Abstract
Despite the development of powerful antiretroviral drugs, HIV-1 associated neurological disorders (HAND) will affect approximately half of those infected with HIV-1. Combined anti-retroviral therapy (cART) targets viral replication and increases T-cell counts, but it does not always control macrophage polarization, brain infection or inflammation. Moreover, it remains difficult to identify those at risk for HAND. New therapies that focus on modulating host immune response by making use of biological pathways could prove to be more effective than cART for the treatment of neuroAIDS. Additionally, while numerous HAND biomarkers have been suggested, they are of little use without methods for appropriate data integration and a systems-level interpretation. Machine learning, could be used to develop multifactorial computational models that provide clinicians and researchers with the ability to identify which factors (in what combination and relative importance) are considered important to outcome.
Keywords: HIV, brain, neuropathogenesis, macrophages, microglia, microRNA, data integration, machine learning
Introduction
Approximately 40 million people worldwide have been infected with HIV-1. In Western cultures, AIDS was first identified in the early 1980s and has evolved from a deadly to treatable disease, usually requiring close management of drug regimes and careful monitoring of biological markers of disease. While treatment has significantly improved and lengthened the lives of those infected with HIV-1, up to half will develop HIV-associated neurological disorder (HAND), which are not effectively treated by combined antiretroviral therapy (cART) and greatly increase morbidity. HAND is a neuroinflammatory disease, a broad term describing a number of events associated with macrophages (the primary immune cell in the brain) in both diseased and aging brains. Biological indicators of neuroinflammation have been identified post-mortem or in animal models; however, little progress has been made in HAND treatment or in the identification of HAND biomarkers for those living with HIV infection. cART is aimed at reducing viral replication, thereby reducing viral load and restoring T-cell counts in lymphoid tissues. However, even with restoration of T cell counts, the role that ongoing macrophage activation plays in HAND is poorly understood. The modulation of macrophage phenotypes via altering host biological pathways is an interesting approach for HAND treatment and has the potential for impacting the brain compartment independent of HIV load measurements. MicroRNAs, small key regulators of cellular machinery, have also been suggested as both potential biomarkers for HAND and as targets for treatment. In this review, we discuss histopathological, cellular, and genetic features of HAND, innovative research aimed at controlling the disease by altering host immune response, and alternative means to supervise or regulate HAND progression. Finally, we discuss how system approaches, computational genomics, and bioinformatics can be used to generate improved classifiers for HAND progression. Furthermore, we discuss how properly mined data and computational modeling could help determine proper therapies for those infected.
1. HIV-associated neurological disorders, past and present
Early in the HIV epidemic, severe neurocognitive conditions, usually resulting in death, were noted in HIV-infected individuals (100). These central nervous system (CNS) conditions were initially identified in persons with advanced HIV-1 infection and called “HIV-Associated Dementia” or HAD (3, 81). At autopsy, histopathological abnormalities in the brains of patients with HAD included disseminated foci of activated microglia, perivascular macrophage infiltration, multinucleated giant cells, and reactive astrogliosis (18-20, 90). Later investigations revealed that milder forms of neurocognitive impairment could be detected in HIV-1-infected persons before the onset of advanced systemic disease (42). Post-mortem findings most strongly associated with HAD severity were the number of activated macrophages present within affected areas of the brain (41). In addition, dendritic loss (72), neuronal loss (7), and brain HIV viral load (13) were relevant for HAD severity however, these did not occur independently of macrophage infiltration.
After the introduction of cART, the incidence of HAD declined dramatically (11), but milder forms of HIV-associated neurological disorders became highly prevalent, identified in up to 50% of HIV-infected individuals (46). The increased prominence of mild impairment in 2007 led to a new disease nosology called “HIV-Associated Neurocognitive Disorders” (HAND). cART-treated patients with HAND differed from pre-cART patients in many ways. In their cerebrospinal fluid (CSF), they are less likely to have detectable HIV-1 RNA or have high levels of certain inflammatory biomarkers (73), and at autopsy, they are less likely to manifest florid neuropathological changes (24, 30). These observations have led to a re-evaluation of the pathogenic mechanisms of HAND, including interest in persistent CNS inflammation, persistent viral reservoirs, and neurotoxicity, all of which are associated with the roles of activated macrophages during long-term HIV infection (2, 9, 32, 57, 66, 107). Importantly, cART does not entirely clear the CNS of HIV-1 (122). In part, this is due to poor drug penetration of some regimens (67). However, the preference of HIV-1 to reside in CNS macrophages and microglia, which harbor a large reservoir of provirus and unintegrated DNA that is unaffected by cART, is also a likely factor (122). Thus, progressive neuronal loss/dysfunction and CNS inflammation can be measured even in well-suppressed patients on long-term therapy (44).
2. The HIV infected brain and cells of the CNS
The brain is an important viral reservoir, a source of viral escape (12) and a barrier to full HIV-1 eradication (80). Brain tissues are difficult to target with current therapies because of the Blood-Brain-Barrier (BBB) and also because it is possible that any current therapies could cause more damage to the brain than the virus itself (80). HIV can enter the brain during early infection (26, 40); however, it is unclear if this is always the case. Moreover, while there have been an abundance of studies on the topic, it remains uncertain if virus entering the brain during early infection can effectively prime the environment for long-term, low-grade inflammation during cART, or if viruses which have evolved and adapted to macrophage-tropic environments (gut, lung, atherosclerotic plaque, bone marrow) are perhaps more apt to set up a significant inflammatory response in the brain (21, 101). Using the SIV-infected simian model for neuroAIDS and green fluorescent staining, researchers have observed that subpopulations of SIV-infected monocytes (precursors of tissue macrophages) in bone marrow travel into brain tissues (21, 116). In a viral evolutionary study, it was demonstrated that the onset of HIV-associated simian encephalitis directly correlated with an increase in viral populations size from bone marrow (101). These findings not only help elucidate how and where neurotropic forms of HIV might evolve before they travel to the brain, but they also indicate that there may be tissues, outside of the brain compartment, that could be targeted with specific therapies aimed at reducing the potential for HAND.
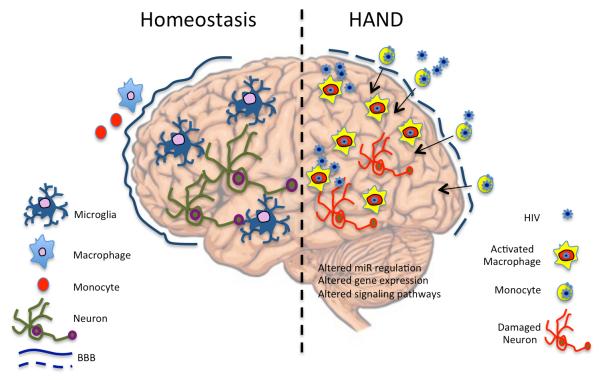
In the brain, subpopulations of blood derived migrant monocytes, macrophages, and resident microglia are infected with HIV (117) (Figure 1). Circulating monocytes enter the CNS and differentiate into macrophages (33, 59). These cells inhabit perivascular, fluid-filled canals that surround perforating arteries and veins in the parenchyma of the brain. Monocyte derived macrophages (MDMs) are phagocytic and antigen-presenting cells that, when activated, can produce a large number of soluble substances important in the pathogenesis of HAND. They are relatively short-lived cells that act as a subtle interface between the systemic circulation and the brain tissues; their population can expand during infection and they can be continually replenished by new blood derived MDMs (115). When healthy brains are examined via autopsy, very few MDMs are present, however in the brains of HAD or HAND patients, MDMs are abundant. Fundamentally, activated macrophages should be considered a major driver of HIV inflammation-associated neuropathogensis and an important target for intervention.
Figure 1. Brain Homeostasis and Infection.
In a healthy brain, microglia are the primary immune cell of the brain and few, if any, perivascular macrophages are present. Microglia monitor and communicate with neurons. During HIV infection, HIV-infected monocytes migrate into the brain and differentiate into activated macrophages. Brain microglia become indistinguishable from perivascular macrophages. Neurons and oligodendrocytes become victims of inflammatory chemicals.
