Abstract
No effective therapy is currently available to promote recovery following ischemic stroke. Stem cells have been proposed as a potential source of new cells to replace those lost due to central nervous system injury, as well as a source of trophic molecules to minimize damage and promote recovery. We undertook a detailed review of data from recent basic science and preclinical studies to investigate the potential application of endogenous and exogenous stem cell therapies for treatment of cerebral ischemia. To date, spontaneous endogenous neurogenesis has been observed in response to ischemic injury, and can be enhanced via infusion of appropriate cytokines. Exogenous stem cells from multiple sources can generate neural cells that survive and form synaptic connections after transplantation in the stroke-injured brain. Stem cells from multiple sources cells also exhibit neuroprotective properties that may ameliorate stroke deficits. In many cases, functional benefits observed are likely independent of neural differentiation, though exact mechanisms remain poorly understood. Future studies of neuroregeneration will require the demonstration of function in endogenously born neurons following focal ischemia. Further, methods are currently lacking to definitively demonstrate the therapeutic effect of newly introduced neural cells. Increased plasticity following stroke may facilitate the functional integration of new neurons, but the loss of appropriate guidance cues and supporting architecture in the infarct cavity will likely impede the restoration of lost circuitry. As such careful investigation of the mechanisms underlying trophic benefits will be essential. Evidence to date suggest that continued development of stem cell therapies may ultimately lead to viable treatment options for ischemic brain injury.
Keywords: stem cells, stroke, neural repair
Introduction
Cerebral Ischemia: The Clinical Challenge
With an incidence of over 700,000 each year in the United States, stroke is the leading cause of disability, and the third leading cause of death in the western world behind heart disease and cancer (Kissela et al., 2001). The number of individuals over the age of sixty-five is expected to double between 2005 and 2030 (American Association on Aging, 2005). Further, the risk of stroke more than doubles for each decade of life over age fifty-five (American Heart Association, 2007). As such, substantial advances in the prevention and treatment of stroke are of paramount importance.
While surgical decompression can reduce mortality in severe cases (Vahedi et al., 2007), the only currently available intervention to reduce the size of the infarct is recombinant tissue plaminogen activator (t-PA) (NINDS Stroke Study Group, 1995). T-PA is approved for use only if administered within 3 hours of the onset of ischemia, though optimal results are observed if given within 90 minutes (Hacke et al., 2004). Unfortunately due to this narrow time window as well as a number of contraindications, t-PA therapy is only available to about 5% of stroke victims evaluated in the emergency room. Of these, t-PA may be expected to yield a ∼30% increase in the number of patients avoiding long-term neurologic deficits (Ropper and Brown, 2005). Advances in endovascular techniques may improve recanalization sufficiently to improve survival, but has yet to be substantiated by randomized clinical trials (Burns, 2008). Unfortunately, although a plethora of neuroprotective compounds have shown promise in animal models, no other treatment to date has shown efficacy in clinical trials (Dirnagl, 2006).
In the background of this imperative clinical need, hundreds of studies have recently been published to investigate the therapeutic potential of either endogenous or transplanted stem cells in laboratory models of stroke. To their advantage, stem cells have the capacity to respond actively to their environment, migrate to areas of injury and secrete neuroprotective compounds, in addition to their potential to generate a variety of new functional cell types. Such properties may afford them therapeutic potential both in the acute phase as well as at later time points after conventional medical therapies would no longer be effective. We here critically review this body of literature, and conclude that although reconstructing normal brain circuitry following stroke via stem cells is not likely in the foreseeable future, and although great care must be taken to ensure safety before considering clinical trials, prelimniary evidence supports the therapeutic potential of certain stem cells for treatment of ischemic brain injury in animal models.
Stem Cells: Substrate for regeneration
Definitions and types of stem cells
Strictly defined, stem cells possess the cardinal features of multipotency and self-renewal. Multipotency is the ability to differentiate into multiple functional cell types. As such, stem cells should be able to functionally reconstitute appropriate tissues in vivo. Self-renewal describes the ability of stem cells to make identical copies of themselves via cell division. At least one daughter cell of a self-renewing cell division must possess the same differentiation and self-renewal potential as the parent cell. As such, stem cells exhibit extensive proliferation and are often presumed to be able to divide indefinitely, yielding a virtually unlimited supply of cells.
A variety of stem cells can be identified that differ in their potency, or the diversity of cell types they can generate. The ultimate example of “potency” is the zygote, a “totipotent” cell that can therefore give rise to both embryonic and extraembryonic tissues (Hyslop et al., 2005). However, given that the zygote is transient and does not self-renew, it is not generally regarded as a stem cell. Embryonic stem cells (ESCs) have the broadest potential of any true stem cell. These cells, isolated from the inner cell mass of the blastocyst, are “pluripotent,” meaning that they can give rise to all cell types within the developing embryo. Remarkably, the adult mammal also contains a large number of stem cells. These more restricted “tissue-specific” stem cells are capable of generating certain local cell types but not those from unrelated tissues. The best studied tissue-specific stem cell is the hematopoietic stem cell, which is capable of generating all blood cell types. Other tissue-specific stem cells have been identified in numerous organs including muscle, skin, gut, liver, pancreas and brain. A recent paper described the derivation of ESC-like cells from mouse testes, perhaps suggesting the possibility of obtaining pluripotent cells from the adult organism (Guan et al., 2006).
Recent studies have demonstrated the induction of pluripotent stem (iPS) cells from terminally differentiated somatic cells via nuclear reprogramming. Takahashi and Yamanaka (2006) systematically transduced mouse fibroblasts with retroviral vectors containing cDNA that encoded one of 24 genes known to be associated with the ESC state. Among these 24 genes they found that transduction with only 4 genes: Oct3/4, Sox2, c-Myc, and Klf4 was sufficient to induce mouse fibroblasts to develop into ES-like cells. Subsequent to this report, Takahashi et al., (2007) were able to extend this finding to human fibroblasts. Thomson and colleagues (Yu et al., 2007) likewise using human fibroblasts, employed a slightly different combination: Oct4, Sox 2, NANOG, and LIN28 to generate iPS cells, importantly avoiding the proto-oncogene c-myc. Although the effects of using iPS cells have yet to be examined in ischemic brain injury, a recent report by Jaenisch and colleagues (Wernig et al., 2008) demonstrates the ability of murine fibroblasts that were reprogrammed to become iPS cells could be induced to differentiate into dopamine neurons and correct neurologic deficits in a rat model of Parkinson's disease. All other indications likewise suggest that iPS cells behave identically to embryo-derived ESCs.
Stem cell behavior
In vivo, “true” self-renewing stem cells generally divide infrequently (Morshead et al., 1994). This feature may help protect the integrity of the genetic material carried by stem cells, as continuous proliferation is associated with increased risk of mutations and possible subsequent tumorgenesis (Miura et al., 2006). Nevertheless, certain adult tumors are now hypothesized to arise from resident stem cells (Dalerba et al., 2007; Nicolis, 2007). In order to generate the number of cells required for in vivo regeneration while minimizing stem cell proliferation, adult stem cells undergo asymmetric self-renewing divisions, giving rise to one stem cell and one committed progenitor cell that can proliferate rapidly and thereby give rise to a large number of more differentiated progeny. Progenitor cells are often unipotent, differentiating into only a single differentiated cell type. In spite of their limited self-renewal capacity, progenitor cells have also been implicated in possible tumorgenesis (Doetsch et al., 2002).
The longstanding dogma of stem cell biology posits that stem and progenitor cells can either self-renew or give rise to more restricted daughter cells, but cannot to move backwards along this developmental sequence or “dedifferentiate.” However, several recent reports have suggested that under specific conditions, certain adult stem cells have the potential to generate cell types from unrelated organs, a phenomenon termed transdifferentiation (Verfaillie, 2002). At present, it is unclear if this phenomenon represents an artifact of in vitro culture conditions (Morshead et al., 2002), or if rare cells with greater potency may in fact exist in vivo. Alternatively, some have suggested that specified stem cells may have the capacity to dedifferentiate (Kondo and Raff, 2000). Many studies describing transdifferentiation have proven difficult to replicate, leading to widespread uncertainty regarding the phenomenon (Giles, 2006). Further, in some cases, initial reports of in vivo transdifferentiation were later challenged by demonstration that certain cell types may fuse with mature cells from unrelated organs (Terada et al., 2002; Wagers et al., 2002; Ying et al., 2002). As such, embryonic stem cells currently remain the gold standard for pluripotency.
Stem Cell Applications
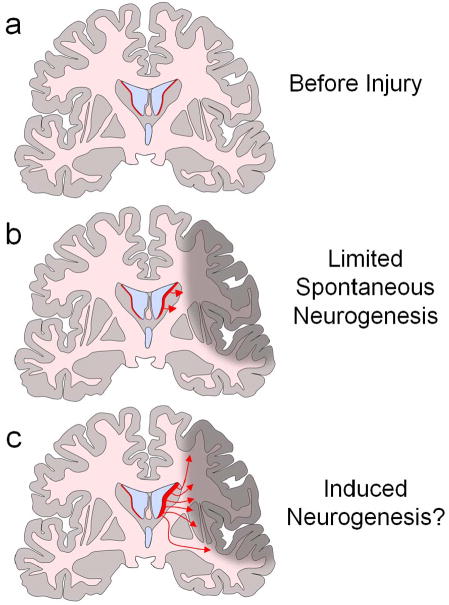
The most commonly discussed role for stem cells involves replacement of cell types lost due to disease or injury, i.e., “cell replacement therapy” (Lindvall and Kokaia, 2006). Such replacement may be achieved either by in vitro differentiation of stem cells into the desired cell type followed by transplantation of the predifferentiated cells into the affected region (Kim et al., 2002), or by direct transplantation of stem cells followed by spontaneous in vivo differentiation of the stem cells into the needed cell types (Bjorklund et al., 2002). The former strategy may be of particular value in situations where a specific cell type is selectively lost and function may be restored by replacement of cells with similar properties, such as diabetes mellitus type 1, ALS or Parkinson's disease. The use of undifferentiated cells however, may be of value when multiple cell types are needed to restore function to a broadly damaged area. For example, undifferentiated multipotent adult progenitor cells (MAPCs) appear to be capable of differentiation into endothelium, smooth muscle and skeletal muscle after delivery in a mouse model of limb ischemia (Aranguren et al., 2008). Likewise, neural stem cells generate glial as well as neural cell types. An alternate cell replacement strategy involves the mobilization of resident tissue specific stem cells to provide the substrate for cell replacement (Chmielnicki et al., 2004; Kolb et al., 2007). In many tissues such as skin, gut and bone marrow, resident stem cells provide a continual source of new cells to replace those lost due to normal wear and tear. Further, resident stem cells in many parts of the body contribute to regeneration after injury. Spontaneous neurogenesis in response to brain injury, though it may occur in some brain regions, is inadequate to allow functional recovery. Thus strategies are being sought to amplify this endogenous regenerative response (Androutsellis-Theotokis et al., 2006), as will be discussed further below.
In addition to cell replacement therapy, it is becoming increasingly understood that stem cells also serve supportive roles. For example, certain stem cells appear to exhibit robust tropism for injury. While most resident cells may die in an injured area, stem cells appear to be attracted to the area of injury and may secrete molecules that promote survival and regeneration (Ourednik et al., 2002). Stem cells may also be recruitment to neoplasms where they are associated with decreased tumor growth via secretion of antiangiogenic and proinflammatory compounds (Yip et al., 2006). Several groups are now looking into harnessing stem cells' tropism for injured areas by delivery of stem cells genetically engineered to deliver therapeutic compounds specifically to the area of injury (Muller et al., 2006). Such an approach may prove particularly relevant for ischemic injuries, in which compromised blood flow may make delivery of such drugs to the affected region more challenging after systemic administration.
Technical considerations for studying neuroregeneration
Neuroregeneration is relatively new science, and with it have come new insights into techniques initially used in its study (Breunig et al, 2007). In some cases, original results obtained using the best methodologies available at the time were subsequently called into question when these methodologies proved capable of yielding misleading results (Priller et al., 2001, Svendsen et al., 2001). Thus, before launching into a discussion of endogenous and exogenous stem cells applications for stroke, we briefly highlight major potential methodological pitfalls, which if not considered may confound the accurate identification of new neurons. Where possible, alternative strategies are suggested that currently appear reliable.
Identification of endogenous neurogenesis
One of the most important techniques used in the study of adult neurogenesis involves the labeling of endogenous dividing cells by systemic administration of thymidine analogs such as bromodeoxyuridine (BrdU) and tritiated thymidine. These incorporate into DNA during S-phase of cell division, and can subsequently be identified in the nucleus of any cell born within a few hours after administration. Co-localization of such markers with neuronal proteins such as NeuN is routinely used to identify newly born neurons. A number of considerations apply to the use of thymidine analogs, including dilution of label with continued cell proliferation, toxicity at high doses, and care to ensure unambiguous co-localization via 3D confocal microscopy (Taupin, 2007). It is also known that normal cells also undergo continual DNA repair, and may thereby take up the label without dividing, though current evidence argues against a detectable level of labeling accumulating through normal DNA repair with short-term administration (Cooper-Kuhn and Kuhn, 2002). Rather, more concerning is that damaged neurons in ischemic brain regions may reenter the cell cycle in a process of abortive DNA synthesis prior to dying via apoptosis (Kuan et al., 2004). As such, BrdU+ neurons in ischemic brain regions cannot simply be assumed to be newly born. Appropriate controls may enable detection of such “false” labeling. For example, expression of mature neuronal markers such as NeuN does not occur until some time after neural cell birth. Thus, analysis within the first 24 hours of BrdU administration will readily reveal such aberrantly labeled neurons, if present. It has previously been thought that such damaged cells always die within 28 days (Kuan et al., 2004), so analysis at longer time-points should eliminate such concerns. However, recent data suggest that some post-mitotic neurons may survive long term after taking up BrdU by DNA synthesis (Burns et al., 2007).
Retroviruses encoding transgenes such as fluorescent proteins have also proven useful for the study of endogenous neurogenesis, and can have the advantage of providing a cytoplasmic label in viable cells that can be used for functional studies in viable tissues (van Praag et al., 2002). Low transduction efficiency, transgene silencing, and the requirement for surgical delivery, however, have made retroviruses less attractive for quantitative studies. Additional strategies currently under development for labeling endogenous neural stem cells include lentiviral systems (Consiglio et al., 2004; Geraerts et al., 2006), as well as mice expressing inducible transgenes under neural stem cell promoters, such as nestin or Sox2 (Imayoshi et al., 2006; Kuo et al., 2006; Legace et al., 2007). The specificity of such techniques, however, remains to be fully investigated.
Identification of transplanted Cells
Similarly problematic has been the accurate identification of transplanted cells. Many cytoplasmic or membrane-permeable labels are easy and convenient to apply prior to transplantation, but can leak out of cells once in the brain, leading to misidentification of host cells as transplanted cells. As such, recommended labels for transplanted cells have included stable transgenic labels, species-specific markers, where available, and “nondiffusible” nuclear markers such as BrdU (Cao et al., 2002). Problems with transgene silencing have led to the popularization of thymidine analog prelabeling of transplanted cells. Thymidine analogs, in theory, should not leak out of grafted cells due to their sequestration within the genetic material of the cell. It is well documented, however, that a high percentage of donor cells die after intracranial transplantation (Sortwell, 2003; Bakshi et al., 2005) We have found that thymidine analogs released from cells that die post-transplantation become incorporated into dividing host cells (Burns et al., 2006). Thus, endogenous neural stem cells and their progeny often contain graft-derived BrdU and are indistinguishable from transplanted cells. As such, thymidine analogs cannot be considered appropriate markers for transplanted cells, and prior studies based on such strategies will need to be re-examined using less fallible techniques (Breunig et al., 2007). Finally, once reliable markers are employed, meaningful assessment of survival and engraftment requires rigorous quantitative techniques such as unbiased stereology. These allow interpretation of differences between experimental groups and individual studies to be made with confidence (Peterson, 1999; Gardi et al., 2008). The development of transgenic MRI-detectable markers may allow rigorous longitudinal quantification and localization of transplanted cells (Deans et al., 2006).
Fusion
Finally, it is now apparent that even transgenic markers are not unequivocal evidence for transdifferentiation. Evidence in the past few years has clearly demonstrated that fusion of two cells can result in the appearance of mature cell types carrying transgenes from unrelated cells (Terada et al., 2002; Wurmser and Gage, 2002; Ying et al., 2002; Alvarez-Dolado et al., 2003; Chen et al., 2006; Jessberger et al., 2007). For example, bone marrow cells were briefly believed to differentiate into Purkinje cells in vivo (Priller et al., 2001), until it was demonstrated that spontaneous fusion between bone marrow cells and Purkinje cells accounted for the results (Alvarez-Dolada et al., 2003; Weimann et al., 2003a, 2003b; Bae et al., 2005). Further, it was reported that neural stem cells may be pluripotent, after injection of neural stem cells into the developing mouse blastocyst yielded mice expressing a NSC-derived transgene in diverse tissues throughout the body (Clarke et al., 2000). Such conclusions, however, had to be abandoned after it was shown that neural stem cells may fuse with host cells of the inner cell mass (Ying et al., 2002). Thus, the burden of proof now requires demonstration of donor transgenes and the absence of host genetic material in order to unambiguously prove the identity of a transplanted or transdifferentiated cell.
