Summary
The harlequin mutant encodes subunit B of plant-specific topoisomerase VI. Transcriptomics showed synergistic effects on expression of adjacent genes, suggesting a broader role in chromatin remodelling during development and stress responses.
Key words: Anthocyanin, cell wall, endoreduplication, hypocotyl, light, oxidative stress, root hairless, skotomorphogenesis, starch.
Abstract
Plant growth is continuous and modular, a combination that allows morphogenesis by cell division and elongation and serves to facilitate adaptation to changing environments. The pleiotropic phenotypes of the harlequin (hlq) mutant, isolated on the basis of ectopic expression of the abscisic acid (ABA)- and auxin-inducible proDc3:GUS reporter gene, were previously characterized. Mutants are skotomorphogenic, have deformed and collapsed epidermal cells which accumulate callose and starch, cell walls abundant in pectins and cell wall proteins, and abnormal and reduced root hairs and leaf trichomes. hlq and two additional alleles that vary in their phenotypic severity of starch accumulation in the light and dark have been isolated, and it is shown that they are alleles of bin3/hyp6/rhl3/Topoisomerase6B. Mutants and inhibitors affecting the cell wall phenocopy several of the traits displayed in hlq. A microarray analysis was performed, and coordinated expression of physically adjacent pairs/sets of genes was observed in hlq, suggesting a direct effect on chromatin. Histones, WRKY and IAA/AUX transcription factors, aquaporins, and components of ubiquitin-E3-ligase-mediated proteolysis, and ABA or biotic stress response markers as well as proteins involved in cellular processes affecting carbon partitioning into secondary metabolites were also identified. A comparative analysis was performed of the hlq transcriptome with other previously published TopoVI mutant transcriptomes, namely bin3, bin5, and caa39 mutants, and limited concordance between data sets was found, suggesting indirect or genotype-specific effects. The results shed light on the molecular mechanisms underlying the det/cop/fus-like pleiotropic phenotypes of hlq and support a broader role for TopoVI regulation of chromatin remodelling to mediate development in response to environmental and hormonal signals.
Introduction
Plants are sessile and have evolved an open pattern of growth and development to give plasticity to their responses to changing environments. A central paradox of plant biology is that there exist only a handful of compounds (phytohormones) that mediate many complex processes in plant growth. A related conundrum is that, despite the elucidation of hormone structures, their biosynthetic pathways, and cognate hormone receptors, an understanding of the mechanisms of hormone action and ‘sensitivity’ remains elusive. Abscisic acid (ABA) is a plant hormone that mediates a myriad of physiological processes in growth and development, including cell division, water use efficiency, and gene expression during seed development and in response to environmental stresses, such as drought, chilling, salt, pathogen attack, UV, and high light (Rock et al., 2010). It was previously shown that the uidA (β-glucuronidase; GUS) reporter, under the transcriptional regulation of the carrot (Daucus carota) Late Embryogenesis-Abundant Dc3 promoter in transgenic Arabidopsis thaliana seedlings, is ABA and auxin inducible within the root zone of elongation and within the vasculature, and inducible in guard cells by ABA (Chak et al., 2000; Rock and Sun, 2005). The abi1-1 and abi2-1 mutations reduce ABA-inducible proDc3:GUS expression in root tissues and interact with (attenuate) auxin signalling leading to proDc3:GUS expression. Furthermore, the aux1 and axr4 mutants, which are affected in auxin transport/homeostasis, show a hypomorphic effect on ABA-inducible proDc3:GUS expression, demonstrating that ABA and IAA signalling pathways interact in roots (Rock and Sun, 2005).
Several reports have established links between ABA and the cell wall. For example, ABA-dependent callose accumulation is required for β-aminobutyric acid-induced resistance to the pathogens Alternaria brassicola and Plectosphaerella cumumerina (Ton and Mauch-Mani, 2004; Ton et al., 2005). ABSCISIC ACID (ABA)-INSENSITIVE-8 is allelic to ELONGATION DEFECTIVE-1 and KOBITO-1, which establishes a link between ABA and the cell wall because mutations at this locus disrupt ABA-regulated gene expression, sugar sensitivity, cell elongation, cellulose synthesis, vascular differentiation, and root meristem maintenance (Cheng et al., 2000; Pagant et al., 2002; Brocard-Gifford et al., 2004). ABI8/KOB1/ELD has recently been shown to regulate movement of transcription factors through plasmodesmata to modulate stomatal patterning (Kong et al., 2012). Mutations in the cellulose synthase gene (CESA8)/IRREGULAR XYLEM1 result in resistance against drought, salt, and osmotic stresses, and in elevated ABA-inducible gene expression (Chen et al., 2005).
Because there is no cell migration in plants, and cell walls are formed concomitant with cell division, the timing and orientation of cell division and expansion, mediated ultimately by hormone signalling to the cell wall, are the primary forces that shape plants. Identification and cloning of mutants that affect plant shape and environmental responses can shed light on the molecular mechanisms that control morphogenesis and hormone action in response to stresses. So far, it has proved difficult to predict the nature of the gene products involved in hormone sensitivity. Previously the harlequin (hlq) mutant was isolated based on ectopic expression of the proDc3:GUS transgene (Subramanian et al., 2002). Here it is reported that hlq, which is de-etiolated in the dark with open cotyledons and short hypocotyls, encodes an allele of brassinosteroid insensitive3/hypocotyl6/root hairless3 (bin3/hyp6/rhl3), the plant-specific homologue of type IIb archaebacterial topoisomerase VI subunit B.
Type II topoisomerases have a catalytic cycle producing double-stranded breaks (as distinct from type I single-stranded breaks) that are associated with the occurrence of transient DNA–topoisomerase covalent complexes implicated in DNA replication, recombination, chromosome segregation, chromatin remodelling, and transcription (Varga-Weisz et al., 1997; Vos et al., 2011). Type II topoisomerases can act as transcriptional repressors, by binding to promoters and blocking the formation of stable pre-initiation complexes, which can be relieved by the addition of sequence-specific transcriptional activators (Brou et al., 1993; McNamara et al., 2008). Topo6B has conserved ATP binding and hydrolysis domains, and a conserved basic motif (B4) of unknown function (Hartung and Puchta, 2001). In addition to the archaeal TOP6A/BIN5/RHL2/SPO11-3 and TOP6B subunits, Arabidopsis TopoVI function requires the activity (Simkova et al., 2012) of two small subunits, At1g48380 /RHL1 (Schneider et al., 1998)/HYP7 (Sugimoto-Shirasu et al., 2005) and At5g24630/BIN4 (Breuer et al., 2007)/MIDGET (MID) (Kirik et al., 2007). The Arabidopsis BIN4/MID subunit of TopoVI can interact in a yeast two-hybrid assay with TFIIB, which is involved in RNA polymerase II recruitment and transcription initiation in eukaryotes (Evans-Roberts et al., 2010; Szklarczyk et al., 2011). Molecular characterization of mutants of bin3/hyp6/rhl3/top6b and the regulatory subunits rhl1/hyp7 and bin4/mid that bind each other, to TOP6A, and to COP1 (Schrader et al., 2013) led to claims of TopoVI as having complex roles in growth and development. Namely, it is essentially involved in brassinosteroid sensitivity (Yin et al., 2002) but probably not hormone cross-talk (Nemhauser et al., 2006), in decatenation of endoreduplicated chromosomes throughout the nucleus (Sugimoto-Shirasu et al., 2002), in DNA replication/repair and coupling to cell cycle arrest that controls cell expansion and proliferation (Hartung et al., 2002; Schrader et al., 2013), including in response to nematode parasites (Vieira et al., 2013). Moreover, TopoVI is involved in transcriptional silencing through chromatin organization (Kirik et al., 2007), and as a genuine component of singlet oxygen retrograde signalling to the nucleus, being both a positive and a negative integrator of different reactive oxygen species (ROS) signals and environmental cues (Simkova et al., 2012). Further studies have shown that the constitutive expression of rice OsTOP6A or OsTOP6B increases the expression of stress-responsive genes, and confers abiotic stress tolerance to transgenic Arabidopsis plants (Jain et al., 2006, 2008). The present phenotypic characterization and transcriptome profiling results for hlq seedlings now demonstrate a fundamental role for TopoVI in plant gene regulation, and provide additional insight into the molecular mechanisms and processes of TopoVI which integrate environmental (e.g. light and dark) and internal (e.g. hormone) signals via chromatin remodelling to control plant growth and development.
Materials and methods
Plant materials
Col-0 (stock # CS60000), abi2-1 (CS23), Ler (CS20), prc1-1 (CS297), bot1-1 (Bichet et al., 2001), kor1-1 (CS298), and T-DNA insertion lines SALK_024455C (subsequently referred to as the hlq-3 allele) and SALK_140704 (hlq-2) were obtained from the Arabidopsis Biological Resource Center (Ohio State University, Columbus OH, USA; http://abrc.osu.edu/). The hlq-1/+ (CS68702), F2 mapping stocks (CS68703, CS68704), hlq-2/+ (CS68705), and hlq-3/+ (CS68706) seed stocks have been deposited at the ABRC. Seeds were sown on plates containing 0.5× Murashige and Skoog salts (Research Products International, Mt. Prospect, IL, USA), 0.8% sucrose, and 0.5% phytagel (Sigma-Aldrich, St. Louis, MO, USA). Plates were kept in the dark at 4 °C for 3 d before being transferred into a growth chamber. The growth conditions were 21 °C with continuous light (~ 100 μE m–2 s–1), except for starch quantitation assays where plants were given a diurnal light cycle of 12h light and 12h dark, and roots and shoots (four biological replicates of ~10mg each) were harvested at the end of the respective cycle. Plates were kept in the chamber for 5 d before scoring for the hlq mutant morphology.
Genetic mapping
Previously, 5100 M1 abi2/abi2 homozygous plants of a line that carried two independent proDc3:GUS reporter genes were mutagenized with ethylmethane sulphonate (EMS), and M2 clonal lines were screened for ABA- or auxin-inducible GUS expression in roots. These studies resulted in isolation of a new allele of rooty/superroot1/hookless3 (Sun, 2003) and two single gene nuclear mutants: hlq and short blue root (sbr) (Subramanian et al., 2002). These mutants have novel ABA- and auxin-inducible proDc3:GUS gene expression phenotypes attributable to ABA and indole acetic acid (IAA) responses, as well as pleiotropic growth phenotypes that suggest a link to ABA and/or auxins. The hlq/+ mutant stock (subsequently termed hlq-1 allele) were backcrossed to the parental line abi2 three times to remove extraneous mutational load caused by the original EMS treatment and to provide supporting evidence that the pleiotropic phenotypes of hlq were due to a single mutation. Due to seedling lethality and sterility, hlq/+ heterozygous plants (in the abi2-1 mutant background) of the Landsberg erecta (Ler) ecotype were crossed with the Columbia (Col-0) ecotype. F2 seeds from a single F1 plant constituted one mapping population. Two F2 lines that segregated for hlq were used for mapping experiments. The segregation in the F2 population for 3:1 wild type to hlq showed that the hlq phenotype was not influenced by the ecotype. No obvious differences in the hlq mutant morphology compared with the Ler parental background suggested that penetrance of the phenotype was complete. DNA was extracted from 2- to 3-week-old mutant seedlings as described (Xin and Chen, 2012). The hlq recombinants were allowed to grow for 3–4 weeks to maximize template DNA yield. The DNA from individual recombinants was used in simple sequence length polymorphism (SSLP) (Bell and Ecker, 1994) and cleaved amplified polymorphic sequence (CAPS) PCR analyses (Konieczny and Ausubel, 1993), or amplified using primers flanking single nucleotide polymorphisms (SNPs) identified from the publicly available Monsanto/Cereon Arabidopsis Ler draft sequence (Jander et al., 2002) [available for download at The Arabidopsis Information Resource (TAIR) (www.arabidopsis.org)]. Repetitive sequences were scanned using the REPEATMASKER program (www.repeatmasker.org). Oligonucleotide primers were designed using ‘Perlprimer’ (http://perlprimer.sourceforge.net/) and synthesized commercially (Sigma; http://sigma.com) and used for PCR amplification using PrimeSTAR HS DNA Polymerase (TAKARA, Shiga, Japan). Amplicons of 100–200bp were targeted to reveal the longest repeats and hence the high probability of polymorphism in agarose gels. Among the different kinds of repeats scanned, TA, CT/CTT, and GA/GAA repeats showed highest abundances and successes for developing useful polymorphic markers at 24, 22, and 14%, respectively, of the total scanned. Information about the position and primer pairs for these markers is given in Supplementary Table S6 available at JXB online. Amplicons were desalted with a Qiaquick PCR Purification Kit (Qiagen, Gaithersburg, MD, USA) and sequenced by the Sanger dideoxynucleotide chain termination method by Beckman Coulter Genomics (Danvers, MA, USA; http://www.beckmangenomics.com/).
Whole-genome resequencing of the hlq/+ heterozygote
Genomic DNA was prepared with a Qiagen DNeasy Plant Minikit (Qiagen) according to the manufacturer’s protocol. Paired-end 100 base Illumina HighSeq reads as fastq files (including quality scores) were mapped to the Col-0 reference genome (TAIR9) using Burrows–Wheeler Aligner (BWA; http://bio-bwa.sourceforge.net/) software with default settings, except the extension gap was set to ‘10’ (Li and Durbin, 2010). This resulted in a slight improvement to 45.7% of all reads successfully mapping to the reference Col-0 genome, and 38.7% of all reads properly paired, yielding an average of 25-fold coverage over all five chromosomes. SAMTools (http://samtools.sourceforge.net/) (Li et al., 2009) was used to index and align the reads to the reference genome, and SnpEff (http://snpeff.sourceforge.net) was used to call SNPs and INDELs (Cingolani et al., 2012). There were 223 homozygous Ler SNPs called in the ~112 kbp interval of interest where hlq mapped in the region chr3: 7213133..7325482, plus 19 deletions and 18 insertions. There were 11 heterozygous Ler SNPs called, one heterozygous insertion, and one heterozygous deletion. A published whole-genome assembly of Ler with >320-fold coverage (Schneeberger et al., 2011) was used to validate the SNP calling methodology. Both heterozygous INDELs were called correctly, as were 92% of the Ler homozygous SNPs/INDELs in the interval (data not shown), whereas an additional 15 SNPs (7%) called by Schneeberger et al. (2011) were not called in the present analysis. It is possible that the extra homozygous SNPs in the data set here and those described but not found in the data are polymorphic within Ler accessions. Notwithstanding, the high concordance between the present results and the published Ler SNPs validated the method.
GUS assays
Plant samples were developed at 37 °C for 12h with a 1mM solution of the indigogenic GUS substrate 5-bromo-4-chloro-3-indolyl β-d-glucuronide (X-Gluc; Rose Scientific, Edmonton, Alberta, Canada) in 50mM KH2PO4 (pH 7.0), 0.1mM EDTA, 0.5mM ferricyanide, 0.5mM ferrocyanide, 0.05% sodium azide, 0.1% Triton X-100. After staining overnight, samples were immersed in 70% ethanol overnight. Samples were then mounted in 30% glycerol on microscope slides for pictures to be taken with a dissecting microscope, or were imaged with an Olympus BX41 light microscope for high magnification detailed imaging using QCapture software (v2.68.6, Silicon Graphics, Fremont, CA, USA).
Analysis of gene expression and genotypes with PCR
Total RNA from roots or leaves of 10-day-old seedlings, or from whole seedlings, was extracted with Iso-RNA Lysis Reagent (5 PRIME, Gaithersburg, MD, USA). For reverse transcription–PCR (RT–PCR), 2 μg of total RNA was digested with RQ1 RNase-free DNase (Promega, Madison, WI, USA) and reverse transcribed by MMLV reverse transcriptase (Promega) with Anchored Oligo-dT (ABgene; Surrey, UK). A cycle number of 30 was used for PCR experiments in Fig. 4B; for Supplementary Fig. S7 at JXB online, 28 cycles were used for SALK_140704, and 31 cycles for SALK_024455. PCR conditions were: 55 °C annealing temperature, 1.5min extension time, 72 °C extension temperature. PCR products were run on an agarose gel with a DNA 1 kbp ladder (Axygen Biosciences, Union City, CA, USA; www.corning.com/axygen/) for calculation of band sizes by linear regression. Primers used in this study are listed in Supplementary Table S6.
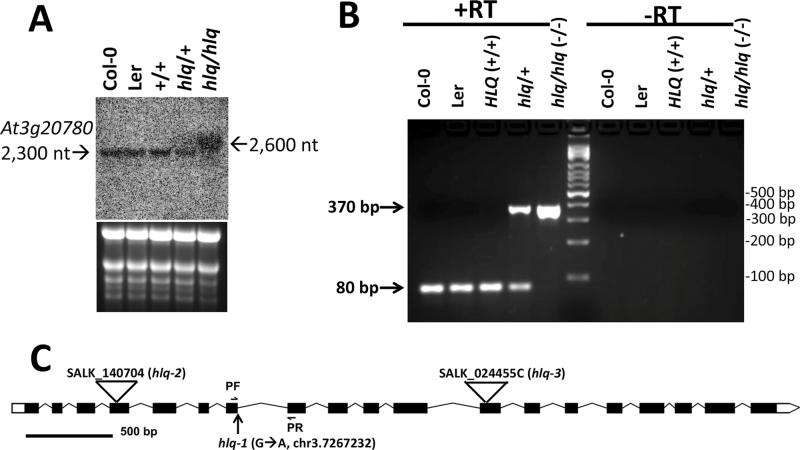
Fig. 4.
Characterization of the At3g20780 mRNA in the hlq mutant demonstrates aberrant intron 7 splicing. (A) RNA gel blot of At3g20780 shows an aberrant mRNA in hlq homozygotes. A 20 μg aliquot of total RNA from seedlings of wild types Col-0, Ler, and HLQ+/+ (abi2-1 parental background), hlq/+ heterozygotes, and the homozygous hlq/hlq mutant were probed with a 1.5 kbp fragment of the At3g20780 cDNA; the lower panel shows equal loadings by ethidium bromide staining of the denaturing agarose gel before blotting. Band sizes of the wild-type and hlq At3g20780 mRNAs are labelled on the left and right sides, respectively. (B) Agarose gel of RT–PCR amplicons from cDNA prepared from total RNAs of control (Col-0, Ler, and HLQ/HLQ), hlq/+ heterozygotes, and hlq/hlq homozygotes (–/–) shows retention of intron 7 (355bp predicted) in hlq genotypes, and absence of intron 7 (~80bp amplicon, 55bp predicted) in controls. ‘+RT’, with reverse transcriptase; ‘–RT’ reverse transcription step omitted from the amplification protocol. (C) Cartoon showing the exon–intron–untranslated region (UTR) structure of At3g20780, the position of primers (PF, PR; horizontal arrows) used for PCR in B, the position of the hlq-1 mutation (vertical arrow) at the intron 7 splice donor site, and positions of T-DNA insertions (triangles) in lines SALK_024455C (hlq-3) and SALK_140704 (hlq-2).