Microglia are resident macrophages of the brain that participate in homeostasis during development, adulthood and ageing. These are long-lived cells that interact closely with neurons and astrocytes (36). They survey the CNS to identify, correct or degrade any sub-optimal functioning cells. Similar to macrophages, microglia also police the CNS for infectious agents or defective cells. They can morph into many different shapes depending on their precise location within the CNS. HIV can also infect Microglia, but it is important to note that microglia do not replicate in the brain, so that they do not represent the bulk of cells that occupy pathologic areas of disease wherein MDMs are prevalent. However, because of their role brain housekeeping processes, HIV infection or destruction of resident brain microglia likely contributes HAND pathogenesis (55, 75). Once activated, microglial cells essentially change morphology and become phenotypically and morphologically indistinguishable from MDMs (88).
Other brain cells such as astrocytes, oligodendrocytes, and neurons are impacted but not necessarily infected by HIV. The literature concerning active HIV infection of astrocytes is conflicting. Studies have suggested that astrocytes are non-productively infected (82), that astrocytes can translate early HIV proteins such as env, nef and tat and that later HIV structural proteins are blocked (84), and that the infection of brain astrocytes compromises the BBB (29). There is minimal evidence for active HIV infection/replication in either neurons or oligodendrocytes, although viral proteins have occasionally been detected in these cells. However, neurons and oligodendrocytes are affected by MDM associated neuroinflammation and neurotoxicity, both directly (from individual neurotoxic viral proteins shed by HIV-1 infected cells), and indirectly (from neurotoxic soluble products in the HIV-1-infected environment) resulting in cognitive, motor, and behavioral changes (1).
3. Macrophage activation and signaling
A comprehensive understanding of HIV-infected macrophages and their effects in the brain may assist in the discovery of therapeutic interventions that are less CNS-toxic than current strategies. Most HIV-1 is transported across the BBB in CD14+/CD16+ monocytes (7). Once differentiated into perivascular macrophages, these cells can infect new cells by forming tight cell-to-cell contacts using a matrix metalloproteinase-9 mechanism (79). MDMs, the true driver of HIV-1 infection in the brain, have three different macrophage activation states: 1) M1 is the IFN-γ classically activated macrophage that displays a pro-inflammatory response, 2) M2 is the macrophage activated by IL-4 and IL-13 that displays an anti-inflammatory response and, 3) dM represents a macrophage deactivated by IL-10 which leads to immune suppression (47). Activation of macrophages into M1 and M2 phenotypes mediates an effective immune response against invading pathogens; however, during HIV-1 infection, the system is reversed and the virus uses M1 and M2 pathways to facilitate viral dissemination and pathogenesis (22). Inflammatory M1 macrophages, recruited to sites of infection, typically have a short half-life and may cause tissue damage. Non-inflammatory M2 macrophages produce immunosuppressive cytokines that counteract M1 signaling (22, 71, 96). Macrophages are extremely plastic and some suggest that the phenotypes overlap, resulting in a spectrum of macrophage populations based on their functions (76). Whether macrophages are HIV-1 infected, or uninfected but activated, they still produce soluble substances that contribute to an increasingly toxic, inflammatory, and dysregulated CNS environment (8, 16). Substantial amounts of unintegrated HIV-1 DNA can persist for long periods in macrophages and can support transcription of viral genes such as nef and tat, and can induce neurotoxic cytokines such as CXCL9 and CXCL10 (103, 119). Exposure to Nef and Tat, and/or platelet-activating factor stimulates macrophages to produce the neurotoxin quinolinic acid in physiologically relevant concentrations (99). The soluble products of HIV-exposed macrophages inhibit long-term signal transmission between neurons in brain slices, indicating yet another possible mechanism for HAND (118).
4. Towards novel therapies for HAND: modulating macrophages via gene targets
An enormous amount of literature describes the interactions of macrophages in the context of inflammation, including hypotheses that implicate macrophages in specific HIV-associated pathologies, such as HAND, lymphoma and atherosclerosis (60-64, 91, 92); however, two important questions remain to be answered in the context of HIV-associated neurocognitive disorders: Can the potential for HAND development be measured? Is there a way to modulate the immune response during cART to reduce the occurrence of HAND? Due to the sensitivity of the brain environment, ideal therapies for HAND would modulate known biological pathways that contribute to macrophage activation outside of the brain, rather than introduce foreign substances into the brain.
Research concerning macrophage-associated biomarkers to track the progression of HAND has identified some surrogate and some potentially causal agents such as quinolinic acid, proinflammatory cytokines and metalloproteinases (39). Some monocyte activation markers in the CSF (sCD14, IL-6, IL-8, CCL2, CCl3, CSCL10, and IFN-γ) are elevated in patients on cART regardless of neurocognitive status, while other appear more strongly associated with HAND (sCD14)(54). However, while these studies show promise, functional proteins are still very difficult to predict from genomic measurements alone and no clear biomarker forerunner for HAND has emerged. The complexity of biomarker development was recently highlighted in a proteomics study where, using only two distinct sets of samples, 3608 proteins from HIV infected and uninfected macrophages were identified, 420 of which were significantly altered upon HIV-1 infection (45).
Macrophage activation states have been modified in animal models using humoral factors. For example, the protein activin A promotes a proinflammatory macrophage phenotype (98). C-reactive protein has been shown to inhibit macrophage transformation to the M2 phenotype (27). Thioredoxin-1 and adiponectin also promote anti-inflammatory macrophages of the M2 phenotype (28, 69). Recently, control of the CSF-1 signaling pathway in macrophages has been suggested as a means to develop inhibitors to treat diseases where infiltrating macrophages contribute to their progression (77, 87). Modulation of transcription factor networks may also represent a means to modulate macrophage polarization. For example, peroxisome proliferator-activated receptors (PPARs) are ligand-activated factors that control lipid and glucose metabolism, as well as the inflammatory response (14). Reactive oxygen species (ROS), derived from NADPH-oxidases, compose a network signaling system known as “redox regulation” that impacts the inflammatory response. Formation of redox signals in classically versus alternatively activated macrophages, their action and interaction at the level of key targets, and the resulting physiology is just beginning to be understood and could represent another approach in modulating biological pathways to inhibit HAND development (17). Targeted inhibition of an enzyme called MLK3 has been presented as a strategy to reverse HAND and rebuild synaptic architecture (37). sCD163 is a marker of HIV activity in both acute and chronically infected patients; in the macaque model for neuropathogenesis, levels of sCD163 correlate with monocyte expansion and can predict those animals that will have more rapid and severe neurological disease (21). A novel candidate drug called PA300 was described recently that decreases the number of activated macrophages in the hearts of macaques with cardiac disease (112). PA300 is a polyamine synthesis inhibitor currently under evaluation only in animal models and its effect in the brain compartment remains unknown.
5. Towards novel therapies for HAND: modulating microRNA pathways
Recent advances in understanding how microRNAs (miRs) might impact the cellular function may yield new insights into an emerging target (macrophages) for regulation of HIV associated diseases such as HAND. MiRs are found in animals, plants, and viruses. They are small, naturally occurring, non-coding RNAs that are key regulators of host machinery because they alter protein expression, which, in turn, can impact a variety of critical cell functions including control of cellular proliferation, apoptosis, and differentiation. Putative miR targets are believed to regulate 10-30% of protein-coding messenger RNAs (mRNA) (10, 48, 51). miRs function post-transcriptionally via the RNA-induced silencing complex (RISC) containing an Argonaut (Ago1-4) protein (10, 34, 52). The general steps of miR regulation, including pre-miR processing and interactions with Dicer, Drosha and RISC have been described extensively in the literature (5, 10, 48, 52). More than 2,000 mature human miRs have been validated and new targets of these regulatory RNAs are continually discovered. miR profiling has been used to detect various human diseases including cancer, as well as organ transplant rejection (70, 86, 102). These discoveries have led to a broader use of miR profiling for biomarker development in a variety of diseases including HAND. Therapeutic strategies related to miRs are also being developed; miR antagonists, such as anti-miRs, can inhibit miRs that acquire a gain-of-function in the diseased tissue, whereas miRs that show a loss-of-function can be restored by using miR mimics. Specific miRs are highly expressed in brain tissues where they play an important role in fine-tuning the balance between the transcriptome and proteome in cells (95).
