Abstract
Nitric oxide (NO) plays an important role in phase-shifting of circadian neuronal activities in the suprachiasmatic nucleus and circadian behavior activity rhythms. In the retina, NO production is increased in a light-dependent manner. While endogenous circadian oscillators in retinal photoreceptors regulate their physiological states, it is not clear whether NO also participates in the circadian regulation of photoreceptors. In the present study, we demonstrate that NO is involved in the circadian phase-dependent regulation of L-type voltage-gated calcium channels (L-VGCCs). In chick cone photoreceptors, the L-VGCCα1 subunit expression and the maximal L-VGCC currents are higher at night, and both Ras-MAPK (mitogen-activated protein kinase)-Erk (extracellular-signal-regulated kinase) and Ras-phosphatidylinositol 3 kinase (PI3K)-protein kinase B (Akt) are part of the circadian output pathways regulating L-VGCCs. The NO-cGMP-protein kinase G (PKG) pathway decreases L-VGCCα1 subunit expression and L-VGCC currents at night, but not during the day, and exogenous NO donor or cGMP decreases the phosphorylation of Erk and Akt at night. The protein expression of neural NO synthase (nNOS) is also under circadian control, with both nNOS and NO production being higher during the day. Taken together, NO/cGMP/PKG signaling is involved as part of the circadian output pathway to regulate L-VGCCs in cone photoreceptors.
Keywords: retina, photoreceptor, circadian, ion channel, signaling, cone
Introduction
Nitric oxide (NO) produced by NO synthase (NOS) functions as an intra- or intercellular messenger in numerous tissues (Bredt 2003). There are three major types of NOS, namely neuronal NOS (nNOS or NOS1), inducible NOS (iNOS or NOS2), and endothelial NOS (eNOS or NOS3)(Alderton et al. 2001). After activation, NOS catalyzes the transformation of arginine to NO that further activates guanylyl cyclase (GC) and leads to cGMP production and activation of protein kinase G (PKG)(Michel & Vanhoutte 2010). In the retina, NO release is increased upon light stimulation and decreased in the dark-adapted state (Sato et al. 2010), and NO is known to affect several types of ion channels, including L-type voltage-gated calcium channels (L-VGCCs) in retinal neurons (Barnes & Jacklet 1997, Barnes & Kelly 2002, Kourennyi et al. 2004). Calcium (Ca2+) influx through the L-VGCCs plays an important role in activation and regulation of different physiological processes, such as secretion, contraction, neurotransmission, and gene expression (Catterall et al. 2005). In the retina, the L-VGCCs are essential for neurotransmitter release from photoreceptors and other retinal neurons (Barnes & Kelly 2002). Neuronal NOS (nNOS) is present in photoreceptors (Cao & Eldred 2001, Crousillac et al. 2003, Neufeld et al. 2000, Shin et al. 2000), and application of NO donors suppresses the L-VGCC currents in cones but enhances Ca2+-currents in rods (Kourennyi et al. 2004). Therefore, NO is crucial for light adaptation of retinal photoreceptors.
Retinal photoreceptors not only respond to acute light or dark signals, they are also capable of initiating more sustained adaptive changes throughout the day, since the visual system must anticipate daily changes in ambient illumination over 10-12 orders of magnitude (Cahill & Besharse 1995, Green & Besharse 2004). The circadian oscillators endogenous to photoreceptors provide a mechanism for such adaptation across 24 hours (Ko et al. 2009a, Ko et al. 2010, Liu et al. 2012), and they are known to regulate retinomotor movement (Burnside 2001, Pierce & Besharse 1985), outer segment disc shedding and membrane renewal (Besharse & Dunis 1983, LaVail 1980), morphological changes at synaptic ribbons (Adly et al. 1999), gene expression (Haque et al. 2002, Korenbrot & Fernald 1989, Pierce et al. 1993), and ion channel activities (Ko et al. 2004a, Ko et al. 2001, Ko et al. 2009b, Ko et al. 2007) among other photoreceptor physiology. Nitric oxide-dependent signaling also participates in circadian rhythms (Ding et al. 1994, Ferreyra & Golombek 2001, Golombek et al. 2004, Melo et al. 1997, Watanabe et al. 1995). In the suprachiasmatic nuclei (SCN), the central circadian clock in mammals, exogenous NO donors produce light-like phase shifts of circadian rhythms of neuronal firing rate in vitro (Ding et al. 1994). Intracerebroventricular injection of a NOS inhibitor blocks the light-induced phase shifts of the circadian behavior rhythm in hamsters (Weber et al. 1995a), while treatment with a NOS inhibitor in cultures phase-shift the circadian rhythms of glucose metabolism (Menger et al. 2007).