RNA gel blot hybridization
For RNA gel blot hybridization, samples of 20 μg of total RNA were resolved on a 1.2% denaturing agarose gel and blotted to a Hybond-N+ membrane (GE Healthcare, Piscataway, NJ, USA) according to the supplier’s protocol. Ambion Millenium RNA markers (GE Healthcare) were run as controls for calculation of band sizes by linear regression. At3g20780 template was amplified using the primers listed in Supplementary Table S6 at JXB online from a cDNA library and was eluted after excision of the band from an agarose gel. Probes were synthesized using the Random Primer DNA Labeling Kit Ver.2 (TAKARA) with [α-32P]dCTP (PerkinElmer, Waltham, MA, USA). Hybridization was carried out with the PerfectHyb Plus hybridization buffer (Sigma-Aldrich) according to the manufacturer’s instructions. A storage phosphor screen (GE Healthcare) was used for autoradiography and it was scanned using a Storm 860 PhosphorImager (GE Healthcare). Band intensities were quantified using the ImageQuant TL software (v2003, GE Healthcare).
Microarray experiment
Three individual pools of total RNA were extracted with Trizol reagent (Invitrogen, Carlsbad, CA, USA) from ~1000 seedlings (14 d old) segregating hlq homozygotes from self-fertilization of the abi2-1/abi2-1-hlq/+ genotype after germination on Petri plates as described and kept vertically under continuous light at 21 °C. The control (wild-type) samples were three pools of normal individuals from the same plates and comprised ‘wild type’ (i.e. abi2-1/abi2-1 homozygous) and abi2-1/abi2-1-hlq/+ heterozygous genotypes used for microarray analysis. In vitro transcription was carried out using an Amino Allyl MessageAmp aRNA Amplification Kit (Ambion; cat#: AM1753) according to the manufacturer’s protocol (Grand Island, NY, USA). A 1000ng aliquot of Cy3- or Cy5-labelled cRNA from different replicate genotype samples was purified separately, combined in equimolar amounts including dye swaps across genotypes, and mixed with hybridization buffer before being applied to three microarrays. Each sample was run on a Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) before and after labelling. The ratio of red (Cy5) and green (Cy3) fluorescence intensities for each spot is indicative of the relative abundance of the corresponding target molecule in the two mRNA target samples. A fourth microarray was hybridized with balanced pooled samples from the same dye-labelled biological replicates as a technical replicate. Hybridizations were performed on the Operon Arabidopsis Long Oligonucleotide Microarrays V3 GEO platform GPL4570. Conditions of hybridization and washing were according to the protocol published by Galbraith et al. (2011). After washing, microarrays were scanned using the GenePix Autoloader 4200AL Scanner (Molecular Devices, Sunnyvale, CA, USA) with laser excitation at 532nm and 635nm at the resolution of 10 pixels μm–1, and saved as 16-bit greyscale multi-image TIFF files. Intensity values were extracted using GenePix Pro 6.0 (Molecular Devices) and saved as ‘gpr’ and ‘txt’ files for each block individually. The data for each array were lowess normalized followed by analysis of variance (ANOVA) of differential gene expression using empirical Bayes methods to moderate the standard deviations between genes. This was done by the software package Linear Models for Microarray Data (limma) (Smyth et al., 2005) written in R language under the BioConductor platform (Gentleman et al., 2004) version R2.5.1 (http://www.r-project.org/). Normalization ‘within’ and ‘between arrays’ scaled the log2-ratios of each two-colour experiment to have the same median-absolute deviation across arrays. A dye effect coefficient was calculated in addition to the genotype coefficient and signal amplitude across all probes and samples. Original MIAME-compliant data are stored at the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) with the locator GSE45806. The key reporters referenced in the literature for retrograde ROS signalling [with fold change (FC) observed in hlq seedlings following in parentheses] are: At5g40010/AAA-ATPase1 (FC=1.08), At3g61190/BONSAI ASSOCIATION PROTEIN1 (FC= 1.68, P<0.0005), and At5g01600/FERRITIN1 (FC= 1.48, P<0.03). The lack of significant up-regulation for AAA-ATPase1 in hlq mutants is consistent with previous results for 5-day-old seedlings of top6a/caa39 (Simkova et al., 2012).
Lignin, callose, pectin, and starch staining/quantitation, dichlobenil and tunicamycin treatments
For staining for lignin with phloroglucinol, the seedlings were treated with a mixture of methanol:acetic acid (9:1) to remove chlorophyll. The seedlings were then cleared in chloral hydrate:water (8:2) for 2–5min and stained with phloroglucinol (1% phloroglucinol in 6 N HCl) for 5min (Zhong et al., 2000). Stained seedlings were observed and photographed using an Axio-vert inverted microscope or Axiophot microscope. Inverse phase contrast was used to better visualize lignin staining. For staining lignin with basic fuchsin, the seedlings were treated with a mixture of methanol:acetic acid (9:1) to remove chlorophyll and then cleared in 10% NaOH at 60 °C for 12h. Cleared seedlings were stained with 0.01% Basic Fuchsin, mounted in 50% glycerol, observed by epifluorescence using a propidium iodide (PI) filter, and photographed.
Callose staining was performed with slight modifications from Buogourd et al. (2000). The seedlings were treated with a mixture of methanol:acetic acid (9:1) to remove chlorophyll and then re-hydrated in water. The seedlings were stained with a 0.025% aqueous solution (1 in 20 dilution of 0.5% stock solution) of aniline blue (Sigma-Aldrich) for 30min. The seedlings were then washed twice with water for 15min each to remove excess aniline blue, mounted in 50% glycerol, observed by epifluorescence, and photographed using a 510nm filter. Callose was quantified from seedlings of different ages according to Kohler et al. (2000). Briefly, the seedlings were weighed, ethanol dehydrated and decolorized for 2 d, homogenized, and extracted with the loading mixture (0.1% aniline blue, 1M glycine/NaOH, 1M HCl). The fluorescence was measured in a fluorescence spectrophotometer at 393nm excitation and 479nm emission wavelengths.
Starch staining was with Lugol’s elemental iodine:potassium iodide (IKI; 5.7mM iodine, 43.4mM potassium iodide) after pigment removal and dissolution of membranes by boiling in 80% ethanol, as described (Kurata and Yamamoto, 1998). Starch quantitation was by solubilization (boiling pulverized frozen material in water after extracting free glucose with 90% ethanol, 60 ºC for 5min), complete conversion with glucan hydrolases to glucose, followed by enzyme-based fluorimetric assay (Abcam; www.abcam.com) according to the manufacturer’s protocol.
For staining pectins, the seedlings were stained with Ruthenium Red (SPI-Chem, Cat. No. 02603-AB) at 0.2% in water with 0.01% Tween for 1h, rinsed twice in distilled water, and viewed under a dissection microscope. Tunicamycin or 2, 6-dichlorobenzonitrile (dichlobenil, DCB; Sigma-Aldrich) was added to the growth medium at a concentration of 1, 2, or 3 μM. Two-day-old seedlings were transferred to tunicamycin-containing medium and allowed for grow for a further 5 d. Their growth was monitored and phenotypes observed on 1-week-old seedlings.
Peroxidase activity
Four-week-old wild-type and hlq mutant plants grown on agar plates were homogenized at 4 °C in homogenization medium [10× vol. g fresh weight (FW)–1] comprising 50mM TRIS-HCl (pH 7.0), 2 μM phenylmethylsulphonyl fluoride, and 1 μg ml–1 of each of the protease inhibitors leupeptin, pepstatin A, and aprotinin (Sigma). The homogenate was centrifuged at 3000 g for 15min to obtain a soluble and crude cell wall fraction. The pellet was washed in homogenization medium and then incubated with 1M KCl (2× vol. g FW–1) in 50mM TRIS-HCl buffer (pH 7.0) for 10min prior to centrifugation for 10min at 3000 g to obtain the KCl extract. The KCl extract was microfuged at 13 000rpm for 7min at 4 °C to remove cell debris and concentrated 10-fold by ultrafitration using a 1.5ml Fugisep (10kDa molecular weight cut-off membrane; Intersep). The KCl extracts were equilibrated in 20mM TRIS-HCl (pH 7.0) by diafiltration using Fugisep-10 membranes (Intersep) prior to loading on a 10% native-polyacrylamide gel (Brownleader et al., 2000). Electrophoresis was performed at 4 °C at 30 mA constant current. The gel was then immersed in a solution of 0.1% guaiacol and 0.03% H2O2 in 50mM potassium acetate buffer, pH 6.0 until bright blue bands appeared (7–10min). The reaction was stopped by immersing in 7% acetic acid.
Microscopy
Light microscopy was performed using a Zeiss stereomicroscope (Göttingen, Germany), and for micro measurements a calibrated microscale was used. For light microscopy of GUS-stained roots, the seedlings were briefly rinsed in 70% ethanol followed by sterile distilled water to remove excessive GUS developer and observed under the microscope. For ‘live and dead’ cell staining, seedling roots were embedded by allowing them to grow into 2% Phytagel minimal medium. Embedded roots in 1–2mm thick slices of gel were transferred to glass slides, immersed in water containing 100 μg ml–1 each of fluorescein diacetate (FDA; Sigma) and PI (Sigma), then incubated in the dark (15min), rinsed twice in sterile distilled water, and epifluorescence was viewed under excitation with a blue filter (450–490nm) by means of a Zeiss Axiophot microscope. The sample was then flooded with GUS developer solution for 16h and photographed in bright field with the same field in view as previously documented. For aniline blue visualization, excitation was with UV and emission was visualized with a 4’,6-diamidino-2-phenylindole (DAPI) filter set.
For scanning electron microscopy, the seedlings were aligned on the conductive paste thinly spread over the microscope stage and immediately frozen under liquid nitrogen. The stage was placed into the cryo-chamber of the microscope and the condensed water removed by vacuum pumping. Then the samples were coated with platinum and observed under a Leica S440 scanning electron microscope (Cambridge, UK) fitted with a Fisons LT7480 cryoprep cryo-stage (Fisons Instruments, UK).
Fourier transform infrared (FTIR) microspectroscopy
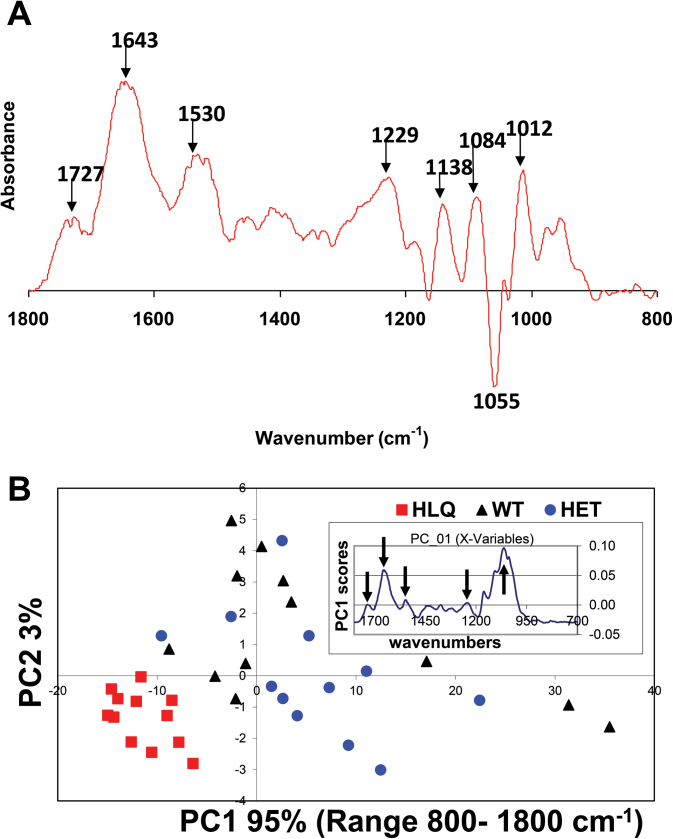
FTIR has been used to ‘fingerprint’ the carbohydrate constituents in the 1200–900cm–1 region, to detect and characterize conformational changes in wall components, and to determine which cross-links between polymers are present. Diagnostic absorbances are as follows: the carboxylic ester group absorbs at ~1740cm–1, amide-stretching bands of protein occur at ~1650cm–1 and 1550cm–1, carboxylic acid groups on pectins absorb at 1610cm–1, phenolics absorb at ~1600cm–1 and 1500cm–1, and carbohydrates absorb between 1200cm–1 and 900cm–1 (McCann et al., 1992). IR spectra of hlq and the wild type were acquired with the Autoimage FT-IR micro-spectroscopy system (PerkinElmer; Norwalk, CT, USA). The microscope includes a camera and a viewing system that magnifies the visible light image of the sample and enables isolation of a region of interest. The image of the sample is displayed on the monitor visible window. The AutoIMAGE software (version 5.0.0 B8) enables the control of the operation of the microscope, and maps and collects the spectra from a sample. The system used a KBr beam splitter and utilized a liquid nitrogen-cooled MCT detector housed in the microscope. The Autoimage was further equipped with an automated XYZ motorized stage that was operated in the auto-focusing (Z-direction) mode under the software control. Both visible images and IR maps were obtained in the transmission mode from an area of 150×150 μm. Samples of cell wall extracts (described below) were placed on a BaF2 window (13mm diameter, 2mm thickness) and air dried at 37 °C for 1h. A background spectrum of clean BaF2 surface was collected before each scan. The IR spectra were recorded using 128 interferograms with 4cm–1 spectral resolution between 4000cm–1 and 700cm–1. Twelve spectra were recorded from each sample. All FTIR spectra were baseline corrected and normalized. Principal component analysis (PCA) is a mathematical technique that is widely used to reduce the dimensionality of data sets. The variability in each individual spectrum relative to the mean of the population is represented as a smaller set of values (axes) termed principal components (PCs). This process concentrates the sources of variability in the data into the first few PCs, and was used in analysis of FTIR spectra from purified cell walls of hlq homo- and heterozygotes, and the wild type.
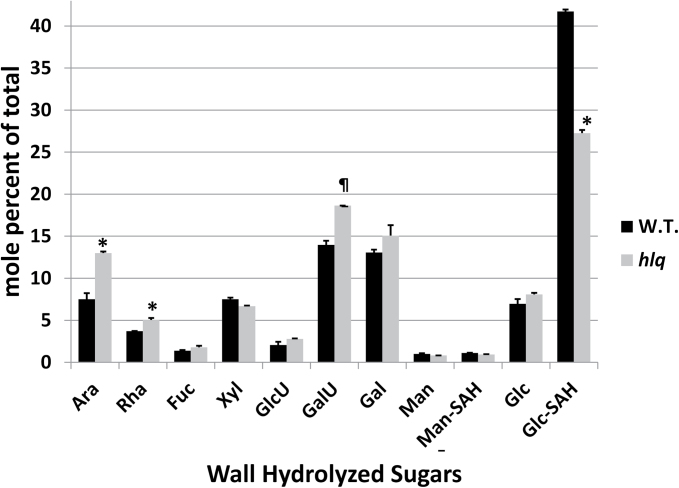
Glycosyl composition analysis of cell walls
Plant tissue (0.7–2g FW) was collected and stored at –70 °C until processed. Frozen tissue was ground into fine powder in liquid N2 with a mortar and pestle and extracted twice in 80% (w/w) phenol:acetic acid:water (5:2:1 v/v/v) to remove protein and other soluble metabolites (Fry, 2000). The pellet after centrifugation was washed with 70% ethanol and extracted twice with 90% (v/v) dimethylsulphoxide (DMSO) to remove starch (Selvendran and O’Neill, 1987). Lipids were removed by washing the pellet twice with chloroform:methanol (2:1 v/v) and acetone (York et al., 1986). The cell wall fraction was dried in air and again in a vacuum desiccator over P2O5. The dry cell wall fraction from hlq mutant and wild-type plants was then analysed at the level of the glycosyl composition of non-cellulosic polysaccharides as previously described (Komalavilas et al., 1991). Briefly, cell walls were subjected to methanolysis in 1.5M methanolic HCl at 80 °C, trimethylsilylation, and gas–liquid chromatography (GC). For analysis including cellulosic polysaccharides (which are not cleaved by methanolysis), an aliquot of the cell wall fraction was pre-swollen in 22 N H2SO4, diluted to 1 N H2SO4, and then hydrolysed at 121 °C (Fry, 2000). After neutralization with Ba(OH)2 and removal of BaSO4, samples were subjected to methanolysis and trimethylsilylation followed by GC-flame ionization detection (FID) on a Hewlett Packard 5890.
Bioinformatics
MAPMAN and PAGEMAN software (Usadel et al., 2005, 2006) (http://mapman.gabipd.org) was used for statistical analysis and graphical representation of metabolic and signalling pathways. Dynamic analysis of the results described herein can be recapitulated by installing MAPMAN on a local computer and uploading the hlq data set in Supplementary Datafile 1 (sheet 1) at JXB online.
Results
The harlequin mutant manifests pleiotropic phenotypes affecting ABA- and auxin-inducible reporter gene expression, cell elongation, epidermal morphogenesis, and carbon partitioning into primary (starch, cell wall) and secondary metabolites (anthocyanins, lignin)
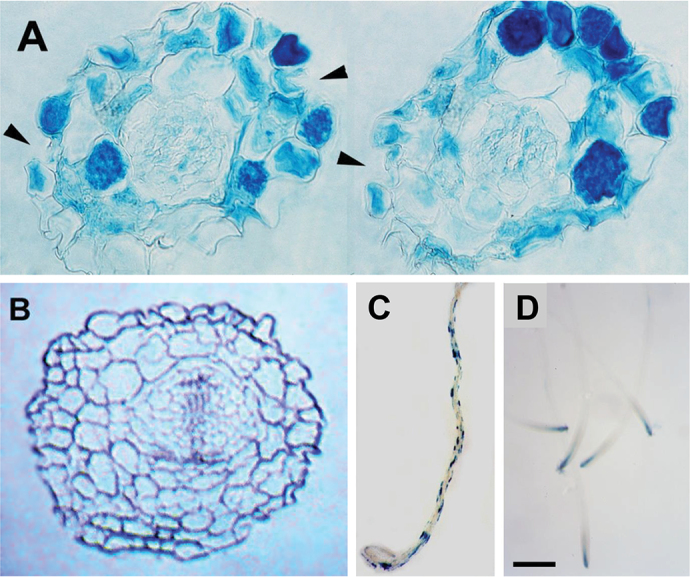
The hlq mutant has an extreme dwarf phenotype, with pleiotropic effects on root and shoot morphology, resulting in brittle leaves and stems and more friable callus (Balasubramanian, 2003), radial root tip swelling, anisotropic epidermal cell expansion, root hair defects, and abnormal cell collapse and associated callose accumulation (Supplementary Fig. S1 at JXB online) (Subramanian et al., 2002). The shape of the cells in the root cortex and also the epidermis were irregular, unlike the regularly shaped spherical cells in the wild-type tissues (Fig. 1). The hlq hypocotyl and root epidermal cell lengths were drastically reduced and the columnar cell files were disrupted (Supplementary Table S1; Fig. S1H), suggesting that the dwarf phenotype of hlq is due to a reduction in cell size, and not in cell number, consistent with the results of others indicating that TopoVI is primarily involved in cell expansion by endoreduplication (Breuer et al., 2007; Sugimoto-Shirasu et al., 2005). The leaf pavement cells were less articulated than the degree of interdigitation seen in the parental type, with the presence of gaps between the pavement cells similar to the gaps found in the epidermis of hlq hypocotyls and roots (Subramanian et al., 2002). Flower organs of hlq plants showed a rough epidermis compared with the smooth surface of the parental-type petals (Supplementary Fig. S1F). These phenotypes are consistent with a role for the HLQ gene product in a developmentally modulated pathway that impinges on ABA, light, and cell wall signalling, similar to ABI8/KOB/ELD1. ProDc3:GUS expression was not specifically confined to either the root trichoblasts or atrichoblasts, as is seen for the WER or GL2 genes (Lee and Schiefelbein, 1999). GUS staining of median cross-sections of the root and hypocotyl of 2-week-old hlq plants indicated that the ectopic proDc3:GUS expression also extended into the cortex (Fig. 1A).