The CD8+ T-cell-depleted and SIV-infected macaque is frequently used for studying the pathogenesis of HIV, especially as it pertains to neuropathogenesis. CD8+ T-cell depletion results in the inability to control viral replication and these macaques rapidly progress to SIV-associated encephalitis. To highlight the use of the macaque model to study miR expression during SIV-induced neuropathogenesis, we used a deep sequencing approach to generate libraries of miR sequences from the temporal lobe of a CD8-depleted macaque during early SIV infection and before the onset of neurological complications (SIVE−) and a CD8-depleted macaque at end-stage disease with SIV-associated encephalitis (SIVE+) (Supplemental material). We subsequently compared these libraries to, 1) a database of known lymphoid-derived macaque (mml) miRs in order to identify miRs that could serve as potential neurodegenerative biomarkers, 2) variation between SIVE− to SIVE+ macaque miRs to identify any changes during progression to SIVE, and 3) miRs derived from humans that are associated with neurological disorders in order to determine their presence and relative abundance (53). Of the 486 published mml-miRs (miRBase release 18), a subset of 315 was identified in brain tissues. Ninety-six of these were found at ≤5.0 RPM. Seventeen upregulated miRs and 27 down-regulated miRs above ≥100 RPM and with a ≥1.5 fold change were identified between early and late samples. Of these, miRs with >200 RPM are shown in Table 1 and were further examined using TarBase (110). TarBase contains more than 65,000 experimentally validated miR-gene interactions (http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=tarbase/index).
Table 1.
Down- and up-regulated temporal lobe Macaque-miRs with greatest fold-change.
| Macaque miRNA | 21 dpi RPM | 75 dpi RPM | Fold Change |
|---|---|---|---|
| mml-miR-24 | 839.93 | 229.02 | −3.67 |
| mml-miR-124a | 4836.71 | 1475.22 | −3.28 |
| mml-miR-30c | 3423.63 | 1364.89 | −2.51 |
| mml-miR-29c | 3267.92 | 1343.23 | −2.43 |
| mml-miR-30a-5p | 9988.39 | 4200.96 | −2.38 |
| mml-miR-30e | 6045.37 | 2603.58 | −2.32 |
| mml-miR-30d | 11423.27 | 5042.82 | −2.27 |
| mml-miR-423-3p | 605.10 | 274.08 | −2.21 |
| mml-miR-92a | 2511.68 | 1228.58 | −2.04 |
| mml-miR-338-3p | 2095.77 | 1025.84 | −2.04 |
| mml-miR-126 | 1051.94 | 526.20 | −2.00 |
| mml-miR-27b | 18489.14 | 10232.94 | −1.81 |
| mml-miR-138 | 785.66 | 456.89 | −1.72 |
| mml-miR-222 | 2824.62 | 1671.89 | −1.69 |
| mml-miR-149 | 803.92 | 485.19 | −1.66 |
| mml-miR-181d | 336.78 | 212.56 | −1.58 |
| mml-miR-148a | 257.66 | 399.99 | 1.55 |
| mml-miR-23b | 875.94 | 1507.85 | 1.72 |
| mml-miR-204 | 316.50 | 558.55 | 1.76 |
| mml-miR-26a | 41420.38 | 73099.72 | 1.76 |
| mml-miR-148b | 345.41 | 630.46 | 1.83 |
| mml-miR-98 | 990.57 | 2173.84 | 2.19 |
| mml-miR-153 | 201.78 | 476.24 | 2.36 |
The highly expressed and up-regulated miRs included miR-153, a miR known to target an anti-apoptotic protein and may increase viral production (BCL-2)(35), miR-98 that targets a protein involved in suppressing cytokine signaling (SOCS)(49), miR-148b that targets a protein (HLA-G) that induces HLA synthesis in response to stressful conditions such as infection (58), miR-26a that targets a protein (EZH2) involved in cell fate decisions (93) and may play a role in central nervous system functions (15), miR-204 that targets homeobox proteins (MEIS1, HOXA10) and may regulate gene expression and hematopoiesis (6), miR-23b that targets chemokine ligand 12 (CXCL12) and is involved in driving lymphocyte cell migration to sites of injury (106) and miR-148a that targets a protein (CDC25B) involved in regulating cell division (68). Six highly expressed and down-regulated miRs also targeted proteins associated with infection. For example, miR-27b targets the Notch pathway, which is involved in controlling gene regulation in the brain during HIV infection (43), miR-30d targets GNAI2, which is known to be increased during HIV infection and associated with increased in CCR5 binding (121), miR-30a-5p targets Beclin1, which is involved in neurodegeneration (78), both miR-30c and miR-30e target UBE2I that modifies ubiquitin, which interacts with HIV-1 nef (50), miR-124a has multiple known targets proteins (EZH2, ROCK2, MPTN, SYCP1, MAPK1, CAV1) that play a role in the nervous system, miR-29c targets BACE1, which is involved in the development of amyloid plaques (97), an early feature of Alzheimer’s disease, and miR-222 that targets a protein (CDKN1B) involved in cell-cycle inhibition (25). Even though Tarbase describes experimentally validated targets, it is important to note that any reported targeting may be tissue/time specific. Interestingly, up-regulated miRs generally targeted mRNAs of genes involved in cell fate decisions/apoptosis and the immune response whereas down-regulated miRs generally targeted mRNAs of genes involved in cell cycle, neurodegeneration, protein degradation, and maintenance of the nervous system.
Specific miRs have been identified with differential expression in the brain during various neurodegenerative diseases (Table 2) (52). Some interesting miR targets in Table 2 include BACE1, which is involved in cerebral deposition of amyloid beta peptide, an early feature of Alzheimer’s disease (31), Pitx3, which is involved in a negative feedback circuit that regulates midbrain function (56) and P250GAP, which plays a key role in the development and refinement of neuronal circuitry (114). Because viruses alter host protein synthesis signaling pathways in cells in order to ensure that ribosomes are recruited to viral messenger RNAs (113), several studies have concluded that HIV/SIV infection also alters miR expression patterns and contributes to neurodegenerative disease (23, 105, 120). Additionally, miR regulation during HIV infection may be quite different than non-infectious neurological disease because HIV encodes the protein TAR, which is capable of sequestering the gene TRBP, an important co-factor in Dicer-mediated miR processing (94) and Tat, which deregulates the levels of several miRs (23).
Table 2.
Neurotropic human miRs identified in macaque brain temporal lobe. Values for miRs were normalized as reads per million (RPM). Highlighted miRs indicate instances where the RPM was >200 and Fold Change was >±1.5.
| Human Neurodegenerative miRs |
Published Findings | Our Findings | |||
|---|---|---|---|---|---|
| Downreglated | Upregulated | SIVE− (RPM) |
SIVE+ (RPM) | Fold Change | |
| hsa-miR-206 | ALS | 3.04 | 0.58 | −5.27 | |
| hsa-miR-133b | Parkinson’s | 194.26 | 51.12 | −3.8 | |
| hsa-miR-338-3p | Prion | 172.96 | 67.87 | −2.55 | |
| hsa-miR-138-5p | AD | 785.66 | 456.89 | −1.72 | |
| hsa-miR-330-3p | Huntington’s | 53.26 | 33.21 | −1.6 | |
| hsa-miR-29b | AD | AD | 162.31 | 103.68 | −1.57 |
| hsa-miR-330-5p | Huntington’s | 290.63 | 200.72 | −1.45 | |
| hsa-miR-103 | AD | 2296.62 | 1642.43 | −1.4 | |
| hsa-miR-125b-5p | AD | 13200.01 | 9514.39 | −1.39 | |
| hsa-miR-29a | AD | AD | 290.63 | 208.52 | −1.39 |
| hsa-miR-342-3p | Prion | 675.6 | 504.54 | −1.34 | |
| hsa-miR-320 | Prion | 259.69 | 195.81 | −1.33 | |
| hsa-let-7b | Prion | 2685.65 | 2015.57 | −1.33 | |
| hsa-miR-9-1 | Huntington’s, AD |
11470.44 | 10139.37 | −1.13 | |
| hsa-miR-132 | Huntington’s | 610.67 | 644.9 | −0.95 | |
| hsa-miR-34c | Parkinson’s | 0 | 0 | 0 | |
| hsa-miR-494 | Prion | 0 | 0.29 | 0 | |
| hsa-miR-328 | Prion | 121.22 | 123.32 | 1.02 | |
| hsa-miR-370 | Prion | 88.76 | 91.84 | 1.03 | |
| hsa-miR-191 | Prion | 12214.51 | 13600.69 | 1.11 | |
| hsa-miR-337-3p | Prion | 1.01 | 1.16 | 1.14 | |
| hsa-let-7d | Prion | 22043.12 | 26685.87 | 1.21 | |
| hsa-miR-107 | AD | 167.38 | 222.67 | 1.33 | |
| hsa-miR-23b | AD | 875.94 | 1507.85 | 1.72 | |
Most neurodegenerative human miRs (ND-miRs) were identified in the macaque temporal lobes, with the exception of four miRs with very low or no expression (Table 2). Some ND-miRs were highly expressed; however, only two of these miRs showed a fold change of ≥1.5 with >200 reads per million (RPM) (Bold Rows, Table 2). In both cases, miR up- or down-regulation was opposite to reports concerning their expression during Alzheimer’s disease. The identification of specific brain miRs associated with neurological disease helps to narrow the field of potential neurodegenerative miR biomarkers that might be missed in routinely collected clinical specimens, such as blood and cerebral spinal fluid, which likely contain a significantly more complex set of miRs associated with multiple and unrelated biological processes.