The L-VGCCs in both retinal photoreceptors and bipolar neurons are under circadian control (Hull et al. 2006, Ko et al. 2009b, Ko et al. 2007). The mRNA and protein expressions of the L-VGCC pore-forming α1 subunit are rhythmic, which leads to larger L-VGCC currents at night (Ko et al. 2009b, Ko et al. 2007). Both MAPK-Erk and PI3K-protein kinase B (Akt) signaling pathways participate in the circadian rhythms of L-VGCCs mainly through regulating the trafficking of L-VGCCα1 subunits (Ko et al. 2009b, Ko et al. 2007). The phosphorylation states of Erk and Akt are also under circadian control. Because inhibition of Erk activity does not affect the circadian rhythm of phosphorylated Akt (pAkt), and vice versa, these pathways work in parallel to regulate the circadian rhythm of L-VGCCs (Ko et al. 2009b, Ko et al. 2007). Since NO modulates L-VGCCs in retinal photoreceptors (Kourennyi et al. 2004), and in the mammalian retina, NOS activity oscillates over the course of a day (Zhang et al. 2005, Llomovatte et al. 1997), we thereby investigated whether NO elicited a circadian phase-dependent modulation of L-VGCCs in avian cone photoreceptors. Using voltage-clamp recordings of L-VGCC currents, Western blots, and pharmacological tools, we further examined how various signaling pathways including NO-cGMP-PKG, MAPK-Erk, and PI3K-Akt were involved in the circadian phase-dependent effect of NO on L-VGCCs in the chick retina.
Materials and methods
Cell cultures and circadian entrainment
Fertilized eggs (Gallus gallus) were obtained from the Poultry Science Department, Texas A&M University (College Station, TX, USA). Chick retinas were dissociated at embryonic day 12 (E12) and cultured for 6 days for photoreceptor enriched cultures in a medium containing Eagle's Minimum Essential Medium (EMEM, Biowhittaker/Cambrex, East Rutherford, NJ, USA), 10% heat-inactivated