Fig. 1.
The hlq mutant has ectopic expression of the proDc3:GUS reporter gene. (A) Cross-section through the hypocotyl of 2-week-old hlq and parental-type (B) plants, stained for GUS. Parental-type plants do not stain for GUS after 10 d. hlq shows ectopic staining not only in the epidermal cells but also in the cortex. The arrows indicate collapsed cells in the epidermal cell file, and the cell shapes are also irregular. (C) Ectopic expression of proDc3:GUS in a 7-day-old hlq mutant primary root. (D) Wild-type expression of proDc3:GUS. Scale bar=500 μm.
The hlq mutant is chlorotic, with only 50% of the chlorophyll content of the parental type when grown in light (Subramanian et al., 2002), suggesting that cellular metabolism may be broadly affected. When hlq pollen was germinated on artificial media, the pollen tubes grew at rates comparable with that of the wild type (data not shown). However, out- or back-crosses of hlq did not yield seeds, nor did manual pollination of hlq mutant flowers with the parental-type pollen. The hlq mutant plants failed to survive on soil or on basal minimal agar media, but under conditions of 0.8% sucrose supplementation, hlq homozygous plants could survive to the flowering stage, although vegetative tissue lost chlorophyll and leaves appeared brown/red in colour (data not shown).
The ability of hlq mutants to survive to flowering when grown on sucrose prompted investigation of carbon partitioning into starch, since it serves as a short-term carbohydrate reservoir. The hlq mutant in the Ler genetic background appeared to accumulate anthocyanins in the shoot meristematic region (Supplementary Fig. S1C at JXB online), reminiscent of the de-etiolated/constitutive photomorphogenesis/fusca class of mutants and transgenic Arabidopsis overexpressing ABI3, ABI4, and ABI5 transcription factors (Finkelstein et al., 2002), but not when in the abi2-1 parental background (data not shown). This is possibly because the abi2 mutation has been shown to suppress sugar induction of the transcription factors PRODUCTION OF ANTHOCYANIN PIGMENT1(PAP1)/MYB75 and PAP2/MYB90 ~6- and 9-fold, respectively, relative to wild-type Ler (Luo et al., 2012).
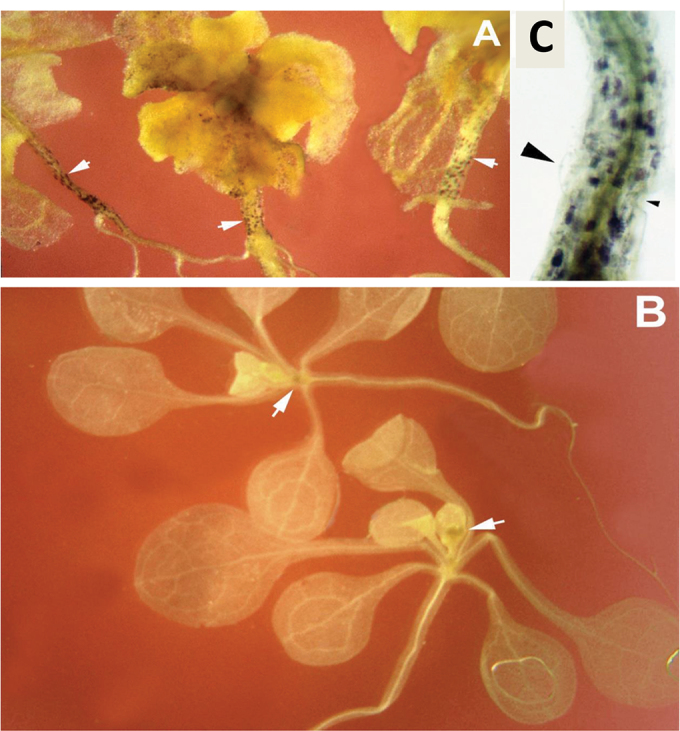
Sugar partitioning into starch was assessed by staining hlq mutants with IKI (Kurata and Yamamoto, 1998) after 10h of darkness. The hlq mutants showed patchy staining of starch granules in the hypocotyls and in the leaves (Fig. 2A), while the wild type was completely devoid of starch (Fig. 2B). The ectopic deposition and relative abundance of starch in shoots of hlq mutants was reminiscent of the proDc3:GUS staining pattern. However, counterstaining the GUS-stained hlq seedlings with IKI showed no obvious concordance between cells expressing GUS and those accumulating starch. Furthermore, the bulged and collapsed hypocotyls cells did not correlate with the presence of starch (Fig. 2C). Together, these results suggested that hlq has either decreased starch breakdown or increased starch synthesis rates, or defects in carbon metabolism or partitioning, which might also affect flux into secondary metabolites such as phenolics (e.g. anthocyanins and lignins). Starch was subsequently quantified in hlq and two new alleles (see below).
Fig. 2.
Ectopic localization of starch in hlq mutants. Staining for starch granules by KI shows ectopic localization of starch in the hypocotyl and leaves of hlq (A, white arrows), whereas there is no staining in the wild-type except at the meristem (B, white arrows). A close-up view of hlq hypocotyl (C) shows that the starch localization does not strictly correspond to hlq specific cell types—neither a bulged, radially expanded cell (large black arrowhead) nor a collapsed cell (small black arrowhead).
Caño-Delgado et al. (2000) showed that cell elongation defects lead to ectopic lignification. Their results were independently confirmed here with the korrigan1 mutant (data not shown) and ectopic lignin was tested for within the proscute1-1/AtCES6 cellulose-deficient mutant (Fagard et al., 2000) and botero1-1, a mutant allele of a katanin-like protein thought to be involved in microtubule stability (Bichet et al., 2001). It was found that both mutants displayed ectopic lignification within the root (Supplementary Fig. S2D, E at JXB online). In order to investigate the relationship between the cell wall and hormone- and stress-regulated gene expression, the expression of the proDc3:GUS reporter was examined in the prc1-1 and bot1-1 cell wall mutants. The proDc3:GUS marker gene was crossed into the prc1-1 and bot1-1 mutant backgrounds using a Ler double insertion line (P series) (Chak et al., 2000) as one parent. In the F2 progeny, the expression patterns of proDc3:GUS in mutant individuals were visualized with the chromogenic substrate X-Gluc. Roots of prc1-1 had varying degrees of constitutive GUS expression (Supplementary Fig. S2B), but were clearly distinguishable from wild-type plants (Supplementary Fig. S2A) on the basis of their proDc3:GUS staining intensity. Individuals of bot1-1 also displayed a range of proDc3:GUS expression phenotypes (Supplementary Fig. S2C). The extent of proDc3:GUS expression correlated with the extent of severity of the dwarf phenotypes in the bot1-1 population (based on root length). These results taken together suggest that cell wall mutants phenocopy the ectopic expression of proDc3:GUS seen in hlq and raise the question of whether the hlq mutant also manifests ectopic lignification.
Significantly, 2 weeks after germination, hlq mutant plants showed conspicuous swelling of the root elongation zone (Supplementary Fig. S1D at JXB online), whereas swelling was less obvious earlier in development (data not shown). The swelling appeared similar to the phenotype of cellulose deficiency for radially swollen1, which encodes a glycosyl transferase (Arioli et al., 1998), and the phenotype of the cytokinesis defective1 mutant, which encodes a GDP-mannose pyrophosphorylase required for N-glycosylation (Lukowitz et al., 2001). Treatment of wild-type seedlings with the herbicide dichlobenil (DCB; 2,6-dichlorobenzonitrile), an inhibitor of cellulose synthesis, phenocopied the swelling observed in the hlq root elongation zone. In the presence of 1 μM DCB, wild-type plants developed a swollen root differentiation zone with few root hairs (Supplementary Fig. S3F). A similar effect was induced by treatment with 1 μM tunicamycin, which interferes with the synthesis of the core glycan chain attached to the Asn-Xaa-Ser/Thr motif of N-linked glycoproteins, which resulted in severe dwarfing, abnormal swelling of the root, and epidermal cell bulging (Supplementary Fig. S3A), as described by Lukowitz et al. (2001). Root hairs emerged closer to the root tip, suggesting inhibition of cell elongation that led to a shorter root elongation zone. The hypocotyls of wild-type plants grown on tunicamycin were also short and radially swollen, with abnormally bulged epidermal cells (Supplementary Fig. S3B) similar to those observed (Fig. 2C) for untreated hlq seedlings. Furthermore, when stained for proDc3:GUS activity, the tunicamycin-treated wild-type plants exhibited ectopic GUS expression (data not shown), consistent with results from cell wall mutants.
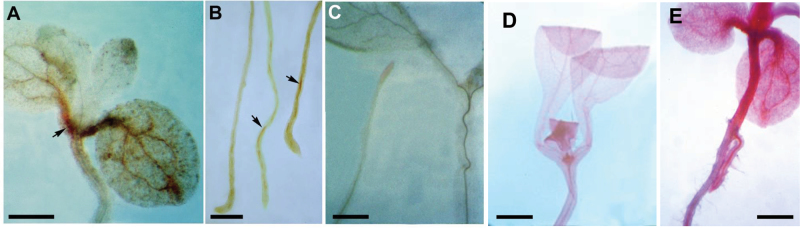
Similar to the phenocopy of hlq for root swelling by inhibitors of cellulose biosynthesis, root tips of tunicamycin-treated seedlings had ectopic lignification, whereas root differentiated regions were normal (Supplementary Fig. S3C at JXB online; compare differentiated root region on the left with root tip on the right). Staining for lignin with phloroglucinol showed ectopic staining in the cotyledons and roots of hlq (Fig. 3A, B), while the wild type showed only a faint staining in the vasculature (Fig. 3C). Histochemical staining of hlq mutants for the presence of suberin using Sudan Red did not show differences from the wild type. Finally, staining with Ruthenium Red, which specifically identifies pectins (Shevell et al., 1994), indicated ectopic pectin accumulation throughout the hypocotyl and cotyledons of hlq mutants (Fig. 3E), which compares with the detection of pectin only in the vasculature, meristem, and leaf primordia of wild-type plants (Fig. 3D). Taken together, these results suggested that the hlq mutant has defects in carbon partitioning to starch and in cell wall biosynthesis impacting primary (cellulose, pectin) and secondary carbon metabolism, for example lignin and anthocyanins.
Fig. 3.
Ectopic expression of lignin and pectin in hlq seedlings. Phloroglucinol staining for lignin in hlq shoot (A) and roots (B) shows ectopic deposition of patches of lignin (arrows). Wild-type (C) staining for lignin showed a faint signal only in the vasculature. Bars=1mm for (A, C); 2mm for (B). (D, E) Ruthenium Red staining for pectin. Ten-day-old wild-type seedlings (D) showed faint staining in the vasculature, meristem, and leaf primordium, whereas there was ectopic deposition of pectin in hlq (E) throughout the vasculature, leaves, and hypocotyl. Bar=1mm.
hlq is an allele of BRASSINOSTEROID INSENSITIVE3/ROOT HAIRLESS3/HYPOCOTYL6/TOPOISOMERASE6 SUBUNIT B
The hlq mutant was previously mapped to the short arm of chromosome 3, ~6.6 cM south of the SSLP marker nga162 (Subramanian et al., 2002). The locus was further fine-mapped by analysing another 3832 F2 recombinant chromosomes segregating as homozygous mutants from two self-pollinated F1 plants (976 and 940 recombinant progeny, respectively) obtained from a cross between hlq/+ heterozygotes and the ecotype Col-0. The results placed hlq in an ~112 kbp interval within bacterial artificial chromosome (BAC) clones F3H11 (>coordinate chr3:7213133), MOE17, and MDF22 (<coordinate chr3:7325482), a region that contains 30 annotated genes. The first indications as to the possible molecular defect in hlq came from the observation that another mutant, hyp6, characterized by K. Sugimoto-Shirasu (personal communication, XIIIth International Conference on Arabidopsis Research) mapped near to hlq and shared similar phenotypes of anisotropic growth, radial swelling of epidermal cells, strongly reduced hypocotyl elongation, immature trichome development, suppressed root hair formation, and enriched cell wall pectins (Balasubramanian, 2003).
A candidate G→A mutation (TAIR10 coordinate chr3: 7267232) that abolished the intron 7 donor consensus (nucleotide 1 of 300) in the pre-mRNA of At3g20780/BIN3/HYP6/RHL3/TOP6B was identified by Illumina HiSeq 2000 whole-genome DNA resequencing of hlq/+ heterozygotes. Supplementary Fig. S4 at JXB online shows the results of Sanger sequencing with a double peak at the mutation site in amplicons from hlq/+ heterozygous template genomic DNA, confirming the Illumina short read analysis. The predicted effect of the mutation, assuming no splicing of intron 7 followed by translation of the aberrant 2512 nucleotide mRNA (based on TAIR10 annotation), is a truncated protein lacking 472 C-terminal amino acid residues of the normal full-length 670 amino acid protein, with the peptide IIIYSYQV added to Met198 before a nonsense UGA stop codon at nucleotide 25 of intron 7. The functional consequence of the translated mutant mRNA would be mutation of a highly conserved aliphatic residue, two residues removed from the GXG motif of the ATP-binding site in the ATPase-like domain (amino acids 49–215; pfam accession PF02518; http://www.ncbi.nlm.nih.gov/Structure/cdd/cddsrv.cgi?uid=238030) and loss of the DNA TopoVI subunit 6B transducer domain (amino acids 398–557; pfam accession PF09239) (Corbett and Berger, 2006).
In order to provide supporting evidence for the G→A transition mutation being causal for the hlq phenotypes, the effect of the mutation was characterized at the mRNA level. Figure 4 shows the results of a total RNA gel blot from seedlings segregating hlq/+ heterozygotes (2:1 ratio with wild-type plants) and homozygous hlq mutant plants, probed with a 1.5 kbp amplicon encompassing most of the At3g20780 mRNA (2212 nucleotides). There was a reduction in the relative abundance of the wild-type At3g20780 mRNA (~2300 nucleotides calculated by linear regression of the mobilities of the RNA size markers) in heterozygotes, and a complete absence of the wild-type mRNA species in hlq homozygous plants. This was replaced by a diffuse band, ~300 nucleotides larger (Fig. 1A), which is consistent with the prediction that the mutation disrupts splicing of the 300 nucleotide intron 7 in At3g20780 pre-mRNA and that the hlq mutation is a null allele. Figure 4B shows the result of an RT–PCR experiment using cDNA templates prepared from wild-type total RNA, hlq/+ heterozygote RNA, and hlq (–/–) homozygous mutant RNA. This further substantiates the previous results, as an amplicon of ~380bp corresponding to the 300bp predicted intron 7 plus adjacent sequences was seen only in hlq/+ heterozygote and hlq homozygote (–/–) genotypes, and a smaller amplicon of ~ 80bp was seen both in hlq/+ heterozygotes and control templates plus reverse transcriptase (+RT). Because the size of the wild-type amplicon was larger than the predicted 55bp and only one cDNA clone (AJ297843) has been described (Hartung and Puchta, 2001), it is plausible that an alternative splice donor found 21 nucleotides downstream in intron 7 might be functional, which would add seven amino acid residues to the translated wild-type Top6B.
Despite performing several backcrosses of the original mutant isolate to the parental line to remove extraneous mutations, because of close linkage, 12 other candidate heterozygous SNPs were found in the mapped interval by whole-genome resequencing of hlq/+ heterozygotes. This formally raises the possibility that the other mutations might contribute to the pleiotropic hlq phenotypes, notwithstanding the fact that none of these SNPs falls within annotated exons or at intron donor–acceptor consensus sites. In order to provide conclusive evidence for a causal link between the hlq mutation and the pleiotropic phenotypes, two T-DNA insertion lines were obtained (SALK_140704 and SALK_024455) which disrupt exons 4 and 12 of At3g20780, respectively. Segregation of dwarf individuals with pleiotropic defects in root hair abundance and epidermal cell morphology was observed (Supplementary Figs S5, S6 at JXB online). Significantly, homozygous mutants of both these lines accumulated anthocyanins in meristems (Supplementary Fig. S5), as seen for hlq (Supplementary Fig. S1C). Based on the nomenclature of Schiefelbein and Somerville (1990), hlq and the tested T-DNA insertion lines had few normal root hairs of at least 100 μm in length, and sparse spike (non-branched) trichomes (Subramanian et al., 2002) were seen on primary leaves. The initiation of root hairs was normal from the basal end of the trichoblasts (towards the root apex), suggesting that the epidermal cells are not impaired in their ability to determine polarity. This observation is in contrast to the mid mutant of the TopoVI regulatory subunit, which has been reported not to express an apical polarity marker ROP2 associated with root hair initiation (Kirik et al., 2007).