As methodology improves, so does the value of forthcoming conclusions. Thus, while research on regenerative medicine is being subjected to ever-increasing scrutiny, the foundation upon which to build regenerative therapies is becoming increasingly solid.
Endogenous Stem Cells and Stroke
Neural Stem Cells
In spite of decades of dogma proclaiming that no new neurons are born in the adult mammalian brain (Ramon y Cajal, 1928), an avalanche of data in the past 15 years has confirmed unequivocally that neural stem cells are in fact present throughout life, and that thousands of new neurons are born daily in the subventricular zone (SVZ) and hippocampus. Neural stem cells can be isolated from the adult brain and cultured in vitro as cellular clusters termed neurospheres in the presence of bFGF and EGF (Reynolds and Weiss, 1992; Reynolds et al., 1996). Upon cytokine withdrawal, these cells give rise to the three neural cell types: neurons, astrocytes and oligodendrocytes (Weiss et al., 1996). Recent in vivo investigations have revealed that neural stem cells possess features of astrocytes and exist at the end of a developmental continuum starting in the neural tube with neuroepithelial cells, followed by radial glia that serve as both parent and radial migratory guide for newly born neurons in the embryonic brain (Alvarez-Buylla et al., 2001). While radial glia in most parts of the brain disappear toward the end of development, some persist as multipotent astrocytes in restricted adult brain regions including the subventricular zone and the subgranular zone of the dentate gyrus, where they are known thereafter as neural stem cells (Doetsch et al., 1999).
Adult Neurogenesis
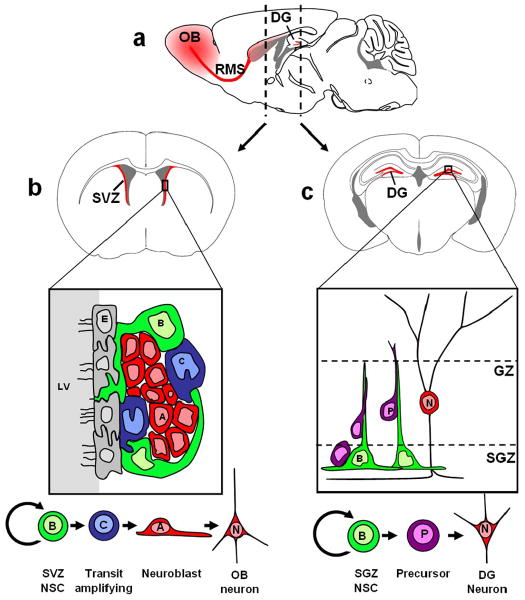
Self-renewing NSCs in the SVZ are slowly dividing GFAP-expressing cells termed “type B cells” (Quinones-Hinojosa et al., 2006). These give rise to rapidly proliferating “transit amplifying” cells termed “type C cells,” which serve to increase the number of progeny. C cells in turn give rise to migrating neuroblasts, “type A cells,” that travel great distances along the rostral migratory stream to the olfactory bulb (Doetsch et al., 1997), where they preferentially differentiate into granular interneurons and functionally integrate into local circuitry (Carleton et al., 2003) (Fig 1a, b). Subsequent evidence has also suggested that resident neural stem cells in the rostral migratory stream give rise to olfactory bulb neurons, preferentially becoming DA+ periglomerular cells (Hack et al., 2005). Although olfactory bulb neurogenesis has been best studied in rodents, there is now evidence that humans likewise harbor NSCs in the SVZ, which migrate along an analogous RMS to a comparatively small olfactory bulb (Curtis et al., 2007). Nevertheless, the true extent of human adult neurogenesis remains controversial (Sanai, et al., 2007). Moreover, the role of olfactory bulb neurogenesis itself remains to be fully elucidated, though correlative evidence in rodents points toward a role in olfactory memory and discrimination.
Figure 1. Neurogenesis persists in adult mammals throughout life.
The parasagital section of a mouse brain in (a) demonstrates the brain regions involved in rodent adult neurogenesis (red). Coronal sections at the approximate regions of the dashed lines are shown in panels (b) and (c). Panel (b) shows the SVZ, where neural stem cells persist throughout life. Slowly dividing type B cells give rise to rapidly dividing type C cells, which in turn give rise to migratory neuroblasts. These reach the olfactory bulb via way of the rostral migratory stream (RMS), and there differentiate into granule and periglomerular neurons. Panel (c) shows the dentate gyrus, where new neurons are also born throughout life. Type B cells in the subgranular zone (SGZ) give rise to precursor cells which migrate up the radial projection of type B cells to become granule neurons in the granular zone (GZ). Parasagital and coronal brain slice images were adapted from Paxinos and Franklin (2004). The subventricular zone diagram in panel (b) illustrates principles from Doetsch (2003).
Although there is some debate as to whether or not neural stem cells in the hippocampus meet the full criteria of “stem cells” (Morshead and van der Kooy, 2004), it is clear that cells with neurogenic capacity give rise to new neurons throughout life in the dentate gyrus (Eriksson et al., 1998) (Fig 1a, c). Intensive investigations have sought to elucidate a possible connection between hippocampal neurogenesis and certain hippocampal-dependent tasks such as learning and memory. Much correlative evidence has been obtained that supports such a role (Kempermann et al., 1998; Shors et al., 2001; Bendel et al., 2005), however, experiments aimed to investigate causality have been difficult to design. Interventions such as growth factor administration or anti-proliferative treatments to increase or decrease hippocampal neurogenesis respectively, may also have effects on the remainder of the hippocampus (Rola et al., 2004). As such, subsequent changes in learning or memory performance could be attributable to indirect effects of the treatment on hippocampal synaptic plasticity rather than neurogenesis. Thus, perhaps not surprisingly, results of studies to establish a causal role of adult neurogenesis in learning and memory have been conflicting (Meshi et al., 2006), though the idea of such a role has gained popularity (Leuner et al., 2006). The development of new transgenic animal models that, for example, allow selective ablation of newly born neurons may be necessary to more definitively address these questions. Such models will also be critical for the assessment of functional gains observed after induction of neurogenesis in the injured brain.
Ischemia-induced neurogenesis
The finding that the adult brain is home to stem cells with neurogenic potential raises the exciting possibility that such cells may be harnessed to restore neurons and glia to areas damaged by CNS disease or injury. In this section, we begin with a discussion of the effects of brain injury on SVZ and SGZ NSCs in situ, then critically review current evidence regarding the presence of newly born neurons in regions of injury. This discussion provides a baseline for subsequent evaluation of techniques to expand and mobilize the neural stem cell pool and thereby augment the endogenous neurogenic response. For the purposes of this discussion, neurogenesis will be defined as the generation of new neurons. Increased proliferation of neural stem cells, while it often leads to increased neurogenesis, is not a direct measure of neurogenesis, as differentiation of the newly born cells toward a neural lineage is also required. Similarly, increased neurogenesis is not a guarantee that an increase in the net number of functional neurons will be observed, as newly born neurons must first be shown to survive–something that is often tied closely to successful synaptic integration into brain circuitry.
Rodent models of brain ischemia
Several rodent models exist for brain ischemia. Transient bilateral carotid artery stenosis leads to “global ischemia” which selectively destroys specific cell types such as CA1 pyramidal cells in the hippocampus. The more common ischemic models, however, are focal, most often involving occlusion of the middle cerebral artery which leads to infarction of the striatum and overlying cortex. Occlusion distal to the striatal branches can be used to generate a selective cortical lesion. Permanent occlusion is accomplished via ligation, cauterization, or laser-induced photothrombosis. Ischemia-reperfusion injury can be modeled via transient occlusion, accomplished by feeding an intraluminal filament through the carotid artery into the proximal MCA. Transient occlusion for a shorter time-period (∼30 min) induces preferential damage to striatal tissue, while occlusion for 90-120 minutes affects both striatum and cortex (Arvidsson et al., 2001).
Increased neurogenesis in ischemic neurogenic regions
Liu et al. first reported increased hippocampal neurogenesis following global ischemia in 1998.(Liu et al., 1998) A 12-fold increase in DG cell birth was observed 1-2 weeks after 10 min of global ischemia. In this case, neurogenesis represented an amplification of normal neurogenesis rather than induction of neurogenesis in a normally non-neurogenic area. Further, such neurogenesis did not serve to replace the CA1 pyramidal cells lost in global ischemia. Interestingly, neurogenesis in this case was not dependent upon actual neuronal cell loss, as ischemic preconditioning, which protects CA1 neurons from subsequent ischemic damage, did not alter the level of increased neurogenesis. Increased NSC proliferation has also been reported in the SVZ following global ischemia (Tonchev et al., 2005). The level of neurogenesis in the DG and SVZ likewise increases in response to focal ischemic injury, again with a peak level of proliferation at around 1-2 weeks after injury (Doetsch et al., 2002; Ardvisson et al., 2002; Parent et al., 2002).
Neurogenesis in ischemic non-neurogenic brain regions
To date, no evidence has been presented to indicate that neural stem cells from the SGZ ever migrate out of the dentate gyrus to replace cells lost in other regions of the brain (Jin et al., 2003b). However, in 2002, two independent groups described that neuroblasts from an expanded ipsilateral SVZ deviate from their normal route toward the olfactory bulb and migrate in chains toward the ischemic penumbra in a rat model of transient MACO (Ardvisson et al., 2002; Parent et al., 2002). Some of these cells persisted and with time developed markers of mature striatal medium spiny neurons of the type lost by infarction. Although a few isolated neuroblasts reached the more distant ischemic cortex, none were shown to generate mature cortical neurons. This contrasted with previous findings that BrdU+ neurons are present in the cortex after ischemia, although failure to demonstrate absence of NeuN/BrdU colocalization immediately after BrdU labeling leaves open questions regarding possible uptake by apoptotic neurons (Gu et al., 2000; Jiang et al., 2001). One subsequent study demonstrated robust migration of DCX+ neuroblasts from the SVZ and RMS into the ischemic cortical penumbra, though survival and maturation of these cells at later time points was not investigated (Jin et al., 2003b). It is now recognized that changes in the SVZ leading to striatal neurogenesis following ischemic injury persist long term, and new neurons continue to be added to the striatum for at least a number of months (Thored et al., 2006; Leker et al., 2007), or even a year (Kokaia et al., 2006) after stroke.
Global ischemia, resulting from events such as myocardial infarction, carbon monoxide inhalation or drowning, is a less common condition in humans. Nevertheless, it is of interest to note that spontaneous regeneration of hippocampal CA1 neurons has also been reported after global ischemia. Using both BrdU administration and retroviral injections into the posterior periventricular region, Nakatomi et al. demonstrated spontaneous regeneration of CA1 neurons lost after global ischemia (2002). The new cells appeared to originate from a ventricular area caudal to the SVZ. This area may correspond to the recently characterized subcallosal zone (SCZ), which contains self-renewing multipotent neural stem cells. SCZ NSCs normally give rise only to oligodendrocytes in vivo, though readily self-renew and generate neurons in vitro (Seri et al., 2006). Bendel et al. subsequently showed that the spontaneous replacement of CA1 neurons correlates with the time course of recovered learning and memory (2005). Given the selective nature of the loss to CA1 neurons in global ischemia, it is likely that the migration and integration of newly born neurons is more readily accomplished in this relatively preserved environment than in the infarct cavity resulting from focal ischemia. Nevertheless, it remains untested to what extent function would improve following global ischemia if CA1 neuron regeneration were inhibited.
The migration of endogenous SVZ-derived cells toward damaged brain regions is not restricted to stroke, and has also been described in Huntington's disease (Curtis et al., 2003), demyelination (Nait-Oumesmar et al., 1999; Picard-Riera et al., 2002), and chromophore-activated synchronous apopotosis (Magavi et al., 2002; Chen et al., 2004). In other cases, however, such as in the 6-OHDA model of Parkinson's disease, no mobilization is observed without concurrent cytokine treatment (Fallon et al., 2000). In perhaps the most provocative example of injury-induced neuroregeneration described to date, selective ablation of specific cortical projection neurons via retrograde uptake of a toxic laser-activated chromophore resulted in robust replacement of the specific neural subtypes ablated. DCX+ cells appeared to migrate from the SVZ and RMS to the cortex via the corpus callosum into appropriate cortical layers before differentiating into projection neurons that could extend axons to appropriate targets in the thalamus or spinal cord. Although over half of the newly born neurons died within 12 weeks, consistent with normal neural development, some survived and maintained long distance connections for over a year (Magavi et al., 2002; Chen et al., 2004). In this model, cells die via apoptosis without inflammation, suggesting that cell death itself must be the initiating signal for neuronal replacement. This is in contrast to other injury models in which neurogenesis may be observed even in the absence of neuronal loss. It remains to be determined if the remarkable fidelity of this regenerative process may reflect the preservation of surrounding parenchyma, but if so, it may suggest that similar results may be more challenging to attain in the cavitating lesions following focal ischemia. Further, these results have so far only been obtained by a single lab, and thus await independent confirmation.
Effect of Age on neuroregeneration
One critique of many rodent studies of stroke is that studies are most frequently performed in young adult animals, while human stroke pathology most frequently occurs in the aged brain environment. It is well established that both SVZ and DG neurogenesis drop precipitously with age. Thus, one may speculate that a less robust neurogenic response to stroke may be observed in old age. However, several lines of evidence suggest that decreased neurogenesis with age may reflect changes in the brain microenvironment rather than alterations in the numbers or properties of neural stem cells themselves. Basal levels of corticosteroids increase with age (Sapolsky, 1992) and have been shown to decrease neural stem cell proliferation (Gould et al., 1998; Montaron et al., 2006). Reversal of this increase by adrenalectomy substantially increases neurogenesis in the dentate gyrus (Cameron et al., 1999; Montaron et al., 1999). The levels of several cytokines, including, FGF2, IGF-1 and VEGF (Shetty et al., 2005) as well as the level of EGF signaling (Enwere et al., 2004) have been shown to decline with age, and infusion of such cytokines in aged brains reverses age-related declines (Lichtenwalner et al., 2001; Jin et al., 2003a). Additionally, similar numbers of neurosphere are obtained upon isolation of NSCs from young and old rodent brains (Tropepe et al., 1997; Seaberg and van der Kooy, 2002). These data suggest that a similar neurogenic potential may in fact be present in the aged brain, available for response to injury. Indeed, when subjected to middle cerebral artery occlusion, 3 and 15 month old rats revealed a similar magnitude of striatal neurogenesis. (Darsalia et al, 2005) urthermore, stroke-induced neurogenesis has recently been observed in the human brain in patients up to 84 years old (Jin et al., 2006; Minger et al., 2007). Cells expressing immature neuronal markers such as DCX and BIII-tubulin as well as ki67, a marker of proliferation, were observed near blood vessels in the penumbra of cortical infarcts. This is in sharp contrast to the normal human cortex in which it has been clearly demonstrated that neurogenesis does not occur in the absence of injury (Bhardwaj et al., 2006).
Regulation and augmentation of ischemia-induced neurogenesis
Although there is now little question that neurogenesis occurs in response to ischemic lesions, it seems evident that this endogenous response would need to be bolstered in order for a meaningful level of regeneration to be achieved (figure 2). Indeed, it remains unclear whether baseline levels of stroke-induced neuroregeneration in mammals is of any functional value at all. Such is not the case in all vertebrates. Amphibians and reptiles exhibit robust regeneration of CNS tissue after injury, leading to a brain post-injury that is structurally and functionally comparable to the pre-injured state. It has been speculated that a paucity of regenerative capacity may be the price paid for increased complexity in the mammalian brain. Nevertheless, detailed understanding of endogenous neurogenic mechanisms may yield insights into possible methods to enhance neurogenesis following injury. A growing list of physiological stimuli, endogenous molecules, and exogenous agents has been shown to regulate adult neurogenesis. (Abrous et al., 2005; Hagg, 2005; and Lichtenwalner et al., 2006). A subset of these has been implicated in the spontaneous endogenous neurogenic response to stroke, while others have been suggested to promote regeneration.
Figure 2. Mobilization of endogenous neural stem cells.
Neural stem cells exist in the subventricular zone of adult humans. (red in panel (a). Proliferation of these neural stem cells increases after injury, and gives rise to a small number of new neurons (b). Mobilization of NSCs such as via administration of cytokines may further promote NSC proliferation, migration, differentiation and survival in rodent models of ischemia. However, the distance to which such cells may migrate in the relatively large human brain remains unclear (c). Pictures drawn based on human MRI images.
Molecules that expand the stem cell pool such as bFGF may be useful for providing adequate cellular substrate for a neuroregenerative response. Indeed, bFGF has been shown to promote cortical cell replacement and functional recovery after cortical injury in neonatal (Monfils et al., 2006) and adult (Leker et al., 2007) rats. In contrast to bFGF, which increases normal neurogenesis in neurogenic regions, EGF also expands the neural stem cell pool, but decreases olfactory bulb neurogenesis (Kuhn et al., 1997). Instead, the generation of astrocytes in the olfactory bulb was increased (Kuhn et al., 1997) and the number of SVZ-derived cells found in adjacent striatal tissue dramatically increased (Craig et al., 1996; Kuhn et al., 1997; Doetsch et al., 2002; Gregg and Weiss, 2003). Similar results have been observed using TGF-alpha, the primary endogenous ligand for the EGF receptor, in a rodent model of Parkinson's disease (Fallon et al., 2000; Chen et al., 2004). BDNF promotes neurogenesis, leading to increased numbers of neurons in the olfactory bulb, in addition to promoting the appearance of new neurons in the striatum (Benraiss et al., 2001; Pencea et al., 2001). The effects of BDNF are enhanced by administration of noggin, which inhibits Wnt signaling – a negative regulator of neurogenesis (Chmielnicki et al., 2004). Erythropoietin (EPO), in addition to regulating RBC production, is upregulated in the SVZ following ischemia and also promotes neurogenesis (Shingo et al., 2001). EPO favors neurogenesis at the expense of NSC self-renewal leading to a decrease in the number of NSCs in the SVZ (Shingo et al., 2001). IGF-1 is another factor upregulated in response to ischemia, and infusion of antibodies to IGF-1 attenuate ischemia-induced proliferation in the SVZ and DG (Dempsey et al., 2003; Yan et al., 2006). Other factors, including VEGF, notch, NO, SCF and G-CSF as well as certain neurotransmitters and chemokines may additionally be implicated in the neurogenic response to ischemia, (Abrous et al., 2005, Lichtenwalner et al., 2006, Chen et al., 2006b), while BDNF, bFGF, and EPO may serve as neurotrophic factors that may promote survival of both injured and newly born neurons (Abrous et al., 2005; Hagg, 2005).