horse serum (Biowhittaker/Cambrex), 20 ng/mL ciliary neurotrophic factor (CNTF; R&D Systems, Minneapolis, MN, USA), 2 mM glutamine (Life Technologies, Carlsbad, CA, USA), 10 μM all-trans retinol (Sigma-Aldrich, St. Louis, MO, USA), 50 U/ml penicillin, and 50 μg/ml streptomycin (Sigma-Aldrich) on poly-D-lysine (Sigma-Aldrich) coated coverslips or culture dishes as described previously (Ko et al. 2004b, Ko et al. 2009b, Ko et al. 2007). Сell culture incubators (maintained at 39°C and 5% CO2) were equipped with lights and timers, which allowed for the entrainment of retinal circadian oscillators to 12 : 12 h light-dark (LD) cycles in vitro. Zeitgeber time (ZT) 0 was designated as when the lights come on, and ZT 12 was the time when the lights turn off. Circadian time (CT) refers to when cultures were kept under constant darkness (DD) after circadian entrainment. Thus, CT 0 - 12 was the “subjective day”, and CT 12 - 24 was the “subjective night”. Electrophysiological experiments were carried out on the sixth day of LD entrainment without exchange of media. Cells were treated with either pharmacological chemicals or vehicle for 2 hr in culture prior to electrophysiological recordings. Some embryos from E10 or E11 were entrained in LD cycles in ovo for 7 days, kept in DD for 2 days, then retinas were harvested at various circadian time points throughout the course of a day for biochemical assays. For some experiments, on the last day of LD entrainment, retinas were dissected, dissociated, cultured on poly-D-lysine coated cultured dishes, and maintained in DD. On the second day of DD, cultures were treated with either pharmacological chemicals or vehicle for 2 hr prior to harvest at CT 4 and CT 16 for biochemical assays. The NO donor S-nitroso-N-acetyl-penicillamine (SNAP; 500 μM) and NOS inhibitor NG-nitro-L-arginine methyl ester (L-NAME, 100 μM) were obtained from Enzo Life Sciences (Farmingdale, NY, USA). The NO donor sodium nitroprusside (SNP, 100 μM) was obtained from T. J. Baker (Radnor, PA, USA). 