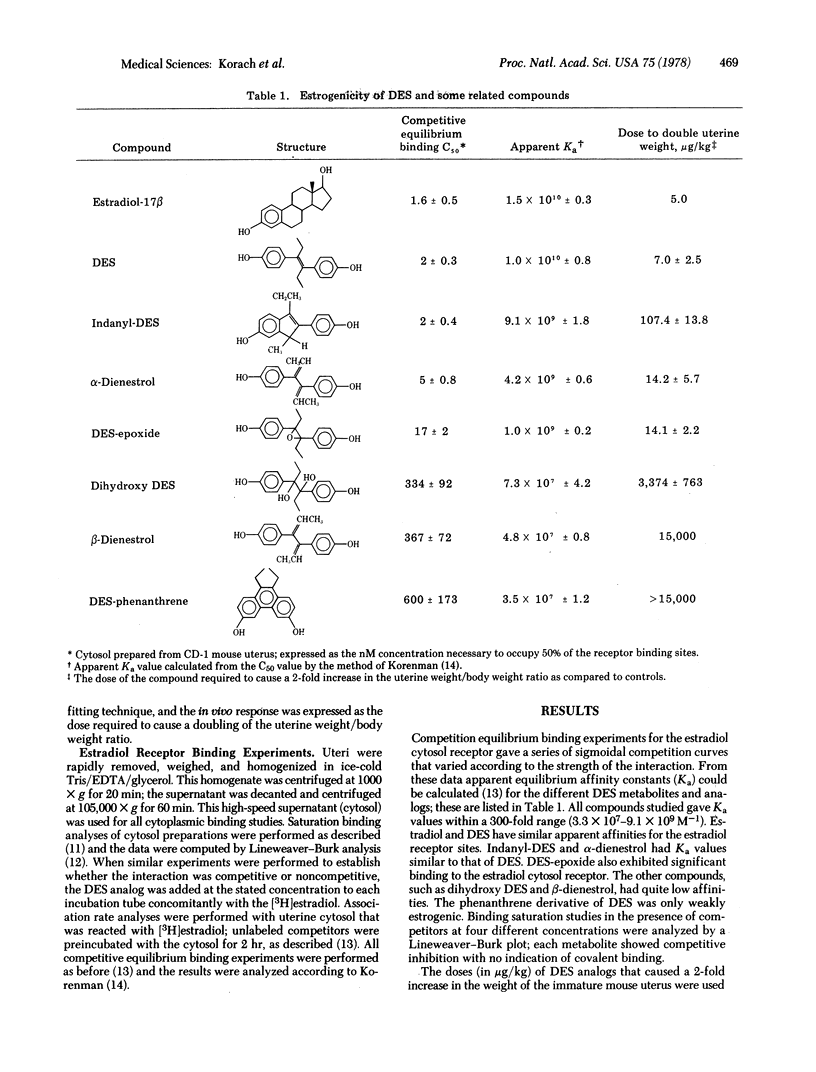
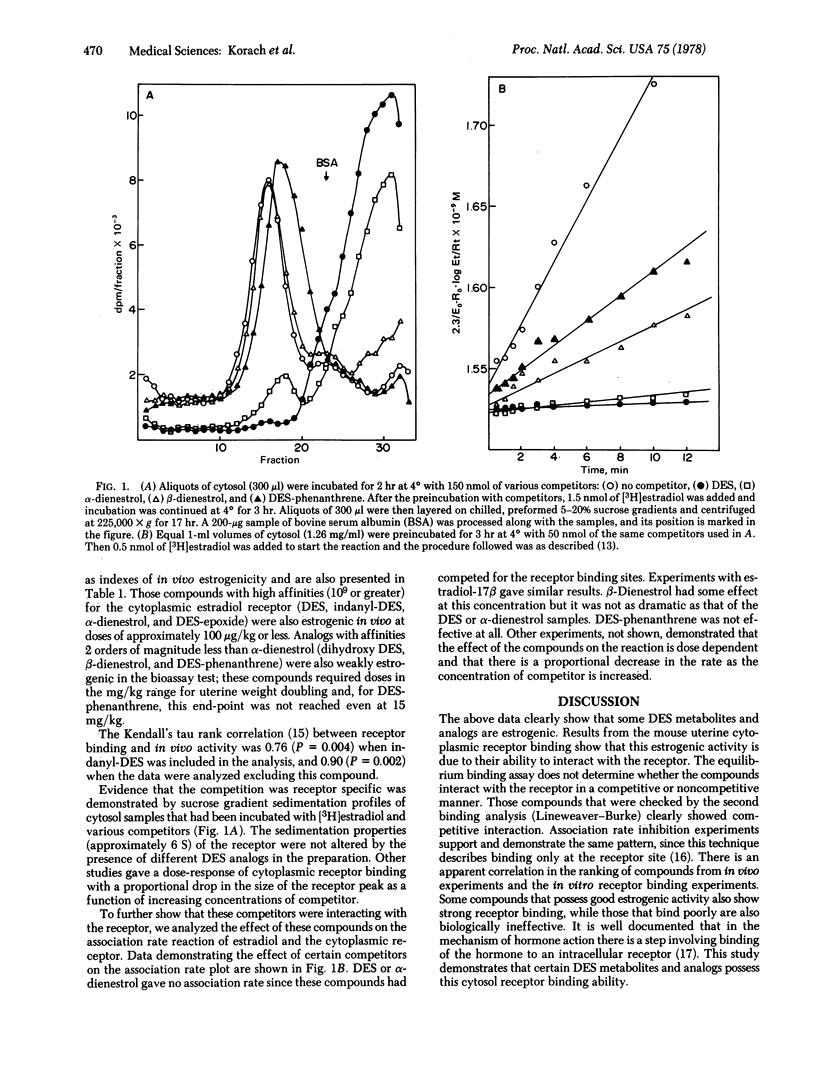
Abstract
The diethylstilbestrol (DES) metabolite (β-dienestrol), which had been identified in mouse, rat, monkey, and human urine, and two proposed metabolic intermediates (diethylstilbestrol α,α′-epoxide and α,α′-dihydroxy DES) were synthesized and their estrogenic activities determined. In addition, three DES analogs, α-dienestrol, DES-dihydroxy diethyl phenanthrene (DES-phenanthrene), and 1-(α-ethyl, 4α-hydroxyphenyl)indanyl-5-ol (indanyl-DES), were studied. Estrogenic activities of the compounds in vivo were determined by the immature mouse uterine weight bioassay; in vitro, their estradiol receptor binding activity (competitive equilibrium binding, sucrose gradient analysis, and association rate inhibition assays) was determined. Results of the mouse uterine weight bioassay gave the following order of estrogenicity: DES > α-dienestrol ≥ DES-epoxide > indanyl-DES > dihydroxy DES > β-dienestrol > DES-phenanthrene. Results of competitive equilibrium binding analyses of these compounds with estradiol-17β for the mouse uterine cytosol receptor followed the same order seen for the bioassay, except for indanyl-DES. DES, indanyl-DES, and α-dienestrol had the greatest affinities (Ka values approximately 0.5-19.1 × 1010 M-1), while DES-phenanthrene had the lowest (Ka = 3.5 × 107 M-1 ± 1.2). Sucrose gradient analysis of the above competition preparations illustrated the displacement of [3H]estradiol from the receptor peak. This displacement was receptor specific and concentration dependent and correlated with the equilibrium binding concentrations. In addition, the most hormonally active substances demonstrated the greatest rate inhibition in the estradiol cytosol receptor association rate reaction (V0). The rank order of estrogenicity of the compounds determined in this study should be useful in evaluating alternative metabolic pathways of DES as well as distinguishing biologically active metabolites from relatively inactive ones.
Keywords: diethylstilbestrol metabolism, estrogen receptor, mouse uterus, hormonal toxicity, structure—activity
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DeSombre E. R., Mohla S., Jensen E. V. Receptor transformation, the key to estrogen action. J Steroid Biochem. 1975 Mar-Apr;6(3-4):469–473. doi: 10.1016/0022-4731(75)90174-0. [DOI] [PubMed] [Google Scholar]