Successful therapeutic strategies will likely require activation of multiple pathways to amplify the progenitor pool, promote neural differentiation, mobilize cells to the site of injury, and enhance the survival of newly born neurons. In recent examples of such multi-pronged approaches, Androuteselli-Theotokis et al. administered a combination of bFGF and notch ligand following MCAO and observed progressive functional recovery over 45 days, which greatly surpassed that observed when either one of the factors was administered independently (2006). Zhao and colleagues have demonstrated that the administration of SCF in combination with GCSF during the acute (Zhao et al., 2007a) or chronic (Zhao et al., 2007b) post stroke periods can enhance neurogenesis and improve limb placement function. In another study, Kolb et al. administered EGF for 7 days followed by EPO for 7 days, resulting in robust replacement of infarcted cortical tissue with neural cells, which, though they lacked normal laminar organization, appeared to facilitate functional recovery on an extensive battery of behavioral measurements (2007). Significant behavioral improvement was also observed if the treatment regimen was not started until 7 days following stroke – a therapeutic window far more generous than the current 3 hours for t-PA. To directly assess the contribution of the newly generated cortical tissue to functional recovery, the authors surgically removed the regenerated cortical area, and interestingly saw loss of behavioral gains at day 7 but not day 1 after removal, suggesting that the new neural tissue may act in a regulatory capacity to promote plasticity or maintain homeostasis of surrounding cortical tissue rather than via direct involvement in motor circuits. Although evidence for mobilization of adult rodent NSCs continues to accumulate, the human brain is much larger and would thus require a far greater number of new neurons, each of which would need to traverse a much greater distance to replace lost cortical tissue (Figure 2).
Perhaps the most impressive example of cytokine-mediated neuroregeneration following stroke was provided by Nakatomi et al., who used combined bFGF and EGF in a global ischemia model of CA1 neuron degeneration to dramatically augment CA1 neuron regeneration, resulting in behavioral performance comparable to the prelesioned state (2002). Here again, robust regeneration may have been facilitated by the relative preservation of other local cell types.
Functional evidence for neuroregeneration
Unlike studies to date of neuroregeneration in focal ischemia models, the overwhelming level of neuroregeneration in Nakatomi et al. permitted prospective labeling of periventricular neural stem cells with a GFP lentivirus, allowing direct demonstration of the functionality of the new neurons via electrophysiological studies (2002). Demonstration of electrophysiological function in neurons born in response to focal ischemia must, by contrast, await further studies, and may require improved techniques to more efficiently label neural stem cells with fluorescent proteins for lineage tracing. Isacson's group (de Chevigny, 2008) recently employed the new Nes-creERT2 mice from Legace et al, (2007) to achieve robust labeling of neuroblasts mobilized in response to TGF-α, suggesting the feasibility ot this approach. Definitively assessing the role of neurogenesis in observed functional recovery will also remain an ongoing challenge. As noted above, compounds employed in the induction of neurogenesis may have multiple functions that may act indirectly of neurogenesis to promote functional recovery. Further, methods employed to prevent neurogenesis such as irradiation and chemotherapy are associated with confounding side-effects. One study to date has employed irradiation to attenuate neurogenesis prior to induction of ischemia. Ischemia-induced cognitive deficits were exacerbated by irradiation, which the authors interpreted as evidence that the inhibition of stroke-induced neurogenesis inhibits normal neurogenesis-dependent functional recovery (Raber et al., 2004). Unfortunately, however, it is difficult to exclude other effects of irradiation, such as contribution to inflammation that may contribute to the behavioral findings in this experiment. As such, direct evidence for the role of neurogenesis in functional recovery must await the development of transgenic methods to specifically inhibit neurogenesis in parallel-treated animals or to selectively induce apoptosis of new neurons born in response to injury.
Safety considerations in endogenous neurogenesis
Although the beneficial effects of various factors for mobilizing neural stem cells is cause for great enthusiasm, the potential for unanticipated adverse effects should be investigated with equal rigor. For example, of the three first cytokines discussed above for the mobilization of neural stem cells, bFGF, EGF and BDNF, each may be associated with additional effects that would warrant careful consideration before proceeding to clinical trials. First, bFGF is known to inhibit the differentiation of oligodendrocyte progenitor cells during development. Direct intraventricular infusion of high concentrations of bFGF in the adult brain has been shown to promote microglial and glial reactivity concurrent with disruption of oligodendrocyte function and myelin production leading to the appearance of demyelinating lesions (Goddard et al., 2001, 2002; Butt and Dinsdale, 2005; Assanah et al., 2006). Second, excessive proliferation of stem cells is well known to increase the risk of genetic alteration and potential tumorgenic transformation. Gliomas are the most common, but also the most aggressive primary brain malignancy, and increasing evidence suggests that gliomas may be derived from endogenous stem or progenitor cells (Singh et al., 2003; Wu et al., 2007; Wu et al., 2008). Thus interventions to actively promote NSC proliferation in vivo should not be undertaken lightly. EGF receptor expression has been proposed as a possible contributor to glioma formation by SVZ progenitors (Doetsch et al., 2002) and infusion of BDNF has been shown to result in highly invasive glioma-like growths (Assanah et al., 2006; Jackson et al., 2006). Finally, infusion of BDNF into the hippocampus has also been shown to lead to spontaneous seizure activity in approximately 25% of treated animals (Scharfman et al., 2002). As such, careful evaluation of the range of possible side effects for any given compound will become an increasingly important aspect of neuroregenerative investigations.
Neural stem cells in non-neurogenic regions?
Many of the above studies have shown convincing evidence of neuroblast migration from the SVZ or RMS to areas of injury. However, in several studies, it has been difficult to exclude the possibility that some newly generated neuronal cells may be born locally. Although there is presently limited evidence for normal neurogenesis in most parts of the brain, cells can be isolated from multiple regions of the brain (including cerebellum, cortex, white matter and spinal cord) that possess NSC properties in vitro, being capable of differentiation into neurons, astrocytes and oligodendrocytes. The exact identity of these cells remains uncertain, and could include quiescent neural stem cells, or glial cells that exhibit neural potential under certain conditions. Indeed, oligodendrocyte precursor cells have been shown to generate neurons in vitro, and endogenous neural stem cells now appear to belong to the astroglial lineage. Recent studies have suggested that genetic manipulation of parenchymal progenitors may promote neurogenesis. Retroviral knock-down of olig2 or overexpression of pax6 in proliferating cortical cells following injury has been shown to induce their transformation into DCX+ cells with neuroblast morphology (Buffo et al., 2005). Further, overexpression of NeuroD in cortical nestin+ cells has also been shown to promote the generation of new GABAergic neurons (Hisatsune, 2006). Cells in the spinal cord with characteristics of OPCs could also be induced to differentiate into immature neurons in the presence of EGF and bFGF, though increased numbers of newly born mature neurons and oligodendrocytes were observed with retroviral overexpression of ngn2 and Mash1 respectively. Thus, although recent work has emphasized the mobilization of endogenous neural stem cells from the subventricular zone, the in vivo reprogramming of other parenchymal progenitors such as oligodendrocyte precursor cells may represent an additional future avenue for neuroregeneration following ischemic injury (Ohori et al., 2006).
A role for endogenous bone marrow in neuroregeneration after stroke?
At least four types of bone marrow-derived cells have interesting relationships with the brain. First, as part of the innate immune system, microglia arise from the hematopoietic lineage and serve roles of immune surveillance in the brain, analogous to macrophages in other organs. These cells rapidly respond to injury in the brain and can demonstrate both pro and anti-inflammatory properties. As discussed in section 1.1.2 above, appropriate regulation of the inflammatory environment following stroke is of utmost importance to minimize injury and promote recovery. Furthermore, the inflammatory environment impacts neural stem cell behavior. While some aspects of the inflammatory response may be involved in mobilization of stem cells to regions of injury, neurogenesis is generally inhibited by inflammation (Monje et al., 2002, 2003), and inhibition of inflammation following stroke has been shown to enhance endogenous neurogenesis (Hoehn et al., 2005). A second group of bone marrow-derived cells are perivascular cells found just inside the basal lamina of vessels which are thought to play an active role in vascular regulation and remodeling (Galimi et al., 2005). As such, these likely play an active role in responding to ischemic injury. Given the robust ability of bone marrow-derived cells to cross the blood brain barrier, especially in regions of injury, some have proposed that bone marrow-derived cells may serve as effective vehicles for delivery of therapeutic genes into the injured brain (Tanaka et al., 2004). Third, the number of circulating endothelial progenitor cells is seen to increase after stroke, and appears to be associated with good prognosis, consistent with the increasingly understood importance of early angiogeneisis following stroke (Sobrino et al., 2007; Chu et al., 2008).
Finally, several controversial reports in recent years have suggested that bone marrow-derived cells may contribute to endogenous neurogenesis. In 2001, Priller et al. reported that donor-derived Purkinje cells could be identified after transplantation of transgene-labeled bone marrow (2001). Additional studies using sex-mismatched bone marrow transplanted (BMT) animals indicated widespread distribution of donor derived neurons (Mezey et al., 2000) and glia (Eglitis and Mezey, 1997) throughout the brain by use of fluorescent in situ hybridization (FISH) to detect the donor sex chromosome. The evidence for plasticity of marrow-derived cells both in the brain and other organs came under heavy fire after multiple groups independently demonstrated that bone marrow derived cells fuse with various somatic cells including Purkinje cells (Terada et al., 2002; Alvarez-Dolada et al., 2003; Weimann et al., 2003b). Furthermore, using transgenic labels, other investigators described the absence of bone marrow-derived neurons and glia in the cerebrum after BMT and various interventions including brain injury (Wehner et al., 2003).
At present, the field remains divided. Proponents of bone marrow plasticity rely heavily on FISH claiming that transgenic labels are likely silenced in the brain and are not sufficiently sensitive for detection of bone marrow-derived neural cells in the brain, while opponents categorically report an absence of bone marrow-derived neurogenesis. A few reports using GFP bone marrow have described that bone marrow-derived cells expressing certain neuronal markers can be found in the brain after bone marrow mobilization or brain injury (Corti et al., 2002; Kawada et al., 2006). However, careful confocal evidence for full differentiation, maturation and integration, along with electrophysiological studies of such cells, all remain to be reported. Furthermore, although some have used FISH to argue against fusion in such situations, transgenic lox-cre strategies have not been employed to confirm the lack of host genes in these cells. Thus, though intriguing, further work will be necessary before the notion of “turning blood into brain” finds widespread acceptance.
Exogenous Stem Cells
Introduction to cell transplantation
The majority of studies to date have shown relatively limited cell replacement from endogenous neural stem cells. Further, the technology, for mobilizing endogenous neural stem cells is relatively new. By contrast work has been in progress for decades to replace lost neural cells by transplantation of either fetal brain tissue (Bjorklund and Stenevi, 1979; Perlow, et al, 1979) or, more recently, stem cells. Potential advantages to this approach may include greater control over cell fate, the ability to deliver any desired number of cells, and reduced risks associated with mitogen infusion. A number of different cell types have been considered for cell transplantation with goals ranging from replacement of host circuitry to delivery of neuroprotective or immunomodulatory compounds. Before expounding upon specific cell types, however, we briefly discuss basic issues related to cell transplantation in general.
Delivery Variables
Several delivery variables must be defined for experiments involving cell transplantation. The goal of optimizing these variables is to maximize the number of surviving functional cells present at the appropriate site of injury. Optimal parameters may vary by cell type as well as whether the predominant goal is cell replacement or neuroprotection.
Timing
Most neuroprotective agents studied to date have a relatively narrow therapeutic time window of only a few hours. Although stem cells have been shown to release certain neuroprotective agents, the therapeutic window for even non-neural stem cells tends to be longer. Many groups transplant cells 1-2 days following injury. However, benefits have been observed with delivery of cells even a full week after focal ischemia – a benefit which did not appear dependent upon forming new graft-derived connections (Zhao et al., 2002). Such findings may suggest mechanisms of action other than cell replacement or neuroprotection, such as enhancement of host neural plasticity. In studies seeking to demonstrate the generation of new graft-derived circuitry, even greater delays may be employed after injury to ensure stable long term behavioral deficits prior to transplantation (Pollock et al., 2006).
Administration Route
Stem cells may be delivered either systemically into the vasculature or locally into the brain (Corti et al., 2005). The migratory properties of many stem cells enable them to effectively traffic toward an area of injury. For example, graft-derived neurons and glia may be seen in the injured spinal cord even after intravenous delivery of neural stem cells (Fujiwara et al., 2004). Bone marrow mononuclear cells are most commonly delivered systemically, while neural cells are more frequently delivered directly into the brain parenchyma. Delivery directly into the infarct core generally yields poor cell survival (Wurmser et al., 2004), though injection into the penumbra routinely yields both surviving cells and neuroprotection (Johnston et al., 2001). Many systemically delivered cells seem to provide significant benefit, even though they may never be directly observed in the brain. For studies desiring optimal systemic distribution, however, direct intraarterial delivery may increase the delivery of cells to the target tissue, due to decreased sequestration in the lungs (Tolar et al., 2006).
Immunosupression
While generally used for experimental xenografts, the role and importance of immunosuppression following allografts remains heavily debated (Barker and Widner, 2004). Neural stem cells may be minimally immunogenic (Modo et al., 2002), whereas MSCs may provoke a robust inflammatory response leading to rapid acute rejection (Coyne et al., 2006). Immunosuppressive drugs such as cyclosporine A may also promote sprouting of host neural cells, potentially leading to functional improvement independent of the grafted cells. Given the serious side effects of immunosuppression, this topic will become of increasing importance as stem cell-based therapies move closer to clinical trials. See Barker and Winder (2004), as well as Chen and Palmer (2008) for more in-depth discussion on this topic.
Pre-Differentiation
The use of stem cells for therapeutic purposes is fundamentally dependent upon stem cells exhibiting different properties prior to transplantation than after transplantation. In preparation for transplantation, extensive proliferation capacity is paramount in order to generate adequate numbers of cells. However, if cells fail to exit the cell cycle at some appropriate point after transplantation, the result may ultimately be fatal. Likewise, although pluripotent or multipotent stem cells may be necessary to ensure capacity to generate the cell types desired after transplantation, uncontrollable inappropriate differentiation may yield undesired cell types in the brain. At the other extreme, fully differentiated neuronal cells may have long projections and be too fragile to transplant. As such, when the goal is to generate functioning neural cells in vivo, cells committed to the neural lineage are generally used. If a specific phenotype is desired, such as dopaminergic neurons for treatment of Parkinson's disease, early postmitotic cells are generally used. When multiple cell types are needed, such as after stroke, multipotent cells may be appropriate. These variables, however, are still being investigated. While undifferentiated NSCs may induce motor recovery in a rat spinal cord injury model, a recent study demonstrated that it also induced aberrant axonal sprouting leading to allodynia-like forepore hypersensitivity (Hofstetter et al., 2005). In this experiment, optimal results were obtained by transducing cells prior to transplantation with Ngn2 which helped prevent astrocytic differentiation of grafted cells and prevented aberrant axonal sprouting.
Pretreatment
The most common fate of transplanted cells is cell death (Bakshi et al., 2005; Burns et al., 2006). While this may be less of a concern with stem cell-derived cells than fetal tissue, which may be in more limited supply, having large numbers of dead cells in the graft may exacerbate stroke-related deficits (Modo et al., 2003). Differentiation toward a neural lineage itself is also directly associated with cell death, and this may be especially true in a non-neurogenic region lacking necessary neurotrophic cues. As such, various methods have been developed to both block apoptotic programs, and to prevent their initiation via provision of various neurotrophic compounds (Sortwell, 2003).
Scaffolds
Even upon the successful differentiation and survival of neurons, the ischemic environment presents particular challenges to functional restoration. A large infarct is associated with massive necrosis and may yield an infarct core that is inhospitable to newly delivered cells due to lack of blood supply and absence of an appropriate extra cellular support. One approach has involved the transplantation of NSC-embedded scaffolding into the infarct cavity to promote the formation of reciprocal connections between graft and host. Though favorable results have been suggested, further studies are needed to more critically assess this technique (Park et al., 2002).