8-Br-cGMP (cGMP; 30 μM), the guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one (ODQ; 2 μM), and DMSO (vehicle; 0.1%) were obtained from Sigma. The protein kinase G (PKG) inhibitor KT5823 (1 μM) was obtained from AG Scientific (San Diego, CA, USA).
Western Immunoblotting analysis
The procedure has been described in detail previously (Ko et al. 2001, Ko et al. 2009b, Ko et al. 2007). Intact retinas were used for time point analysis (Figure 6) while dissociated cone cultures were used for drug treatment experiments (Figures 3-5). Samples were homogenized in RIPA buffer, denatured at 95°C with 2x Laemmli buffer, separated on 10% SDS-PAGE gels, and transferred to nitrocellulose membranes. The primary antibodies used in the studies were a polyclonal antibody insensitive to the phosphorylation state of Erk (total Erk, served as the loading control, Santa Cruz Biochemicals, Santa Cruz, CA, USA), a monoclonal antibody sensitive to the phosphorylation state of Erk (Sigma), a monoclonal antibody sensitive to the phosphorylation state of Akt (Cell Signaling, Danvers, MA, USA), a monoclonal antibody against nNOS (Sigma-Aldrich), a monoclonal antibody against eNOS (Cell Signaling, Danvers, MA, USA), and a polyclonal antibody for L VGCCα1D (Alomone Labs, Jerusalem, Israel). Blots were visualized using appropriate HRP-conjugated secondary antibodies (Cell Signaling) and an ECL detection system (Pierce, Rockford, IL, USA). The ratio of target protein to total Erk for each sample was determined by densitometry using Scion Image (NIH, Bethesda, MD, USA).
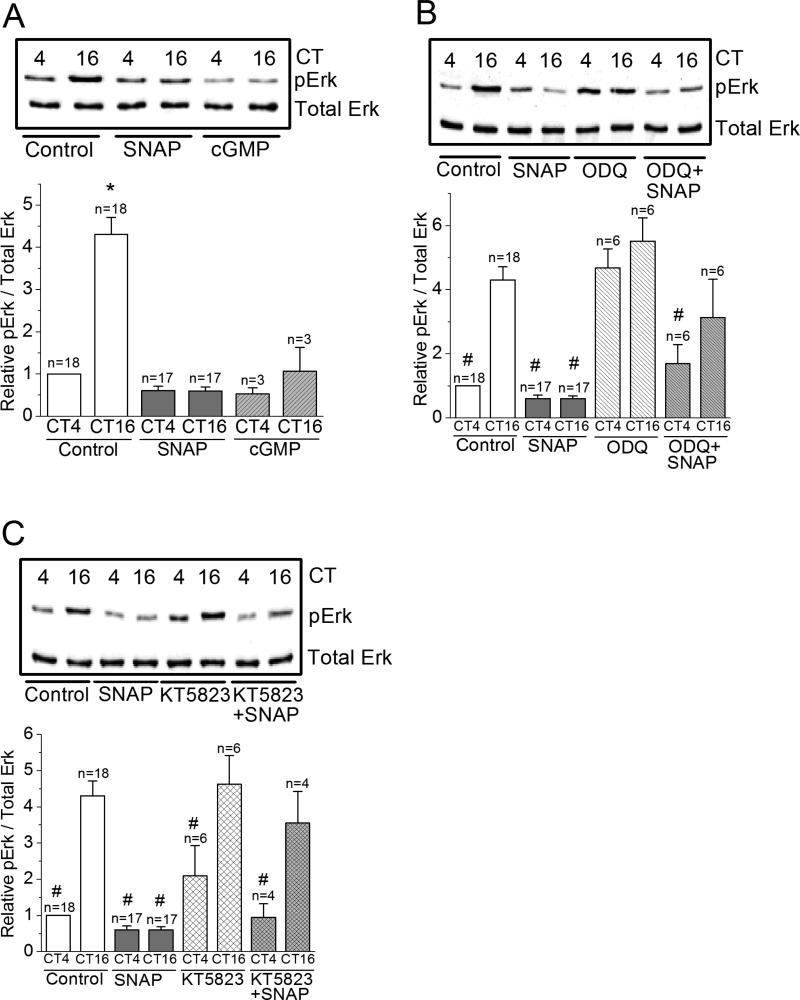
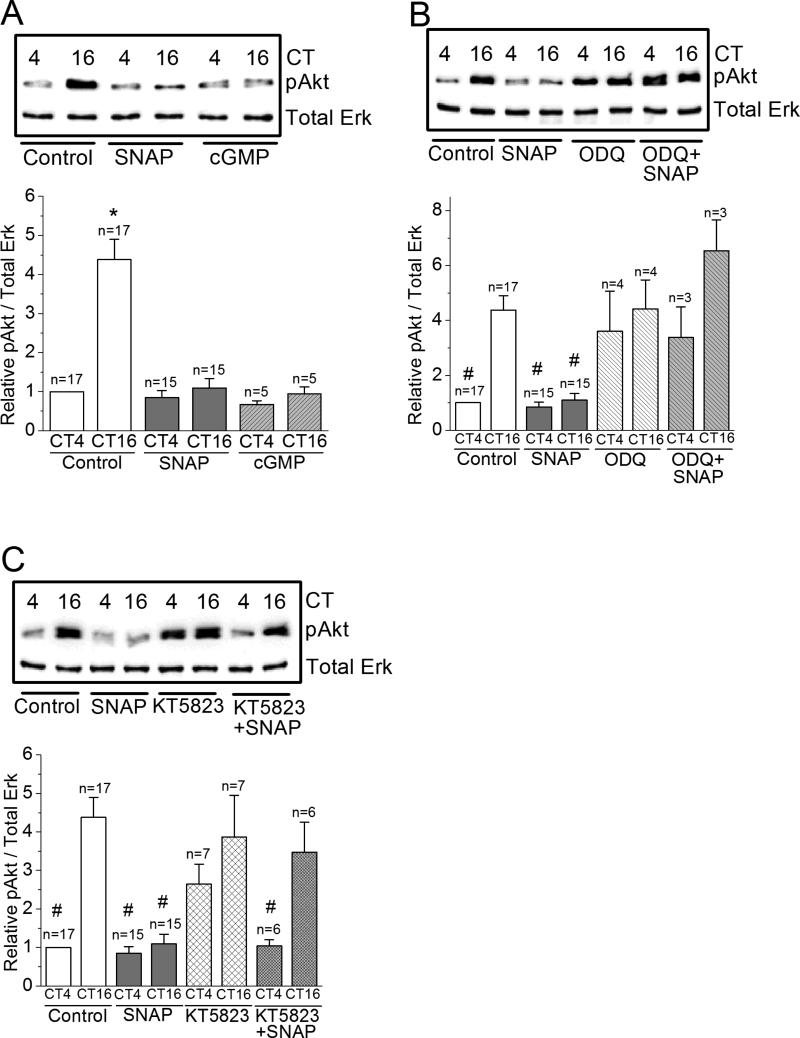
Figure 6. NO-cGMP-PKG signaling is upstream of Erk.