Certain human miRs are involved in the processes of activating macrophages and microglia during inflammation. During active inflammation in the CNS, both microglia and macrophages express M1 markers and have low levels of miR-124, while macrophages in normal CNS or during disease recovery upregulate miR-124 (89). miR-414, miR-155, and miR-124 also contribute to the generation of monocytes in bone marrow (74). miR-155 is significantly upregulated in macrophages in response to lipopolysaccharide, suggesting that it is important for macrophage activation (83). MiR-124 was altered threefold in the simian experiment described above. In another study, interleukin-27 treated human macrophages induced the expression of novel miRs with antiviral properties (104). These studies clearly demonstrate that miRs contribute to the adaptation of macrophages to the local environment and the regulation of inflammation. Since activation of macrophages is the most predominant feature of HAND, the use of anti-inflammatory miRs such as miR-124 and inhibitors for pro-inflammatory miRs such as miR-155 that modulate macrophage activation could be useful for controlling HAND in HIV-infected individuals.
6. Summary
There is considerable interest by medical research agencies to fully understand HIV brain infection/inflammation and to develop novel treatment methods in the context of HIV eradication. In the National Center for Biontechnology Information’s (NCBI) BioSystems database (https://www.ncbi.nlm.nih.gov/biosystems/), 1419 genes and over 3000 proteins are associated with the macrophage inflammatory response. In NCBI’s Geo Proteins database, over 150,000 genes are associated with macrophage response to virus infection and each record has links to profile and sequence neighbors (http://www.ncbi.nlm.nih.gov/geo/). The National NeuroAIDS Tissue Consortium (NNTC) (http://www.nntc.org/) recently performed brain gene expression arrays to elucidate the pathophysiologies of HIV-1-associated neurocognitive disorders and profiled 24 human patients with varied degrees of HAND and controls and found that over 1,900 gene probes were regulated (38). The Macrophage research is inundated with literature that describes various aspects of the inflammatory process but fails to link them in a universal fashion that has clinical translation as its central goal (111). Furthermore, proteomic studies often incorporate in vitro experiments when in vivo work may reveal an entirely different set of outcomes. The NNTC and the AIDS and Cancer Specimen Resource (ACSR) (http://acsr.ucsf.edu/)(4) are important biorepositories for HIV-infected human biospecimens from a wide spectrum of HIV-related or associated diseases, including cancer, and from appropriate HIV-negative controls that were established as a resource for investigators working in the fields of HIV/AIDS immunology and pathology—such resources are instrumental for investigators to further the field. Biobanks and tissue repositories for studies with HIV-infected animal models are also needed in the research community (108, 109).
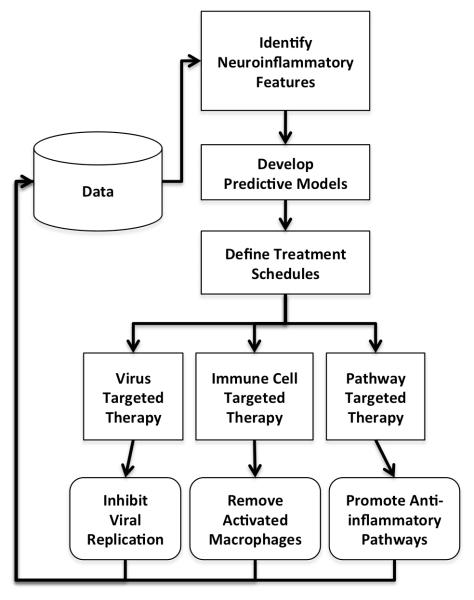
An improved understanding of the systems biology that leads to HAND coupled with the rapidly increasing amount of data being generated on the topic suggests an opportunity to develop molecular biomarkers for HAND, and patient specific treatments for HAND in light of sufficient data (Figure 2). These approaches could make use of machine learning or computational intelligence approaches similar to tools that allow for HIV tropism prediction (65) or microarray analysis (85). However these computational approaches will have to make use of data from largely different sources, over different time scales, at different system levels from genes to miRs to proteins to metabolomics, even demographics. However, the nonlinear classifiers that result have the opportunity to not only assist with improved personalized therapies for patient care but to help elucidate which features (and their combination) that are of most utility for prediction. An understanding of these features establishes a feedback loop of learning that generates more data, additional features, refined modeling, and improved understanding of the systems biology that leads to HIV-associated neuropathogenesis.
Figure 2. A data-driven systems approach to therapy.
In light of sufficient data, predictive models for HAND could be generated using computational intelligence. These models could construct treatment schedules that target the virus, immune cells or biological pathways as needed in light of a complex combination of neuroinflammatory biomarkers. The system would generate additional data on which feature-model-therapy combinations worked best. Repeated cycles of this approach would generate improved therapy and systems that link features to clinical actions. (no color reproduction necessary)
Supplementary Material
Acknowledgements
This project was supported by National Institutes of Health grants R01 NS063897-01A2, P50 GM103297-01 and R01 MH100984-01.
Footnotes
The authors have no conflicting financial interest.