Controls
It remains true that the results of any experiment are only as good as the controls. As noted in section 1.3 above, the challenges involved in obtaining accurate results in experiments involving neuroregeneration are far from trivial. In general, a control cell type presumed not to have the desired properties of neural differentiation or neuroprotection is indicated. While saline injections may control for the effects of needle injection (Batchelor et al., 2002), live cells such as fibroblasts may also allow for possible transfer of cell labels. Dead cells have been used by some, but have been suggested to exacerbate stroke deficits (Modo et al., 2003). Cell fusion may be cell type-dependent and is hence difficult to control for, and may require use of cre/loxP technology (Novak et al., 2000). The ultimate goal of any treatment is improved functional recovery. Promising results obtained in preclinical trials have been notoriously difficult to reproduce in the clinical arena. Thus, extra care should be applied to the design of preclinical studies to maximize their validity—a topic carefully reviewed by Dirnagl (2006).
Genetic stability
Fetal cells have been used for decades in animal studies of Parkinson's disease, ischemic brain injury, and numerous other pathologies (Onifer and Low, 1990; Jansen et al., 1997). Several human clinical trials have moved ahead and revealed that in the case of dopamine cell transplants, grafts may survive and function for periods of up to 14 years after transplantation. (Piccini et al., 1999; Mendez et al., 2008). Limited availability of fetal tissue, however, has fueled the search for alternate sources of cells, including stem cells. Stem cells are typically cultured for extended periods of time in vitro, and may be exposed to several fold more cell divisions than would be experienced by any normal cell in the body. Greater potential thus exists for “mistakes” to be made during DNA replication and cell division. Pluripotent cells such as germ cells and embryonic stem cells express high levels of telomerase that permit theoretically unlimited proliferation. In vivo, the genetic payload of germ cells is maintained in pristine condition from generation to generation in spite of having accumulated untold numbers of cell divisions over the millennia. However, such is not true for embryonic stem cells, which may accumulate substantial karyotypical abnormalities after extended passaging in vitro. This situation may be exacerbated in adult stem cells, which, while they may express some telomerase, do not express enough to prevent telomere shortening (Hiyama and Hiyama, 2007). Substantial karyotypical instability may thus result in stem cells cultured for extensive periods in vitro, which in some cases, may lead to malignant transformation (Miura et al., 2006). Significant aneuploidy may develop in mouse ESCs after 20 passages (Longo et al., 1997), though human ESCs may tend to be somewhat more stable (Hoffman et al., 2005). In order to facilitate in vitro self renewal while minimizing occurrence of karyotypic changes, many investigators favor the use of cells that have been passaged a minimal number of times, using specific passaging techniques (Thomson et al., 2008), or cells that have been immortalized via overexpression of proto-oncogenes such as Ras, or even direct overexpression of telomerase (Roy et al., 2004). Immortalized cell lines are often selected for favorable growth and differentiation characteristics that may differ substantially from non-immoralized NSCs. For example, certain immortalized NSC lines are able to generate DA neurons, whereas primary NSCs generally do not. Findings obtained with immortalized cells therefore cannot be generalized to the parent stem cell population. The clinical utility of immortalized cells remains unclear. Some suggest that immortalized cell lines offer the best way to ensure use of well characterized, stable cells, however, others believe that immortalization renders an unacceptably increased risk for subsequent malignant transformation. To address this concern, some groups have generated “conditionally immortalized” cell lines that are immortal only in the presence of specific agents (Pollock et al., 2006). Finally, any genetic modification may carry the risk of disrupting certain genes involved in cell cycle regulation. Thus, transgene integration sites and cell behavior should be carefully characterized before clinical application of genetically modified cells.
Neural stem cells
Neural stem cells can be isolated from many regions of the central nervous system of embryonic as well as adult mammals. They can be propagated in culture in the presence of EGF and/or bFGF as proliferative clusters of cells termed neurospheres. Recent studies have demonstrated that rather than being homogeneous aggregates of stem cells, neurospheres actually represent a heterogeneous collection of cells including true stem cells committed progenitors and differentiated progeny. This is in contrast to embryonic stem cells, which in the presence of appropriate signaling molecules can be maintained as a relatively homogeneous population of stem cells. NSCs also differ from ESCs in terms of the variety of neurons they can generate. Profiles of NSC gene expression tend to point to NSCs expanded as neurospheres in EGF and bFGF as adopting a forebrain profile. Consistent with this, attempts to differentiate NSCs into cells from other regions of the CNS, such as dopaminergic neurons, cerebellar Purkinje cells or motorneurons have in most cases been unsuccessful. Nevertheless, neural stem cells can be successfully differentiated into representative cell types in parts of the brain most commonly affected by stroke, such as cortical projection neurons (Englund et al., 2002), interneurons (Scheffler et al., 2005) and hippocampal pyramidal neurons (Corti et al., 2005). Retrograde labeling, synaptic integration and action potential generation from NSC-derived neurons has been demonstrated in vivo (Englund et al., 2002). Recent studies have suggested that more diverse fates, including motorneurons (Wu et al., 2002), may be obtained from neural stem cells cultured in monolayer, most commonly on a substrate of laminin. This may reflect the capacity for maintenance of a less mature cell type using this technique. Recent work from the laboratory of Austin Smith has suggested that neural stem cells from embryonic stem cells or from fetal or adult brain may be maintained as radial glia via this technique (Conti et al., 2005; Pollard et al., 2006). Alternatively, improved access to bFGF and EGF in the monolayer system may minimize spontaneous differentiation that could otherwise restrict the potential of neural stem cells grown as neurospheres.
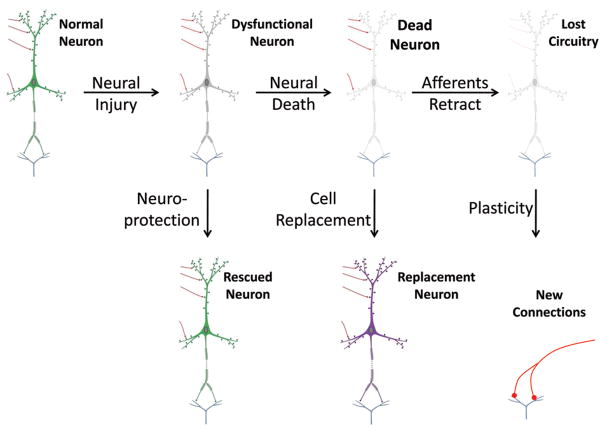
That NSCs can differentiate into neurons (Song et al., 2002a, 2002b; Kelly et al., 2004), astrocytes (Winkler et al., 1998; Herrera et al., 1999; Eriksson et al., 2003) oligodendrocytes (Yandava et al., 1999; Pluchino et al., 2003) and possibly even endothelium (Wurmser et al., 2004) would suggest that NSCs should be capable of replacing most of the cell types affected by an ischemic injury. Actual results in preclinical studies, however, have been quite varied. Neural stem cells, including human, can clearly survive after transplantation, have a tendency to migrate toward areas of infarct (Kelly et al., 2004), and can generate functional neurons (Englund et al., 2002) that may form connections with host cells (Park et al., 2002). While several studies have found NSCs to predominantly differentiate into glia after transplantation into normally non-neurogenic regions (Winkler et al., 1998; Herrera et al., 1999; Eriksson et al., 2003), robust neural differentiation has been observed after transplantation of cells cultured on laminin (Wu et al., 2002; Yan et al., 2007). Most studies have not observed substantial changes in infarct size after NSC transplantation (Kelly et al., 2004; Pollock et al., 2006), however, neuroprotective (Ourednik et al., 2002; Lee et al., 2007) and immunomodulatory (Fujiwara et al., 2004; Pluchino et al., 2005) effects of neural stem cells in addition to their potential for at least some cell replacement (Sinden et al., 1997) have collectively yielded beneficial effects in multiple animal models of neurodegeneration and brain injury, including stroke (Sinden et al., 1997; Chu et al., 2004; Pollock et al., 2006). Some of the potential mechanisms via which transplanted cells such as neural stem cells may yield functional benefits are summarized in Figure 3.
Figure 3. Mechanisms of exogenous stem cell action.
Exogenous stem cells may act via multiple mechanisms to restore function following brain injury. Neuroprotective actions, which could include modulation of inflammation or secretion of neuroprotective compounds, may rescue dysfunctional neurons, thereby preserving existing neural circuitry. Once a host neuron has died, stem cells may conceivably be used to replace the lost neuron, however, effective cell replacement may be dependent upon preservation of surrounding cytoarchitecture. Assuming original circuits cannot be maintained due to retraction of afferent projections after the neuron dies, or due to cavitation of the injury site, stem cells may still participate in the formation of new circuits. This may occur directly via synaptic incorporation into new circuitry, or indirectly by promoting the formation or maintanence of new connections by host neurons. In either case, such generation of new synaptic connections is termed synaptic plasticity. A causal link has not yet been established between functional benefit after cell transplantation following stroke and the synaptic integration of grafted cells. Images adapted from Squire et al (2003).
Embryonic stem cells
Embryonic stem cells (ESCs), including the more recently described iPS cells, comprise the most versatile stem cells currently available, being capable of differentiation into all adult cell types, including functional neurons (Benninger et al., 2003) and glia (Brustle et al., 1999; Scheffler et al., 2003). Electrophysiological evidence of synaptic integration has been obtained in vivo after transplantation of mouse (Brustle et al., 1997; Harkany et al., 2004; Wernig et al., 2004) and human (Muotri et al., 2005; Roy et al., 2006) ESC-derived cells. As such, embryonic stem cells can provide an almost unlimited supply of desired cells for transplantation. However, such potency comes at a price, as embryonic stem cells also form teratomas after transplantation in vivo (Bjorklund et al., 2002; Carson et al., 2006). Indeed teratoma formation is one of the primary functional tests to verify the potency of embryonic stem cells. As such, before ESC-derived cells can be considered for transplantation, it is critical that the graft be free of undifferentiated pluripotent cells. Considerable effort has been exerted to try to harness the potential of ESCs and guide their differentiation toward the neural lineage. Although numerous differentiation techniques yield a high percentage of neural cells (Zhang et al., 2001; Liour and Yu, 2003; Glaser and Brustle, 2005; Kim et al., 2006; Lowell et al., 2006), few methods yield a perfectly homogenous cell population completely devoid of undifferentiated cells with teratoma-forming potential (Sanchez-Pernaute et al., 2005; Carson et al., 2006). As such additional steps such as antibiotic selection (Li et al., 1998) and FACS (Wernig et al., 2002; Chung et al., 2006) are often used during or after differentiation to purify the neural cells. Perhaps the most robust differentiation protocol described to date that inherently eliminates pluripotent cells involves the spontaneous differentiation at low density, followed by propagation as a monolayer in EGF and bFGF (Conti et al., 2005). Whether resulting cells are any more potent than NSCs cultured under similar conditions, however, remains to be determined.
In order to avoid teratoma formation, most studies of ES-derived cell transplantation have employed cells pre-differentiated toward either neurons or neural precursors. Although for some disease models, ESCs have allowed the generation of cell types difficult to obtain from neural stem cells such as dopaminergic neurons (Bjorklund et al., 2002; Kim et al., 2002; Sanchez-Pernaute et al., 2005; Takagi et al., 2005; Morizane et al., 2006; Roy et al., 2006) and motorneurons (Wichterle et al., 2002; Plachta et al., 2004), results after transplantation of ESC-derived cells for stroke (Buhnemann et al., 2006; Hayashi et al., 2006) have been similar to those obtained using brain-derived neural stem cells, though perhaps yielding a higher percentage of neurons. After transplantation into the ischemic brain, ESC-derived neurons have met criteria of electrophysiological function and synaptic connectivity (Buhnemann et al., 2006), however, a clear correlation between the generation of new ES-derived neurons and behavioral recovery after stroke remains to be demonstrated.
Teratocarcinoma cells
A variant of embryonic stem cells are teratocarcinoma cells. Derived from an immortalized cell line, these cells differentiate into a pure population of neurons upon exposure to retinoic acid, and are the only cells that have been tested in human clinical trials for the treatment of stroke. On the basis of preliminary preclinical studies that suggested long term integration of teratocarcinoma cells in rodents (Kleppner et al., 1995), Kondoziolka et al. transplanted 1-3 million cells into patients with chronic stable deficits following basal ganglia stroke in an open label phase 1 trial (2000). Safety at the 12 month time point was established with this technique, and some patients appeared to show some benefit based on the European Stroke Scale score. A subsequent small non-blinded trial failed to demonstrate significant benefit in treated patients compared to controls, though a trend toward improvement was observed (Kondziolka et al., 2005). Surviving graft-derived cells were observed 27 months after transplantation from the first trial (Nelson et al., 2002) with no evidence of neoplasm. However, the level of integration of grafted cells into host circuitry was not clear in the absence of a cytoplasmic label for the grafted cells. Although continued refinement could conceivably yield therapeutic benefit from this cell type, teratocarcinoma cells will likely continue to raise questions of safety, based on the tumorigenic nature of the donor cells (Buchan et al., 2001). Predifferentiation of these cells prior to transplantation reportedly gives rise to uniformly differentiated, postmitotic neuronal cells (Trojanowski et al., 1997), however, mature neurons are more susceptible to cell death following transplantation than less mature cells.
Bone Marrow and Cord Blood-derived Cells
A variety of cell types can be obtained from bone marrow, many of which have been suggested to have beneficial effects after transplantation into the ischemic brain. Cord blood contains an enriched fraction of the progenitor cells normally found in bone marrow, and has, like bone marrow, been shown to contain cells with multipotent potential. The normal role of bone marrow derived cells in the brain was discussed above (section 2.5), and will not be reviewed further, except to note that delivery of bone marrow-derived cells (Willing et al., 2003a; Shyu et al., 2006) and umbilical cord cells (Willing et al., 2003b; Vendrame et al., 2004; Nan et al., 2005; Xiao et al., 2005) particularly including the CD34+ fraction (Taguchi et al., 2004) have been shown to yield dramatic decreases in infarct size and improvements in behavioral function. In a complementary experiment, selective genetic ablation of activated microglia significantly exacerbated ischemic brain damage (Kleppner et al., 1995). Clinically, in addition to transplantation of bone marrow cells, endogenous bone marrow cells can be mobilized by G-CSF (Sprigg et al., 2006), and this too has led to dramatically improved outcomes following stroke in animal models (Kawada et al., 2006; Dunac et al., 2007). It should be noted, however, that G-CSF also has direct neuroprotective actions (Schneider et al., 2005; Schabitz et al., 2007) and promotes neurogenesis (Schneider et al., 2005). Thus, results may represent a combined effect. Clinical trials to assess G-CSF safety and efficacy after stroke are currently underway (Sprigg et al., 2006; Schabitz et al., 2008).
Marrow stromal cells (MSCs) are perhaps the most commonly employed bone marrow-derived cells in animal models of stroke. Several studies have suggested that MSCs, like bone marrow mononuclear cells and umbilical cord cells, may generate neurons both in vitro and in vivo. These data, however, have often been based on morphological criteria in vitro (Woodbury et al., 2000; Deng et al., 2001) and in vivo studies employing transferable labels (Kopen et al., 1999; Chen et al., 2000, Li et al., 2001; Munoz-Elias et al., 2004; Shyu et al., 2006). These substandard methodologies together with non-stringent histological criteria to define a neuron make these studies difficult to interpret (Svedsen et al., 2001; Lu et al., 2004; Burns et al., 2006; Coyne et al., 2006). Nevertheless, substantial functional benefits have been observed after both intracranial and intravascular delivery (Chen et al., 2001a, 2001b, 2003; Honma et al., 2006). Although MSCs migrate toward injury-associated signals in vitro (Son et al., 2006; Ponte et al., 2007; Menon et al., 2007), MSCs appear to exhibit rather poor survival after transplantation into the brain (Coyne et al., 2006). As such, the mechanism's underlying observed functional benefits have remained elusive. Nevertheless, this fact alone should not detract from their potential therapeutic value which is being investigated in several clinical trials for various diseases (Giordano et al., 2007). On a cautionary note, both mouse (Miura et al., 2006; Tolar et al., 2007) and human (Zaghloul et al., 2001; Serakinci et al., 2004; Rubio et al., 2005) MSCs may exhibit malignant transformation after extended culture in vitro, a concern that may be exacerbated by their immunosuppressive properties (Djouad et al., 2003; Ryan et al., 2005; Zhu et al., 2006). Thus, as with all stem cell populations, care will be needed to accurately assess safety, including demonstration of a normal karyotype prior to clinical applications.
Although originally described as MSCs, multipotent adult progenitor cells (MAPCs) have emerged as bone marrow-derived cells with greater potential than normal MSCs (Reyes and Verfaillie, 2001; Jiang et al., 2002). Specifically, MAPCs have been reported to generate functional cell types from all 3 embryonic germ layers, including neuronal cells that exhibited mature electrophysiological properties in vitro (Schwartz et al., 2002; Ross et al., 2006; Zeng et al., 2006; Serafini et al., 2007). Like MSCs, MAPCs have been shown to improve behavioral recovery in a rodent model of stroke (Zhao et al., 2002), however, in vivo neural differentiation of these cells after postnatal transplantation has not been demonstrated (Burns et al., 2006). A number of other multipotent stem cell populations from bone marrow with properties similar to MAPCs have also been described (D'Ippolito et al., 2004; Kogler et al., 2004; Yoon et al., 2005; Kucia et al., 2006; Anjos-Afonso and Bonnet, 2007). To our knowledge, however, none of these have demonstrated mature electrophysiological function after neural differentiation, nor been tested in models of ischemic brain injury.