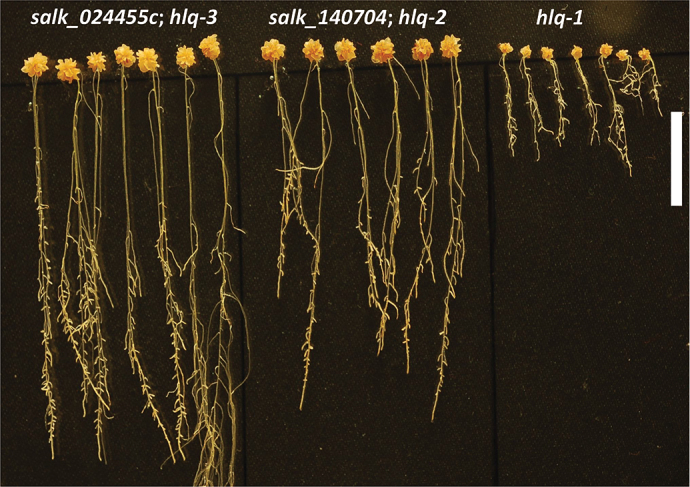
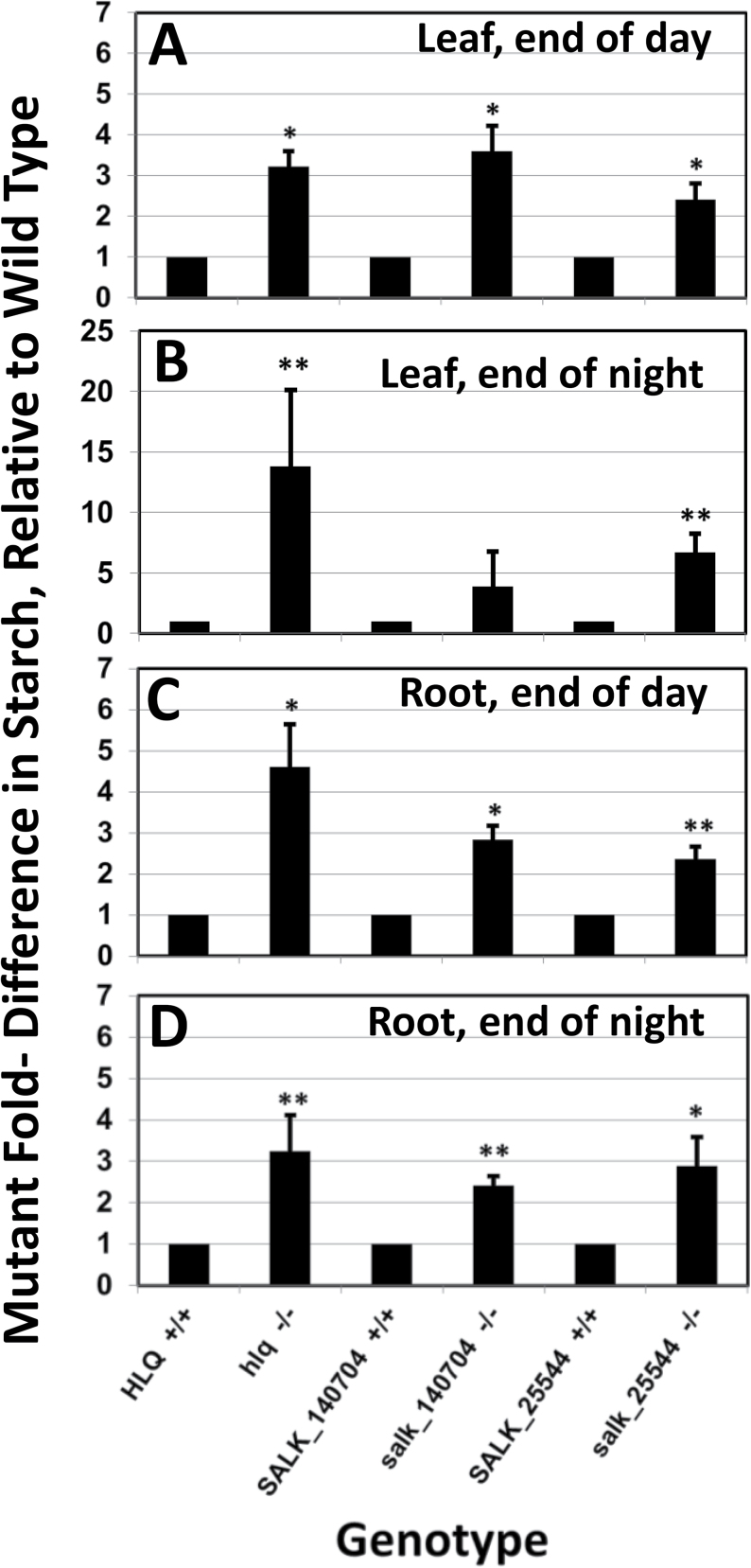
Qualitatively the root hairs of hlq (not shown) and the T-DNA insertion lines were observed to bulge or branch (Supplementary Fig. S6 at JXB online). The roots and shoots of the knockout mutants had a visually rough appearance, and the root differentiation zone and the distal region of the elongation zone were often swollen (Supplementary Figs S5D, S6). Homozygotes of both T-DNA insertion lines had very similar pleiotropic phenotypes to those of hlq mutants, but not as severe in terms of dwarfing of roots and shoots (Fig. 5). Interestingly, the presumed homozygous line SALK_024455C was found not to be homozygous, with only a few seeds out of ~60 obtained from the stock centre showing the dwarf phenotype. After verifying heterozygous lines, complementation tests were performed by crossing to hlq/+ heterozygotes, and non-complementation (predicted 3:1 wild type:mutant segregation) was observed in F1 seedlings (Supplementary Fig. S7). PCR genotyping of these individual plants showed that they were heterozygous for the T-DNA insertions, which rules out self-pollination of hlq/+ as an alternative interpretation of the results. χ2 analysis of the numbers of observed segregating mutants in the F1 generation from crosses of heterozygous lines for the T-DNA insertions of SALK_024455C and SALK_140704 with hlq/+ provided conclusive evidence that hlq is an allele of BIN3/HYL6/RHL3/TOP6B (Table 1). Since the T-DNA alleles have not been described previously, they are designated based on decreasing phenotypic dwarf severities (Fig. 5) as hlq-2/salk_140704 and hlq-3/salk_024455c, respectively (Fig. 4C). Analyses of absolute (Table 2) and relative (Fig. 6) starch accumulations in leaves and roots of the hlq-1, hlq-2, and hlq-3 mutants compared with isogenic controls validated prior staining results (Fig. 2A, C), and further showed that excess starch accumulation in mutants is not abated during the night, when starch was normally metabolized to give an ~7-fold drop in wild-type controls (Table 2). The strong correlations between fold starch accumulations in mutant leaves (Table 2) or roots (Fig. 6C) taken together with the observed severities of dwarf phenotypes (Fig. 5) supports the interpretation that hlq-1 is a null allele (Fig. 4A) and that hlq-2 and hlq-3 are somewhat leaky or hypomorphic alleles.
Fig. 5.
T-DNA knockout lines SALK_140704 and SALK_024455C identify two new hypomorphic alleles of hlq based on severity of shoot and root dwarfism and other pleiotropic effects on morphogenesis. Segregating mutants were plated on 0.8% sucrose minimal media and grown for 6 weeks. Bar=1cm.
Table 1.
χ2 analysis of mutant phenotype segregation (non-complementation) observed in F1 progeny of crosses between SALK_24455C/+ and SALK_140704/+ with hlq/+ heterozygous genotypes supports that HLQ is an allele of BIN3/HYP6/RHL3/TOP6B
| Phenotype | Observed SALK_24455a | Expectedb | Observed SALK_140704c | Expectedb |
|---|---|---|---|---|
| Mutant | 9 | 12 | 47 | 48.75 |
| Wild type | 39 | 36 | 148 | 146.25 |
| Total individuals | 48 | 195 | ||
| χ2 P-value | 0.32 | 0.77 |
a Six separate crosses.
b1 mutant:3 wild type segregation null hypothesis for non-complementation, df=1.
c Eleven separate crosses.
Table 2.
Quantitation of starch (mg starch per g FW) in different organs and in response to light in a series of hlq mutant alleles
| Genotype | Tissue, and time of harvest | Ratio of leaf starch in mutant/wild type (fold effect) | ||||
|---|---|---|---|---|---|---|
| Leaf, end of day | Leaf, end of night | Root, end of day | Root, end of night | End of day | End of night | |
| hlq-1 (–/–) homozygote | 15.94 | 13.56 | 5.63 | 4.41 | 4.1 | 28.6 |
| HLQ (+/–) heterozygote/WT | 3.89 | 0.47 | 2.62 | 1.74 | ||
| salk_140704/hlq-2 (–/–) homozygote | 17.18 | 9.76 | 2.65 | 1.72 | 2.8 | 12.6 |
| SALK_140704 (+/–) het/WT | 6.09 | 0.78 | 1.43 | 1.10 | ||
| salk_25544c/hlq-3 (–/–) homozygote | 9.88 | 9.81 | 3.25 | 2.28 | 1.9 | 11.9 |
| SALK_25544C (+/–) het/WT | 5.14 | 0.82 | 1.58 | 1.06 | ||
| Wild type average (±SEM) | 5.04±0.64 | 0.69±0.11 | 1.88±0.37 | 1.30±0.22 | ||
| Mutant average(±SEM) | 14.33±2.25 | 11.04±1.26 | 3.84±0.91 | 2.80±0.82 | ||
Fig. 6.
Relative starch accumulation in leaves (A, B) and roots (C, D) of hlq mutant alleles compared with isogenic controls at the end of day (A, C) and end of night (B, D) diurnal cycles. Asterisks indicate significantly different from the wild type (*P<0.05; **P<0.01, Student’s paired t-test, equal variance assumed). Error bars are the SEM, n=4.
Transcriptome profiling of the hlq mutant and meta-analysis of other TopoVI mutant data sets reveals pleiotropy at the levels of chromatin and signalling of light, ROS, hormone, sugar, and calcium affecting carbon metabolism
Since the HLQ/BIN3/HYL6/RHL3/TOP6B gene has previously been described as regulating several molecular processes and signalling, it was endeavoured to take a systems approach to better understand the nature of HLQ effects, by analysing deregulated gene expression in hlq mutant seedlings using microarrays. The data are provided in Supplementary Datafile 1 at JXB online. Of the 25 673 genes on the microarray, ~23% were significantly (P<0.05) misregulated in hlq compared with control, with 305 and 365 genes being down-regulated and up-regulated, respectively, >2-fold (average P<0.002). Using an arbitrary cut-off for fold effects of 1.74 (log2=0.8), 514 genes were identified as down-regulated and 559 up-regulated in hlq (average P<0.003). Supplementary Fig. S8 shows a ‘volcano plot’ for FC of all probes as a function of statistical significance (P-values of FC).
The Java desktop application ‘Exploratory Gene Association Networks (EGAN) (Paquette and Tokuyasu, 2010), which provides a contextual graph visualization of transcriptome results, was employed and an intriguing observation was made: excluding 24 cases of gene duplications (where co-regulation is assumed to be due to duplicated regulatory elements), there was a statistically significant chromosomal adjacency for 27 sets/pairs of up-regulated and 24 sets/pairs of down-regulated genes, or ~5% of the 1073 most differentially expressed genes (P<10–21, χ2 test using 1078 ‘control’ genes with average FC symmetrically distributed about zero; Supplementary Table S2 at JXB online). This number of gene pairs was more than three times the number of cases that would be predicted based on observed discordant pairs (14 pairs of adjacent differentially expressed genes that had opposite signs for FC, which fit well to a binomial model of chance occurrence). This observation suggested that the TopoVI complex containing HLQ/TOP6B acts in part by chromatin remodelling of proximal clusters of two and three genes, as has been observed for the differentiation of haematopoietic stem cells to erythroid and neutrophil cell types (Kosak et al., 2007). Consistent with this interpretation is that 56% of those immediately adjacent co-regulated genes are encoded on opposite strands, ruling out co-transcriptional control per se (e.g. by read-through of RNA polymerase II). The average distance between the distal ends of the co-regulated genes was 8 kbp (Supplementary Table S2). Previous studies showed that the TopoIIα-like RHL1 and BIN4/MID proteins bind each other, TOP6A (but not TOP6B), and DNA (Breuer et al., 2007; Sugimoto-Shirasu et al., 2005). It is of note that Simkova et al. (2012) showed by chromatin immunoprecipitation that Arabidopsis TopoVI subunits RHL1 and TOP6A directly bind to the promoter/transcription initiation sites of a pair of adjacent genes encoding unknown domains, At1g24145 and At1g24147, claiming that the TopoVI complex functions directly in initiation and elongation of transcription for different classes of genes subject to regulation by singlet and peroxide pathways of ROS.
Given the recent claim that TOP6A/CAA39/AtSPO11-3/RHL2/BIN5 is an integrator of singlet oxygen and H2O2 stress response pathways and therefore regulates expression of several thousand genes in Arabidopsis (Gadjev et al., 2006), it was first sought to compare those reported 1O2- and H2O2-responsive genes subject to regulation by TOP6A/CAA39 (Simkova et al., 2012) with first-generation transcriptome microarray studies on bin5/top6a and bin3/top6b (Yin et al., 2002) as a framework for interpretation of the transcriptome results with hlq/top6b. Of the 255 genes claimed to be down-regulated in the caa39/top6a mutant, 13 genes (or only 5%) were strictly concordant with the 314 genes claimed to be down-regulated in bin5/top6a (Supplementary Table S3 at JXB online) (but with the caveats that bin5 results were from a smaller number of genes interrogated by the Affymetrix 8.3K GeneChip®, and caa39 is claimed to be a weak allele). This observation raised questions about interpretations of specificity drawn by previous microarray studies of TopoVI mutants.
Transfer from dark to light of 5-day-old top6a/caa39 mutants in the fluorescent in blue light (flu) mutant background triggers photo-oxidative stress, resulting in misexpression of 1093 genes (Simkova et al., 2012), similar to the number of significantly misregulated genes that were observed in hlq. This situation permits a reasonable analysis for agreement across experiments. Supplementary Table S3 at JXB online provides a comparison of genes displaying altered transcript levels for top6a/caa39 (Simkova et al., 2012) with the present results for hlq/top6b. For the 51 genes (cluster 1) reported to be up-regulated in top6a/caa39 mutants unrelated to singlet oxygen signalling (and enriched for genes related to DNA repair), 13 were significantly up-regulated in hlq and one was significantly down-regulated; this is a very good agreement in terms of qualitative effect, but represents only about a quarter of the results that would be expected, assuming reproducibility.
For the 113 genes claimed to be induced by 1O2 in a TopoVI-dependent manner (cluster 4), 22 genes were observed to be significantly up-regulated and 12 genes significantly down-regulated, for an overall concordance of 30% differential expression, but not strictly (the null hypothesis of reproducibility was down-regulation in hlq, but more genes were up-regulated). For the cluster of 55 genes hyperactivated by 1O2 in top6a/caa39 mutants (cluster 5, indicated as TopoVI-repressed), 12 up-regulated and two down-regulated genes were observed in hlq, a similar concordance (25%) with the TopoVI-specific effects described above. Similarly for cluster 2, in which were indicated 25 down-regulated genes in top6a/caa39 not subject to singlet oxygen regulation, four down-regulated and two up-regulated genes were observed. This generally fits the null hypothesis, but with a low concordance (24%) as observed for the singlet oxygen-specific genes claimed to interact with top6a/caa39. For the 199 genes (clusters 6 and 8) indicated as singlet oxygen inducible and up-regulated in top6a/caa39 (i.e. indicated to be TopoVI repressible), good concordance was observed with only five down-regulated genes and 90 up-regulated genes (48% of the total).
Turning attention to further data sets, an analysis of ~5500 genes in which 316 genes were reported as down-regulated in both bin3/top6b and bin5/top6a mutants (Yin et al., 2002) was examined. Supplementary Table S3 at JXB online provides the hlq/WT log2FC results for genes identified in that list. Good concordance was observed, with 137 genes being significantly misregulated in the hlq/top6b mutant (43%), and very good reproducibility, with only 6% (20) of these genes being up-regulated and 117 genes down-regulated in hlq, as hypothesized. Taken together, this meta-analysis supports the hypothesis that TopoVI mutants reproducibly alter expression of specific gene sets that can provide clues to the underlying cross-talk in gene networks controlling growth and development.
Finally, those genes (clusters 3 and 7) reported as down- and up-regulated, respectively, by singlet oxygen produced after a shift to high light conditions in the flu background, but which were claimed not to interact with the top6a/caa39 genotype (Simkova et al., 2012), were compared. In this case, the null hypothesis in the meta-analysis is non-concordance (low reproducibility) for the hlq/top6b-specific effects. Of the 203 genes reported as down-regulated by a shift to high light, 39 were significantly up-regulated in hlq, whereas 24 genes were significantly down-regulated (total concordance 31%), similar to or higher than the overall percentage seen for the observed concordance for TopoVI-specific effects (clusters 1, 2, 4, 5, 6, and 8).
Similarly, for cluster 7 (365 genes up-regulated by light shift but independent of top6a/caa39), 132 significantly up-regulated and 24 down-regulated genes were observed in hlq/top6b, a very good agreement of 43% overall and strict reproducibility in terms of expected up-regulation. Taken together, these results showing high hlq/top6b concordance, vis-à-vis claimed top6a/caa39 non-specific effects, suggest that the TopoVI modes of action include pathways other than the oxidative stress response. Despite the relatively good agreement for differentially expressed genes observed between the present results and two independent experiments that reported similar numbers (several hundred) of TopoVI-dependent down-regulated genes, meta-analysis across all three microarray experiments found only seven consistently misregulated genes, and strict concordance was marginal (Table 3). This finding reinforces the notion that the pleiotropic nature of TopoVI mutants defies easy descriptions of function, which raises questions of whether TopoVI is indeed a key integrator of the principal pathways of brassinosteroids (Yin et al., 2002), cell cycle control (Hartung et al., 2002; Sugimoto-Shirasu et al., 2002), or singlet oxygen (Simkova et al., 2012) signalling as previously claimed. Since hlq came out of a screen for misregulated ABA-inducible gene expression (Subramanian et al., 2002), TopoVI activity probably targets many different signalling pathways and processes, either directly or indirectly.
Table 3.
Genes significantly misregulated in three independent microarray experimentsa in TopoVI mutants
| AGI | Annotation, gene ontology process/function | top6a/caa39 b | hlq/top6b log2FC (this work) | Concordance? |
|---|---|---|---|---|
| At5g42650 | CYP74A/DELAYED DEHISCENCE2; allene oxide synthase | Singlet induction TOP6A dependent | –0.67 | Yes |
| At5g47370 | HAT2, homeodomain transcription factor induced by auxin; root morphogenesis related | Singlet repressed, TOP6A independent | –0.29 | Yes |
| At5g57560c | TOUCH4/cell wall modifying; rapidly induced by environmental stimuli | TOP6A repressed, independent of singlet induction | 1.29 | Not for bin3 |
| At1g01120 | KCS1, 3-ketoacyl-CoA synthase, critical for fatty acid elongation in wax biosynthesis | Singlet induced, TOP6A independent | –0.49 | Not for singlet |
| At2g44940 | DREB subfamily A-4 of Ethylene Response Factor/APETELA2 domain transcription factor | Singlet induced, TOP6A independent | –0.49 | Not for singlet |
| At4g20780 | CALMODULIN LIKE 42, EF hand domain calcium and protein binding; involved in trichome branching, abiotic stress responses | Singlet induced, TOP6A independent | –0.27 | Not for singlet |
| At2g17880 | Chaperone DnaJ heat shock protein, response to sugar | Singlet repressed, TOP6A independent | 0.25 | No |
a Originally reported (Yin et al., 2002) as down-regulated >2-fold in both bin3/top6b and bin5/top6a mutants.
b Genes classified (Simkova et al., 2012) as ‘topo6a/caa39 induced’ (cluster 1); ‘topo6a repressed’ (cluster 2); ‘singlet repressed, TOP6A independent’ (cluster 3); ‘singlet induced TOP6A dependent’ (cluster 4); ‘singlet induced TOP6A repressed’ (cluster 5); ‘TOP6A repressed, independent of singlet induction’ (clusters 6, 8); ‘singlet induced, TOP6A independent’ (cluster 7).
c Reported (Yin et al., 2002) as down-regulated; brassinosteroid induction dependent on BIN3/TOP6B and BIN5/TOP6A.