References
- 1.Alter G, Suscovich TJ, Teigen N, Meier A, Streeck H, Brander C, Altfeld M. Single-stranded RNA derived from HIV-1 serves as a potent activator of NK cells. J Immunol. 2007;178:7658–7666. doi: 10.4049/jimmunol.178.12.7658. [DOI] [PubMed] [Google Scholar]
- 2.Annunziata P. Blood-brain barrier changes during invasion of the central nervous system by HIV-1. Old and new insights into the mechanism. J Neurol. 2003;250:901–906. doi: 10.1007/s00415-003-1159-0. [DOI] [PubMed] [Google Scholar]
- 3.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayers LW, Silver S, McGrath MS, Orenstein JM. The AIDS and Cancer Specimen Resource: role in HIV/AIDS scientific discovery. Infectious agents and cancer. 2007;2:7. doi: 10.1186/1750-9378-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bei L, Huang W, Wang H, Shah C, Horvath E, Eklund E. HoxA10 activates CDX4 transcription and Cdx4 activates HOXA10 transcription in myeloid cells. J Biol Chem. 2011;286:19047–19064. doi: 10.1074/jbc.M110.213983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell JE. The neuropathology of adult HIV infection. Rev Neurol (Paris) 1998;154:816–829. [PubMed] [Google Scholar]
- 8.Bergamini A, Faggioli E, Bolacchi F, Gessani S, Cappannoli L, Uccella I, Demin F, Capozzi M, Cicconi R, Placido R, Vendetti S, Colizzi GM, Rocchi G. Enhanced production of tumor necrosis factor-alpha and interleukin-6 due to prolonged response to lipopolysaccharide in human macrophages infected in vitro with human immunodeficiency virus type 1. J Infect Dis. 1999;179:832–842. doi: 10.1086/314662. [DOI] [PubMed] [Google Scholar]
- 9.Bergonzini V, Calistri A, Salata C, Del Vecchio C, Sartori E, Parolin C, Palu G. Nef and cell signaling transduction: a possible involvement in the pathogenesis of human immunodeficiency virus-associated dementia. J Neurovirol. 2009;15:238–248. doi: 10.1080/13550280902939748. [DOI] [PubMed] [Google Scholar]
- 10.Berkhout B, Jeang KT. RISCy business: MicroRNAs, pathogenesis, and viruses. J Biol Chem. 2007;282:26641–26645. doi: 10.1074/jbc.R700023200. [DOI] [PubMed] [Google Scholar]
- 11.Bhaskaran K, Mussini C, Antinori A, Walker AS, Dorrucci M, Sabin C, Phillips A, Porter K. Changes in the incidence and predictors of human immunodeficiency virus-associated dementia in the era of highly active antiretroviral therapy. Ann Neurol. 2008;63:213–221. doi: 10.1002/ana.21225. [DOI] [PubMed] [Google Scholar]
- 12.Bingham R, Ahmed N, Rangi P, Johnson M, Tyrer M, Green J. HIV encephalitis despite suppressed viraemia: a case of compartmentalized viral escape. Int J STD AIDS. 2011;22:608–609. doi: 10.1258/ijsa.2011.010507. [DOI] [PubMed] [Google Scholar]
- 13.Boni J, Emmerich BS, Leib SL, Wiestler OD, Schupbach J, Kleihues P. PCR identification of HIV-1 DNA sequences in brain tissue of patients with AIDS encephalopathy. Neurology. 1993;43:1813–1817. doi: 10.1212/wnl.43.9.1813. [DOI] [PubMed] [Google Scholar]
- 14.Bouhlel MA, Staels B, Chinetti-Gbaguidi G. Peroxisome proliferator-activated receptors--from active regulators of macrophage biology to pharmacological targets in the treatment of cardiovascular disease. Journal of internal medicine. 2008;263:28–42. doi: 10.1111/j.1365-2796.2007.01892.x. [DOI] [PubMed] [Google Scholar]
- 15.Bracken AP, Dietrich N, Pasini D, Hansen KH, Helin K. Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes Dev. 2006;20:1123–1136. doi: 10.1101/gad.381706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brew BJ, Bhalla RB, Paul M, Gallardo H, McArthur JC, Schwartz MK, Price RW. Cerebrospinal fluid neopterin in human immunodeficiency virus type 1 infection. Ann Neurol. 1990;28:556–560. doi: 10.1002/ana.410280413. [DOI] [PubMed] [Google Scholar]
- 17.Brune B, Dehne N, Grossmann N, Jung M, Namgaladze D, Schmid T, von Knethen A, Weigert A. Redox control of inflammation in macrophages. Antioxidants & redox signaling. 2013;19:595–637. doi: 10.1089/ars.2012.4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Budka H. The definition of HIV-specific neuropathology. Acta Pathol Jpn. 1991;41:182–191. doi: 10.1111/j.1440-1827.1991.tb01645.x. [DOI] [PubMed] [Google Scholar]
- 19.Budka H, Costanzi G, Cristina S, Lechi A, Parravicini C, Trabattoni R, Vago L. Brain pathology induced by infection with the human immunodeficiency virus (HIV). A histological, immunocytochemical, and electron microscopical study of 100 autopsy cases. Acta Neuropathol. 1987;75:185–198. doi: 10.1007/BF00687080. [DOI] [PubMed] [Google Scholar]
- 20.Budka H, Wiley CA, Kleihues P, Artigas J, Asbury AK, Cho ES, Cornblath DR, Canto M. C. Dal, DeGirolami U, Dickson D, et al. HIV-associated disease of the nervous system: review of nomenclature and proposal for neuropathology-based terminology. Brain Pathol. 1991;1:143–152. doi: 10.1111/j.1750-3639.1991.tb00653.x. [DOI] [PubMed] [Google Scholar]
- 21.Burdo TH, Lackner A, Williams KC. Monocyte/macrophages and their role in HIV neuropathogenesis. Immunological reviews. 2013;254:102–113. doi: 10.1111/imr.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cassol E, Cassetta L, Alfano M, Poli G. Macrophage polarization and HIV-1 infection. J Leukoc Biol. 2010;87:599–608. doi: 10.1189/jlb.1009673. [DOI] [PubMed] [Google Scholar]
- 23.Chang JR, Mukerjee R, Bagashev A, Del Valle L, Chabrashvili T, Hawkins BJ, He JJ, Sawaya BE. HIV-1 Tat protein promotes neuronal dysfunction through disruption of microRNAs. J Biol Chem. 2011;286:41125–41134. doi: 10.1074/jbc.M111.268466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cherner M, Masliah E, Ellis RJ, Marcotte TD, Moore DJ, Grant I, Heaton RK. Neurocognitive dysfunction predicts postmortem findings of HIV encephalitis. Neurology. 2002;59:1563–1567. doi: 10.1212/01.wnl.0000034175.11956.79. [DOI] [PubMed] [Google Scholar]
- 25.Clark E, Santiago F, Deng L, Chong S, de La Fuente C, Wang L, Fu P, Stein D, Denny T, Lanka V, Mozafari F, Okamoto T, Kashanchi F. Loss of G(1)/S checkpoint in human immunodeficiency virus type 1-infected cells is associated with a lack of cyclin-dependent kinase inhibitor p21/Waf1. J Virol. 2000;74:5040–5052. doi: 10.1128/jvi.74.11.5040-5052.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis LE, Hjelle BL, Miller VE, Palmer DL, Llewellyn AL, Merlin TL, Young SA, Mills RG, Wachsman W, Wiley CA. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42:1736–1739. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- 27.Devaraj S, Jialal I. C-reactive protein polarizes human macrophages to an M1 phenotype and inhibits transformation to the M2 phenotype. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:1397–1402. doi: 10.1161/ATVBAHA.111.225508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Hadri K, Mahmood DF, Couchie D, Jguirim-Souissi I, Genze F, Diderot V, Syrovets T, Lunov O, Simmet T, Rouis M. Thioredoxin-1 promotes anti-inflammatory macrophages of the M2 phenotype and antagonizes atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:1445–1452. doi: 10.1161/ATVBAHA.112.249334. [DOI] [PubMed] [Google Scholar]
- 29.Eugenin EA, Clements JE, Zink MC, Berman JW. Human immunodeficiency virus infection of human astrocytes disrupts blood-brain barrier integrity by a gap junction-dependent mechanism. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:9456–9465. doi: 10.1523/JNEUROSCI.1460-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Everall I, Vaida F, Khanlou N, Lazzaretto D, Achim C, Letendre S, Moore D, Ellis R, Cherner M, Gelman B, Morgello S, Singer E, Grant I, Masliah E. Cliniconeuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. J Neurovirol. 2009;15:360–370. doi: 10.3109/13550280903131915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang M, Wang J, Zhang X, Geng Y, Hu Z, Rudd JA, Ling S, Chen W, Han S. The miR-124 regulates the expression of BACE1/beta-secretase correlated with cell death in Alzheimer’s disease. Toxicol Lett. 2012;209:94–105. doi: 10.1016/j.toxlet.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 32.Fiala M, Rhodes RH, Shapshak P, Nagano I, Martinez-Maza O, Diagne A, Baldwin G, Graves M. Regulation of HIV-1 infection in astrocytes: expression of Nef, TNF-alpha and IL-6 is enhanced in coculture of astrocytes with macrophages. J Neurovirol. 1996;2:158–166. doi: 10.3109/13550289609146878. [DOI] [PubMed] [Google Scholar]
