Abstract
Introduction
Sleep curtailment is an endemic behavior in modern society. Well-controlled laboratory studies have shown that sleep loss in young adults is associated with increased desire for high-calorie food and obesity risk. However, the relevance of these laboratory findings to real life is uncertain. We conducted a 3 week, within-participant, intervention study to assess the effects of extended bedtimes on sleep duration and food desire under real life conditions in individuals who are at risk for obesity.
Methods
Ten overweight young adults reporting average habitual sleep duration of less than 6.5 hours were studied in the home environment. Habitual bedtimes for 1-week (baseline) were followed by bedtimes extended to 8.5 hours for 2-weeks (intervention). Participants were unaware of the intervention until after the baseline period. Participants received individualized behavioural counselling on sleep hygiene on the first day of the intervention period. Sleep duration was recorded by wrist actigraphy throughout the study. Participants rated their sleepiness, vigor and desire for various foods using visual analog scales at the end of baseline and intervention periods.
Results
On average, participants obtained 1.6 hours more sleep with extended bedtimes (5.6 vs. 7.1; p<0.001) and reported being less sleepy (p=0.004) and more vigorous (p=0.034). Additional sleep was associated with a 14% decrease in overall appetite (p=0.030) and a 62% decrease in desire for sweet and salty foods (p=0.017). Desire for fruits, vegetables and protein-rich nutrients was not affected by added sleep.
Conclusions
Sleep duration can be successfully increased in real life settings and obtaining adequate sleep is associated with less desire for high calorie foods in overweight young adults who habitually curtail their sleep.
Keywords: Sleep extension, appetite, food desire, obesity, sleep hygiene
Introduction
Sleep curtailment has become an increasingly prevalent behavior in modern society. It is estimated that average sleep duration has decreased by 1.5 to 2 hours in the past half century. Today, as many as one-third of American adults report obtaining less than 7 hours of sleep. According to a recent survey by the National Sleep Foundation (1), roughly one-third of Americans reported, “not getting enough sleep” by comparing the hours of sleep they say they need to the hours of sleep they are actually getting on workdays or weekdays. Overall, more than half of them agreed that “not getting enough sleep” affects their job performance, ability to carry out household duties, relationship with family or friends, and ability to perform everyday activities.
Substantial evidence from population studies suggests that young adults reporting short habitual sleep durations are at increased risk of developing obesity (2-4). Well-controlled laboratory studies have demonstrated that sleep restriction in young adults is associated with alterations in appetite regulation, particularly with more desire for high calorie foods (5-8), which may increase the risk for weight gain. However, the relevance of these laboratory findings to real life has not been studied. In other words, there has been no intervention study so far that has investigated whether sleep time can be improved in real-life settings and whether additional sleep has any beneficial effects in individuals who are at risk for obesity. This may be because it is commonly believed that increasing sleep duration may be difficult to achieve in real life where individuals have priorities and other responsibilities competing with sleep.
Therefore, we designed our study using a home-based intervention aimed at extending bedtimes and evaluated its effects on sleep duration and food desire in at-risk individuals, while they live in their usual environment. We hypothesized that sleep duration can be increased in real life settings with a behavioral intervention to extend bedtimes through individualized sleep hygiene counseling. We further hypothesized that additional sleep has beneficial effects on appetite and decreases cravings for weight-promoting, high calorie foods in overweight young adults who habitually curtail their sleep.
Methods
Design Overview
The study was approved by the University of Chicago institutional review board. We conducted a within participant, intervention study under real life conditions, beginning with habitual bedtimes (baseline period; nights N01-N07) for one week immediately followed by extended bedtimes (intervention period; nights N08-N21) for two weeks. Participants were unaware of the intervention until after the habitual bedtime period to ensure that they did not modify their habitual sleep-wake behaviour, and thus their habitual sleep patterns were effectively captured at baseline. Participants were told that the purpose of the study was to collect information on their sleep-wake patterns at home. They were also told that they may be asked to modify the timing of sleep, but not that this change would result in a sleep extension. The advertisements stated that the study involves completing questionnaires and wearing a wrist watch at home for 3 weeks. Participants received individualized behavioural counselling about sleep hygiene on the first day of the intervention period. We objectively monitored sleep-wake patterns by continuous wrist actigraphy during the entire 3-week study. Participants rated their sleepiness, vigor and desire for various food items at the end of the baseline and intervention periods.