Given the pleiotropic nature of hlq, MAPMAN software (Usadel et al., 2005) (http://mapman.gabipd.org) was used to analyse empirically the transcriptome data set in terms of metabolic and regulatory pathways affected in hlq mutants. Supplementary Fig. S9 and Supplementary Datafile 1 at JXB online provide, in the form of a relative heat map and lists, respectively, the number and degree of 2575 metabolism-related genes that are significantly misregulated in hlq (average P<0.005 for visibly coloured genes in Supplementary Fig. S9). Genes for photosynthesis, photorespiration, terpenoid biosynthesis, nitrogen assimilation, tetrapyrrole/chlorophyll/haem/phytochrome chromophore biosynthesis, the reductive pentose phosphate Calvin/Benson/Bassham cycle, and starch biosynthesis were uniformly down-regulated in hlq seedlings. This generally correlated with observed chlorosis in the mutant (Fig. 5), but was inversely correlated with starch accumulation (Figs 2, 6), an unexpected finding that suggests feedback regulation of carbon metabolism. Supplementary Datafile 2 documents the findings of specific genes, pathways, and processes significantly altered at the transcriptome level in hlq.
It was of particular interest that one of the down- regulated (2.38 FC, P<0.00001) tetrapyrrole genes is At5g13630/GENOMES UNCOUPLED5/Mg-CHELATASE-H, encoding an enzyme of chlorophyll biosynthesis variously described as involved in plastid to nucleus retrograde signalling (Mochizuki et al., 2001; Susek et al., 1993) and a mediator of ABA signal transduction, possibly as an ABA receptor modulating expression of WRKY-domain transcriptional repressors, ROS homeostasis, and lipid β-oxidation (Shen et al., 2006; Wu et al., 2009; Shang et al., 2010; Jiang et al., 2011; Tsuzuki et al., 2011; Xu et al., 2012). Remarkably, the downstream targets of GUN5, At2g33150/3-ketoacyl-CoA thiolase/KAT2 and At1g80840/WRKY40, are implicated in ABA signalling (Shang et al., 2010; Jiang et al., 2011; Liu et al., 2012), and both are significantly up-regulated in hlq mutants (2.96 FC, P<0.0001; and 1.96 FC, P<0.002, respectively (Table 4). KAT2 is categorized in Supplementary Fig. S9 at JXB online as involved in branched-chain amino acid catabolism, which was over-represented with significantly down-regulated biosynthetic genes and up-regulated catabolic genes (P=0.003: Supplementary Datafile 1). Interestingly, several of the WRKY genes up-regulated in hlq (Supplementary Table S4) are also expressed specifically in trichomes (WRKY8, -15, -18, and -33) (Jakoby et al., 2008) and involved in hormone signalling cross-talk and oxidative stress response (WRKY25) (Hu and Ma, 2006). Similar to mutants that do not produce mature trichomes (Marks et al., 2009), many abiotic stress effectors were misregulated in hlq (Supplementary Datafile 1), including genes for ABA biosynthesis and metabolism, members of the PYRABACTIN-RESISTANT1/REGULATORY COMPONENTS OF ABA RECEPTOR11/RCAR11 class of ABA receptors, and genes with functional evidence as effectors of ABA signalling and biosynthesis (Table 4).
Table 4.
ABA biosynthesis and signalling genes with significantly altered expression in hlq mutant seedlings
| AGI | Gene name | Annotation | hlq/WT log2FC | P-value |
|---|---|---|---|---|
| At1g15520 | ABCG40 | ABC transporter; ABA importer | 1.91 | 0.0001 |
| At2g47130 | SDR3 | Short-chain dehydrogenase/reductase ABA2-like | 1.73 | 0.0001 |
| At2g33150 | KAT2/PED1 | Peroxisomal 3-ketoacyl-CoA thiolase, fatty acid β-oxidation; positive ABA effector | 1.56 | 0.0001 |
| At1g80840 | WRKY40 | Pathogen-induced transcription factor; ABA repressor | 0.97 | 0.001 |
| At5g67300 | MYB44 | R2R3 MYB transcription factor; ABA sensitivity | 0.85 | 0.01 |
| At1g56070 | LOS1 | Low response to Osmotic Stress1; translation elongation factor 2-like; cold stress response | 0.52 | 0.001 |
| At5g58670 | PLC1 | Phospholipase C1; ABA, drought, salt, cold response | 0.45 | 0.05 |
| At4g26080 | ABI1 | ABA insensitive1; protein phosphatase 2C | 0.35 | 0.008 |
| At3g57530 | CPK32 | Calcium-dependent protein kinase32; phosphorylates ABA RESPONSE FACTOR4 | 0.31 | 0.01 |
| At1g33560 | ADR1 | ACTIVATED DISEASE RESISTANCE1; NBS-LRR; interacts with ABI1 in drought response | 0.31 | 0.03 |
| At1g69260 | AFP1 | ABI5-Binding Protein; domain unknown function | 0.28 | 0.03 |
| At3g24650 | ABI3 | ABA insensitive3; B3 domain transcription factor | 0.26 | 0.02 |
| At2g40220 | ABI4 | ABA insensitive4; AP2 domain transcription factor | 0.23 | 0.03 |
| At4g01026 | PYL7 | Pyrabactin-Like7; ABA receptor | 0.22 | 0.04 |
| At5g45870 | PYL12 | Pyrabactin-Like12; ABA receptor | 0.22 | 0.05 |
| At3g25010 | RLP41 | Receptor Like Protein41; ABA hypersensitive to chlorosis | –0.21 | 0.04 |
| At2g32860 | LOS15 | AtBGL2; Beta glucosidase33; ABA-GE hydrolase | –0.24 | 0.02 |
| At1g80080 | TMM/RPL17 | Too Many Mouths; stomatal development; ABA insensitive to chlorosis | –0.26 | 0.02 |
| At1g35515 | HOS10/MYB8 | High response to Osmotic Stress10; ABA hypersensitive | –0.33 | 0.02 |
| At5g45340 | CYP707A3 | ABA 8’-hydroxylase; phaseic acid synthesis | –0.46 | 0.03 |
| At1g73000 | PYL3 | Pyrabactin-Like3; ABA receptor | –0.46 | 0.02 |
| At4g17870 | PYR1 | Pyrabactin Resistent1; ABA receptor | –0.58 | 0.01 |
| At5g53160 | PYL8 | Pyrabactin-Like8; ABA receptor | –0.63 | 0.0008 |
| At3g63520 | NCED1 | Nine-cis-epoxycarotenoid dioxygenase | –0.75 | 0.0005 |
| At5g67030 | ABA1 | Zeaxanthin epoxidase; mutant ABA deficient | –0.84 | 0.002 |
| At4g15560 | CLA1 | 1-Deoxyxylulose 5-phosphate synthase; chloroplastos alterados1; ABA deficient | –0.87 | 0.0002 |
| At1g52400 | AtBG1 | Beta glucosidase18; ABA-GE hydrolase | –1.01 | 0.0002 |
| At2g41070 | DPBF4/EEL | Dc3-Pro Binding Fctr4; bZIP; Enhanced Em Level | –1.24 | 0.0002 |
| At1g64670 | CED1 | 9-CIS EPOXYCAROTENOID DIOXYGENASE DEFECTIVE; BODYGUARD1; epidermal wax biosynthesis; alpha-beta hydrolase | –1.60 | 0.0008 |
Supporting the observed phenotypes of lignin and anthocyanin accumulation in hlq (Fig. 3A, B; Supplementary Figs S1C, S5D at JXB online), many genes involved in aromatic amino acid and phenylpropanoid/lignin biosynthesis were up-regulated in hlq, whereas flavonoid biosynthetic genes were both up- and down-regulated (Supplementary Fig. S9; Supplementary Datafile 1), some of which are mono-oxygenases that have haem cofactors (see above). The strong up-regulation of NITRILASE2, which functions in an alternative pathway of wound- and pathogen-induced auxin (IAA) biosynthesis from indole-3-acetonitrile (Huh et al., 2012) and degradation of tryptophan-derived glucosinolates, indicates that auxin or tryptophan amino acid homeostasis may be affected in hlq, or that the hlq defect in trichome development may be responsible for the observed (Supplementary Fig. S9) misregulation of glucosinolate biosynthetic genes (Jakoby et al., 2008). Since trichome-specific expression of the cell cycle checkpoint CYCLINB1;2 results in rescue of the top6a/rhl2 and mid mutant trichome defects but not of their dwarf phenotypes (Kirik et al., 2007), it is likely that the misregulation of glucosinolate pathways in hlq mutants is a direct effect of TopoVI on carbon partitioning processes.
With regards to the pleiotropic cell wall phenotypes of hlq, several genes encoding proteins (EXPANSINS, ARABINOGALACTAN PROTEINS) that modify or contribute to wall structure and/or metabolism were up-regulated (Table 5), providing correlative evidence for the functions of those genes in the cell wall phenotypes of hlq, including the increased abundance of pectins (Fig. 3E) and radial swelling of epidermal cells (Fig. 2C), which was phenocopied by the N-glycosylation inhibitor tunicamycin (Supplementary Fig. S3A, B at JXB online). Arabinogalactan proteins are involved in root and stem expansion (Willats and Knox, 1996; Ding and Zhu, 1997; Park et al., 2003) and broadly implicated in plant development including programmed cell death (Gao and Showalter, 1999) similar to the observed hlq phenotype, and possibly in ABA-inducible gene expression (Desikan et al., 1999).
Table 5.
List of cell wall genes most up- and down-regulated in hlq seedlings by transcriptome profiling
| AGI | Annotation | hlq/WT log2FC | P-value |
|---|---|---|---|
| Up-regulated wall biosynthesis | |||
| At2g18660 | EXPANSIN-LIKE B3 | 3.32 | <0.0002 |
| At3g45970 | EXPANSIN-LIKE A2 | 1.29 | <0.0007 |
| At2g22470 | ARABINOGALACTAN PROTEIN 2 | 2.21 | <0.0002 |
| At1g35230 | ARABINOGALACTAN PROTEIN 5 | 1.14 | <0.0007 |
| At4g09030 | ARABINOGALACTAN PROTEIN 10 | 1.07 | <0.0003 |
| At5g64310 | ARABINOGALACTAN PROTEIN 1 | 1.00 | 0.002 |
| At2g45220 | PECTINESTERASE | 2.73 | <0.0009 |
| At3g48580 | ENDO-XYLOGUCAN TRANSFERASE | 1.20 | <0.0003 |
| At5g57560 | XYLOGUCAN TRANSFERASE/TOUCH4 | 1.29 | <0.0002 |
| At4g23990 | CELLULOSE SYNTHASE/CSLG3 | 0.84 | <0.0006 |
| At3g02230 | UDP-l-Ara MUTASE/RGP1a | 0.94 | <0.0006 |
| At4g25810 | XYLOGUCAN ENDO-TRANSGLYCOSYLASE6/XTR6 | 1.06 | <0.0004 |
| At3g07160 | GSL10; GLUCAN SYNTHASE-LIKE10, β-1,3 CALLOSE | 0.35 | <0.006 |
| Up-regulated wall catabolism | |||
| At3g57510 | POLYGALACTURONASE/ADPG1 | 1.42 | <0.02 |
| At4g13210 | PECTATE LYASE | 1.06 | <0.0003 |
| At4g30270 | MANNAN-XYLOSE HYDROLASE/MERISTEM5B | 1.07 | <0.0003 |
| At5g20950 | β-1,4-GLUCANASE | 0.90 | <0.0008 |
| Down-regulated wall metabolism | |||
| At2g06850 | ENDO-XYLOGUCAN TRANSFERASE/EXGT-A1 | –1.43 | <0.0006 |
| At4g37800 | ENDO-XYLOGUCAN TRANSFERASE | –1.20 | <0.0004 |
| At5g39310 | EXPANSIN 24 | –0.92 | <0.002 |
| At2g37640 | EXPANSIN A3 | –0.73 | <0.002 |
| At4g28250 | EXPANSIN B3 | –0.72 | <0.003 |
a Annotated in Rautengarten et al. (2011). RGP1, REVERSIBLY GLYCOSYLATED POLYPEPTIDE1.
Characterization of hlq whole cell wall composition by infrared spectroscopy and quantitation of glycosyl composition
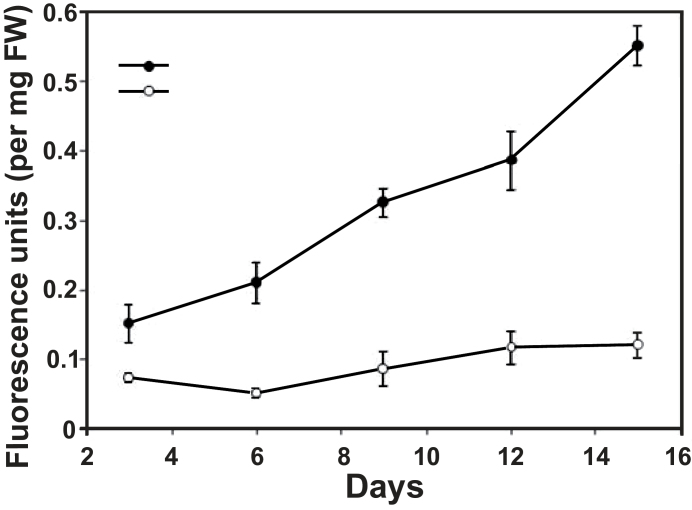
The metabolism of callose (β-1,3-glucan) within plant cell walls is essential to many developmental, physiological, and stress-related processes (Chen and Kim, 2009). Production of callose is induced by mechanical stress and wounding, and under conditions of cellulose deficiency (e.g. in cell wall mutants). Some dwarf mutants ectopically deposit callose (Lukowitz et al., 2001), pectin (His et al., 2001), and the phenolic polymers suberin (Cheng et al., 2000) and lignin (Burk et al., 2001), thereby compromising the stability and flexibility of the cell wall. Treatment of wild-type plants with tunicamycin resulted in the appearance of patches of callose accumulation within the root elongation zone (Supplementary Fig. S3D at JXB online), similar to the phenotype of hlq mutants (Subramanian et al., 2002). A comparison of callose content in hlq and the wild type during growth on sucrose-supplemented agar media indicated that the hlq mutants showed a steady increase in callose accumulation over 15 d, with mutants accumulating up to five times the callose content of wild-type plants (Fig. 7).
Fig. 7.
Callose content of wild-type versus hlq during growth of seedlings. Mutants of hlq had five times more callose than the wild type after 15 d of growth. Error bars represent ±SEM of three replicates. The results presented are for the abi2-1/hlq double mutant; however, the phenotypes described were independently verified on Ler/hlq single mutants.
Yariv reagent binds arabinogalactan proteins and triggers accumulation of callose by induction of the callose synthase gene At3g07160/GLUCAN-SYNTHASE-LIKE10 and the transcription factor gene WRKY40 (Guan and Nothnagel, 2004), which were both significantly up-regulated in hlq (Tables 4, 5). Similarly, the transcription factor gene MYB44 was up-regulated in hlq (Table 4) and has been shown to regulate callose production in Arabidopsis (Lü et al., 2011). Since the present results suggested that hlq mutants have a generalized perturbation in cell wall composition, hlq mutant cell walls were characterized by spectroscopic and biochemical methods.
Along with soluble expansins and structural proteins including hydroxyproline-rich extensins and arabinogalactan proteins, four primary and secondary polymer classes make up the plant cell wall: cellulose, hemicellulose, pectin, and lignin. FTIR microspectroscopy, which measures the energy of asymmetric molecular bond vibrations, is a rapid, non-invasive method for detecting a range of polysaccharides affecting cell wall architecture in muro, based on their functional groups, including, but not limited to, carboxylic esters, phenolic esters, protein amides, and carboxylic acids. A digital subtraction plot of the hlq FTIR spectrum minus that of the wild-type spectrum from extracted cell walls is provided in Fig. 8A. The frequency–structure peak at wavenumber 1727cm–1 corresponds to the C=O stretch of esterified pectins, consistent with the qualitative result of pectin staining showing elevated pectins in hlq leaves (Fig. 3E) (Alonso-Simon et al., 2011). Secondary amide bonds attributed to cell wall peptides (McCann et al., 1992) were observed at 1643cm–1 corresponding to the C=O stretch, at 1530cm–1 for the N-H amide, and at 1229cm–1 for the C-N stretch. The peaks observed at 1138cm–1 and 1084cm–1 are probably due to the C-O-C glycosidic bonds of various polysaccharides that differ between hlq and the wild type. Importantly, the negative peak at 1055cm–1 probably corresponds to the ring C-O of cellulose (Alonso-Simon et al., 2011) and homogalacturonan, whereas the positive peak at 1010cm–1 probably represents the ring C-C stretch of various polysaccharides (pectins). These results suggest that there is a deficiency of cellulose or hemicelluloses and an increased abundance of pectins in the hlq mutant as compared with the wild type.
Fig. 8.
Fourier transform infrared (FTIR) microspectroscopy analysis of dehydrated cell wall extracts of hlq and the wild type. (A) Digital subtraction frequency–structure correlation chart. Difference (Δ) spectrum of hlq minus wild-type FTIR spectra. The peak at wavenumber 1727cm–1 corresponds to the C=O stretch of esterified pectins; peaks at 1643cm–1 (C=O stretch), 1530cm–1 (N-H amide), and 1229cm–1 (C-N stretch) correspond to secondary amide bonds attributed to cell wall peptides, or lignin for 1643cm–1 (McCann et al., 1992); peaks at 1138cm–1 and 1084cm–1 are probably due to the C-O-C glycosidic bonds of various polysaccharides which differ between hlq and the wild type; the negative peak at 1055cm–1 is the ring C-O of cellulose, and 1010cm–1 is probably the ring C-C. (B) Principal component analysis of FTIR spectra separates the hlq and controls (WT and heterozygote samples) into two non-overlapping clusters which account for 98% of the variation. Inset: plot of the PC1 loadings versus wavenumber, showing that wavenumbers 1735, 1657, 1546, and 1244cm–1 (esterified pectins, lignin/peptides) are dominants in addition to cellulose at 1063cm–1 (arrows).
The results of PCA discriminatory analysis on the hlq and wild-type wall spectra are shown in Fig. 8B. Most of the variation (98%) in FTIR spectral differences between cell wall extracts of hlq, hlq/+ heterozygotes, and wild-type walls could be attributed to two PCs, with replicate hlq homozygous samples clustering separately in the lower left quadrant from hlq/+ heterozygotes, and wild-type samples clustering broadly together in the other three quadrants (Fig. 8B). Plotting the PC that accounted for 95% of the variation as a function of wavenumber (Fig. 8B, inset arrows) showed peaks at 1735cm–1 (esterified pectins), 1657cm–1 (lignin), 1546cm–1 (secondary peptide amide), 1244cm–1 (C-N peptide amide), and 1063cm–1 (cellulose or homogalacturonan), corresponding well to previous reports (McCann et al., 1992). It is concluded from this analysis that the hlq mutant is deficient in cellulose or hemicelluloses, or both, and has elevated levels of pectins, lignins, and cell wall proteins. This conclusion was further supported by the microarray results for cell wall structural proteins EXPANSIN-LIKE and arabinogalactan proteins (Table 5).