- 33.Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L’Heureux D, Regulier EG, Richardson MW, Amini S, Morgello S, Khalili K, Rappaport J. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol. 2001;7:528–541. doi: 10.1080/135502801753248114. [DOI] [PubMed] [Google Scholar]
- 34.Fukaya T, Tomari Y. PABP is not essential for microRNA-mediated translational repression and deadenylation in vitro. EMBO J. 2011;30:4998–5009. doi: 10.1038/emboj.2011.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao J, Cai Q, Lu J, Jha HC, Robertson ES. Upregulation of cellular Bcl-2 by the KSHV encoded RTA promotes virion production. PLoS One. 2011;6:e23892. doi: 10.1371/journal.pone.0023892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garden GA. Microglia in human immunodeficiency virus-associated neurodegeneration. Glia. 2002;40:240–251. doi: 10.1002/glia.10155. [DOI] [PubMed] [Google Scholar]
- 37.Gelbard HA, Dewhurst S, Maggirwar SB, Kiebala M, Polesskaya O, Gendelman HE. Rebuilding synaptic architecture in HIV-1 associated neurocognitive disease: a therapeutic strategy based on modulation of mixed lineage kinase. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2010;7:392–398. doi: 10.1016/j.nurt.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gelman BB, Chen T, Lisinicchia JG, Soukup VM, Carmical JR, Starkey JM, Masliah E, Commins DL, Brandt D, Grant I, Singer EJ, Levine AJ, Miller J, Winkler JM, Fox HS, Luxon BA, Morgello S. The National NeuroAIDS Tissue Consortium brain gene array: two types of HIV-associated neurocognitive impairment. PloS one. 2012;7:e46178. doi: 10.1371/journal.pone.0046178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gendelman HE. Biomarkers, laboratory, and animal models for the design and development of adjunctive therapies for HIV-1 dementia and other neuroinflammatory disorders. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2007;2:8–13. doi: 10.1007/s11481-006-9050-2. [DOI] [PubMed] [Google Scholar]
- 40.Georgsson G, Stahl-Hennig C, Tenner-Racz K, Uberla K, Stoiber H, Uguccioni M, Dierich M, Ignatius R, Steinman RM, Racz P. The central nervous system in mucosal vaccination of rhesus macaques with simian immunodeficiency virus Deltanef. Neuropathol Appl Neurobiol. 2007;33:644–657. doi: 10.1111/j.1365-2990.2007.00859.x. [DOI] [PubMed] [Google Scholar]
- 41.Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- 42.Grant I, Atkinson JH, Hesselink JR, Kennedy CJ, Richman DD, Spector SA, McCutchan JA. Evidence for early central nervous system involvement in the acquired immunodeficiency syndrome (AIDS) and other human immunodeficiency virus (HIV) infections. Studies with neuropsychologic testing and magnetic resonance imaging. Ann Intern Med. 1987;107:828–836. doi: 10.7326/0003-4819-107-6-828. [DOI] [PubMed] [Google Scholar]
- 43.Grigorian A, Hurford R, Chao Y, Patrick C, Langford TD. Alterations in the Notch4 pathway in cerebral endothelial cells by the HIV aspartyl protease inhibitor, nelfinavir. BMC Neurosci. 2008;9:27. doi: 10.1186/1471-2202-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, Alger J, Singer E, Campbell T, Yiannoutsos C, Cohen R, Navia B. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. Aids. 2011;25:625–633. doi: 10.1097/QAD.0b013e3283427da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haverland NA, Fox HS, Ciborowski P. Quantitative Proteomics by SWATH-MS Reveals Altered Expression of Nucleic Acid Binding and Regulatory Proteins in HIV-1-Infected Macrophages. Journal of proteome research. 2014 doi: 10.1021/pr4012602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heaton RK, Clifford DB, Franklin DR, Jr., Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herbein G, Varin A. The macrophage in HIV-1 infection: from activation to deactivation? Retrovirology. 2010;7:33. doi: 10.1186/1742-4690-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Houzet L, Jeang KT. MicroRNAs and human retroviruses. Biochim Biophys Acta. 2011;1809:686–693. doi: 10.1016/j.bbagrm.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu G, Zhou R, Liu J, Gong AY, Chen XM. MicroRNA-98 and let-7 regulate expression of suppressor of cytokine signaling 4 in biliary epithelial cells in response to Cryptosporidium parvum infection. J Infect Dis. 2010;202:125–135. doi: 10.1086/653212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin YJ, Cai CY, Zhang X, Burakoff SJ. Lysine 144, a ubiquitin attachment site in HIV-1 Nef, is required for Nef-mediated CD4 down-regulation. J Immunol. 2008;180:7878–7886. doi: 10.4049/jimmunol.180.12.7878. [DOI] [PubMed] [Google Scholar]
- 51.John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. Human MicroRNA targets. PLoS Biol. 2004;2:e363. doi: 10.1371/journal.pbio.0020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Junn E, Mouradian MM. MicroRNAs in neurodegenerative diseases and their therapeutic potential. Pharmacol Ther. 2011 doi: 10.1016/j.pharmthera.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Junn E, Mouradian MM. MicroRNAs in neurodegenerative diseases and their therapeutic potential. Pharmacology & therapeutics. 2012;133:142–150. doi: 10.1016/j.pharmthera.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kamat A, Lyons JL, Misra V, Uno H, Morgello S, Singer EJ, Gabuzda D. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. Journal of acquired immune deficiency syndromes. 2012;60:234–243. doi: 10.1097/QAI.0b013e318256f3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaushik DK, Gupta M, Basu A. Microglial response to viral challenges: every silver lining comes with a cloud. Frontiers in bioscience. 2011;16:2187–2205. doi: 10.2741/3847. [DOI] [PubMed] [Google Scholar]
- 56.Kim J, Inoue K, Ishii J, Vanti WB, Voronov SV, Murchison E, Hannon G, Abeliovich A. A MicroRNA feedback circuit in midbrain dopamine neurons. Science. 2007;317:1220–1224. doi: 10.1126/science.1140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koedel U, Kohleisen B, Sporer B, Lahrtz F, Ovod V, Fontana A, Erfle V, Pfister HW. HIV type 1 Nef protein is a viral factor for leukocyte recruitment into the central nervous system. J Immunol. 1999;163:1237–1245. [PubMed] [Google Scholar]
- 58.Kulkarni S, Savan R, Qi Y, Gao X, Yuki Y, Bass SE, Martin MP, Hunt P, Deeks SG, Telenti A, Pereyra F, Goldstein D, Wolinsky S, Walker B, Young HA, Carrington M. Differential microRNA regulation of HLA-C expression and its association with HIV control. Nature. 2011;472:495–498. doi: 10.1038/nature09914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumar S, Jack R. Origin of monocytes and their differentiation to macrophages and dendritic cells. J Endotoxin Res. 2006;12:278–284. doi: 10.1179/096805106X118861. [DOI] [PubMed] [Google Scholar]