Participants and Setting
Overweight adults (age range: 21 to 40 years; body mass index range: 25.0 to 29.9 kg/m2) reporting an average habitual sleep duration of <6.5 hours were recruited through local advertisements. Exclusion criteria were insomnia, regular napping, shift work, extreme chronotype, travel across time-zones within the past 4 weeks, history of eating or psychiatric disorders, acute or chronic medical condition, alcohol abuse, smoking, pregnancy or childbirth (past year), any prescription medications, and current enrolment in diet or exercise programs. Eligibility was established by a structured survey and a brief interview. Eleven participants, who met the eligibility criteria, were enrolled. Wrist actigraphy recordings failed and were incomplete in one woman, who was excluded from the analysis. Data from the remaining 10 participants (5men, 5 women), who completed the study, are presented. Throughout the entire study, the participants followed their daily routine activities and slept in their usual home environment.
Intervention: Extended Bedtimes
During the first week of the study, participants were asked to continue their habitual bedtimes at home. On the first day of the intervention period, participants met with the study investigators in an office setting to receive individualized behavioural counselling on sleep hygiene through a structured interview. First, all social and environmental factors related to habitual sleep patterns were discussed in detail. Actigraphy data from baseline period was briefly reviewed. Next, individualized behavioural counselling on sleep hygiene was provided with the goal of accommodating the extended bedtimes in the participant's lifestyle in the best possible way. Participants also received counselling about potential modifiable factors and other barriers in their lifestyle that may prevent them from extending bedtime duration. As necessary, factors related to sleep partner, children, other family members and pets were considered and individual recommendations were provided to better implement extended bedtimes into the daily routine. At the end of the interview, participants were provided with individualized recommendations to follow at home for 2 weeks, aiming to extend bedtime duration to 8.5 hours (with the intention to increase sleep duration to the healthier length of 7-8 hours per night). Bedtimes and wake-up times were individually designed, taking into account personal schedules and priorities. At the end of the first week of the intervention period, the participants returned for a brief follow-up visit. Actigraphy data from the first week of the intervention period was reviewed and further counselling was provided, as needed.
Data collection
Participants were asked to wear a wrist activity monitor (Actiwatch 64, Mini-Mitter Respironics, Inc) on the non-dominant arm throughout the study. This monitor detects participants' movement via accelerometers and has a built-in event marker. Participants were asked to press the event-marker button when they went to bed to sleep each night and when they got out of bed each morning. Participants also kept daily sleep logs to indicate the times when they went to bed and got out of bed. At the beginning of the study, participants completed the Pittsburgh Sleep Quality Index (9) to assess overall sleep quality and Morningness-Eveningness questionnaire (10) to determine chronotypes.
At the end of each study period, participants completed validated visual analog scales of vigor (11) and appetite (5) in the morning before eating their breakfast. Sleepiness was determined from the response to the question “How sleepy do you feel?” on a 10-cm scale (with “very little” and “very much” as limits). Food desire was assessed by asking the participants to mark their ratings of how much they would enjoy eating various food items on a 10-cm scale (with “not at all” and “very much” as limits). Participants were asked to provide their ratings at the moment, without concern for calories, fat, or a healthy diet.
Data Analysis and Statistics
Sleep was automatically scored by Actiware Version 5 software (Respironics, Inc), an actigraphy-based sleep-scoring program using previously described and validated algorithms (12). Sleep duration was calculated as the sum of all epochs scored as sleep during the time in bed. Variability across nights in a participant's sleep duration was summarized using the coefficient of variation. Sleep data were averaged across nights in each participant for each study period. Sleep efficiency (reported as percentage) was defined as the total sleep time divided by the total time spent in bed multiplied by 100. Sleep latency was defined as the time in minutes before sleep onset following the bedtime. Comparisons between habitual bedtime and extended bedtime periods were performed using a two-sided paired t-test with a significance level of 0.05 (JMP 9.0.2, SAS Institute). Results are reported as mean±SE.