Cell transplantation for delivery of trophic molecules
Finally, given the migratory potential of certain cell types towards areas of injury, stem cells may serve as appropriate vehicles for delivery of specific molecules that may be difficult to deliver at adequate concentration into the ischemic area via systemic administration (Muller et al., 2006). Candidates for such ex-vivo gene therapy may include anti-inflammatory, pro-angiogenic and pro-survival molecules. Molecules to promote endogenous neurogenesis may also prove to be useful. Indeed, given the low percentage of adult-born neurons that survive, delivery of pro-angiogenic and pro-survival drugs may indirectly promote the survival of migrating neuroblasts that find themselves at the infarct site. Many of the trophic effects exhibited by neural and bone marrow-derived stem cells are poorly understood, thus the delivery of such stem cells in conjunction with specific gene delivery may provide additional benefit (Chen et al., 2001a; Horita et al., 2006; Liu et al., 2006; Gamm et al., 2007).
Conclusions
While we may never be able to “regrow” large areas of brain lost to stroke, stem cell technology is a rapidly evolving field that will likely significantly impact the future treatment of ischemic brain injury. It is now clear that neural stem cells exist in the brain and undergo limited neurogenesis in response to stroke. Proof of principle exists that this endogenous response may be substantially augmented through the delivery of appropriate cytokines. Exogenous stem cells offer complementary advantages of being available in unlimited numbers with additional control over fate, cell number, timing and site of delivery. The fact that substantial functional gains have been observed in animal models after delivery of cells of both neural and non-neural origin in pre-clinical models of ischemic brain injury is encouraging. Nevertheless, caution is indicated to ensure the highest standards of safety and scientific rigor as this exciting field moves forward.
Acknowledgments
Support for this work was provided by the 3M science and technology fellowship, the NIH MSTP program, Minnesota Medical Foundation, and the University of Minnesota doctoral dissertation fellowship, and grants from the National Institutes of Health.
Abbreviations
- 6-OHDA
6-hydroxydopamine
- BDNF
brain derived neurotrophic factor
- bFGF
basic fibroblast growth factor
- BMT
bone marrow transplant
- BrdU
bromodeoxyuridine
- CA1
cornu ammon region 1
- DA
dopamine
- DCX+
double cortin
- DG
dentate gyrus
- EGF
epidermal growth factor
- EPO
erythropoietin
- ESC
embryonic stem cell
- FACS
fluorescent-activated cell sorting
- FGF2
fibroblast growth factor 2
- FISH
fluorescent in situ hybridization
- GABA
gamma-aminobutryric acid
- G-CSF
granulocyte colony-stimulating factor
- GFAP
glial cell acidic protein
- GFP
green fluorescent protein
- GZ
granular zone
- IGF-1
insulin growth factor
- MAPC
multipotent adult progenitor cell
- MCAO
middle cerebral artery occlusion
- MSC
marrow stromal cell / mesenchymal stem cell
- Ngn2
neurogenin 2
- NSC
neural stem cell
- OPC
oligodendrocyte precursor cell
- RBC
red blood cell
- RMS
rostral migratory stream
- SCZ
subcallosal zone
- SGZ
subgranular zone
- SVZ
subventricular zone
- t-PA
tissue plaminogen activator
- VEGF
vascular endothelial growth factor
Literature Cited
- Population Division. U.S. Census Bureau; 2005. Interim State Population Projections, 2005. American Association on Aging. [Google Scholar]
- American Heart Association. [Accessed April 8, 2007];2007 www.americanheart.org.
- Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network physiology. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buyalla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD. Notch signaling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–826. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- Anjos-Afonso F, Bonnet D. Nonhematopoietic/endothelial SSEA-1+ cells define the most primitive progenitors in the adult murine bone marrow mesenchymal compartment. Blood. 2007;109:1298–1306. doi: 10.1182/blood-2006-06-030551. [DOI] [PubMed] [Google Scholar]
- Aranguren XL, McCue JD, Hendrickx B, Zhu XH, Du F, Chen E, Pelacho B, Penuelas I, Abizanda G, Uriz M, Frommer SA, Ross JJ, Schroeder BA, Seaborn MS, Adney JR, Hagenbrock J, Harris NH, Zhang Y, Zhang X, Nelson-Holte MH, Jiang Y, Billiau AD, Chen W, Prosper F, Verfaillie CM, Lutten A. Multipotent adult progenitor cells sustain function of ischemic limbs in mice. J Clin Invest. 2008;118:505–514. doi: 10.1172/JCI31153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson A, Kokaia Z, Lindvall O. N-methyl-D-aspartate receptor-mediated increase of neurogenesis in adult rat dentate gyrus following stroke. Eur J Neurosci. 2001;14:10–18. doi: 10.1046/j.0953-816x.2001.01611.x. [DOI] [PubMed] [Google Scholar]
- Assanah M, Lochhead R, Ogden A, Bruce J, Goldman J, Canoll P. Glial progenitors in adult white matter are driven to form malignant gliomas by platelet-derived growth factor-expressing retroviruses. J Neurosci. 2006;26:6781–6790. doi: 10.1523/JNEUROSCI.0514-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae JS, Furuya S, Shinoda Y, Endo S, Schuchman EH, Hirabayashi Y, Jin HK. Neurodegeneration augments the ability of bone marrow-derived mesenchymal stem cells to fuse with Purkinje neurons in Niemann-Pick type C mice. Hum Gene Ther. 2005;16:1006–1011. doi: 10.1089/hum.2005.16.1006. [DOI] [PubMed] [Google Scholar]
- Bakshi A, Keck CA, Koshkin VS, LeBold DG, Siman R, Snyder EY, McIntosh TK. Caspase-mediated cell death predominates following engraftment of neural progenitor cells into traumatically injured rat brain. Brain Res. 2005;1065:8–19. doi: 10.1016/j.brainres.2005.09.059. [DOI] [PubMed] [Google Scholar]
- Barker RA, Widner H. Immune problems in central nervous system cell therapy. NeuroRx. 2004;1:472–481. doi: 10.1602/neurorx.1.4.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor PE, Porritt MJ, Martinello P, Parish CL, Liberatore GT, Donnan GA, Howells DW. Macrophages and microglia produce local trophic gradients that stimulate axonal sprouting torward but not beyond the wound edge. Mol Cell Neurosci. 2002;21:436–453. doi: 10.1006/mcne.2002.1185. [DOI] [PubMed] [Google Scholar]
- Bendel O, Bueters T, Von Euler M, Ove Ogren S, Sandin J, von Euler G. Reappearance of hippocampal CA1 neurons after ischemia is associated with recovery of learning and memory. J Cereb Blood Flow Metab. 2005;25:1586–1595. doi: 10.1038/sj.jcbfm.9600153. [DOI] [PubMed] [Google Scholar]
- Benninger F, Beck H, Wernig M, Tucker KL, Brustle O, Scheffler B. Functional integration of embryonic stem cell-derived neurons in hippocampal slice cultures. J Neurosci. 2003;23:7075–7083. doi: 10.1523/JNEUROSCI.23-18-07075.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benraiss A, Chmielnicki E, Lerner K, Roh D, Goldman SA. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J Neurosci. 2001;21:6718–6731. doi: 10.1523/JNEUROSCI.21-17-06718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj RD, Curtis MA, Spalding KL, Buchholz BA, Fink D, Bjork-Eriksson T, Nordborg C, Gage FH, Druid H, Eriksson PS, Frisen J. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci U S A. 2006;103:12564–12568. doi: 10.1073/pnas.0605177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund A, Stenevi U. Reconstruction of the nigrostriatal dopamine pathway by intracerebral nigral transplants. 1979;177:555–560. doi: 10.1016/0006-8993(79)90472-4. [DOI] [PubMed] [Google Scholar]
- Bjorklund LM, Sanchez-Pernaute R, Chung S, Andersson T, Chen IY, McNaught KS, Brownell AL, Jenkins BG, Wahlestedt C, Kim KS, Isacson O. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci U S A. 2002;99:2344–2349. doi: 10.1073/pnas.022438099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breunig JJ, Arellano JI, Macklis JD, Rakic P. Everything that Glitters Isn't Gold: A Critical Review of Postnatal Neural Precursor Analyses. Cell Stem Cell. 2008;1:612–627. doi: 10.1016/j.stem.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Brustle O, Spiro AC, Karram K, Choudhary K, Okabe S, McKay RD. In vitro-generated neural precursors participate in mammalian brain development. Proc Natl Acad Sci U S A. 1997;94:14809–14814. doi: 10.1073/pnas.94.26.14809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustle O, Jones KN, Learish RD, Karram K, Choudhary K, Wiestler OD, Duncan ID, McKay RD. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999;285:754–756. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- Buchan AM, Warren D, Burstein R. Transplantation of cultured human neuronal cells for patients with stroke. Neurology. 2001;56:821–822. doi: 10.1212/wnl.56.6.820-a. [DOI] [PubMed] [Google Scholar]
- Buffo A, Vosko MR, Erturk D, Hamann GF, Jucker M, Rowitch D, Gotz M. Expression pattern of the transcription factor Olig2 in response to brain injuries: implication for neuronal repair. Proc Natl Acad Sci U S A. 2005;102:18183–18188. doi: 10.1073/pnas.0506535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhnemann C, Scholz A, Bernreuther C, Malik CY, Braun H, Schachner M, Reymann KG, Dihne M. Neuronal differentiation of transplanted embryonic stem cell-derived precursors in stroke lesions of adult rats. Brain. 2006;129:3238–3248. doi: 10.1093/brain/awl261. [DOI] [PubMed] [Google Scholar]
- Burns KA, Ayoub AE, Breunig JJ, Adhami F, Weng WL, Colbert MC, Rakic P, Kuan CY. Nestin-CreER mice reveal DNA synthesis by nonapoptotic neurons following cerebral ischemia-hypoxia. Cereb Cortex. 2007;17:3585–3592. doi: 10.1093/cercor/bhl164. [DOI] [PubMed] [Google Scholar]
- Burns TC, Ortiz-Gonzalez XR, Gutierrez-Perez M, Keene CD, Sharda R, Demorest ZL, Jiang Y, Nelson-Holte M, Soriano M, Nakagawa Y, Luquin MR, Garcia-Verdugo JM, Prosper F, Low WC, Verfaillie CM. Thymidine analogs are transferred from prelabeled donor to host cells in the central nervous system after transplantation: a word of caution. Stem Cells. 2006;24:1121–1127. doi: 10.1634/stemcells.2005-0463. [DOI] [PubMed] [Google Scholar]
- Burns TC, Rodriguez GJ, Patel S, Hussein HM, Georgiadis AL, Lakshminarayan K, Qureshi AI. Endovascular Interventions Following Intravenous Thrombolysis May Improve Survival and Recovery in Patients with Acute Ischemic Stroke: A Case Control Study. American Journal of Neuroradiology. 2008 doi: 10.3174/ajnr.A1236. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AM, Dinsdale J. Fibroblast growth factor 2 induces loss of adult oligodendrocytes and myelin in vivo. Exp Neurol. 2005;192:125–133. doi: 10.1016/j.expneurol.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- Cao QL, Onifer SM, Whittemore SR. Labeling stem cells in vitro for identification of their differentiated phenotypes after grafting into the CNS. Methods Mol Biol. 2002;198:307–318. doi: 10.1385/1-59259-186-8:307. [DOI] [PubMed] [Google Scholar]
- Carleton A, Petreanu LT, Lansford R, Alvarez-Buyalla A, Lledo PM. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6:507–518. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- Carson CT, Aigner S, Gage FH. Stem cells: the good, bad, and barely in control. Nat Med. 2006;12:1237–1238. doi: 10.1038/nm1106-1237. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Chopp M. Intracerebral transplantation of bone marrow with BDNF after MCAo in rat. Neuropharmacology. 2000;39:711–716. doi: 10.1016/s0028-3908(00)00006-x. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001a;189:49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001b;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- Chen J, Magavi SS, Macklis JD. Neurogenesis of corticospinal motor neurons extending spinal projections in adult mice. Proc Natl Acad Sci U S A. 2004;101:16357–16362. doi: 10.1073/pnas.0406795101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KA, Laywell ED, Marshall G, Walton N, Zheng T, Steindler DA. Fusion of neural stem cells in culture. Exp Neurol. 2006;198:129–135. doi: 10.1016/j.expneurol.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Chen J, Zacharek A, Li Y, Li A, Wang L, Katakowski M, Roberts C, Lu M, Chopp M. N-cadherin mediates nitric oxide-induced neurogenesis in young and retired breeder neurospheres. Neuroscience. 2006b;140(2):377–88. doi: 10.1016/j.neuroscience.2006.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Palmer TD. Cellular repair of CNS disorders: an immunological perspective. Hum Mol Genet. 2008;17(R1):R84–92. doi: 10.1093/hmg/ddn104. [DOI] [PubMed] [Google Scholar]
- Chmielnicki E, Benraiss A, Economides AN, Goldman SA. Adenovirally expressed noggin and brain-derived neurotrophic factor cooperate to induce new medium spiny neurons from resident progenitor cells in the adult striatal ventricular zone. J Neurosci. 2004;24:2133–2142. doi: 10.1523/JNEUROSCI.1554-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K, Kim M, Park KI, Jeong SW, Park HK, Jung KH, Lee ST, Kang L, Lee K, Park DK, Kim SU, Roh JK. Human neural stem cells improve sensorimotor deficits in the adult rat brain with experimental focal ischemia. Brain Res. 2004;1016:145–153. doi: 10.1016/j.brainres.2004.04.038. [DOI] [PubMed] [Google Scholar]
- Chu K, Jung KH, Lee ST, Park HK, Sinn DI, Kim JM, Kim DH, Kim JH, Kim SJ, Song EC, Kim M, Lee SK, Roh JK. Circulating endothelial progenitor cells as a new marker of endothelial dysfunction or repair in acute stroke. Stroke. 2008;39(5):1441–7. doi: 10.1161/STROKEAHA.107.499236. [DOI] [PubMed] [Google Scholar]
- Chung S, Shin BS, Hedlund E, Pruszak J, Ferree A, Kang UJ, Isacson O, Kim KS. Genetic selection of sox1GFP-expressing neural precursors removes residual tumorigenic pluripotent stem cells and attenuates tumor formation after transplantation. J Neurochem. 2006;97:1467–1480. doi: 10.1111/j.1471-4159.2006.03841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke DL, Johansson CD, Wilbertz J, Veress B, Nilsson E, Karlstrom H, Lendahl U, Frisen J. Generalized potential of adult neural stem cells. Science. 2000;288:1660–1663. doi: 10.1126/science.288.5471.1660. [DOI] [PubMed] [Google Scholar]
- Consiglio A, Gritti A, Dolcetta D, Follenzi A, Bordignon C, Gage FH, Vescovi AL, Naldini L. Robust in vivo gene transfer into adult mammalian neural stem cells by lentiviral vectors. Proc Natl Acad Sci U S A. 2004;101:14835–14840. doi: 10.1073/pnas.0404180101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti L, Pollard SM, Gorba T, Reitano E, Toselli M, Biella G, Sun Y, Sanzone S, Ying QL, Cattaneo E, Smith A. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol. 2005;3:e283. doi: 10.1371/journal.pbio.0030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper-Kuhn CM, Kuhn HG. Is it all DNA repair? Methodological considerations for detecting neurogenesis in the adult brain. Brain Res Dev Brain Res. 2002;134:13–21. doi: 10.1016/s0165-3806(01)00243-7. [DOI] [PubMed] [Google Scholar]
- Corti S, Locatelli F, Strazzer S, Salani S, Del Bo R, Soligo D, Bossolasco P, Bresolin N, Scarlato G, Comi GP. Modulated generation of neuronal cells from bone marrow by expansion and mobilization of circulating stem cells with in vivo cytokine treatment. Exp Neurol. 2002;177:443–452. doi: 10.1006/exnr.2002.8004. [DOI] [PubMed] [Google Scholar]
- Corti S, Locatelli F, Papadimitriou D, Donadoni C, Del Bo R, Fortunato F, Strazzer S, Salani S, Bresolin N, Comi GP. Multipotentiality, homing properties, and pyramidal neurogenesis of CNS-derived LeX(ssea-1)+/CXCR4+ stem cells. FASEB J. 2005;19:1860–1862. doi: 10.1096/fj.05-4170fje. [DOI] [PubMed] [Google Scholar]
- Coyne TM, Marcus AJ, Woodbury D, Black IB. Marrow stromal cells transplanted to the adult brain are rejected by an inflammatory response and transfer donor labels to host neurons and glia. Stem Cells. 2006;24:2483–2492. doi: 10.1634/stemcells.2006-0174. [DOI] [PubMed] [Google Scholar]
- Craig CG, Tropepe V, Morshead CM, Reynolds BA, Weiss S, van der Kooy D. In vivo growth factor expansion of endogenous subependymal neural precursor cell populations in the adult mouse brain. J Neurosci. 1996;16:2649–2658. doi: 10.1523/JNEUROSCI.16-08-02649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MA, Penney EB, Pearson AG, van Roon-Mom WM, Butterworth NJ, Dragunow M, Connor B, Faull RL. Increased cell proliferation and neurogenesis in the adult human Huntington's disease brain. Proc Natl Acad Sci U S A. 2003;100:9023–9027. doi: 10.1073/pnas.1532244100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelson C, Holtas S, van Roon-Mom WM, Bjork-Eriksson T, Nordborg C, Frisen J, Dragunow M, Faull RL, Eriksson PS. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- D'Ippolito G, Diabira S, Howard G, Menei P, Roos BA, Schiller PC. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extension expansion and differentiation potential. J Cell Sci. 2004;117:2971–2981. doi: 10.1242/jcs.01103. [DOI] [PubMed] [Google Scholar]
- Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–284. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- Darsalia V, Heldmann U, Lindvall O, Kokaia Z. Stroke-induced neurogenesis in aged brain. Stroke. 2005;36(8):1790–5. doi: 10.1161/01.STR.0000173151.36031.be. [DOI] [PubMed] [Google Scholar]
- Deans AE, Wadghiri YZ, Bernas LM, Yu X, Rutt BK, Turnbull DH. Cellular MRI contrast via coexpression of transferrin receptor and ferritin. Magn Reson Med. 2006;56(1):51–9. doi: 10.1002/mrm.20914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chevigny A, Cooper O, Vinuela A, Reske-Nielsen C, Lagace DC, Eisch AJ, Isacson O. Fate Mapping and Lineage Analyses Demonstrate the Production of a Large Number of Striatal Neuroblasts after TGF{alpha} and Noggin Striatal Infusions into the Dopamine-depleted Striatum. Stem Cells. 2008 doi: 10.1634/stemcells.2008-0080. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey RJ, Sailor KA, Bowen KK, Tureyen K, Vemuganti R. Stroke-induced progenitor cell proliferation in adult spontaneously hypertensive rat brain: effect of exogenous IGF-1 and GDNF. J Neurochem. 2003;87:586–597. doi: 10.1046/j.1471-4159.2003.02022.x. [DOI] [PubMed] [Google Scholar]
- Deng W, Obrocka M, Fischer I, Prockop DJ. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem Biophys Res Commun. 2001;282:148–152. doi: 10.1006/bbrc.2001.4570. [DOI] [PubMed] [Google Scholar]
- Dirnagl U. Bench to bedside: the quest for quality in experimental stroke research. J Cereb Blood Flow Metab. 2006;26:1465–1478. doi: 10.1038/sj.jcbfm.9600298. [DOI] [PubMed] [Google Scholar]
- Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, Noel D, Jordensen C. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–3844. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;6:1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- Dunac A, Frelin C, Popolo-Blondeau M, Chatel M, Mahagne MH, Philip PJ. Neurological and functional recovery in human stroke are associated with peripheral blood CD34+ cell mobilization. J Neurol. 2007;254:327–332. doi: 10.1007/s00415-006-0362-1. [DOI] [PubMed] [Google Scholar]
- Eglitis MA, Mezey E. Hematopoetic cells differentiate into both microglia and macroglia in the brain of adult mice. Proc Natl Acad Sci U S A. 1997;94:4080–4085. doi: 10.1073/pnas.94.8.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund U, Bjorklund A, Wictorin K, Lindvall O, Kokaia M. Grafted neural stem cells develop into functional pyramidal neurons and integrate into host cortical circuitry. Proc Natl Acad Sci U S A. 2002;99:17089–17094. doi: 10.1073/pnas.252589099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enwere E, Shingo T, Gregg C, Fujikawa H, Ohta S, Weiss S. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci. 2004;24:8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Eriksson C, Bjorklund A, Wictorin K. Neuronal differentiation following transplantation of expanded mouse neurosphere cultures derived from different embryonic forebrain regions. Exp Neurol. 2003;184:615–635. doi: 10.1016/S0014-4886(03)00271-1. [DOI] [PubMed] [Google Scholar]
- Fallon J, Reid S, Kinyamu R, Opole I, Opole R, Baratta J, Korc M, Endo TL, Duong A, Nguyen G, Karkehabadhi M, Twardzik D, Patel S, Loughlin S. In vivo induction of massive proliferation, directed migration, and differentiation of neural cells in the adult mammalian brain. Proc Natl Acad Sci U S A. 2000;97:14686–14691. doi: 10.1073/pnas.97.26.14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara Y, Tanaka N, Ishida O, Fujimoto Y, Murakami T, Kajihara H, Yasunaga Y, Ochi M. Intravenously injected neural progenitor cells of transgenic rats can migrate to the injured spinal cord and differentiate into neurons, astrocytes and oligodendrocytes. Neurosci Lett. 2004;366:287–291. doi: 10.1016/j.neulet.2004.05.080. [DOI] [PubMed] [Google Scholar]
- Galimi F, Summers RG, van Praag H, Verma IM, Gage FH. A role for bone marrow-derived cells in the vasculature of noninjured CNS. Blood. 2005;105:2400–2402. doi: 10.1182/blood-2004-02-0612. [DOI] [PubMed] [Google Scholar]
- Gamm DM, Wang S, Lu B, Girman S, Holmes T, Bischoff N, Shearer RL, Sauve Y, Capowski E, Svendsen CN, Lund RD. Protection of visual functions by human neural progenitors in a rat model of retinal disease. PLoS ONE. 2007;2:e338. doi: 10.1371/journal.pone.0000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardi JE, Nyengaard JR, Gundersen HJ. Automatic sampling for unbiased and efficient stereological estimation using the proportionator in biological studies. J Microsc. 2008;230(Pt 1):108–20. doi: 10.1111/j.1365-2818.2008.01963.x. [DOI] [PubMed] [Google Scholar]
- Geraerts M, Eggermont K, Hernandez-Acosta P, Garcia-Verdugo JM, Baekelandt V, Debyser Z. Lentiviral vectors mediate efficient and stable gene transfer in adult neural stem cells in vivo. Hum Gene Ther. 2006;17:635–650. doi: 10.1089/hum.2006.17.635. [DOI] [PubMed] [Google Scholar]
- Giles J. The trouble with replication. Nature. 2006;442:344–347. doi: 10.1038/442344a. [DOI] [PubMed] [Google Scholar]
- Giorgano A, Galderisi U, Marino IR. From the laboratory bench to the patient's bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007;211:27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- Glaser T, Brustle O. Retinoic acid induction of ES-cell-derived neurons: the radial glia connection. Trends Neurosci. 2005;28:397–400. doi: 10.1016/j.tins.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Goddard DR, Berry M, Kirvell SL, Butt AM. Fibroblast growth factor-2 inhibits myelin production by oligodendrocytes in vivo. Mol Cell Neurosci. 2001;18:556–569. doi: 10.1006/mcne.2001.1025. [DOI] [PubMed] [Google Scholar]
- Goddard DR, Berry M, Kirvell SL, Butt AM. Fibroblast growth factor-2 induces astroglial and microglial reactivity in vivo. J Anat. 2002;200:57–67. doi: 10.1046/j.0021-8782.2001.00002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci U S A. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg C, Weiss S. Generation of functional radial glial cells by embryonic and adult forebrain neural stem cells. J Neurosci. 2003;23:11587–11601. doi: 10.1523/JNEUROSCI.23-37-11587.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Brannstrom T, Wester P. Cortical neurogenesis in adult rats after reversible photothrombotic stroke. J Cereb Blood Flow Metab. 2000;20:1166–1173. doi: 10.1097/00004647-200008000-00002. [DOI] [PubMed] [Google Scholar]
- Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, Nolte J, Wolf F, Li M, Engel W, Hasenfuss G. Plutipotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–1203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- Hack MA, Saghatelyan A, de Chevigny A, Pfeifer A, Ashery-Padan R, Lledo PM, Gotz M. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat Neurosci. 2005;8:865–872. doi: 10.1038/nn1479. [DOI] [PubMed] [Google Scholar]
- Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick JP, Brott T, Frankel M, Grotta JC, Haley EC, Jr, Kwiatkowski T, Levine SR, Lewandowski C, Lu M, Lyden P, Marler JR, Patel S, Tilley BC, Albers G, Bluhmski E, Wilhelm M, Hamilton S. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. ATLANTIS Trials Investigators, ECASS Trials Investigators, NINDS rt-PA Study Group Investigators. [DOI] [PubMed] [Google Scholar]
- Hagg T. Molecular regulation of adult CNS neurogenesis: an integrated view. Trends Neurosci. 2005;28:589–595. doi: 10.1016/j.tins.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Harkany T, Andang M, Kingma HH, Gorcs TJ, Holmgren CD, Zilberter Y, Ernfors P. Region-specific generation of functional neurons from naïve embryonic stem cells in adult brain. J Neurochem. 2004;88:1229–1239. doi: 10.1046/j.1471-4159.2003.02243.x. [DOI] [PubMed] [Google Scholar]
- Hayashi J, Takagi Y, Fukuda H, Imazato T, Nishimura M, Fujimoto M, Takahashi J, Hashimoto N, Nozaki K. Primate embryonic stem cell-derived neuronal progenitors transplanted into ischemic brain. J Cereb Blood Flow Meta. 2006;26:906–914. doi: 10.1038/sj.jcbfm.9600247. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A. Adult-derived neural precursors transplanted into multiple regions in the adult brain. Ann Neurol. 1999;46:867–877. doi: 10.1002/1531-8249(199912)46:6<867::aid-ana9>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hisatsune T. Regeneration of cortical neurons from nestin + adult progenitor cells. Exp Neurol. 2006;198:571. [Google Scholar]
- Hiyama E, Hiyama K. Telomere and telomerase in stem cells. Br J Cancer. 2007;96:1020–1024. doi: 10.1038/sj.bjc.6603671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn BD, Palmer TD, Steinberg GK. Neurogenesis in rats after focal cerebral ischemia is enhanced by indomethacin. Stroke. 2005;36:2718–2724. doi: 10.1161/01.STR.0000190020.30282.cc. [DOI] [PubMed] [Google Scholar]
- Hoffman LM, Carpenter MK. Characterization and culture of human embryonic stem cells. Nat Biotechnol. 2005;23(6):699–708. doi: 10.1038/nbt1102. [DOI] [PubMed] [Google Scholar]
- Hofstetter CP, Holmstrom NA, Lilja JA, Schweinhardt P, Hao J, Spenger C, Wiesenfeld-Hallin Z, Kurpad SN, Frisen J, Olson L. Allodynia limits the usefulness of intraspinal neural stem cell grants; directed differentiation improves outcome. Nat Neurosci. 2005;8:346–353. doi: 10.1038/nn1405. [DOI] [PubMed] [Google Scholar]
- Honma T, Honmou O, Iihoshi S, Harada K, Houkin K, Hamada H, Kocsis JD. Intravenous infusion of immortalized human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Exp Neurol. 2006;199:56–66. doi: 10.1016/j.expneurol.2005.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horita Y, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. Intravenous administration of glial cell line-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in the adult rat. J Neurosci Res. 2006;84:1495–1504. doi: 10.1002/jnr.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyslop L, Stojkovic M, Armstrong L, Walter T, Stojkovic P, Przyborski S, Herbert M, Murdoch A, Strachan T, Lako M. Downregulation of NANOG induces differentiation of human embryonic stem cells to extraembryonic lineages. Stem Cells. 2005;23:1035–1043. doi: 10.1634/stemcells.2005-0080. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Ohtsuka T, Metzger D, Chambon P, Kageyama R. Temporal regulation of Cre recombinase activity in neural stem cells. 2006;44:233–238. doi: 10.1002/dvg.20212. [DOI] [PubMed] [Google Scholar]
- Jackson EL, Garcia-Verdugo JM, Gil-Perotin S, Roy M, Quinones-Hinohosa A, VandenBerg S, Alvarez-Buylla A. PDGFR alpha-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron. 2006;51:187–199. doi: 10.1016/j.neuron.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Jansen EM, Solberg L, Underhill S, Wilson S, Cozzari C, Hartman BK, Faris P, Low WC. Transplantation of fetal neocortex ameliorates sensorimotor and locomotor deficits following neonatal ischemic-hyposix brain injury in rats. Exp Neurol. 1997;147:487–497. doi: 10.1006/exnr.1997.6596. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Clemenson GD, Jr, Gage FH. Spontaneous fusion and nonclonal growth of adult neural stem cells. Stem Cells. 2007;25:871–874. doi: 10.1634/stemcells.2006-0620. [DOI] [PubMed] [Google Scholar]
- Jiang W, Gu W, Brannstrom T, Rosqvist R, Wester P. Cortical neurogenesis in adult rats after transiet middle cerebral artery occlusion. Stroke. 2001;32:1201–1207. doi: 10.1161/01.str.32.5.1201. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonazlez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Jin K, Sun Y, Xie L, Batteur S, Mao XO, Smelick C, Logvinova A, Greenberg DA. Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell. 2003a;2:175–183. doi: 10.1046/j.1474-9728.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- Jin K, Sun Y, Xie L, Peel A, Mao XO, Batteur S, Greenberg DA. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci. 2003b;24:171–189. doi: 10.1016/s1044-7431(03)00159-3. [DOI] [PubMed] [Google Scholar]
- Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, Shen J, Mao Y, Banwait S, Greenberg DA. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci U S A. 2006;103:13198–13202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RE, Dillon-Carter O, Freed WJ, Borlongan CV. Trophic factor secreting kidney cell lines: in vitro characterization and functional effects following transplantation in ischemic rats. Brain Res. 2001;900:268–276. doi: 10.1016/s0006-8993(01)02327-7. [DOI] [PubMed] [Google Scholar]
- Kawada H, Takizawa S, Takanashi T, Morita Y, Fujita J, Fukuda K, Takagi S, Okano H, Ando K, Hotta T. Administration of hematopoietic cytokines in the subacute phase after cerebral infarction is effective for functional recovery facilitation proliferation of intrinsic neural stem/progenitor cells and transition of bone marrow-derived neuronal cells. Circulation. 2006;113:701–710. doi: 10.1161/CIRCULATIONAHA.105.563668. [DOI] [PubMed] [Google Scholar]
- Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, Masel J, Yenari MA, Weissman IL, Uchida N, Palmer T, Steinberg GK. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci U S A. 2004;101:11839–11844. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent detate gyrus. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DW, Chung S, Hwang M, Ferree A, Tsai HC, Park JJ, Chung S, Nam TS, Kang UK, Isacson O, Kim KS. Stromal cell-derived inducing activity, Nurr1, and signaling molecules synergistically induce dopaminergic neurons from mouse embryonic stem cells. Stem Cells. 2006;24:557–567. doi: 10.1634/stemcells.2005-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Auerbach JM, Rodriguez-Gomez JA, Velasco I, Gavin D, Lumelski N, Lee SH, Nguyen J, Sanchez-Pernaute R, Bankiewicz K, McKay R. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 2002;418:50–56. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- Kissela B, Broderick J, Woo D, Kothari R, Miller R, Khoury J, Brott T, Pancioli A, Jauch E, Gebel J, Shukla R, Alwell K, Tomsick T. Greater Cincinnati/Northern Kentucky stroke study: volume of first-ever ischemic stroke among blacks in a population-based study. Stroke. 2001;32:1285–1290. doi: 10.1161/01.str.32.6.1285. [DOI] [PubMed] [Google Scholar]
- Kleppner SR, Robinson KA, Trojanowski JQ, Lee VM. Transplanted human neurons derived from a teratocarcinoma cell line (NTera-2) mature, integrate, and survive for over 1 year in the nude mouse brain. J Comp Neurol. 1995;357:618–632. doi: 10.1002/cne.903570410. [DOI] [PubMed] [Google Scholar]
- Kogler G, Sensken S, Airey JA, Trapp T, Muschen M, Feldhahn N, Liedtke S, Sorg RV, Fisher J, Rosenbaum C, Greschat S, Knipper A, Bender J, Degistirici O, Gao J, Caplan AI, Colletti EJ, Almeida-Porada G, Muller HW, Zanjani E, Wernet P. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123–135. doi: 10.1084/jem.20040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokaia Z, Thored P, Arvidsson A, Lindvall O. Regulation of stroke-induced neurogenesis in adult brain – recent scientific progress. Cereb Cortex. 2006;161(Suppl 1):i162–i167. doi: 10.1093/cercor/bhj174. [DOI] [PubMed] [Google Scholar]
- Kolb B, Morshead C, Gonzalez C, Kim M, Gregg C, Shingo T, Weiss S. Growth factor-stimulated generation of new cortical tissue and functional recovery after stroke damage to the motor cortex of rats. J Cereb Blood Flow Metab. 2007;27:983–997. doi: 10.1038/sj.jcbfm.9600402. [DOI] [PubMed] [Google Scholar]
- Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotent CNS stem cells. Science. 2000;238:1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- Kondziolka D, Wechsler L, Goldstein S, Meltzer C, Thulborn KR, Gebel J, Jannetta P, DeCesare S, Elder EM, McGrogan M, Reitman MA, Bynum L. Transplantation of cultured human neuronal cells for patients with stroke. Neurology. 2000;55(4):565–9. doi: 10.1212/wnl.55.4.565. [DOI] [PubMed] [Google Scholar]
- Kondziolka D, Steinberg GK, Wechsler L, Meltzer CC, Elder E, Gebel J, Decesare S, Jovin T, Zafonte R, Lebowitz J, Flickinger JC, Tong D, Marks MP, Jamieson C, Luu D, Bell-Stephens T, Teraoka J. Neurotransplantation for patients with subcortical motor stroke: a phase 2 randomized trial. J Neurosurg. 2005;103:38–45. doi: 10.3171/jns.2005.103.1.0038. [DOI] [PubMed] [Google Scholar]
- Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan CY, Schloemer AJ, Lu A, Burns KA, Weng WL, Williams MT, Strauss KI, Vorhees CV, Flavell RA, Davis RJ, Sharp FR, Rakic P. Hypoxia-ischemia induces DNA synthesis without cell proliferation in dying neurons in adult rodent brain. J Neurosci. 2004;24:10763–10772. doi: 10.1523/JNEUROSCI.3883-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J, Ratajczak MZ. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells indentified in adult bone marrow. Leukemia. 2006;20:857–869. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FG. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CT, Mirzadeh Z, Soriano-Navarro M, Rasin M, Wang D, Shen J, Sestan N, Garcia-Verdugo J, Alvarez-Buylla A, Jan LY, Jan YN. Postnatal deletion of Numb/Numblike reveals repair and remodeling capacity in the subventricular neurogenic niche. Cell. 2006;127:1253–1264. doi: 10.1016/j.cell.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JP, Jeyakumar M, Gonzalez R, Takahashi H, Lee PJ, Baek RC, Clark D, Rose H, Fu G, Clarke J, McKercher S, Meerloo J, Muller FJ, Park KI, Butters TD, Dwek RA, Schwartz P, Tong G, Wenger D, Lipton SA, Seyfriend TN, Platt FM, Snyder EY. Stem cells act through multiple mechanisms to benefit mice with neurodegenerative metabolic disease. Nat Med. 2007;13:439–447. doi: 10.1038/nm1548. [DOI] [PubMed] [Google Scholar]
- Lagace DC, Whitman MC, Noonan MA, Ables JL, DeCarolis NA, Arguello AA, Donovan MH, Fischer SJ, Farnbauch LA, Beech RD, DiLeone RJ, Greer CA, Mandyam CD, Eisch AJ. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci. 2007;27(46) doi: 10.1523/JNEUROSCI.3812-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]; Stem cells act through multiple mechanisms to benefit mice with neurodegenerative metabolic disease. Nat Med. 13:439–447. doi: 10.1038/nm1548. [DOI] [PubMed] [Google Scholar]
- Leker RR, Soldner F, Velasco I, Gavin DK, Androutsellis-Theotokis A, McKay RD. Long-lasting regeneration after ischemia in the cerebral cortex. Stroke. 2007;38:153–161. doi: 10.1161/01.STR.0000252156.65953.a9. [DOI] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Li M, Pevny L, Lovell-Badge R, Smith A. Generation of purified neural precursors from embryonic stem cells by lineage selection. Curr Biol. 1998;8:971–974. doi: 10.1016/s0960-9822(98)70399-9. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Chopp M. Adult bone marrow transplantation after stroke in adult rats. Cell Transplant. 2001;10:31–40. [PubMed] [Google Scholar]
- Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Parent JM. Adult neurogenesis and the ischemic forebrain. J Cereb Blood Flow Metab. 2006;26:1–20. doi: 10.1038/sj.jcbfm.9600170. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441:1094–1096. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- Liour SS, Yu RK. Differentiation of radial glia-like cells from embryonic stem cells. Glia. 2003;42:109–117. doi: 10.1002/glia.10202. [DOI] [PubMed] [Google Scholar]
- Liu H, Honmou O, Harada K, Nakamura K, Houkin K, Hamada H, Kocsis JD. Neuroprotection by PIGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain. 2006;129:2734–2745. doi: 10.1093/brain/awl207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998;18:7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo L, Bygrave A, Grosveld FG, Pandolfi PP. The chromosome make-up of mouse embryonic stem cells is predictive of somatic and germ cell chimaerism. Transgenic Res. 1997;6(5):321–8. doi: 10.1023/a:1018418914106. [DOI] [PubMed] [Google Scholar]
- Lowell S, Benchoua A, Heavey B, Smith AG. Notch promotes neural lineage entry by pluripotent embryonic stem cells. PLoS Biol. 2006;4:3121. doi: 10.1371/journal.pbio.0040121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Blesch A, Tuszynski MH. Induction of bone marrow stromal cells to neurons: differentiation, transdifferentiation, or artifact? J Neurosci Res. 2004;77:174–191. doi: 10.1002/jnr.20148. [DOI] [PubMed] [Google Scholar]
- Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- Mendez I, Viñuela A, Astradsson A, Mukhida K, Hallett P, Robertson H, Tierney T, Holness R, Dagher A, Trojanowski JQ, Isacson O. Dopamine neurons implanted into people with Parkinson's disease survive without pathology for 14 years. Nat Med. 2008;(5):507–9. doi: 10.1038/nm1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon LG, Picinich S, Koneru R, Gao H, Lin SY, Koneru M, Mayer-Kuckuk P, Glod J, Banerjee D. Differentiation gene expression associated with migration of mesenchymal stem cells to conditioned medium from tumor cells or bone marrow cells. Stem Cells. 2007;25:520–528. doi: 10.1634/stemcells.2006-0257. [DOI] [PubMed] [Google Scholar]
- Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, Malapani C, Moore H, Hen R. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci. 2006;9:729–731. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- Mezey E, Chandross KH, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- Minger SL, Ekonomou A, Carta EM, Chinoy A, Perry RH, Ballard CG. Endogenous neurogenesis in the human brain following cerebral infarction. Regen Med. 2007;2:69–74. doi: 10.2217/17460751.2.1.69. [DOI] [PubMed] [Google Scholar]
- Miura M, Miura Y, Padilla-Nash HM, Molinolo AA, Fu B, Patel V, Seo BM, Sonoyama W, Zheng JJ, Baker CC, Chen W, Ried T, Shi S. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 2006;24:1095–1103. doi: 10.1634/stemcells.2005-0403. [DOI] [PubMed] [Google Scholar]
- Modo M, Rezaie P, Heuschling P, Patel S, Male DK, Hodges H. Transplantation of neural stem cells in a rat model of stroke: assessment of short-term graft survival and acute host immunological response. Brain Res. 2002;958:70–82. doi: 10.1016/s0006-8993(02)03463-7. [DOI] [PubMed] [Google Scholar]
- Modo M, Stroemer RP, Tang E, Patel S, Hodges H. Effects of implantation site of dead stem cells in rats with stroke damage. Neuroreport. 2003;14:39–42. doi: 10.1097/00001756-200301200-00007. [DOI] [PubMed] [Google Scholar]
- Monfils MH, Driscoll I, Kamitakahara H, Wilson B, Flynn C, Teskey GC, Kleim JA, Kolb B. FGF-2-induced cell proliferation stimulates anatomical, neurophysiological and functional recovery from neonata motor cortex injury. Eur J Neurosci. 2006;24:739–749. doi: 10.1111/j.1460-9568.2006.04939.x. [DOI] [PubMed] [Google Scholar]
- Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockage restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Montaron MF, Petry KG, Rodriguez JJ, Marinelli M, Aurousseau C, Rougon G, Le Moal M, Abrous DN. Adrenalectomy increases neurogenesis but not PSA-NCAM expression in aged dentate gyrus. Eur J Neurosci. 1999;11:1479–1485. doi: 10.1046/j.1460-9568.1999.00579.x. [DOI] [PubMed] [Google Scholar]
- Montaron MF, Drapeau E, Dupret D, Kitchener P, Aurousseau C, Le Moal M, Pizza PV, Abrous DN. Lifelong corticosterone level determines age-related decline in neurogenesis and memory. Neurobiol Aging. 2006;27:645–654. doi: 10.1016/j.neurobiolaging.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Morizane A, Takahashi J, Shinoyama M, Ideguchi M, Takagi Y, Fukuda H, Koyanagi M, Sasai Y, Hashimoto N. Generation of graftable dopaminergic neuron progenitors from mouse ES cells by a combination of coculture and neurosphere methods. J Neurosci Res. 2006;83:1015–1027. doi: 10.1002/jnr.20799. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Benveniste P, Iscove NN, van der Kooy D. Hematopoietic competence is a rare property of neural stem cells that may depend on genetic and epigenetic alterations. Nat Med. 2002;8:268–273. doi: 10.1038/nm0302-268. [DOI] [PubMed] [Google Scholar]
- Morshead CM, van der Kooy D. Disguising adult neural stem cells. Curr Opin Neurobiol. 2004;14:125–131. doi: 10.1016/j.conb.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Muller FJ, Snyder EY, Loring JF. Gene therapy: can neural stem cells deliver? Nat Rev Neurosci. 2006;7:75–84. doi: 10.1038/nrn1829. [DOI] [PubMed] [Google Scholar]
- Munoz-Elias G, Marcus AJ, Coyne TM, Woodbury D, Black IB. Adult bone marrow stromal cells in the embryonic brain: engraftment, migration, differentiation, and long-term survival. J Neurosci. 2004;24:4585–4595. doi: 10.1523/JNEUROSCI.5060-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muotri AR, Nakashima K, Toni N, Sandler VM, Gage FH. Development of functional human embryonic stem cell-derived neurons in mouse brain. Proc Natl Acad Sci U S A. 2005;102:18644–18648. doi: 10.1073/pnas.0509315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- Nan Z, Grande A, Sanberg CD, Sanberg PR, Low WC. Infusion of human umbilical cord blood ameliorates neurologic deficits in rats with hemorrhagic brain injury. Ann NY Acad Sci. 2005;1049:84–96. doi: 10.1196/annals.1334.009. [DOI] [PubMed] [Google Scholar]
- Nati-Oumesmar B, Decker L, Lachapelle F, Avellana-Adalid V, Bachelin C, Van Evercooren AB. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur J Neurosci. 1999;11:4357–4366. doi: 10.1046/j.1460-9568.1999.00873.x. [DOI] [PubMed] [Google Scholar]
- Tissue plasmiogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. National Institute of Neurological Disorders and Stroke rt-PA Study Group. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Kondziolka D, Wechsler L, Goldstein S, Gebel J, DeCesare S, Elder EM, Zhang PJ, Jacobs A, McGrogan M, Lee VM, Trojanowski JQ. Cloncal human (hNT) neuron grafts for stroke therapy: neuropathology in a patient 27 months after implantation. Am J Pathol. 2002;160:1201–1206. doi: 10.1016/S0002-9440(10)62546-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolis SK. Cancer stem cells and “stemness” genes in neuron-oncology. Neurobiol Dis. 2007;25:217–229. doi: 10.1016/j.nbd.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reported mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- Ohori Y, Yamamoto S, Nagao M, Sugimori M, Yamamoto N, Nakamura K, Nakafuku M. Growth factor treatment and genetic manipulation stimulate neurogenesis and oligodendrogenesis by endogenous neural progenitors in the injured adult spinal cord. J Neurosci. 2006;26:11948–11960. doi: 10.1523/JNEUROSCI.3127-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onifer SM, Low WC. Spatial memory deficit resulting from ischemia-induced damage to the hippocampus is ameliorated by intra-hippocampal transplants of fetal hippocampal neurons. Prog Brain Res. 1990;82:359–366. doi: 10.1016/s0079-6123(08)62623-0. [DOI] [PubMed] [Google Scholar]
- Ourednik J, Ourednik V, Lynch WP, Schachner M, Snyder EY. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat Biotechnol. 2002;20:1103–1110. doi: 10.1038/nbt750. [DOI] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Park KI, Teng YD, Snyder EY. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat Biotechnol. 2002;20:1111–1117. doi: 10.1038/nbt751. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2. Boston: Elsevier Academic Press; 2004. [Google Scholar]
- Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlow MJ, Freed WJ, Hoffer BJ, Seiger A, Olson L, Wyatt RJ. Brain grafts reduce motor abnormalities produced by destruction of nigrostriatal dopamine system. Science. 1979;204:643–647. doi: 10.1126/science.571147. [DOI] [PubMed] [Google Scholar]
- Peterson DA. Quantitative histology using confocal microscopy: implementation of unbiased stereology procedures. Methods. 1999;18(4):493–507. doi: 10.1006/meth.1999.0818. [DOI] [PubMed] [Google Scholar]
- Picard-Riera N, Decker L, Delarasse C, Goude K, Nait-Oumesmar B, Liblau R, Pham-Dihn D, Evercooren AB. Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc Natl Acad Sci U S A. 2002;99:13211–13216. doi: 10.1073/pnas.192314199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccini P, Brooke DJ, Bjorklund A, Gunn RN, Grasby PM, Rimoldi O, Brundin P, Hagell P, Rehncrona S, Widner H, Lindvall O. Dopamine release from nigral transplants visualized in vivo in a Parkinson's patient. Nat Neurosci. 1999;2:1137–1140. doi: 10.1038/16060. [DOI] [PubMed] [Google Scholar]
- Plachta N, Bibel M, Tucker KL, Barde YA. Developmental potential of defined neural progenitors derived from mouse embryonic stem cells. Development. 2004;131:5449–5456. doi: 10.1242/dev.01420. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, Furlan R, Comi G, Vescovi AL, Martino G. Injectino of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, Martinello M, Cattalini A, Bergami A, Furlan R, Comi G, Constantin G, Martino G. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005;436:266–271. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- Pollard SM, Conti L, Sun Y, Goffredo D, Smith A. Adherent neural stem (NS) cells from fetal and adult forebrain. Cereb Cortex. 2006;16(Suppl 1):i112–i120. doi: 10.1093/cercor/bhj167. [DOI] [PubMed] [Google Scholar]
- Pollock K, Stroemer P, Patel S, Stevanato L, Hope A, Miljan E, Dong Z, Hodges H, Price J, Sinden JD. A conditionall immortal clonal stem line from human cortical neuroepithelium for the treatment of ischemic stroke. Exp Neurol. 2006;199:143–155. doi: 10.1016/j.expneurol.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Ponte AL, Marais E, Gallay N, Langonne A, Delorme B, Herault O, Charbord P, Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: Comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- Priller J, Persons DA, Klett FF, Kempermann G, Kreutzberg GW, Dirnagl U. Neogenesis of cerebellar Purkinje neurons from gene-marked bone marrow cells in vivo. J Cell Biol. 2001;155:733–738. doi: 10.1083/jcb.200105103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones-Hinojosa A, Sanai S, Soriano-Naravvao M, Gonzalez-Perez O, Mirzadeh Z, Gil-Perotin S, Romero-Rodriguez R, Berger MS, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and cytoarchitecture of the adult human subventricular zone: a nice of neural stem cells. J Comp Neruol. 2006;494:415–434. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- Raber J, Fan Y, Matsumori Y, Liu Z, Weinstein PR, Fike JR, Liu J. Irradiation attenuates neurogenesis and exacerbates ischemia-induced deficits. Ann Neurol. 2004;44:381–389. doi: 10.1002/ana.10853. [DOI] [PubMed] [Google Scholar]
- Ramon Y, Cajal S. Degeneration and regeneration of the nervous system. New York: Hafner; 1928. [Google Scholar]
- Reyes M, Verfaillie CM. Characterization of multipotent adult progenitor cells, a subpopulation of mesenchymal stem cells. Ann NY Acad Sci. 2001;938:231–233. doi: 10.1111/j.1749-6632.2001.tb03593.x. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol. 1996;175:1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, Fike JR. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Ropper AH, Brown RH. Adams and Victor's Principles of Neurology. 8. New York: McGraw-Hill; 2005. [Google Scholar]
- Ross JJ, Hong Z, Willenbring B, Zeng L, Isenberg B, Lee EH, Reyes M, Keirstead SA, Weir EK, Tranquillo RT, Verfaillie CM. Cytokine-induced differentiation of multipotent adult progenitor cells into functional smooth muscle cells. J Clin Invest. 2006;116:3139–3149. doi: 10.1172/JCI28184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy NS, Nakano T, Keyoung HM, Windrem M, Rashbaum WK, Alonso ML, Kang J, Peng W, Carpenter MK, Lin J, Nedergaard M, Goldman SA. Telomerase immortalization of neuronally restricted progenitor cells derived from the human fetal spinal cord. Nat Biotechnol. 2004;22:297–305. doi: 10.1038/nbt944. [DOI] [PubMed] [Google Scholar]
- Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006;12:1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- Rubio D, Garcia-Castro J, Martin MC, de la Fuente R, Cigudosa JC, Lloyd AC, Bernad A. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–3039. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond) 2005;2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Pernaute R, Studer L, Ferrari D, Perrier A, Lee H, Vinuela A, Isacson O. Long-term survival of dopamine neurons derived from parthenogenetic primate embryonic stem cells (cyno-1) after transplantation. Stem Cells. 2005;23:914–922. doi: 10.1634/stemcells.2004-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanai N, Berger MS, Garcia-Verdugo JM, Alvarez-Buylla A. Comment on “Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension”. Science. 2007 Oct 19;318(5849):393. doi: 10.1126/science.318.5849.393a. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Do glucocorticoid concentrations rise with age in the rat? Neurobiol Aging. 1992;13:171–174. doi: 10.1016/0197-4580(92)90025-s. [DOI] [PubMed] [Google Scholar]