In order to differentiate between the effects of hlq on cellulose and hemicellulose levels, the glycosyl composition of whole cell wall extracts subjected to methanolysis and trimethylsilylation combined with GC with or without prior swelling and hydrolysis of cellulose (SAH) using concentrated sulphuric acid was analysed. On a mole percentage basis of constituent sugar residues, the whole cell wall fraction of hlq seedlings contained significantly more abundant components of pectic arabinogalactans than the wild type, in particular arabinose (5.5 mol% increase), rhamnose (1.3 mol% increase), and galacturonic acid (4.6 mol% increase), whereas hlq mutant walls contained significantly less cellulosic glucose (–14.5 mol% decrease) than those of the wild type (Fig. 9). This supports the finding that hlq accumulates more pectin in leaves than the wild type (Fig. 3E) and has a wall architecture deficient in cellulose (Fig. 8), and the conclusion that cellulose deficiency and/or pectin overabundance resulted in severely compromised epidermal cell wall structural integrity (Fig. 2C; Supplementary Figs S1H, S6 at JXB online).
Fig. 9.
Glycosyl composition (mol%) of the whole cell wall fraction from wild type (WT) and hlq mutant Arabidopsis seedlings, without and with prior H2SO4 swelling and hydrolysis (SAH) to differentiate cellulose and tightly bound mannosyl polymers from extractable (e.g. hemicellulose) polysaccharides. Cell walls of hlq had significantly decreased cellulose and compensatory increases in arabinosyl, rhamnosyl, and galactosyl residues typical of pectic arabinogalactans. Sugar residues were measured as TMS methylglycosides by GC-FID after methanolysis of whole walls (before and after SAH). Glc-SAH and Man-SAH represent the additional residues detected due to SAH. Error bars indicate ±SEM (n=2). Asterisks (*) indicate significantly different from both wild-type and hlq/+ heterozygote samples, P<0.02 (Student’s two-sided t-test, equal variance assumed). ¶ indicates significantly different from wild-type samples, P<0.02.
Discussion
Although previous claims (Hartung et al., 2002; Sugimoto-Shirasu et al., 2002; Schrader et al., 2013) on the role of TopoVI, found only in plants and archaebacteria, in the decatenation of endoreplicated chromosomes during cell growth are not under debate, based on the results obtained on hlq mutants, a more complex role for TopoVI in transducing at the level of chromatin environmental and internal signals controlling growth and development is proposed. Others have also recognized this possibility (Sugimoto-Shirasu et al., 2002; Yin et al., 2002; Breuer et al., 2007; Kirik et al., 2007). The pleiotropic phenotypes of hlq were previously characterized, in particular that mutants are skotomorphogenic, with deformed epidermal cells which stain for callose, abnormal and reduced root hairs and leaf trichomes, and stochastic ectopic expression of an ABA- and auxin-inducible transgene. Here hlq mutants were further characterized at the physiological and transcriptome levels as accumulating starch, callose, and anthocyanins, with cell walls abundant in pectins, lignin, and cell wall proteins. Moreover, despite the absence of endoreplication in Arabidopsis inflorescences (Galbraith et al., 1991), it was shown here (Supplementary Fig. S1B, F at JXB online), as has been shown by others (Sugimoto-Shirasu et al., 2002), that hlq/top6b mutants can produce mostly normal flowers but neither homozygous male nor female hlq sporophyte organs functioned properly in reproduction, notwithstanding artificial nutrient supplementation or the previous claims that the hyp6/top6b, rhl2/top6a/atspo11-3, and bin3-1 alleles are null yet produce viable seeds (Sugimoto-Shirasu et al., 2002; Yin et al., 2002). Consistent with the notion that TopoVI is important for reproduction is the observation that in rice the TopoVI subunits are expressed maximally in pre-pollinated flowers (Jain et al., 2006). Here hlq and two additional alleles that vary in their phenotypic severity were isolated and it was shown that they are alleles of bin3/hyp6/rhl3/Topoisomerase 6B.
The rhl3 allele of TOP6B is documented in TAIR (http://www.arabidopsis.org) as a mutation of the splice acceptor in intron 17, which is stated to result in the transcription of an mRNA with at least three altered sizes that create a premature stop codon in the last exon. Hartung et al. (2002) described an unnamed T-DNA mutant allele that disrupted exon 12 and deleted 268bp of the TOP6B gene including exon13. On the basis of root calli death 3 weeks after growth induction, they argued that deficiency of AtTOP6B results in a general growth defect. Because of the absence, in the present case, of a correctly spliced intron 7 mRNA (Fig. 4) and based on the prediction that the hlq mutation sits adjacent to a highly conserved aliphatic residue two residues distal to the GXG motif of the ATP binding site in the ATPase-like domain (Corbett and Berger, 2006), it is argued here the hlq-1 mutation is a bona fide null allele. Phenotypic analysis of two new hypomorophic hlq alleles (Fig. 5) supports this contention. The various descriptions by others of presumed null alleles demonstrate that, due to the pleiotropic nature of top6B mutants, limited phenotypic characterizations can give misleading interpretations of gene function. This notion is supported by the contradictory reports of bin3/hyp6/top6b mutant effects on chromosome ploidy levels for several cell types (Hartung et al., 2002; Sugimoto-Shirasu et al., 2002; Yin et al., 2002) and the broad lack of concordance from meta-analysis of the hlq transcriptome with other previously published TopoVI mutant transcriptomes (Table 3; Supplementary Table S3 at JXB online), including other claimed null alleles.
The Arabidopsis genome encodes a second type II topoisomerase (At3g23890) that is differentially regulated from TopoVI (Breuer et al., 2007) and probably functions in mitosis, T-DNA integration, and cell cycle regulation (Xie and Lam, 1994; Makarevitch and Somers, 2006), and may have some overlapping functions with TopoVI (Sugimoto-Shirasu et al., 2005). The claims that BIN4/MID, a DNA-binding component of TopoVI, functions primarily as a DNA damage response effector during post-mitotic endocycles (Breuer et al., 2007) and transcriptional silencing (Kirik et al., 2007) mediated through the cell cycle checkpoint effector Ataxia Telangiectasia-mutated and Rad3-related (At5g40820/ATR) and ectopically expressed markers At2g31320/poly (ADP-ribose) polymerase1/PARP1, At4g02390/PARP2, AT4G21070/BRCA1, At5g20850/RAD51, and AT4G37490/CYCLINB1;1 was not supported by the present transcriptome analysis of hlq, as none of these genes was altered (see Supplementary Datafile 1). Conclusions drawn from a meta-analysis of different microarray data sets are not absolute. It cannot be ruled out that genotype-, tissue-, or methodology-specific differences between the present experiments and those of others contributed to lack of agreement at the transcriptome level. However, it is also noted that contradictory results have been reported for the role of BIN4/MID in transcriptional silencing of heterochromatin (Breuer et al., 2007; Kirik et al., 2007). Since BIN4/MID does not physically interact with TOP6B/HLQ (Breuer et al., 2007), the lack of similar molecular phenotypes for DNA damage markers in hlq mutants suggests that alternative mechanisms exist for regulation of TopoVI activities independent of BIN4/MID.
In this context, it can be recalled that hlq mutants were isolated on the basis of ectopic expression of the ABA- and auxin-inducible proDc3:GUS reporter gene in the epidermal cells of roots and hypocotyls (Subramanian et al., 2002). Previous results showed that only a subset of hlq root epidermal cells stained strongly with the viable stain fluorescein diacetate (FDA). Remarkably proDc3:GUS staining exactly coincided with the FDA staining patterns, suggesting that FDA-marked epidermal cells specifically express proDc3:GUS and that these cells are metabolically hyperactive. The brassinolide insensitivity, skotomorphogenesis/hypocotyl elongation defect, and root hairless phenotypes of the hlq mutant were previously described (Subramanian et al., 2002), consistent with the descriptions of other independent mutant allele phenotypes. The fundamental question is thus framed as ‘chicken versus egg’: is the hlq/bin3/hyp6/rhl3/top6B mutation causal for the expression of proDc3:GUS, which would thus serve as a bona fide marker for Top6B function in gene regulation, or rather is proDc3:GUS expression a downstream consequence of indirect internal stresses imposed by cell viability, wall defects/callose synthesis, or other secondary processes disrupted in the hlq mutant? The present results showing altered biosynthesis of primary metabolites such as starch (Figs 2, 6; Table 2) and cell walls (Figs 3, 7–9) in hlq, as well as proDc3:GUS expression in cell wall mutants of different classes (Supplementary Fig. S2 at JXB online) and following inhibition of peptidoglycan and cellulose biosynthesis (Supplementary Fig. S3) are consistent with a primary (direct) effect of hlq on gene expression. It is also possible that the lignin accumulation phenotype (Fig. 3) could cause alterations in cell–cell communication and thereby disturb hormonal signals or sensitivities.
The present meta-analysis of other transcriptome results showing the concordance of bin5/top6a and caa39/top6a with the hlq/top6b results further supports the chromatin remodelling model, because numerous differentially expressed unrelated adjacent genes could be identified (Supplementary Datafile 1 at JXB online) such as At4g37250/LRR-NBS receptor kinase and At4g37260/MYB73; At5g23340/F-box and At5g23350/GRAM domain ABA responsive; At1g24140/matrixin metallopeptidase and At1g24150/formin homologue4; At1g28370/ERF-AP2 domain11 and At1g28380/necrotic spotted lesions1; At1g66080/unknown and At1g66090/LRR-NBS receptor kinase; At1g74930/DREB subfamily A-5 and At1g74940/DUF581; At2g18690/unknown and At2g18700/TSP11; AT3G25600/ EF-hand family protein and AT3G25610/haloacid dehalogenase; AT4G27652 and AT4G27654 (unrelated unknowns); At4g33040/thioredoxin and At4g33050/embryo sac defective39; and At5g13180/NAC083 and At5g13190/LITAF domain. All these gene pairs were coordinately regulated, supporting the generalized observation that hlq/top6b can impact chromatin remodelling, leading to transcription of unrelated adjacent genes. As previously noted (Simkova et al., 2012) in the context of top6a/caa39 characterization, there is in vivo molecular evidence that human TopoIIβ can generate transient double-stranded DNA breaks that permit exchanges in nucleosome-specific histone H1-HMGB proteins, which promotes local changes of chromatin structure and leads to the transcriptional activation of target genes (Ju et al., 2006). Topoisomerase-mediated chromatin factor exchange represents an attractive mechanism for the transcriptional activation and repression of different sets of genes. More broadly, the present findings also raise questions about the forces exerted by chromatin remodelling factors in shaping plant genomes during evolution, for example if TopoVI was acquired by lateral gene transfer from archaebacteria instead of having been lost from the animal and fungal kingdoms. The finding of statistically significant misregulation of histone genes in the hlq mutant (Supplementary Fig. S13, Datafile S1, Table S4 at JXB online) including At1g06760/HISTONE H1 is consistent with a chromatin factor exchange model and implies that there may be a feedback mechanism for TopoVI to regulate partner histone expression. The present systems approach of transcriptome meta-analysis revealed that hlq mutants misregulate a subset of ontologically unrelated genes based on proximity, which may help to explain the pleiotropic phenotypes of bin3/hyp6/rhl3/top6b/hlq.
Remarkably, mutants and inhibitors affecting the cell wall phenocopy several of the traits displayed in hlq. Plant cell walls are complex carbohydrate polymers, and it is estimated that ~10% of plant genomes are devoted to cell wall biogenesis. However, knowledge of their biogenesis and functions in signal transduction of environmental stimuli and plant development is limited, even in model organisms. Starch is the major non-structural carbohydrate in plants. It serves as an important store of carbon that fuels plant metabolism and growth when they are unable to photosynthesize. Arabidopsis has proven to be a powerful genetic system for discovering how starch is synthesized and degraded (Streb and Zeeman, 2012), and the present results (Figs 2, 6; Supplementary Fig. S9 at JXB online) suggest that HLQ/TOP6B can be added to the list of genes important for regulating starch and cell wall biosynthesis. However, caution should be exercised when inferring regulatory mechanisms because studies at the transcriptome level do not address post-translational regulation of metabolic pathways. Changes in sugar levels or other metabolic intermediates might serve as the trigger for the fluxes into and out of the starch pool. It was proposed that macro-autophagy may play a role in degrading non-functional chloroplasts in mutants of starch metabolism (Stettler et al., 2009). Hartung et al. (2002) also observed, at the ultrastructural level in the cytoplasm of top6b/spo11-2 and top6a/atspo11-3 mutants, agglomerations of protein bodies, peroxisomes, and small vacuoles indicative of autophagy. There is genetic evidence that accumulation of starch degradation intermediates, such as disaccharides, causes severe cellular phenotypes such as chlorosis and chloroplast lysis, because mutations in β-amylase2 and β-amylase3, which are strongly down-regulated in hlq, prevent the release of maltose from the starch granule and decrease the severe cellular chlorosis observed in the maltose exporter1/dpe1 double mutant (Stettler et al., 2009). Upon export to the cytosol, maltose is metabolized by a glucosyl transfer reaction catalysed by the cytosolic disproportionating enzyme At2g40840/DPE2. Mutants of dpe2 accumulate starch and 200 times more maltose than the wild type, which causes severe chlorosis (Chia et al., 2004; Stettler et al., 2009), similar to the observed hlq gene expression phenotypes including significantly reduced DPE2 expression (P=0.009; Supplementary Datafile 1), and elevated expression of several autophagy genes (Supplementary Fig. S11). The observation of significantly elevated expression of the two Glc6P/Pi transporter genes (At5g54800/GPT1 and At1g61800/GPT2, P<0.003; Supplementary Datafile 1) is consistent with a model of starch metabolism intermediates functioning as regulators of carbon partitioning. Transcriptome profiling of Arabidopsis sucrose-inducible gene expression after 30min relief from starvation also revealed similar affected processes to those reported here: up-regulation of GPT2 and differential expression of 21 transcription regulators (43% concordance), 15 ubiquitin-targeting proteins (60% concordance, but opposite FC), trehalose phosphate synthases (50% concordance, but opposite FC), autophagy protein 8e (opposite FC), several glutaredoxins (75% concordance, plus one At1g28480/GRX480, P<0.001), and carbon-scavenging enzymes for proline and myoinositol (all opposite FC: At4g39800/Inositol-3-phosphate synthase/IPS1; At3g30775/proline oxidase; and myoinositol oxygenases MIOX2/4/At2g19800/At4g26260) (Osuna et al., 2007). That study also reported strong repression of At2g17880/DnaJ and of lipid catabolism genes after sucrose addition that mirrored the decrease of fatty acids in carbon-starved seedlings and their gradual recovery after sucrose addition. Of the handful of genes in Table 3 identified from meta-analysis as highly significant, one is a down-regulated lipid biosynthetic gene and another is At2g17880/DnaJ. Furthermore, the pervasive down-regulation of over-represented GDSL-motif lipases (P=0.01; Supplementary Fig. S12; Supplementary Datafile 1) suggests that lipids are being oxidized to fuel gluconeogenesis. These correlations strongly support the interpretation that hlq seedlings are starving, especially when taken together with results (Fig. 6, Table 2) that show that hlq leaves accumulate starch in the dark. Alternatively, the observed strong statistical association of hlq differentially expressed genes with protein degradation (Supplementary Fig. S10) is consistent with the phenotypes of the det/cop/fus class of mutants disrupting the COP9 signalosome, which is structurally and functionally similar to the 19S regulatory particle of the 26S proteosome and interacts with SCF-type E3 ubiquitin ligases to regulate targeted protein degradation (Smalle and Vierstra, 2004; Gusmaroli et al., 2007).
AGP2, the regulatory subunit of the rate-limiting starch biosynthetic enzyme, is inactivated under oxidizing conditions (Streb and Zeeman, 2012), a state that the present transcriptome data and previous results (Simkova et al., 2012) indicate exists in hlq/top6b and top6a/caa39 mutants. Soluble starch synthase1 (At5g24300/SS1) has also been proposed to be regulated by the redox state (Glaring et al., 2012), and it was observed here to be up- regulated (P=0.005; Supplementary Datafile 1 at JXB online) in hlq, which is an incongruity compared with other starch biosynthesis genes. In potato, high levels of glucose and sucrose increase both the AGP activation state and starch synthesis. The signalling processes for AGP regulation have been proposed to occur through hexokinase for glucose and through SnRK1 kinases for sucrose (Tiessen et al., 2003). HEXOKINASE1/GLUCOSE-INSENSITIVE2 was slightly up-regulated in hlq (P<0.05; Supplementary Datafile S1), whereas SnRK1 kinases were not affected. However, several of the 35 SnRK2/3 genes were significantly up-regulated (P<0.04; SnRK2.3; SnRK3.14/CIPK6/SOS3-INTERACTING3; SnRK3.10/CIPK7; SnRK3.15/CIPK14/PSK24; Supplementary Datafile S1) or down-regulated (P<0.008; SnRK2.1/ASK2; SnRK3.12/CIPK9/PKS6; SnRK3.17/CIPK3). Interestingly, these particular SnRKs have been shown to function in ABA, osmotic, and salt stress, flavonoid/sugar and calcium signalling, and auxin responses (Luan et al., 2002; Kolukisaoglu et al., 2004; Luan, 2009; Tripathi et al., 2009; Heyndrickx and Vandepoele, 2012), all processes described herein as affected by the hlq mutation and manifest in the pleiotropic phenotypes. Likewise, the hlq phenotypes of abnormal root hair development, sterility, and biotic stress responses through LRR-NBS receptor kinases is known to be controlled by calcium and ROS (Monshausen et al., 2008, 2009), processes significantly altered in hlq (Supplementary Fig. S11; Supplementary Datafile S1). Furthermore, there is evidence that the disaccharide trehalose-6-phosphate (Tre6P) acts as a signalling intermediate in the sucrose-dependent activation of AGP (Lunn et al., 2006), which is strongly down-regulated in hlq (Supplementary Datafile S1). The finding that the trehalose marker PAD4 and Tre6P biosynthetic gene TREHALOSE PHOSPHATE SYNTHASE11 were strongly up-regulated in hlq supports this model. However, the details of this mechanism remain to be elucidated, and the unexpected down-regulated expression of the AGP2 gene in hlq is not understood. Further analyses of trehalose and Tre6P in hlq and hypomorphic alleles could reveal the connections between the pathways of starch metabolism and the signalling pathways that regulate them, and provide insights into cell wall integrity-related mechanisms underlying environmental sensing, cell expansion, and morphogenesis (Hématy et al., 2007; Boisson-Dernier et al., 2011; Cheung and Wu, 2011).