Results
Participants had a mean age of 28.6 ±1.7 years and mean body mass index of 28.0 ±0.6 kg/m2. Five had full-time and 2 had part-time jobs, 2 were working from home, and 1 was a student. The sample was comprised of 3 Caucasian, 4 African-American, 1 Asian and 2 Hispanic participants. At the beginning of the study, the average Pittsburgh Sleep Quality Index score was 5.1 ±0.5. None of the subjects were extreme chronotypes as assessed by the Morningness-Eveningness questionnaire with an average score of 50.0 ±1.8.
Overall, participants slept 1.6 hours more with extended bedtimes as compared to habitual bedtimes (Table 1). Night-to-night variability in sleep duration was similar between study periods (p=0.33) with a mean coefficient of variation of 13.6% (range 6.1 to 25.5%) during habitual bedtimes and 11.1% (range 6.7 to 21.4%) during extended bedtimes (Figure 1A). Bedtime duration was increased by 1.8 (0.2) hours with the intervention (Table 1). On average, the participants went to bed ∼1 hour 15 min earlier with the intervention (hh:mm; 00:54 ± 00:17 during habitual bedtimes vs 23:38 ± 00:13 during extended bedtimes) and got up ∼30min later (hh:mm; 07:19 ± 00:23 during habitual bedtimes vs 07:52 ± 00:17 during extended bedtimes). Sleep efficiency and latency were similar during both study periods, confirming that the participants were habitually sleep deprived. The intervention resulted in increased sleep duration in all 10 participants (Figure 1B). As expected, the magnitude of increase in sleep duration varied among participants (ranging from 37min to 2h 44min) with an increase of at least 1 hour in 8 out of 10 participants. Sleep duration increased by an average of 1.4 ±0.1 hours on weekday nights and 1.9 ±0.3 hours on weekend nights (Figure 1C). Sleep extension, relative to habitual sleep, was associated with an ∼7% increase in average daytime activity (Table 1)
Table 1. Effect of Bedtime Extension on Actigraphy-Based Sleep Characteristics.
| Characteristic | Habitual Bedtimes | Extended Bedtimes | p value |
|---|---|---|---|
| Sleep duration (hour) | 5.6 (0.1) | 7.1 (0.1) | < 0.001 |
| Bedtime duration (hour) | 6.4 (0.2) | 8.2 (0.1) | < 0.001 |
| Sleep efficiency (%) | 86.5 (1.4) | 86.5 (1.3) | 0.995 |
| Sleep latency (minute) | 8.2 (1.8) | 10.6 (1.7) | 0.282 |
| Daytime activity (counts/minute) * | 394 (25) | 423 (24) | 0.020 |
Data are mean (SE)
Calculated as the average activity counts from the wrist actigraphy recordings during the periods when subjects were not in bed to sleep
Figure 1. Sleep Duration During Habitual Bedtime and Extended Bedtime.
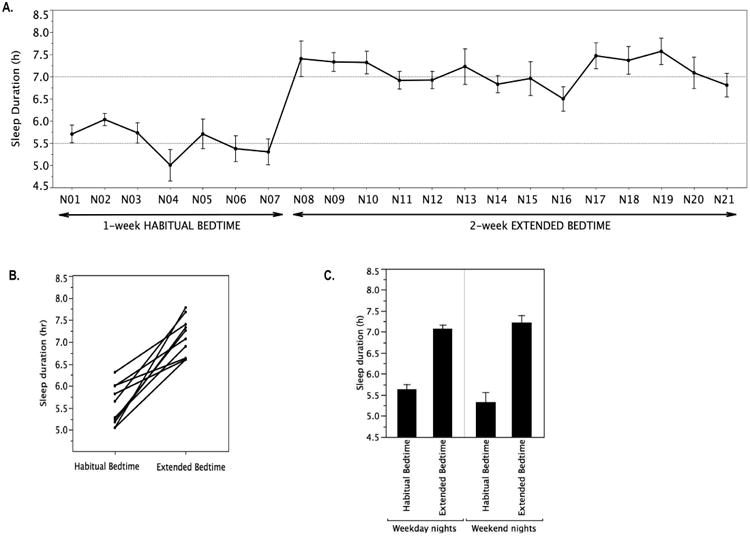
A) Mean (SE) night-by-night sleep duration during the habitual bedtime period (nights N01 to N07) and extended bedtime period (nights N08 to N21). B) Individual changes in average sleep duration between habitual and extended bedtimes. C) Mean ±SE sleep duration on the weekday nights (Sunday through Thursday) and the weekend nights (Friday and Saturday).