- Schabitz WR, Scheiner A. New targets for established proteins: exploring G-CSF for the treatment of stroke. Trends Pharmacol Sci. 2007;28:157–161. doi: 10.1016/j.tips.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Schabitz WR, Kruger C, Pitzer C, Weber D, Laage R, Gassler N, Aronowski J, Mier W, Kirsch F, Dittgen T, Bach A, Sommer C, Schneider A. A neuroprotective function for the hematopoietic protein granulocyte-macrophage colony stimulating factor. J Cereb Blood Flow Metab. 2008;28:29–43. doi: 10.1038/sj.jcbfm.9600496. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, Sollas AL, Cross SD. Spontaneous limbic seizures after intrahippocampal infusion of brain-derived neurotrophic factor. Exp Neurol. 2002;174:201–214. doi: 10.1006/exnr.2002.7869. [DOI] [PubMed] [Google Scholar]
- Scheffler B, Schmandt T, Schroder W, Steinfarz B, Husseini L, Wellmer J, Seifert G, Karram K, Beck H, Blumcke I, Wiestler OD, Steinhauser C, Brustle O. Functional network integration of embryonic stem cell-derived astrocytes in hippocampal slice cultures. Development. 2003;130:5533–5541. doi: 10.1242/dev.00714. [DOI] [PubMed] [Google Scholar]
- Scheffler B, Walton NM, Lin DD, Goetz AK, Enikolopov G, Roper SN, Steindler DA. Phenotypic and functional characterization of adult brain neuropoiesis. Proc Natl Acad Sci U S A. 2005;102:9355–9358. doi: 10.1073/pnas.0503965102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Kruger C, Steigleder T, Weber D, Pitzer C, Laage R, Aronowski J, Maurer MH, Gassler N, Mier W, Hasselblatt M, Kollmar R, Schwab S, Sommer C, Bach A, Kuhn HG, Schabitz WR. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest. 2005;115:2083–2098. doi: 10.1172/JCI23559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291–1302. doi: 10.1172/JCI15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaberg RM, van der Kooy D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the detate gyrus contains restricted progenitors. J Neurosci. 2002;22:1784–1793. doi: 10.1523/JNEUROSCI.22-05-01784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini M, Dylla SJ, Oki M, Heremans Y, Tolar J, Jiang Y, Buckley SM, Pelacho B, Burns TC, Frommer S, Rossi DJ, Bryder D, Panoskaltsis-Mortari A, O'Shaughnessy MJ, Nelson-Holte M, Fine GC, Weissman IL, Blazar BR, Verfaillie CM. Hematopoietic reconstitution by multipotent adult progenitor cells: precursors to long-term hematopoietic stem cells. J Exp Med. 2007;204:129–139. doi: 10.1084/jem.20061115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serakinci N, Guldberg P, Burns JS, Abdallah B, Schrodder H, Jensen T, Kassem M. Adult human mesenchymal stem cell as a target for neoplastic transformation. Oncogene. 2004;23:5095–5098. doi: 10.1038/sj.onc.1207651. [DOI] [PubMed] [Google Scholar]
- Seri B, Herrera DG, Gritti A, Ferron S, Collado L, Vescovi A, Garcia-Verdugo JM, Alvarez-Buylla A. Composition and organization of the SCZ: a large germinal layer containing neural stem cells in the adult mammalian brain. Cereb Cortex. 2006;16(Suppl 1):i103–i111. doi: 10.1093/cercor/bhk027. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005;51:173–186. doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- Shingo T, Sorokan ST, Shimazaki T, Weiss S. Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J Neurosci. 2001;21:9733–9743. doi: 10.1523/JNEUROSCI.21-24-09733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Shyu WC, Lin SZ, Chiang MF, Su CY, Li H. Intracerebral peripherally blood stem cell (CD34+) implantation induces neuroplasticity by enhancing beta1 integrin-mediated angiogenesis in chronic stroke rats. J Neurosci. 2006;26:3444–3453. doi: 10.1523/JNEUROSCI.5165-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden JD, Rashid-Doubell F, Kershaw TR, Nelson A, Chadwick A, Jat PS, Noble MD, Hodges H, Gray JA. Recovery of spatial learning by grafts of a conditionally immortalized hippocampal neuroepithelial cell line into the ischaemia-lesioned hippocampus. Neuroscience. 1997;81:599–608. doi: 10.1016/s0306-4522(97)00330-8. [DOI] [PubMed] [Google Scholar]
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- Sobrino T, Hurtado O, Moro MA, Rodríguez-Yáñez M, Castellanos M, Brea D, Moldes O, Blanco M, Arenillas JF, Leira R, Dávalos A, Lizasoain I, Castillo J. The increase of circulating endothelial progenitor cells after acute ischemic stroke is associated with good outcome. Stroke. 2007;38(10):2759–64. doi: 10.1161/STROKEAHA.107.484386. [DOI] [PubMed] [Google Scholar]
- Son BR, Marquez-Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J, Ratajczak MZ, Janowska-Wieczorek A. Migration of bone marrow nad cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254–1264. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- Song HJ, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002a;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Song HJ, Stevens CF, Gage FH. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat Neurosci. 2002b;5:438–445. doi: 10.1038/nn844. [DOI] [PubMed] [Google Scholar]
- Sortwell CE. Strategies for the augmentation of grafted dopamine neuron survival. Front Biosci. 2003;8:s522–s532. doi: 10.2741/1096. [DOI] [PubMed] [Google Scholar]
- Sprigg N, Bath PM, Zhao L, Willmot MR, Gray LJ, Walker MF, Dennis MS, Russel N. Granulocyte-colony-stimulating factor mobilizes bone marrow stem cells in patients with subacute ischemic stroke: the Stem cell Trial of recovery EnhanceMent after Stroke (STEMS) pilot randomized, controlled trial (ISRCTN 16784092) Stroke. 2006;37:2979–2983. doi: 10.1161/01.STR.0000248763.49831.c3. [DOI] [PubMed] [Google Scholar]
- Squire LR, Roberts JL, Spitzer NC, Zigmond MJ, McConnell SK, Bloom FE, editors. Fundamental Neuroscience. 2nd. Academic Press; San Diego, CA: 2003. [Google Scholar]
- Svendsen CN, Bhattacharyya A, Tai YT. Neurons from stem cells: preventing an identity crisis. Nat Rev Neurosci. 2001;2:831–834. doi: 10.1038/35097581. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, Tsukamoto Y, Iso H, Fujimori Y, Stern DM, Naritomi H, Matsuyama T. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi Y, Takahashi J, Saiki H, Morizane A, Hayashi T, Kishi Y, Fukuda H, Okamoto Y, Koyanagi M, Ideguchi M, Hayashi H, Imazato T, Kawasaki H, Suemori H, Omachi S, Iida H, Itoh N, Nakatsuji N, Sasia Y, Hashimoto N. Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest. 2005;115:102–109. doi: 10.1172/JCI21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kitagawa K, Sugiura S, Matsuoka-Omura E, Sasaki T, Yagita Y, Hori M. Infiltrating microphages as in vivo targets for intravenous gene delivery in cerebral infarction. Stroe. 2004;35:1968–1973. doi: 10.1161/01.STR.0000133685.59556.a7. [DOI] [PubMed] [Google Scholar]
- Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev. 2007;53:198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- Thomson A, Wojtacha D, Hewitt Z, priddle H, Sottiele V, Di Domenico A, Fletcher J, Waterfall M, Corrales NL, Ansell R, McWhir J. Human embryonic stem cells passaged using enzymatic methods retain a normal karyotype and express CD30. Cloning Stem Cells. 2008;10(1):89–106. doi: 10.1089/clo.2007.0072. [DOI] [PubMed] [Google Scholar]
- Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- Tolar J, O'shaughnessy MJ, Panoskaltsis-Mortari A, McElmurry RT, Bell S, Riddle M, McIvor RS, Yant SR, Kay MA, Krause D, Verfaillie CM, Blazar BR. Host factors that impact the biodistribution and persistence of multipotent adult progenitor cells. Blood. 2006;107:4182–4188. doi: 10.1182/blood-2005-08-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolar J, Nauta AJ, Osborne MJ, Panoskaltsis-Mortari A, McElmurry RT, Bell S, Xia L, Zhou N, Riddle M, Schroeder TM, Westendorf JJ, McIvor RS, Hogendoorn PC, Szuhai K, Oseth L, Hirsch B, Yant SR, Kay MA, Peister A, Prockop DJ, Fibbe WE, Blazar BR. Sarcoma derived from cultured mesenchymal stem cells. 2007;25:371–379. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- Tonchev AB, Yamashima T, Sawamoto K, Okano H. Enhanced proliferation of progenitor cells in the subventricular zone and limited neuronal production in the striatum and neocortex of adult macaque monkeys after global cerebral ischemia. J Neurosci Res. 2005;81:776–788. doi: 10.1002/jnr.20604. [DOI] [PubMed] [Google Scholar]
- Trojanowski JQ, Kleppner SR, Hartley RS, Miyazono M, Fraser NW, Kesari S, Lee VM. Transfectable and transplantable postmitotic human neurons: a potential “platform” for gene therapy of nervous sytem diseases. Exp Neruol. 1997;144:92–97. doi: 10.1006/exnr.1996.6393. [DOI] [PubMed] [Google Scholar]
- Tropepe V, Craig CG, Morshead CM, van der Kooy D. Transformation growth factor-alpha null and senescent mice show decreased neural progenitor cell proliferation in the forebrain subependyma. J Neurosci. 1997;17:7850–7859. doi: 10.1523/JNEUROSCI.17-20-07850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, Amelink GJ, Schmiedeck P, Schwab S, Rothwell PM, Bousser MG, van der Worp HB, Hacke W. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6:215–222. doi: 10.1016/S1474-4422(07)70036-4. DECIMAL, DESTINY, and HAMLET investigators. [DOI] [PubMed] [Google Scholar]
- Van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendrame M, Cassady J, Newcomb J, Butler T, Pennypacker KR, Zigova T, Sanberg CD, Sanberg PR, Willing AE. Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke. 2004;35:2390–2395. doi: 10.1161/01.STR.0000141681.06735.9b. [DOI] [PubMed] [Google Scholar]
- Verfaillie CM. Adult stem cells: assessing the case for pluripotency. Trends Cell Biol. 2002;12:502–508. doi: 10.1016/s0962-8924(02)02386-3. [DOI] [PubMed] [Google Scholar]
- Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- Wehner T, Bontert M, Eyupoglu I, Prass K, Prinz M, Klett FF, Heinze M, Bechmann I, Nitsch R, Kirchhoff F, Kettenmann H, Dirnagl U, Priller J. Bone marrow-derived cells expressing green fluorescent protein under the control of the glial fibrillary acidic protein promoter do not differentiate into astrocytes in vitro and in vivo. J Neurosci. 2003;23(12):5004–5011. doi: 10.1523/JNEUROSCI.23-12-05004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimann JM, Johansson CB, Trejo A, Blau HM. Stable reprogrammed heterokaryons form spontaneously in Purkinje neurons after bone marrow transplant. Nat Cell Biol. 2003a;5:959–966. doi: 10.1038/ncb1053. [DOI] [PubMed] [Google Scholar]
- Weimann JM, Charlton CA, Brazelton TR, Hackman RC, Blau HM. Contribution of transplanted bone marrow cells to Purkinje neurons in human adult brains. Proc Natl Acad Sci U S A. 2003b;100:2088–2093. doi: 10.1073/pnas.0337659100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC, Reynolds BA. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996;16:7599–7609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig M, Tucker KL, Gornik V, Schneiders A, Buschwald R, Wiestler OD, Barde YA, Brustle O. Tau EGFP embryonic stem cells: an efficient tool for neuronal lingeage selection and transplantation. J Neurosci Res. 2002;69:918–24. doi: 10.1002/jnr.10395. [DOI] [PubMed] [Google Scholar]
- Wernig M, Benninger F, Schmandt T, Rade M, Tucker KL, Bussow H, Beck H, Brustle O. Functional integration of embryonic stem cell-derived neurons in vivo. J Neurosci. 2004;24:5258–5268. doi: 10.1523/JNEUROSCI.0428-04.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F, Broccoli V, Constantine-Paton M, Isacson O, Jaenisch R. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc Natl Acad Sci U S A. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Willing AE, Vendrame M, Mallery J, Cassady CJ, Davis CD, Sanchez-Ramos J, Sanberg PR. Mobilized peripheral blood cells administered intravenously produce functional recovery in stroke. Cell Transplant. 2003a;12:449–454. doi: 10.3727/000000003108746885. [DOI] [PubMed] [Google Scholar]
- Willing AE, Lixian J, Milliken M, Poulos S, Zigova T, Song S, Hart C, Sanchez-Ramos J, Sanberg PR. Intravenous versus intrastriatal cord blood administration in a rodent model of stroke. J Neurosci Res. 2003b;73:296–307. doi: 10.1002/jnr.10659. [DOI] [PubMed] [Google Scholar]
- Winkler C, Fricker RA, Gates MA, Olsson M, Hammang JP, Carpenter MK, Bjorklund A. Incorporation and glial differentiation of mouse EGF-responsive neural progenitor cells after transplantation into the embryonic rat brain. Mol Cell Neurosci. 1998;11:99–116. doi: 10.1006/mcne.1998.0674. [DOI] [PubMed] [Google Scholar]
- Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Wu A, Wiesner S, Xiao J, Ericson K, Chen W, Hall WA, Low WC, Ohlfest JR. Expression of MHC I and NK ligands on human CD133+ gliomas cells: possible targets of immunotherapy. J Neurooncol. 2007;83:121–131. doi: 10.1007/s11060-006-9265-3. [DOI] [PubMed] [Google Scholar]
- Wu A, Oh W, Wiesner S, Ericson K, Chen L, Hall WA, Champoux PE, Low WC, Ohlfest JR. Persistence of CD133+ cells in human and mouse gliomas cell lines: detailed characterization of GL261 glioma cells with cancer stem cell-like properties. 2008 doi: 10.1089/scd.2007.0133. [DOI] [PubMed] [Google Scholar]
- Wu P, Tarasenko YI, Gu Y, Huang LY, Coggeshall RE, Yu Y. Region-specific generation of cholinergic neurons from fetal human neural stem cells grafted in adult rat. Nat Neruosci. 2002;5:1271–1278. doi: 10.1038/nn974. [DOI] [PubMed] [Google Scholar]
- Wurmser AE, Gage FH. Stem cells: cell fusion causes confusion. Nature. 2002;416:485–487. doi: 10.1038/416485a. [DOI] [PubMed] [Google Scholar]
- Wurmser AE, Nakashima K, Summers RG, Toni N, D'Amour KA, Lie DC, Gage FH. Cell fusion-independent differentiation of neural stem cells to the endothelial lineage. Nature. 2004;430:350–356. doi: 10.1038/nature02604. [DOI] [PubMed] [Google Scholar]
- Xiao J, Nan Z, Motooka Y, Low WC. Transplantation of a novel cell line population of umbilical cord blood stem cells ameliorates neurological deficits associated with ischemic brain injury. Stem Cells Dev. 2005;14:722–733. doi: 10.1089/scd.2005.14.722. [DOI] [PubMed] [Google Scholar]
- Yan J, Xu L, Welsh AM, Hatfield G, Hazel T, Johe K, Koliatsos VE. Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. PLoS Med. 2007;4:e39. doi: 10.1371/journal.pmed.0040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan YP, Sailor KA, Vemuganti R, Dempsey RJ. Insulin-like growth factor-1 is an endogenous mediator of focial ischemia-induced neural progenitor proliferation. Eur J Neurosci. 2006;24:45–54. doi: 10.1111/j.1460-9568.2006.04872.x. [DOI] [PubMed] [Google Scholar]
- Yandava BD, Billinghurst LL, Snyder EY. “Global” cell replacement is feasible via neural stem cell transplantation: evidence from the dysmyelinated shiverer mouse brain. Proc Natl Acad Sci U S A. 1999;96:7029–7034. doi: 10.1073/pnas.96.12.7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying QL, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature. 2002;416:545–548. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- Yip S, Sabetrasekh R, Sidman RL, Snyder EY. Neural stem cells as novel cancer therapeutic vehicles. Eur J Cancer. 2006;42:1298–1308. doi: 10.1016/j.ejca.2006.01.046. [DOI] [PubMed] [Google Scholar]
- Yoon YS, Wecker A, Heyd L, Park JS, Tkebuchava T, Kusano K, Hanley A, Scadova H, Qin G, Cha DH, Johnson KL, Aikawa R, Asahara T, Losordo DW. Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. J Clin Invest. 2005;115:326–338. doi: 10.1172/JCI22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zaghloul AA, el-Shattawy HH, Kassem AA, Ibrahim EA, Reddy IK, Khan MA. Honey, a prospective antibiotic: extraction, formulation, and stability. Pharmazie. 2001;56:643–647. [PubMed] [Google Scholar]
- Zeng L, Rahrmann E, Hu Q, Lund T, Sandquist L, Felten M, O'Brien TD, Zhang J, Verfaillie C. Multipotent adult progenitor cells from swine bone marrow. Stem Cells. 2006;24:2355–2366. doi: 10.1634/stemcells.2005-0551. [DOI] [PubMed] [Google Scholar]
- Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie CM, Low WC. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol. 2002;174:11–20. doi: 10.1006/exnr.2001.7853. [DOI] [PubMed] [Google Scholar]
- Zhao LR, Singhal S, Duan WM, Mehta J, Kessler JA. Brain repair by hematopoietic growth factors in a rat model of stroke. Stroke. 2007a;38:2584–2591. doi: 10.1161/STROKEAHA.106.476457. [DOI] [PubMed] [Google Scholar]
- Zhao LR, Berra HH, Duan WM, Singhal S, Mehta J, Apkarian AV, Kessler JA. Beneficial effects of hematopoietic growth factor therapy in chronic ischemic stroke in rats. Stroke. 2007b;38:2804–2811. doi: 10.1161/STROKEAHA.107.486217. [DOI] [PubMed] [Google Scholar]
- Zhu W, Xu W, Jiang R, Qian H, Chen M, Hu J, Cao W, Han C, Chen Y. Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Exp Mol Pathol. 2006;80:267–274. doi: 10.1016/j.yexmp.2005.07.004. [DOI] [PubMed] [Google Scholar]