The observed 1.9-fold up-regulation (P<0.0003) of At2g03770/ST1-Like sulfotransferase, involved in brassinosteroid metabolism (Marsolais et al., 2007), is noted in connection with bin3/hlq skotomorphogenesis phenotypes. Similarly, the hlq mutant showed significant down-regulation (P<0.02) of a brassinosteroid biosynthetic gene At5g05690/constitutive photomorphogenic dwarf/cabbage3/cyp90a1. Mutants of cpd/cbb3/cyp90a1 display de-etiolation and de-repression of light-induced genes in the dark, dwarfism, male sterility, and activation of stress-regulated genes in the light. Likewise, the observed strong down-regulation in hlq (Table 5) of ABA1, CLA1, CED1, and NCED1 involved in ABA biosynthesis, skotomorphogenesis via cartenoid metabolism, and regulation of cell elongation and morphogenesis (Barrero et al., 2008) is intriguing. Like hlq, mutants of ced1/bodyguard/alpha-beta hydrolase are dwarfed, extremely sensitive to osmotic stress, and have abnormal leaves, collapsed cells, and reduced numbers of trichomes (Wang et al., 2011). Taken together with the observed misregulation of lipid and isoprenoid metabolism pathways in hlq (Supplementary Fig. S9, Supplementary Datafile S1), it is speculated that TopoVI regulation of genes controlling wax or lipid metabolism may be the molecular mechanism underlying the unexpected genetic link (Wang et al., 2011) between cuticle biogenesis and ABA and osmotic stress responses. It is also speculated that TopoVI regulation of AT1G01550/BYPASS1 and At4g01360/BYPASS3 (significantly up-regulated in hlq; P<0.001, Supplementary Datafile S1) underlies the pleiotropic phenotypes of bps1/3 mutants affecting root production of a mobile shoot morphogen of unknown structure derived from carotenoids (Lee et al., 2012).
The findings that hlq/top6b mutants resemble the det/cop/fus class of mutants which accumulate the secondary metabolites anthocyanins parallels several recent lines of genetic evidence that responses to environmental cues are tightly coupled through sensors that regulate carbon partitioning between primary (aromatic amino acid) and secondary (polyphenolic) metabolites (Tzin and Galili, 2010). In addition to being a primary metabolite for protein synthesis, phenylalanine availability is rate limiting for production of phenylpropanoids important for physiology, UV protectants, fragrances/flavours related to reproduction, defence, hormonal pathways, and the synthesis of cell wall material that can comprise up to 50% of captured photosynthetic carbon, depending on the species. One possible insight into the cell elongation, lignin and anthocyanin accumulation phenotypes of hlq (Fig. 3A, B; Supplementary Figs S1C, S5D at JXB online) is that stem elongation, biomass, and lignin biosynthesis in vascular bundles and fibre cells are controlled by stem- and root-specific expression of chloroplastic arogenate dehydratases At3g44720/ADT4 and At5g22630/ADT5 (Corea et al., 2012), which were significantly up-regulated in hlq mutants (P<0.0007; Supplementary Datafile 1). Another interesting facet is the link between ethylene and auxin signalling and transport, root hair development, cell elongation, and flavonols (Lewis et al., 2011): At2g20610/SUPERROOT1/HOOKLESS3/ROOTY is an auxin overexpressor encoding an aminotransferase that functions in cross-talk between auxin and ethylene and is a marker for glucosinolate signalling (Clay et al., 2009), and it was significantly down-regulated in hlq (P<0.01; Supplementary Datafile 1). Remarkably dc3 overexpressor2 (dor2) was isolated and shown that it was a new allele of sur1/rty from the same screen that produced hlq (Sun, 2003). Further analysis and classification of up- and down-regulated genes in other TopoVI subunit mutants and characterization at the genetic, protein, and molecular levels of a series of hypomorphic TopoVI subunit B alleles (Fig. 5) may reveal the core function(s) of TopoVI. Studies on regulation of phenylalanine and polyphenolic biosynthetic pathways in both the chloroplast and cytosol, including plasma membrane-localized processes, should help unravel the complexities of lignin, cellulose, and polyphenolic contributions to structural integrity and cellular functions at the transcriptional, metabolic, and tissue levels.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Pleiotropic phenotypes of the hlq mutant.
Figure S2. Ectopic proDc3:GUS expression and lignification in two cell-wall related mutants procuste1-1 and botero1-1.
Figure S3. Cell wall inhibitors tunicamycin and DCB phenocopy epidermal traits of hlq.
Figure S4. Sanger sequencing results on both strands of independent amplicons spanning the hlq point mutation at chr3: 7267232, confirming the Illumina whole-genome re-sequencing result of G→A transition.
Figure S5. Dwarf, chlorotic, and anthocyanin-accumulation phenotypes of homozygotes for T-DNA insertion lines in At3g20780 and that disrupt exons 4 and 12.
Figure S6. Pleiotropic root hair defects of anisotropic growth, bulging, and branching for T-DNA insertion lines in At3g20780/TOP6B.
Figure S7. Non-complementation (segregating mutants) in F1 progeny of crosses between heterozygous hlq/+ and heterozygous T-DNA insertion lines, validated by PCR.
Figure S8. Volcano plot of the hlq transcriptome profiling experiment, showing the relationship between fold change effects of the hlq genotype compared with the wild type and statistical significance based on three replicates for ~25 000 genes on the microarray.
Figure S9. Transcriptome profiling of hlq seedlings for general metabolic pathway effects using MAPMAN (Usadel et al., 2005).
Figure S10. Transcriptome profiling of hlq seedlings for ubiquitin- and autophagy- dependent protein degradation pathways using MAPMAN.
Figure S11. Transcriptome profiling of hlq seedlings to ‘Gene Regulation Overview’ using MAPMAN.
Figure S12. Transcriptome profiling of hlq seedlings to (A) ‘Cellular Response Overview’ and (B) ‘Large Enzyme Families’ pathways using MAPMAN.
Figure S13. Transcriptome profiling of hlq seedlings to specific families of transcription factors using MAPMAN.
Datafile S1. Transcriptome Microarray log2FC of hlq/WT, with statistical significance for differential expression (sheet 1). Sheet 2: overview of statistically significant over-represented processes (Benjamini–Hochberg corrected) affected by hlq, based on Wilcoxon Rank Sum Test of the transcriptome data set calculated by MAPMAN. Sheet 3: list of genes in statistically over-represented bins.
Data file S2. Detailed characterization of the hlq transcriptome.
Table S1. Cell length parameters of wild-type and hlq mutant hypocotyl and roots.
Table S2 (Datafile 1, sheet 4). Analysis of the 1079 most differentially expressed genes in the hlq mutant for chromosomal adjacency and co-regulation, compared with a comparable number of ‘control’ genes with the smallest FC symmetrically distributed about FC=0.
Table S3 (Datafile 1, sheet 5). Concordance between hlq transcriptome results and previously published lists of mis-regulated genes in bin3/top6b (Yin et al., 2002) and top6a/caa39 (Simkova et al., 2012).
Table S4. List of HISTONE, WRKY, and AUX/IAA genes significantly mis-regulated in hlq mutant.
Table S5 (Datafile 1, sheet 6). Analysis of concordance between hlq misregulated genes compared with published transcriptome data sets for ABA RESPONSI VE17-(PYRABACTIN-RESISTANCE-LIKE12 homologous) overexpressing transgenic plants (Krishnaswamy et al., 2008) and ABA-regulated genes (Matsui et al., 2008).
Table S6. Primers for new markers developed during fine-mapping the hlq mutant, and other primers.
Acknowledgements
The authors thank Terry Thomas for providing the proDc3:GUS reporter lines 7-2 and 11-1, Ralph Quatrano for support, Lucy Smith for back-crossing hlq, the SEM facility at the Department of Pathology, University of Hong Kong for technical assistance with scanning electron microscopy, Thomas Altmann, Shibo Li, and Megan Sweeney for assistance with microarrays, Bruce Roe and Scott Dowd for preliminary sequencing and data analysis, respectively, Ruobai Sun for assistance with Illumina short read data sets, an anonymous reviewer for helpful suggestions on the manuscript, and the TTU High Performance Computing Center for support of the Hrothgar computer cluster. This work was supported by grant #2006-34186-16976-sub-J55-Q01097 from the USDA Southwest Consortium for Plant Genetics and Water Resources to CDR and DWG. The funders had no role in the study design, in the collection, analysis, or interpretation of data, or in the writing of the manuscript or decision to submit the manuscript for publication.
References
- Alonso-Simon A, Garcia-Angulo P, Melida H, Encina A, Alvarez JM, Acebes JL. 2011. The use of FTIR spectroscopy to monitor modifications in plant cell wall architecture caused by cellulose biosynthesis inhibitors. Plant Signaling and Behavior 6, 1104–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arioli T, Peng L, Betzner AS, et al. 1998. Molecular analysis of cellulose biosynthesis in Arabidopsis. Science 279, 717–720 [DOI] [PubMed] [Google Scholar]
- Balasubramanian R. 2003. Harlequin (hlq): an Arabidopsis mutant that ectopically expresses Dc3-GUS and shows defects in cell wall morphogenesis. PhD Thesis, Hong Kong University of Science and Technology. http://ustlib.ust.hk/ [Google Scholar]
- Barrero JM, Rodriguez PL, Quesada V, Alabadi D, Blazquez MA, Boutin JP, Marion-Poll A, Ponce MR, Micol JL. 2008. The ABA1 gene and carotenoid biosynthesis are required for late skotomorphogenic growth in Arabidopsis thaliana . Plant, Cell and Environment 31, 227–234 [DOI] [PubMed] [Google Scholar]
- Bell CJ, Ecker JR. 1994. Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics 19, 137–144 [DOI] [PubMed] [Google Scholar]
- Bichet A, Desnos T, Turner S, Grandjean O, Höfte H. 2001. BOTERO1 is required for normal orientation of cortical microtubules and anisotropic cell expansion in Arabidopsis. The Plant Journal 25, 137–148 [DOI] [PubMed] [Google Scholar]
- Boisson-Dernier A, Kessler SA, Grossniklaus U. 2011. The walls have ears: the role of plant CrRLK1Ls in sensing and transducing extracellular signals. Journal of Experimental Botany 62, 1581–1591 [DOI] [PubMed] [Google Scholar]
- Bougourd S, Marrison J, Haseloff J. 2000. An aniline blue staining procedure for confocal microscopy and 3D imaging of normal and perturbed cellular phenotypes in mature Arabidopsis embryos. The Plant Journal 24, 543–550 [DOI] [PubMed] [Google Scholar]
- Breuer C, Stacey NJ, West CE, Zhao Y, Chory J, Tsukaya H, Azumi Y, Maxwell A, Roberts K, Sugimoto-Shirasu K. 2007. BIN4, a novel component of the plant DNA topoisomerase VI complex, is required for endoreduplication in Arabidopsis. The Plant Cell 19, 3655–3668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocard-Gifford I, Lynch TJ, Garcia ME, Malhotra B, Finkelstein RR. 2004. The Arabidopsis thaliana ABSCISIC ACID-INSENSITIVE8 locus encodes a novel protein mediating abscisic acid and sugar responses essential for growth. The Plant Cell 16, 406–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brou C, Kuhn A, Staub A, Chaudhary S, Grummt I, Davidson I, Tora L. 1993. Sequence-specific transactivators counteract topoisomerase II-mediated inhibition of in vitro transcription by RNA polymerases I and II. Nucleic Acids Research 21, 4011–4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownleader MD, Hopkins J, Mobasheri A, Dey PM, Jackson P, Trevan M. 2000. Role of extensin peroxidase in tomato (Lycopersicon esculentum Mill.) seedling growth. Planta 210, 668–676 [DOI] [PubMed] [Google Scholar]
- Burk DH, Liu B, Zhong R, Morrison WH, Ye Z-H. 2001. A katanin-like protein regulates normal cell wall biosynthesis and cell elongation. The Plant Cell 13, 807–827 [PMC free article] [PubMed] [Google Scholar]
- Caño-Delgado AI, Metzlaff K, Bevan MW. 2000. The eli1 mutation reveals a link between cell expansion and secondary cell wall formation in Arabidopsis thaliana . Development 127, 3395–3405 [DOI] [PubMed] [Google Scholar]
- Chak RKF, Thomas TL, Quatrano RS, Rock CD. 2000. The genes ABI1 and ABI2 are involved in abscisic acid- and drought-inducible expression of the Daucus carota Dc3 promoter in guard cells of transgenic Arabidopsis thaliana (L.) Heynh. Planta 210, 875–883 [DOI] [PubMed] [Google Scholar]
- Chen X-Y, Kim J-Y. 2009. Callose synthesis in higher plants. Plant Signaling and Behavior 4, 489–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZZ, Hong XH, Zhang HR, Wang YQ, Li X, Zhu JK, Gong ZZ. 2005. Disruption of the cellulose synthase gene, AtCesA8/IRX1, enhances drought and osmotic stress tolerance in Arabidopsis. The Plant Journal 43, 273–283 [DOI] [PubMed] [Google Scholar]
- Cheng J-C, Lertpiriyapong K, Wang S, Sung ZR. 2000. The role of the Arabidopsis ELD1 gene in cell development and photomorphogenesis in darkness. Plant Physiology 123, 509–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AY, Wu H-M. 2011. THESEUS 1, FERONIA and relatives: a family of cell wall-sensing receptor kinases? Current Opinion in Plant Biology 14, 632–641 [DOI] [PubMed] [Google Scholar]
- Chia T, Thorneycroft D, Chapple A, Messerli G, Chen J, Zeeman SC, Smith SM, Smith AM. 2004. A cytosolic glucosyltransferase is required for conversion of starch to sucrose in Arabidopsis leaves at night. The Plant Journal 37, 853–863 [DOI] [PubMed] [Google Scholar]
- Cingolani P, Platts A, Wang LL, Coon M, Nguyen T, Wang L, Land SJ, Lu X, Ruden DM. 2012. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w 1118; iso-2; iso-3 . Fly 6, 80–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay NK, Adio AM, Denoux C, Jander G, Ausubel FM. 2009. Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 323, 95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett KD, Berger JM. 2006. Structural basis for topoisomerase VI inhibition by the anti-Hsp90 drug radicicol. Nucleic Acids Research 34, 4269–4277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corea ORA, Ki C, Cardenas CL, Kim S-J, Brewer SE, Patten AM, Davin LB, Lewis NG. 2012. Arogenate dehydratase isoenzymes profoundly and differentially modulate carbon flux into lignins. Journal of Biological Chemistry 287, 11446–11459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R, Hagenbeek D, Neill SJ, Rock CD. 1999. Flow cytometry and surface plasmon resonance analyses demonstrate that the monoclonal antibody JIM19 interacts with a rice cell surface component involved in abscisic acid signalling in protoplasts. FEBS Letters 456, 257–262 [DOI] [PubMed] [Google Scholar]
- Ding L, Zhu J-K. 1997. A role for arabinogalactan-proteins in root epidermal cell expansion. Planta 203, 289–294 [DOI] [PubMed] [Google Scholar]
- Evans-Roberts KM, Breuer C, Wall MK, Sugimoto-Shirasu K, Maxwell A. 2010. Arabidopsis thaliana GYRB3 does not encode a DNA gyrase subunit. PLoS One 5, e9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagard M, Desnos T, Desprez T, Goubet F, Refregier G, Mouille G, McCann M, Rayon C, Vernhettes S, Höfte H. 2000. PROCUSTE1 encodes a cellulose synthase required for normal cell elongation specifically in roots and dark-grown hypocotyls of Arabidopsis. The Plant Cell 12, 2409–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Gampala SSL, Rock CD. 2002. Abscisic acid signaling in seeds and seedlings. The Plant Cell 14, S15–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC. 2000. The growing plant cell wall: chemical and metabolic analysis , reprint edn. Caldwell, NJ: The Blackburn Press [Google Scholar]
- Gadjev I, Vanderauwera S, Gechev TS, Laloi C, Minkov IN, Shulaev V, Apel K, Inze D, Mittler R, Van Breusegem F. 2006. Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiology 141, 436–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith DW, Harkins KR, Knapp S. 1991. Systemic endopolyploidy in Arabidopsis thaliana . Plant Physiology 96, 985–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith DW, Janda J, Lambert GM. 2011. Multiparametric analysis, sorting, and transcriptional profiling of plant protoplasts and nuclei according to cell type. Methods in Molecular Biology 699, 407–429 [DOI] [PubMed] [Google Scholar]
- Gao M, Showalter AM. 1999. Yariv reagent treatment induces programmed cell death in Arabidopsis cell cultures and implicates arabinogalactan protein involvement. The Plant Journal 19, 321–331 [DOI] [PubMed] [Google Scholar]
- Gentleman R, Carey V, Bates D, et al. 2004. Bioconductor: open software development for computational biology and bioinformatics. Genome Biology 5, R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaring MA, Skryhan K, Kotting O, Zeeman SC, Blennow A. 2012. Comprehensive survey of redox sensitive starch metabolising enzymes in Arabidopsis thaliana . Plant Physiology and Biochemistry 58, 89–97 [DOI] [PubMed] [Google Scholar]
- Guan Y, Nothnagel EA. 2004. Binding of arabinogalactan proteins by Yariv phenylglycoside triggers wound-like responses in Arabidopsis cell cultures. Plant Physiology 135, 1346–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusmaroli G, Figueroa P, Serino G, Deng XW. 2007. Role of the MPN subunits in COP9 signalosome assembly and activity, and their regulatory interaction with Arabidopsis Cullin3-based E3 ligases. The Plant Cell 19, 564–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hématy K, Sado P-E, Van Tuinen A, Rochange S, Desnos T, Balzergue S, Pelletier S, Renou J-P, Höfte H. 2007. A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Current Biology 17, 922–931 [DOI] [PubMed] [Google Scholar]
- Hartung F, Angelis KJ, Meister A, Schubert I, Melzer M, Puchta H. 2002. An archaebacterial topoisomerase homolog not present in other eukaryotes is indispensable for cell proliferation of plants. Current Biology 12, 1787–1791 [DOI] [PubMed] [Google Scholar]
- Hartung F, Puchta H. 2001. Molecular characterization of homologues of both subunits A (SPO11) and B of the archaebacterial topoisomerase 6 in plants. Gene 271, 81–86 [DOI] [PubMed] [Google Scholar]
- Heyndrickx KS, Vandepoele K. 2012. Systematic identification of functional plant modules through the integration of complementary data sources. Plant Physiology 159, 884–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- His I, Driouich A, Nicol F, Jauneau A, Höfte H. 2001. Altered pectin composition in primary cell walls of korrigan, a dwarf mutant of Arabidopsis deficient in a membrane-bound endo-1,4-β-glucanase. Planta 212, 348–358 [DOI] [PubMed] [Google Scholar]
- Hu W, Ma H. 2006. Characterization of a novel putative zinc finger gene MIF1: involvement in multiple hormonal regulation of Arabidopsis development. The Plant Journal 45, 399–422 [DOI] [PubMed] [Google Scholar]
- Huh SU, Lee SB, Kim HH, Paek KH. 2012. ATAF2, a NAC transcription factor, binds to the promoter and regulates NIT2 gene expression involved in auxin biosynthesis. Molecules and Cells 34, 305–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M, Tyagi AK, Khurana JP. 2006. Overexpression of putative topoisomerase 6 genes from rice confers stress tolerance in transgenic Arabidopsis plants. FEBS Journal 273, 5245–5260 [DOI] [PubMed] [Google Scholar]