Participants reported being less sleepy (p=0.004) and more vigorous (p=0.034) with extended bedtimes relative to habitual bedtimes (Table 2). More sleep was associated with a 14% decrease in overall appetite ratings when all food categories were considered. Desire for sweet and salty foods was decreased by 62%, whereas desire for fruits, vegetables and protein-rich nutrients were not affected by additional sleep.
Table 2. Effect of Bedtime Extension on Vigor and Appetite Ratingsa.
| Characteristic | Habitual Bedtimes | Extended Bedtimes | p value |
|---|---|---|---|
| Vigor Ratings | |||
| Sleepy | 6.9 (0.9) | 2.4 (0.8) | 0.004 |
| Global Vigor | 4.5 (0.8) | 6.9 (0.6) | 0.034 |
| Appetite Ratings | |||
| Overall appetite | 4.0 (0.6) | 3.5 (0.6) | 0.030 |
| Sweet & Salty foods (cake, candy, cookies, ice cream, pastry, chips, salted nuts, pickles, and olives) | 2.4 (0.6) | 0.9 (0.2) | 0.017 |
| Starchy food (bread, pasta, cereal, and potatoes) | 5.1 (1.0) | 4.1 (1.0) | 0.156 |
| Fruits and fruit juices | 6.4 (1.1) | 6.1 (1.1) | 0.632 |
| Vegetables | 2.1 (1.1) | 2.5 (1.0) | 0.478 |
| Meat, poultry, fish, and eggs | 5.0 (1.1) | 5.2 (0.9) | 0.764 |
| Dairy (milk, cheese, and yogurt) | 4.8 (1.1) | 4.5 (1.1) | 0.785 |
Data are mean (SE)
Average ratings on a visual analog scale from 0- to 10-cm
Discussion
We demonstrated that a 2-week home-based behavioral intervention to extend bedtimes results in increased sleep duration in overweight young adults who habitually curtail their sleep. We report for the first time that additional sleep has beneficial effects on food desirability with decreased cravings for weight promoting sweet and salty foods in this at-risk population. These findings demonstrate the feasibility of sleep extension in real life settings, where individuals have other priorities competing with sleep, and suggest that obtaining adequate sleep might have beneficial effects on food desire in individuals who are at risk for obesity.
We have used a home-based intervention through counselling on sleep hygiene. Behavioral modifications that were recommended to extend bedtimes were individualized to meet each participant's needs and life-style. Based on participants' self-report at the end of the study, the restriction of electronic media exposure (i.e. television and internet use) too close to bedtime or when in bed before going to sleep, was a key behavioral component for the success of the intervention in all 10 participants. In agreement with our data, cross-sectional studies have shown that TV viewing and computer use is associated with delayed bedtimes and reduced sleep duration (13, 14). A recent study also suggests that promoting household routines, particularly increasing sleep duration and reducing TV viewing, may be an effective approach to reduce body mass index among pre-school aged children (15).
Our finding that sleep extension (objectively measured) is feasible in real life settings is consistent with two recent reports where extending sleep duration (assessed by self-reported sleep diaries) had beneficial effects on neurocognitive function in habitually short sleeping healthy and obese individuals (16, 17). On average, our participants obtained 1.6 hours more sleep with extended bedtimes, which is comparable in magnitude to the difference in sleep duration reported in prior studies as having adverse effects of sleep restriction on diabetes and obesity risk (18-21). Thus, the amount of additional sleep obtained in real life settings could conceivably have important metabolic implications to reduce obesity and diabetes risk. Consistent with this hypothesis, a longitudinal study reported that a spontaneous shift in sleep duration from short to healthier amount (≤6 h to 7-8 h) was associated with attenuated fat gain over 6 years (22).