- Doyle T. D., Benson W. R., Filipescu N. Photocyclization of diethylstilbestrol. Isolation of a stable, self-trapping dihydrophenanthrene intermediate. J Am Chem Soc. 1976 May 26;98(11):3262–3267. doi: 10.1021/ja00427a035. [DOI] [PubMed] [Google Scholar]
- Engel L. L., Weidenfield J., Merriam G. R. Metabolism of diethylstilbestrol by rat liver: a preliminary report. J Toxicol Environ Health Suppl. 1976;1:37–44. [PubMed] [Google Scholar]
- Gunning J. E. The DES story. Obstet Gynecol Surv. 1976 Nov;31(11):827–833. doi: 10.1097/00006254-197611000-00026. [DOI] [PubMed] [Google Scholar]
- Herbst A. L., Ulfelder H., Poskanzer D. C. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med. 1971 Apr 15;284(15):878–881. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- Jensen E. V., DeSombre E. R. Mechanism of action of the female sex hormones. Annu Rev Biochem. 1972;41:203–230. doi: 10.1146/annurev.bi.41.070172.001223. [DOI] [PubMed] [Google Scholar]
- Korach K. S., Muldoon T. G. Characterization of the interaction between 17 beta-estradiol and its cytoplasmic receptor in the rat anterior pituitary gland. Biochemistry. 1974 Apr 23;13(9):1932–1938. doi: 10.1021/bi00706a024. [DOI] [PubMed] [Google Scholar]
- Korach K. S., Muldoon T. G. Inhibition of anterior pituitary estrogen-receptor complex formation by low-affinity interaction with 5 alpha-dihydrotestosterone. Endocrinology. 1975 Jul;97(1):231–236. doi: 10.1210/endo-97-1-231. [DOI] [PubMed] [Google Scholar]
- Korach K. S., Muldoon T. G. Studies on the nature of the hypothalamic estradiol-concentrating mechanism in the male and female rat. Endocrinology. 1974 Mar;94(3):785–793. doi: 10.1210/endo-94-3-785. [DOI] [PubMed] [Google Scholar]
- McLachlan J. A. Prenatal exposure to diethylstilbestrol in mice: toxicological studies. J Toxicol Environ Health. 1977 Jan;2(3):527–537. doi: 10.1080/15287397709529453. [DOI] [PubMed] [Google Scholar]
- Metzler M. Metabolic activation of carcinogenic diethylstilbestrol in rodents and humans. J Toxicol Environ Health Suppl. 1976;1:21–35. [PubMed] [Google Scholar]
- Shah H. C., McLachlan J. A. The fate of diethylstilbestrol in the pregnant mouse. J Pharmacol Exp Ther. 1976 Jun;197(3):687–696. [PubMed] [Google Scholar]