- Jain M, Tyagi AK, Khurana JP. 2008. Constitutive expression of a meiotic recombination protein gene homolog, OsTOP6A1, from rice confers abiotic stress tolerance in transgenic Arabidopsis plants. Plant Cell Reports 27, 767–778 [DOI] [PubMed] [Google Scholar]
- Jakoby MJ, Falkenhan D, Mader MT, Brininstool G, Wischnitzki E, Platz N, Hudson A, Hülskamp M, Larkin J, Schnittger A. 2008. Transcriptional profiling of mature Arabidopsis trichomes reveals that NOECK encodes the MIXTA-Like transcriptional regulator MYB106 . Plant Physiology 148, 1583–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jander G, Norris SR, Rounsley SD, Bush DF, Levin IM, Last RL. 2002. Arabidopsis map-based cloning in the post-genome era. Plant Physiology 129, 440–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Zhang X-F, Wang X-F, Zhang D-P. 2011. Arabidopsis 3-Ketoacyl-CoA Thiolase-2 (KAT2), an enzyme of fatty acid beta-oxidation, is involved in ABA signal transduction. Plant and Cell Physiology 52, 528–538 [DOI] [PubMed] [Google Scholar]
- Ju B-G, Lunyak VV, Perissi V, Garcia-Bassets I, Rose DW, Glass CK, Rosenfeld MG. 2006. A topoisomerase IIb-mediated dsDNA break required for regulated transcription. Science 312, 1798–1802 [DOI] [PubMed] [Google Scholar]
- Kirik V, Schrader A, Uhrig JF, Hülskamp M. 2007. MIDGET unravels functions of the Arabidopsis Topoisomerase VI complex in DNA endoreduplication, chromatin condensation, and transcriptional silencing. The Plant Cell 19, 3100–3110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler A, Schwindling S, Conrath U. 2000. Extraction and quantitative determination of callose from Arabidopsis leaves. Biotechniques 28, 1084–1086 [DOI] [PubMed] [Google Scholar]
- Kolukisaoglu U, Weinl S, Blazevic D, Batistic O, Kudla J. 2004. Calcium sensors and their interacting protein kinases: genomics of the Arabidopsis and rice CBL–CIPK signaling networks. Plant Physiology 134, 43–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komalavilas P, Zhu JK, Nothnagel EA. 1991. Arabinogalactan-proteins from the suspension-culture medium and plasma-membrane of rose cells. Journal of Biological Chemistry 266, 15956–15965 [PubMed] [Google Scholar]
- Kong D, Karve R, Willet A, Chen M-K, Oden J, Shpak ED. 2012. Regulation of plasmodesmatal permeability and stomatal patterning by the glycosyltransferase-like protein KOBITO1. Plant Physiology 159, 156–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczny A, Ausubel FM. 1993. A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. The Plant Journal 4, 403–410 [DOI] [PubMed] [Google Scholar]
- Kosak ST, Scalzo D, Alworth SV, Li F, Palmer S, Enver T, Lee JSJ, Groudine M. 2007. Coordinate gene regulation during hematopoiesis is related to genomic organization. PLoS Biology 5, e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata T, Yamamoto KT. 1998. petit1, a conditional growth mutant of Arabidopsis defective in sucrose-dependent elongation growth. Plant Physiology 118, 793–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D-K, Van Norman JM, Murphy C, Adhikari E, Reed JW, Sieburth LE. 2012. In the absence of BYPASS1-related gene function, the bps signal disrupts embryogenesis by an auxin-independent mechanism. Development 139, 805–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MM, Schiefelbein J. 1999. WEREWOLF, a MYB-related protein in Arabidopsis, is a position-dependent regulator of epidermal cell patterning. Cell 99, 473–483 [DOI] [PubMed] [Google Scholar]
- Lewis DR, Ramirez MV, Miller ND, Vallabhaneni P, Ray WK, Helm RF, Winkel BSJ, Muday GK. 2011. Auxin and ethylene induce flavonol accumulation through distinct transcriptional networks. Plant Physiology 156, 144–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Durbin R. 2010. Fast and accurate long-read alignment with Burrows–Wheeler transform. Bioinformatics 26, 589–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup. 2009. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z-Q, Yan L, Wu Z, Mei C, Lu K, Yu Y-T, Liang S, Zhang X-F, Wang X-F, Zhang D-P. 2012. Cooperation of three WRKY-domain transcription factors WRKY18, WRKY40, and WRKY60 in repressing two ABA-responsive genes ABI4 and ABI5 in Arabidopsis . Journal of Experimental Botany 63, 6371–6392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü B, Sun W, Zhang S, Zhang C, Qian J, Wang X, Gao R, Dong H. 2011. HrpNEa-induced deterrent effect on phloem feeding of the green peach aphid Myzus persicae requires AtGSL5 and AtMYB44 genes in Arabidopsis thaliana . Journal of Bioscience 36, 123–137 [DOI] [PubMed] [Google Scholar]
- Luan S. 2009. The CBL–CIPK network in plant calcium signaling. Trends in Plant Science 14, 37–42 [DOI] [PubMed] [Google Scholar]
- Luan S, Kudla J, Rodriguez-Concepcion M, Yalovsky S, Gruissem W. 2002. Calmodulins and calcineurin-like proteins: calcium sensors for specific signal response coupling in plants. The Plant Cell 14, S389–S400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowitz W, Nickle TC, Meinke DW, Last RL, Conklin PL, Somerville CR. 2001. Arabidopsis cyt1 mutants are deficient in a mannose-1-phosphate guanylyltransferase and point to a requirement of N-linked glycosylation for cellulose biosynthesis. Proceedings of the National Academy of Sciences, USA 98, 2262–2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn JE, Feil R, Hendriks JHM, Gibon Y, Morcuende R, Osuna D, Scheible W-R, Carillo P, Hajirezaei M-R, Stitt M. 2006. Sugar-induced increases in trehalose 6-phosphate are correlated with redox activation of ADPglucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana . Biochemical Journal 397, 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q-J, Mittal A, Jia F, Rock C. 2012. An autoregulatory feedback loop involving PAP1 and TAS4 in response to sugars in Arabidopsis. Plant Molecular Biology 80, 117–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarevitch I, Somers DA. 2006. Association of Arabidopsis topoisomerase IIA cleavage sites with functional genomic elements and T-DNA loci. The Plant Journal 48, 697–709 [DOI] [PubMed] [Google Scholar]
- Marks MD, Wenger JP, Gilding E, Jilk R, Dixon RA. 2009. Transcriptome analysis of Arabidopsis wild-type and gl3-sst sim trichomes identifies four additional genes required for trichome development. Molecular Plant 2, 803–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsolais F, Boyd J, Paredes Y, Schinas A-M, Garcia M, Elzein S, Varin L. 2007. Molecular and biochemical characterization of two brassinosteroid sulfotransferases from Arabidopsis, AtST4a (At2g14920) and AtST1 (At2g03760). Planta 225, 1233–1244 [DOI] [PubMed] [Google Scholar]
- McCann MC, Hammouri M, Wilson R, Belton P, Roberts K. 1992. Fourier transform infrared microspectroscopy is a new way to look at plant cell walls. Plant Physiology 100, 1940–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara S, Wang H, Hanna N, Miller WH. 2008. Topoisomerase IIb negatively modulates retinoic acid receptor a function: a novel mechanism of retinoic acid resistance. Molecular and Cellular Biology 28, 2066–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki N, Brusslan JA, Larkin R, Nagatani A, Chory J. 2001. Arabidopsis genomes uncoupled 5 (GUN5) mutant reveals the involvement of Mg-chelatase H subunit in plastid-to-nucleus signal transduction. Proceedings of the National Academy of Sciences, USA 98, 2053–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monshausen GB, Bibikova TN, Weisenseel MH, Gilroy S. 2009. Ca2+ regulates reactive oxygen species production and pH during mechanosensing in Arabidopsis roots. The Plant Cell 21, 2341–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monshausen GB, Messerli MA, Gilroy S. 2008. Imaging of the Yellow Cameleon 3.6 indicator reveals that elevations in cytosolic Ca2+ follow oscillating increases in growth in root hairs of Arabidopsis. Plant Physiology 147, 1690–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemhauser JL, Hong FX, Chory J. 2006. Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell 126, 467–475 [DOI] [PubMed] [Google Scholar]
- Osuna D, Usadel B, Morcuende R, Gibon Y, et al. 2007. Temporal responses of transcripts, enzyme activities and metabolites after adding sucrose to carbon-deprived Arabidopsis seedlings. The Plant Journal 49, 463–491 [DOI] [PubMed] [Google Scholar]
- Pagant S, Bichet A, Sugimoto K, Lerouxel O, Desprez T, McCann M, Lerouge P, Vernhettes S, Höfte H. 2002. KOBITO1 encodes a novel plasma membrane protein necessary for normal synthesis of cellulose during cell expansion in Arabidopsis. The Plant Cell 14, 2001–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette J, Tokuyasu T. 2010. EGAN: exploratory gene association networks. Bioinformatics 26, 285–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MH, Suzuki Y, Chono M, Knox JP, Yamaguchi I. 2003. CsAGP1, a gibberellin-responsive gene from cucumber hypocotyls, encodes a classical arabinogalactan protein and is involved in stem elongation. Plant Physiology 131, 1450–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautengarten C, Ebert B, Herter T, Petzold CJ, Ishii T, Mukhopadhyay A, Usadel B, Scheller HV. 2011. The interconversion of UDP-arabinopyranose and UDP-arabinofuranose is indispensable for plant development in Arabidopsis. The Plant Cell 23, 1373–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock CD, Sakata Y, Quatrano RS. 2010. Stress signaling I: the role of abscisic acid. In: Pareek A, Sopory SK, Bohnert HJ, Govindjee, eds. Abiotic stress adaptation in plants: physiological, molecular and genomic foundation. Dordrecht, The Netherlands: Springer, 33–73 [Google Scholar]
- Rock CD, Sun X. 2005. Crosstalk between ABA and auxin signaling pathways in roots of Arabidopsis thaliana (L.) Heynh. Planta 222, 98–106 [DOI] [PubMed] [Google Scholar]
- Schiefelbein JW, Somerville C. 1990. Genetic control of root hair development in Arabidopsis thaliana. The Plant Cell 2, 235–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger K, Ossowski S, Ott F, et al. 2011. Reference-guided assembly of four diverse Arabidopsis thaliana genomes. Proceedings of the National Academy of Sciences, USA 108, 10249–10254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K, Mathur J, Boudonck K, Wells B, Dolan L, Roberts K. 1998. The ROOT HAIRLESS 1 gene encodes a nuclear protein required for root hair initiation in Arabidopsis. Genes and Development 12, 2013–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader A, Welter B, Hülskamp M, Hoecker U, Uhrig JF. 2013. MIDGET connects COP1-dependent development with endoreduplication in Arabidopsis thaliana . The Plant Journal 75, 67–79 [DOI] [PubMed] [Google Scholar]
- Selvendran RR, O’Neill MA. 1987. Isolation and analysis of cell walls from plant material. Methods in Biochemical Analysis 32, 25–153 [DOI] [PubMed] [Google Scholar]
- Shang Y, Yan L, Liu Z-Q, et al. 2010. The Mg-chelatase H subunit of Arabidopsis antagonizes a group of WRKY transcription repressors to relieve ABA-responsive genes of inhibition. The Plant Cell 22, 1909–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y-Y, Wang X-F, Wu F-Q, et al. 2006. The Mg-chelatase H subunit is an abscisic acid receptor. Nature 443, 823–826 [DOI] [PubMed] [Google Scholar]
- Shevell DE, Leu W-M, Gillmor CS, Xia G, Feldmann KA, Chua N-H. 1994. EMB30 is essential for normal cell division, cell expansion, and cell adhesion in Arabidopsis and encodes a protein that has similarity to Sec7. Cell 77, 1051–1062 [DOI] [PubMed] [Google Scholar]
- Simkova K, Moreau F, Pawlak P, Vriet C, Baruah A, Alexandre C, Hennig L, Apel K, Laloi C. 2012. Integration of stress-related and reactive oxygen species-mediated signals by Topoisomerase VI in Arabidopsis thaliana . Proceedings of the National Academy of Sciences, USA 109, 16360–16365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalle J, Vierstra RD. 2004. The ubiquitin 26S proteasome proteolytic pathway. Annual Review of Plant Biology 55, 555–590 [DOI] [PubMed] [Google Scholar]
- Smyth GK, Michaud J, Scott HS. 2005. Use of within-array replicate spots for assessing differential expression in microarray experiments. Bioinformatics 21, 2067–2075 [DOI] [PubMed] [Google Scholar]
- Stettler M, Eicke S, Mettler T, Messerli G, Hortensteiner S, Zeeman SC. 2009. Blocking the metabolism of starch breakdown products in Arabidopsis leaves triggers chloroplast degradation. Molecular Plant 2, 1233–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb S, Zeeman SC. 2012. Starch metabolism in Arabidopsis. The Arabidopsis Book. 10, e0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, Rajagopal B, Rock CD. 2002. Harlequin (hlq) and short blue root (sbr), two Arabidopsis mutants that ectopically express an abscisic acid- and auxin-inducible transgenic carrot promoter and have pleiotropic effects on morphogenesis. Plant Molecular Biology 49, 93–105 [DOI] [PubMed] [Google Scholar]
- Sugimoto-Shirasu K, Roberts GR, Stacey NJ, McCann MC, Maxwell A, Roberts K. 2005. RHL1 is an essential component of the plant DNA topoisomerase VI complex and is required for ploidy-dependent cell growth. Proceedings of the National Academy of Sciences, USA 102, 18736–18741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto-Shirasu K, Stacey NJ, Corsar J, Roberts K, McCann MC. 2002. DNA topoisomerase VI is essential for endoreduplication in Arabidopsis. Current Biology 12, 1782–1786 [DOI] [PubMed] [Google Scholar]
- Sun X. 2003. Characterization of an auxin- and abscisic acid-inducible reporter gene: Dc3-GUS in reported auxin mutants, and mutant screening based on auxin responsive Dc3-GUS expression. MSc thesis. Hong Kong University of Science and Technology. http://ustlib.ust.hk/ [Google Scholar]
- Susek RE, Ausubel FM, Chory J. 1993. Signal transduction mutants of Arabidopsis uncouple nuclear CAB and RBCS gene expression from chloroplast development. Cell 74, 787–799 [DOI] [PubMed] [Google Scholar]
- Szklarczyk D, Franceschini A, Kuhn M, et al. 2011. The STRING database in 2011: functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Research 39, D561–D568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiessen A, Prescha K, Branscheid A, Palacios N, McKibbin R, Halford NG, Geigenberger P. 2003. Evidence that SNF1-related kinase and hexokinase are involved in separate sugar-signalling pathways modulating post-translational redox activation of ADP-glucose pyrophosphorylase in potato tubers. The Plant Journal 35, 490–500 [DOI] [PubMed] [Google Scholar]
- Ton J, Jakab G, Toquin V, Flors V, Iavicoli A, Maeder MN, Metraux JP, Mauch-Mani B. 2005. Dissecting the beta-aminobutyric acid-induced priming phenomenon in Arabidopsis. The Plant Cell 17, 987–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton J, Mauch-Mani B. 2004. beta-amino-butyric acid-induced resistance against necrotrophic pathogens is based on ABA-dependent priming for callose. The Plant Journal 38, 119–130 [DOI] [PubMed] [Google Scholar]
- Tripathi V, Parasuraman B, Laxmi A, Chattopadhyay D. 2009. CIPK6, a CBL-interacting protein kinase is required for development and salt tolerance in plants. The Plant Journal 58, 778–790 [DOI] [PubMed] [Google Scholar]
- Tsuzuki T, Takahashi K, Inoue S-I, Okigaki Y, Tomiyama M, Hossain M, Shimazaki K-I, Murata Y, Kinoshita T. 2011. Mg-chelatase H subunit affects ABA signaling in stomatal guard cells, but is not an ABA receptor in Arabidopsis thaliana . Journal of Plant Research 124, 527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzin V, Galili G. 2010. New insights into the shikimate and aromatic amino acids biosynthesis pathways in plants. Molecular Plant 3, 956–972 [DOI] [PubMed] [Google Scholar]
- Usadel B, Nagel A, Steinhauser D, et al. 2006. PageMan: an interactive ontology tool to generate, display, and annotate overview graphs for profiling experiments. BMC Bioinformatics 7, 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usadel B, Nagel A, Thimm O, et al. 2005. Extension of the visualization tool MapMan to allow statistical analysis of arrays, display of corresponding genes, and comparison with known responses. Plant Physiology 138, 1195–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga-Weisz PD, Wilm M, Bonte E, Dumas K, Mann M, Becker PB. 1997. Chromatin-remodelling factor CHRAC contains the ATPases ISWI and topoisomerase II. Nature 388, 598–602 [DOI] [PubMed] [Google Scholar]
- Vieira P, Kyndt T, Gheysen G, Engler JD. 2013. An insight into critical endocycle genes for plant-parasitic nematode feeding sites establishment. Plant Signaling and Behavior 8, e24223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos SM, Tretter EM, Schmidt BH, Berger JM. 2011. All tangled up: how cells direct, manage and exploit topoisomerase function. Nature Reviews Molecular Cell Biology 12, 827–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z-Y, Xiong L, Li W, Zhu J-K, Zhu J. 2011. The plant cuticle is required for osmotic stress regulation of abscisic acid biosynthesis and osmotic stress tolerance in Arabidopsis. The Plant Cell 23, 1971–1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willats WGT, Knox JP. 1996. A role for arabinogalactan-proteins in plant cell expansion: evidence from studies on the interaction of β-glucosyl Yariv reagent with seedlings of Arabidopsis thaliana . The Plant Journal 9, 919–925 [DOI] [PubMed] [Google Scholar]
- Wu F-Q, Xin Q, Cao Z, et al. 2009. The magnesium-chelatase H subunit binds abscisic acid and functions in abscisic acid signaling: new evidence in Arabidopsis. Plant Physiology 150, 1940–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S, Lam E. 1994. Abundance of nuclear DNA topoisomerase II is correlated with proliferation in Arabidopsis thaliana . Nucleic Acids Research 22, 5729–5736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Z, Chen J. 2012. A high throughput DNA extraction method with high yield and quality. Plant Methods 8, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y-H, Liu R, Yan L, Liu Z-Q, Jiang S-C, Shen Y-Y, Wang X-F, Zhang D-P. 2012. Light-harvesting chlorophyll a/b-binding proteins are required for stomatal response to abscisic acid in Arabidopsis . Journal of Experimental Botany 63, 1095–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Cheong H, Friedrichsen D, Zhao Y, Hu J, Mora-Garcia S, Chory J. 2002. A crucial role for the putative Arabidopsis topoisomerase VI in plant growth and development. Proceedings of the National Academy of Sciences, USA 99, 10191–10196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- York WS, Darvill AG, McNeil M, Stevenson TT, Albersheim P. 1986. Isolation and characterization of plant cell walls and cell wall components. Methods in Enzymology 118, 3–40 [Google Scholar]
- Zhong R, Ripperger A, Ye Z-H. 2000. Ectopic deposition of lignin in the pith of stems of two Arabidopsis mutants. Plant Physiology 123, 59–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.