Our participants also reported being less sleepy and more vigorous when they obtained additional sleep, which could potentially lead to increased physical activity. Consistent with this hypothesis, we have found that our participants had increased daytime activity (based on wrist monitors) during the intervention period as compared to the baseline period. Indeed, observational data in free-living adults at risk for diabetes show that persons who habitually curtail their sleep have more sedentary behavior, where sleep loss related declines in physical activity strongly correlate with reductions in subjective vigor (23).
We have found that additional sleep in the home environment was associated with decreased overall subjective appetite and a 62% reduction in desire for sweet and salty foods. Erstwhile desire for fruits, vegetables and protein-rich nutrients was not affected by added sleep. These findings suggest that added sleep may help individuals to make healthier food choices as recommended by the USDA guidelines on the MyPlate designation (24). Our data is consistent with anecdotal reports of less cravings for sweet and salty snacks in obese adults who extended their habitual sleep duration (25) as well as with the findings from previous short-term laboratory studies in healthy volunteers, in which sleep restriction was associated with increased appetite ratings, particularly more cravings for calorie-dense foods high in fat and sugar (5-7) and increased consumption of carbohydrates from snacks (20). A recent laboratory study involving brain imaging in sleep deprived healthy young volunteers has identified alterations in specific brain regions that may be involved in increased desire for high-calorie foods, and thus provides a potential biological mechanism by which sleep restriction may lead to poor food choices (7). Indeed, multiple neural systems and pathways are involved in appetitive drive and food intake (26, 27). Of interest, future mechanistic studies could investigate how sleep extension affects the lateral hypothalamic orexin neurons, which are known to play a key role in the interactions between sleeping and feeding behaviors (28). Our finding that sleep extension is associated with reduced desire for high calorie foods is in agreement with a laboratory study in healthy volunteers showing that transitioning from an insufficient to adequate/recovery sleep decreased energy intake, especially of fats and carbohydrates (29).
Our study has several limitations. This was a single center study with a small sample size in overweight individuals with selective eligibility criteria, which may limit the generalizability to more diverse populations. The exclusion of sleep disorders was based on self-report and not a laboratory sleep study. Extended bedtimes were implemented over a short period of 2-weeks, and thus the study does not provide information on the potential effects of an intervention sustained over a longer period (e.g. months to years). Nevertheless, at the end of the study, we asked the participants to complete a brief survey inquiring how easy or difficult it was to implement sleep extension in their daily life and whether they are likely to continue extending their bedtimes in the long-term. Seven out of 10 participants found the intervention fairly easy and 9 out of 10 participants said that they are likely to continue to obtain more sleep in the long-term because they have noticed several beneficial effects (e.g. more energy, better mood, more alertness etc.). Another limitation is that we did not collect information on meal frequency and timing and assessed food desirability only in the morning hours before breakfast. Previous studies reported an increase in appetite and caloric intake in the morning (breakfast), and to a greater extend in the evening hours (20, 30, 31). We thus predict that the effects on food desirability that were observed in this study after extended bedtimes would have been larger if measurements were repeated later during the day. We did not collect data on actual energy intake or assess the changes in appetite regulating hormones in this outpatient study, and thus the subjectivity of appetite measures that we report could be perceived as a limitation. Yet, the only available method to objectively track energy intake in free-living individuals involves a combination of the measurements of total energy expenditure by doubly labeled water method with quantification of changes in body energy stores. We believe that such rigorous studies in free-living environment would be the next logical step towards addressing the question as to whether changes in appetite following sleep extension would result in behavioural changes in food consumption. Finally, we have chosen a study design where participants served as their own control beginning with habitual bedtimes followed by extended bedtimes. Importantly, participants were blinded to the behavioral recommendations until the beginning of the intervention period. Our finding with regard to the positive shifts in attitude toward longer sleep times and the participants' desire to continue this behavior after the 2-week intervention period suggest that future research efforts may not be able to use a randomized order of sleep interventions in outpatient studies. After experiencing the positive effects of extending sleep to the healthy range, it is unlikely that these behavioral changes will”washout” prior to a control period. Nevertheless, it is important to note that our study did not include a randomized design with a separate control arm (e.g. randomized controlled parallel group design), and thus we cannot necessarily exclude additional environmental factors (not assessed in our study) that may have contributed to our findings.
