelak J. Enzymatic synthesis of two fucose-containing glycolipids with fucosyltransferases of human serum. Eur J Biochem. 1976 May 1;64(2):499–506. doi: 10.1111/j.1432-1033.1976.tb10328.x. [DOI] [PubMed] [Google Scholar]
- Prasad K. N., Hsie A. W. Morphologic differentiation of mouse neuroblastoma cells induced in vitro by dibutyryl adenosine 3':5'-cyclic monophosphate. Nat New Biol. 1971 Sep 29;233(39):141–142. doi: 10.1038/newbio233141a0. [DOI] [PubMed] [Google Scholar]
- Rauvala H. The fucoganglioside of human kidney. FEBS Lett. 1976 Feb 15;62(2):161–164. doi: 10.1016/0014-5793(76)80043-9. [DOI] [PubMed] [Google Scholar]
- Schenkel-Brunner H., Chester M. A., Watkins W. M. Alpha-L-fucosyltransferases in human serum from donors of different ABO, secretor and Lewis blood-group phenotypes. Eur J Biochem. 1972 Oct;30(2):269–277. doi: 10.1111/j.1432-1033.1972.tb02095.x. [DOI] [PubMed] [Google Scholar]
- Schubert D., Jacob F. 5-bromodeoxyuridine-induced differentiation of a neuroblastoma. Proc Natl Acad Sci U S A. 1970 Sep;67(1):247–254. doi: 10.1073/pnas.67.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeds N. W., Gilman A. G., Amano T., Nirenberg M. W. Regulation of axon formation by clonal lines of a neural tumor. Proc Natl Acad Sci U S A. 1970 May;66(1):160–167. doi: 10.1073/pnas.66.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomiany B. L., Slomiany A. Branched blood group A-active fucolipids of hog gastric mucosa. Biochim Biophys Acta. 1977 Mar 25;486(3):531–540. doi: 10.1016/0005-2760(77)90103-5. [DOI] [PubMed] [Google Scholar]
- Sundsmo J. S., Hakomori S. Lacto-N-neotetraosylceramide ("paragloboside") as a possible tumor-associated surface antigen of hamster NILPY tumor. Biochem Biophys Res Commun. 1976 Feb 9;68(3):799–806. doi: 10.1016/0006-291x(76)91216-x. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Ishizuka I., Yamakawa T. Isolation and characterization of a ganglioside containing fucose from boar testis. J Biochem. 1975 Nov;78(5):947–954. doi: 10.1093/oxfordjournals.jbchem.a131001. [DOI] [PubMed] [Google Scholar]
- Tumilowicz J. J., Nichols W. W., Cholon J. J., Greene A. E. Definition of a continuous human cell line derived from neuroblastoma. Cancer Res. 1970 Aug;30(8):2110–2118. [PubMed] [Google Scholar]
- Watanabe K., Matsubara T., Hakomori S. alpha-L-Fucopyranosylceramide, a novel glycolipid accumulated in some of the human colon tumors. J Biol Chem. 1976 Apr 25;251(8):2385–2387. [PubMed] [Google Scholar]
- Wiegandt H. Gangliosides of extraneural organs. Hoppe Seylers Z Physiol Chem. 1973 Sep;354(9):1049–1056. doi: 10.1515/bchm2.1973.354.2.1049. [DOI] [PubMed] [Google Scholar]
- YAMAKAWA T., IIDA T. Immunochemical study on the red blood cells. I. Globoside, as the agglutinogen of the ABO system on erythrocytes. Jpn J Exp Med. 1953 Aug;23(4):327–331. [PubMed] [Google Scholar]
- Yang H. J., Hakomori S. I. A sphingolipid having a novel type of ceramide and lacto-N-fucopentaose 3. J Biol Chem. 1971 Mar 10;246(5):1192–1200. [PubMed] [Google Scholar]