- 60.Lamers SL, Fogel GB, Huysentruyt LC, McGrath MS. HIV-1 nef protein visits B-cells via macrophage nanotubes: a mechanism for AIDS-related lymphoma pathogenesis? Current HIV research. 2010;8:638–640. doi: 10.2174/157016210794088209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lamers SL, Fogel GB, Singer EJ, Salemi M, Nolan DJ, Huysentruyt LC, McGrath MS. HIV-1 Nef in macrophage-mediated disease pathogenesis. International reviews of immunology. 2012;31:432–450. doi: 10.3109/08830185.2012.737073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lamers SL, Poon AF, McGrath MS. HIV-1 nef protein structures associated with brain infection and dementia pathogenesis. PloS one. 2011;6:e16659. doi: 10.1371/journal.pone.0016659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lamers SL, Salemi M, Galligan DC, de Oliveira T, Fogel GB, Granier SC, Zhao L, Brown JN, Morris A, Masliah E, McGrath MS. Extensive HIV-1 intra-host recombination is common in tissues with abnormal histopathology. PloS one. 2009;4:e5065. doi: 10.1371/journal.pone.0005065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lamers SL, Salemi M, Galligan DC, Morris A, Gray R, Fogel G, Zhao L, McGrath MS. Human immunodeficiency virus-1 evolutionary patterns associated with pathogenic processes in the brain. J Neurovirol. 2010;16:230–241. doi: 10.3109/13550281003735709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lamers SL, Salemi M, McGrath MS, Fogel GB. Prediction of R5, X4, and R5X4 HIV-1 coreceptor usage with evolved neural networks. IEEE/ACM Trans Comput Biol Bioinform. 2008;5:291–300. doi: 10.1109/TCBB.2007.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lehmann MH, Masanetz S, Kramer S, Erfle V. HIV-1 Nef upregulates CCL2/MCP-1 expression in astrocytes in a myristoylation- and calmodulin-dependent manner. J Cell Sci. 2006;119:4520–4530. doi: 10.1242/jcs.03231. [DOI] [PubMed] [Google Scholar]
- 67.Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Morgello S, Simpson D, Grant I, Ellis RJ. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liffers ST, Munding JB, Vogt M, Kuhlmann JD, Verdoodt B, Nambiar S, Maghnouj A, Mirmohammadsadegh A, Hahn SA, Tannapfel A. MicroRNA-148a is down-regulated in human pancreatic ductal adenocarcinomas and regulates cell survival by targeting CDC25B. Lab Invest. 2011;91:1472–1479. doi: 10.1038/labinvest.2011.99. [DOI] [PubMed] [Google Scholar]
- 69.Lovren F, Pan Y, Quan A, Szmitko PE, Singh KK, Shukla PC, Gupta M, Chan L, Al-Omran M, Teoh H, Verma S. Adiponectin primes human monocytes into alternative anti-inflammatory M2 macrophages. American journal of physiology. Heart and circulatory physiology. 2010;299:H656–663. doi: 10.1152/ajpheart.00115.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 71.Mantovani A, Sica A, Allavena P, Garlanda C, Locati M. Tumor-associated macrophages and the related myeloid-derived suppressor cells as a paradigm of the diversity of macrophage activation. Hum Immunol. 2009;70:325–330. doi: 10.1016/j.humimm.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 72.Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, Achim CL, McCutchan JA, Nelson JA, Atkinson JH, Grant I. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann Neurol. 1997;42:963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- 73.McArthur JC, McDermott MP, McClernon D, St Hillaire C, Conant K, Marder K, Schifitto G, Selnes OA, Sacktor N, Stern Y, Albert SM, Kieburtz K, deMarcaida JA, Cohen B, Epstein LG. Attenuated central nervous system infection in advanced HIV/AIDS with combination antiretroviral therapy. Arch Neurol. 2004;61:1687–1696. doi: 10.1001/archneur.61.11.1687. [DOI] [PubMed] [Google Scholar]
- 74.Mishima T, Mizuguchi Y, Kawahigashi Y, Takizawa T. RT-PCR-based analysis of microRNA (miR-1 and -124) expression in mouse CNS. Brain research. 2007;1131:37–43. doi: 10.1016/j.brainres.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 75.Mocchetti I, Campbell LA, Harry GJ, Avdoshina V. When human immunodeficiency virus meets chemokines and microglia: neuroprotection or neurodegeneration? Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2013;8:118–131. doi: 10.1007/s11481-012-9353-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mouchemore KA, Pixley FJ. CSF-1 signaling in macrophages: pleiotrophy through phosphotyrosine-based signaling pathways. Critical reviews in clinical laboratory sciences. 2012;49:49–61. doi: 10.3109/10408363.2012.666845. [DOI] [PubMed] [Google Scholar]
- 78.Munz C. Beclin-1 targeting for viral immune escape. Viruses. 2011;3:1166–1178. doi: 10.3390/v3071166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Muratori C, Sistigu A, Ruggiero E, Falchi M, Bacigalupo I, Palladino C, Toschi E, Federico M. Macrophages transmit human immunodeficiency virus type 1 products to CD4-negative cells: involvement of matrix metalloproteinase 9. Journal of virology. 2007;81:9078–9087. doi: 10.1128/JVI.00675-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nath A, Clements JE. Eradication of HIV from the brain: reasons for pause. AIDS. 2011;25:577–580. doi: 10.1097/QAD.0b013e3283437d2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Navia BA, Jordan BD, Price RW. The AIDS dementia complex: I. Clinical features. Ann Neurol. 1986;19:517–524. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- 82.Neumann M, Felber BK, Kleinschmidt A, Froese B, Erfle V, Pavlakis GN, Brack-Werner R. Restriction of human immunodeficiency virus type 1 production in a human astrocytoma cell line is associated with a cellular block in Rev function. Journal of virology. 1995;69:2159–2167. doi: 10.1128/jvi.69.4.2159-2167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.O’Connell RM, Rao DS, Chaudhuri AA, Boldin MP, Taganov KD, Nicoll J, Paquette RL, Baltimore D. Sustained expression of microRNA-155 in hematopoietic stem cells causes a myeloproliferative disorder. The Journal of experimental medicine. 2008;205:585–594. doi: 10.1084/jem.20072108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Overholser ED, Coleman GD, Bennett JL, Casaday RJ, Zink MC, Barber SA, Clements JE. Expression of simian immunodeficiency virus (SIV) nef in astrocytes during acute and terminal infection and requirement of nef for optimal replication of neurovirulent SIV in vitro. Journal of virology. 2003;77:6855–6866. doi: 10.1128/JVI.77.12.6855-6866.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peng B, Zhu D, Ander BP, Zhang X, Xue F, Sharp FR, Yang X. An integrative framework for Bayesian variable selection with informative priors for identifying genes and pathways. PloS one. 2013;8:e67672. doi: 10.1371/journal.pone.0067672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peng S, Zeng X, Li X, Peng X, Chen L. Multi-class cancer classification through gene expression profiles: microRNA versus mRNA. J Genet Genomics. 2009;36:409–416. doi: 10.1016/S1673-8527(08)60130-7. [DOI] [PubMed] [Google Scholar]
- 87.Pixley FJ. Macrophage Migration and Its Regulation by CSF-1. International journal of cell biology. 2012;2012:501962. doi: 10.1155/2012/501962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ponomarev ED, Shriver LP, Maresz K, Dittel BN. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. Journal of neuroscience research. 2005;81:374–389. doi: 10.1002/jnr.20488. [DOI] [PubMed] [Google Scholar]
- 89.Ponomarev ED, Veremeyko T, Weiner HL. MicroRNAs are universal regulators of differentiation, activation, and polarization of microglia and macrophages in normal and diseased CNS. Glia. 2013;61:91–103. doi: 10.1002/glia.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pumarola-Sune T, Navia BA, Cordon-Cardo C, Cho ES, Price RW. HIV antigen in the brains of patients with the AIDS dementia complex. Ann Neurol. 1987;21:490–496. doi: 10.1002/ana.410210513. [DOI] [PubMed] [Google Scholar]
- 91.Salemi M, Lamers SL, Huysentruyt LC, Galligan D, Gray RR, Morris A, McGrath MS. Distinct patterns of HIV-1 evolution within metastatic tissues in patients with non-Hodgkins lymphoma. PloS one. 2009;4:e8153. doi: 10.1371/journal.pone.0008153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Salemi M, Lamers SL, Yu S, de Oliveira T, Fitch WM, McGrath MS. Phylodynamic analysis of human immunodeficiency virus type 1 in distinct brain compartments provides a model for the neuropathogenesis of AIDS. Journal of virology. 2005;79:11343–11352. doi: 10.1128/JVI.79.17.11343-11352.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sander S, Bullinger L, Klapproth K, Fiedler K, Kestler HA, Barth TF, Moller P, Stilgenbauer S, Pollack JR, Wirth T. MYC stimulates EZH2 expression by repression of its negative regulator miR-26a. Blood. 2008;112:4202–4212. doi: 10.1182/blood-2008-03-147645. [DOI] [PubMed] [Google Scholar]