In summary, a two-week home-based intervention to extend bedtimes can successfully increase sleep duration in real life conditions and obtaining adequate sleep increases vigor and decreases cravings for weight promoting sweet and salty foods in overweight young adults who habitually curtail their sleep. These findings have important implications for current efforts to reduce the burden of obesity and diabetes. Future intervention studies of longer duration with robust assessments of energy balance and metabolism under free-living conditions are warranted to investigate whether obtaining adequate sleep, which could be implemented in clinical scenarios, is an effective strategy to reduce metabolic risk and to improve the success of adherence to lifestyle regimens for the prevention and treatment of obesity and diabetes. The demonstration of a clear benefit of additional sleep on energy metabolism in real life settings would provide a strong incentive to adopt healthy sleep habits in individuals who are overweight or obese, and may benefit millions of Americans.
Highlights.
Sleep extension is feasible in real life settings despite other priorities competing with sleep.
Adequate sleep is associated with less desire for sweet and salty foods in overweight young adults
Adequate sleep may help individuals to make healthier food choices
Acknowledgments
This work was supported by an NIH grant to the National Center for Advancing Translational Sciences (CTSA-UL1 TR000430) at the University of Chicago.
The funding sources had no role in the study design or concept; the collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all participants for being included in the study.
This work described in this article has not been published previously and is not under consideration for publication elsewhere and its publication is approved by all authors.
Footnotes
Statements: Authors Esra Tasali, Florian Chapotot, Kristen Wroblewski and Dale Schoeller declare that they have no conflict of interest relevant to this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention. Morbidity and Mortality Weekly Report. 2011 Mar 4; 2011. [Google Scholar]
- 2.Nielsen LS, Danielsen KV, Sorensen TI. Short sleep duration as a possible cause of obesity: critical analysis of the epidemiological evidence. Obes Rev. 2011;12(2):78–92. doi: 10.1111/j.1467-789X.2010.00724.x. [DOI] [PubMed] [Google Scholar]
- 3.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16(3):643–53. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNeil J, Doucet E, Chaput JP. Inadequate sleep as a contributor to obesity and type 2 diabetes. Can J Diabetes. 2013;37(2):103–8. doi: 10.1016/j.jcjd.2013.02.060. [DOI] [PubMed] [Google Scholar]
- 5.Spiegel K, Tasali E, Penev P, Van Cauter E. Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141(11):846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 6.Schmid SM, Hallschmid M, Jauch-Chara K, Born J, Schultes B. A single night of sleep deprivation increases ghrelin levels and feelings of hunger in normal-weight healthy men. J Sleep Res. 2008 doi: 10.1111/j.1365-2869.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 7.Greer SM, Goldstein AN, Walker MP. The impact of sleep deprivation on food desire in the human brain. Nat Commun. 2013;4:2259. doi: 10.1038/ncomms3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaput JP, Klingenberg L, Sjodin AM. Sleep restriction and appetite control: waking to a problem? Am J Clin Nutr. 2010;91(3):822–3. doi: 10.3945/ajcn.2009.29011. author reply 3-4. [DOI] [PubMed] [Google Scholar]
- 9.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 10.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110. [PubMed] [Google Scholar]
- 11.Monk TH. A Visual Analog Scale technique to measure global vigor and affect. Psychiatr Res. 1989;27:89–99. doi: 10.1016/0165-1781(89)90013-9. [DOI] [PubMed] [Google Scholar]
- 12.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 13.Nuutinen T, Ray C, Roos E. Do computer use, TV viewing, and the presence of the media in the bedroom predict school-aged children's sleep habits in a longitudinal study? BMC Public Health. 2013;13:684. doi: 10.1186/1471-2458-13-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fossum IN, Nordnes LT, Storemark SS, Bjorvatn B, Pallesen S. The Association Between Use of Electronic Media in Bed Before Going to Sleep and Insomnia Symptoms, Daytime Sleepiness, Morningness, and Chronotype. Behav Sleep Med. 2013 doi: 10.1080/15402002.2013.819468. [DOI] [PubMed] [Google Scholar]
- 15.Haines J, McDonald J, O'Brien A, Sherry B, Bottino CJ, Schmidt ME, et al. Healthy habits, happy homes: randomized trial to improve household routines for obesity prevention among preschool-aged children. JAMA Pediatr. 2013;167(11):1072–9. doi: 10.1001/jamapediatrics.2013.2356. [DOI] [PubMed] [Google Scholar]
- 16.Gumenyuk V, Korzyukov O, Roth T, Bowyer SM, Drake CL. Sleep extension normalizes ERP of waking auditory sensory gating in healthy habitually short sleeping individuals. PLoS ONE. 2013;8(3):e59007. doi: 10.1371/journal.pone.0059007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lucassen EA, Piaggi P, Dsurney J, de Jonge L, Zhao XC, Mattingly MS, et al. Sleep extension improves neurocognitive functions in chronically sleep-deprived obese individuals. PLoS ONE. 2014;9(1):e84832. doi: 10.1371/journal.pone.0084832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darukhanavala A, Booth JN, 3rd, Bromley L, Whitmore H, Imperial J, Penev PD. Changes in insulin secretion and action in adults with familial risk for type 2 diabetes who curtail their sleep. Diabetes Care. 2011;34(10):2259–64. doi: 10.2337/dc11-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nedeltcheva AV, Kessler L, Imperial J, Penev PD. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab. 2009;94(9):3242–50. doi: 10.1210/jc.2009-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89(1):126–33. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robertson MD, Russell-Jones D, Umpleby AM, Dijk DJ. Effects of three weeks of mild sleep restriction implemented in the home environment on multiple metabolic and endocrine markers in healthy young men. Metabolism. 2013;62(2):204–11. doi: 10.1016/j.metabol.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Chaput JP, Despres JP, Bouchard C, Tremblay A. Longer sleep duration associates with lower adiposity gain in adult short sleepers. Int J Obes (Lond) 2012;36(5):752–6. doi: 10.1038/ijo.2011.110. [DOI] [PubMed] [Google Scholar]
- 23.Bromley LE, Booth JN, 3rd, Kilkus JM, Imperial JG, Penev PD. Sleep restriction decreases the physical activity of adults at risk for type 2 diabetes. Sleep. 2012;35(7):977–84. doi: 10.5665/sleep.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.USDA's MyPlate. United States Department of Agriculture. 2011 http://www.choosemyplate.gov.
- 25.Cizza G, Marincola P, Mattingly M, Williams L, Mitler M, Skarulis M, et al. Treatment of obesity with extension of sleep duration: a randomized, prospective, controlled trial. Clin Trials. 2010;7(3):274–85. doi: 10.1177/1740774510368298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berthoud HR. The neurobiology of food intake in an obesogenic environment. Proc Nutr Soc. 2012;71(4):478–87. doi: 10.1017/S0029665112000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berthoud HR, Munzberg H, Richards BK, Morrison CD. Neural and metabolic regulation of macronutrient intake and selection. Proc Nutr Soc. 2012;71(3):390–400. doi: 10.1017/S0029665112000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakurai T. Roles of orexin/hypocretin in regulation of sleep/wakefulness and energy homeostasis. Sleep Med Rev. 2005;9(4):231–41. doi: 10.1016/j.smrv.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci U S A. 2013;110(14):5695–700. doi: 10.1073/pnas.1216951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr. 2010;91(6):1550–9. doi: 10.3945/ajcn.2009.28523. [DOI] [PubMed] [Google Scholar]
- 31.Spaeth AM, Dinges DF, Goel N. Effects of Experimental Sleep Restriction on Weight Gain, Caloric Intake, and Meal Timing in Healthy Adults. Sleep. 2013;36(7):981–90. doi: 10.5665/sleep.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]