- 94.Sanghvi VR, Steel LF. The cellular TAR RNA binding protein, TRBP, promotes HIV-1 replication primarily by inhibiting the activation of double-stranded RNA-dependent kinase PKR. J Virol. 2011;85:12614–12621. doi: 10.1128/JVI.05240-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saugstad JA. MicroRNAs as effectors of brain function with roles in ischemia and injury, neuroprotection, and neurodegeneration. J Cereb Blood Flow Metab. 2010;30:1564–1576. doi: 10.1038/jcbfm.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18:349–355. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 97.Siegenthaler BM, Rajendran L. Retromers in Alzheimer’s disease. Neurodegener Dis. 2012;10:116–121. doi: 10.1159/000335910. [DOI] [PubMed] [Google Scholar]
- 98.Sierra-Filardi E, Puig-Kroger A, Blanco FJ, Nieto C, Bragado R, Palomero MI, Bernabeu C, Vega MA, Corbi AL. Activin A skews macrophage polarization by promoting a proinflammatory phenotype and inhibiting the acquisition of anti-inflammatory macrophage markers. Blood. 2011;117:5092–5101. doi: 10.1182/blood-2010-09-306993. [DOI] [PubMed] [Google Scholar]
- 99.Smith DG, Guillemin GJ, Pemberton L, Kerr S, Nath A, Smythe GA, Brew BJ. Quinolinic acid is produced by macrophages stimulated by platelet activating factor, Nef and Tat. Journal of neurovirology. 2001;7:56–60. doi: 10.1080/135502801300069692. [DOI] [PubMed] [Google Scholar]
- 100.Snider WD, Simpson DM, Nielsen S, Gold JW, Metroka CE, Posner JB. Neurological complications of acquired immune deficiency syndrome: analysis of 50 patients. Ann Neurol. 1983;14:403–418. doi: 10.1002/ana.410140404. [DOI] [PubMed] [Google Scholar]
- 101.Strickland S, Veras N, Prosperi M, Burdo T, Nolan D, Suchard M, Williams K, Salemi M. Correlating Neuropathogenesis with Brain Infection Dynamics in the SIVmac251-infected CD8-depleted Rhesus Macaque Model Using Bayesian Phylogeography. 20th Conference on retroviruses and Opportunistic Infection; Atlanta, GA. 2013. [Google Scholar]
- 102.Sui W, Dai Y, Huang Y, Lan H, Yan Q, Huang H. Microarray analysis of MicroRNA expression in acute rejection after renal transplantation. Transpl Immunol. 2008;19:81–85. doi: 10.1016/j.trim.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 103.Sui Y, Stehno-Bittel L, Li S, Loganathan R, Dhillon NK, Pinson D, Nath A, Kolson D, Narayan O, Buch S. CXCL10-induced cell death in neurons: role of calcium dysregulation. Eur J Neurosci. 2006;23:957–964. doi: 10.1111/j.1460-9568.2006.04631.x. [DOI] [PubMed] [Google Scholar]
- 104.Swaminathan S, Hu X, Zheng X, Kriga Y, Shetty J, Zhao Y, Stephens R, Tran B, Baseler MW, Yang J, Lempicki RA, Huang D, Lane HC, Imamichi T. Interleukin-27 treated human macrophages induce the expression of novel microRNAs which may mediate anti-viral properties. Biochemical and biophysical research communications. 2013;434:228–234. doi: 10.1016/j.bbrc.2013.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tatro ET, Scott ER, Nguyen TB, Salaria S, Banerjee S, Moore DJ, Masliah E, Achim CL, Everall IP. Evidence for Alteration of Gene Regulatory Networks through MicroRNAs of the HIV-infected brain: novel analysis of retrospective cases. PLoS One. 2010;5:e10337. doi: 10.1371/journal.pone.0010337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Timotijevic G, Stojkovic M. Mostarica, Miljkovic D. CXCL12: Role in neuroinflammation. Int J Biochem Cell Biol. 2012 doi: 10.1016/j.biocel.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 107.van Marle G, Henry S, Todoruk T, Sullivan A, Silva C, Rourke SB, Holden J, McArthur JC, Gill MJ, Power C. Human immunodeficiency virus type 1 Nef protein mediates neural cell death: a neurotoxic role for IP-10. Virology. 2004;329:302–318. doi: 10.1016/j.virol.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 108.Vaught J, Rogers J, Myers K, Lim MD, Lockhart N, Moore H, Sawyer S, Furman JL, Compton C. An NCI perspective on creating sustainable biospecimen resources. Journal of the National Cancer Institute. Monographs. 2011;2011:1–7. doi: 10.1093/jncimonographs/lgr006. [DOI] [PubMed] [Google Scholar]
- 109.Vaught JB, Henderson MK, Compton CC. Biospecimens and biorepositories: from afterthought to science. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21:253–255. doi: 10.1158/1055-9965.EPI-11-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vergoulis T, Vlachos IS, Alexiou P, Georgakilas G, Maragkakis M, Reczko M, Gerangelos S, Koziris N, Dalamagas T, Hatzigeorgiou AG. TarBase 6.0: capturing the exponential growth of miRNA targets with experimental support. Nucleic acids research. 2012;40:D222–229. doi: 10.1093/nar/gkr1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vodovotz Y, Constantine G, Faeder J, Mi Q, Rubin J, Bartels J, Sarkar J, Squires RH, Jr., Okonkwo DO, Gerlach J, Zamora R, Luckhart S, Ermentrout B, An G. Translational systems approaches to the biology of inflammation and healing. Immunopharmacology and immunotoxicology. 2010;32:181–195. doi: 10.3109/08923970903369867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Walker J, Burdo T, Miller A, Misgin K, Sulciner M, McGrath M, Williams K. Macrophages in Hearts of SIV+ Rhesus Macaques with Cardiac Disease Are Decreased Using PA300. Conference on Retroviruses and Opportunistic Infection; Atlanta, GA. 2013. [Google Scholar]
- 113.Walsh D, Mohr I. Viral subversion of the host protein synthesis machinery. Nat Rev Microbiol. 2011;9:860–875. doi: 10.1038/nrmicro2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wayman GA, Davare M, Ando H, Fortin D, Varlamova O, Cheng HY, Marks D, Obrietan K, Soderling TR, Goodman RH, Impey S. An activity-regulated microRNA controls dendritic plasticity by down-regulating p250GAP. Proc Natl Acad Sci U S A. 2008;105:9093–9098. doi: 10.1073/pnas.0803072105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Williams K, Alvarez X, Lackner AA. Central nervous system perivascular cells are immunoregulatory cells that connect the CNS with the peripheral immune system. Glia. 2001;36:156–164. doi: 10.1002/glia.1105. [DOI] [PubMed] [Google Scholar]
- 116.Williams K, Burdo TH. Monocyte mobilization, activation markers, and unique macrophage populations in the brain: observations from SIV infected monkeys are informative with regard to pathogenic mechanisms of HIV infection in humans. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2012;7:363–371. doi: 10.1007/s11481-011-9330-3. [DOI] [PubMed] [Google Scholar]
- 117.Williams KC, Hickey WF. Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annu Rev Neurosci. 2002;25:537–562. doi: 10.1146/annurev.neuro.25.112701.142822. [DOI] [PubMed] [Google Scholar]
- 118.Xiong H, Zeng YC, Zheng J, Thylin M, Gendelman HE. Soluble HIV-1 infected macrophage secretory products mediate blockade of long-term potentiation: a mechanism for cognitive dysfunction in HIV-1-associated dementia. Journal of neurovirology. 1999;5:519–528. doi: 10.3109/13550289909045381. [DOI] [PubMed] [Google Scholar]
- 119.Yao H, Bethel-Brown C, Li CZ, Buch SJ. HIV neuropathogenesis: a tight rope walk of innate immunity. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2010;5:489–495. doi: 10.1007/s11481-010-9211-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yelamanchili SV, Chaudhuri AD, Chen LN, Xiong H, Fox HS. MicroRNA-21 dysregulates the expression of MEF2C in neurons in monkey and human SIV/HIV neurological disease. Cell Death Dis. 2010;1:e77. doi: 10.1038/cddis.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhao J, Ma L, Wu YL, Wang P, Hu W, Pei G. Chemokine receptor CCR5 functionally couples to inhibitory G proteins and undergoes desensitization. J Cell Biochem. 1998;71:36–45. doi: 10.1002/(sici)1097-4644(19981001)71:1<36::aid-jcb4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 122.Zhao L, Galligan DC, Lamers SL, Yu S, Shagrun L, Salemi M, McGrath MS. High level HIV-1 DNA concentrations in brain tissues differentiate patients with post-HAART AIDS dementia complex or cardiovascular disease from those with AIDS. Sci China C Life Sci. 2009;52:651–656. doi: 10.1007/s11427-009-0085-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.