(A) The level of phosphorylated Erk (pErk) is significantly higher at night (CT 16) than during the day (CT 4). Treatment with SNAP or 8-Br-cGMP (cGMP) significantly decreases the level of pErk at night (CT 16). * indicates that the pErk level of controls at night (CT 16) is significantly higher than all other groups. (B) Treatment with ODQ alone increases pErk levels at CT 4. Treatment with ODQ reverses the effect of SNAP on pErk. # indicates that these groups are significantly different from the control at CT 16. There is no difference among control at CT 16, ODQ treated groups at CT 4 and CT 16, and ODQ+SNAP group at CT 16. (C) Treatment with KT5823 shows similar results in pErk as ODQ. # indicates that these groups are significantly different from the control at CT 16. *, # p<0.05.
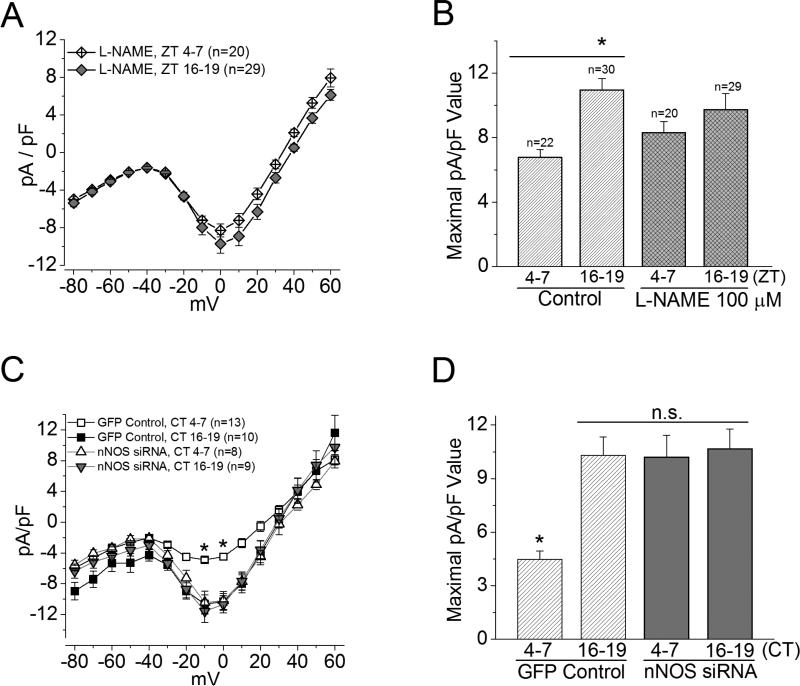
Figure 3. Inhibition of endogenous NOS affects L-VGCCs in cone photoreceptors.
(A) and (B) Treatment with a NOS inhibitor, L-NAME (100 μM), for 2 hr prior to recordings only slightly changed L-VGCC current densities at both day (ZT 4-7) and night (ZT 16-19) times. (A) The average current density-voltage relationship of L-NAME treated photoreceptors is shown. (B) Both L-NAME treated groups (ZT 4-7 and ZT 16-19) are not significantly different from respective control groups. * indicates that the current densities of control cells recorded at ZT 16-19 are significantly different from the control recorded at ZT 4-7 in maximal current density values elicited at 0 mV. *p<0.05. (C) and (D) L-VGCCs were recorded from photoreceptors transfected with GFP (control) or co-transfected with GFP and Nos1 siRNA (nNOS siRNA). (C) The average current density-voltage relationships of the GFP control and the nNOS siRNA are shown. (D) The maximal current density from the GFP control recorded at ZT 4-7 is significantly lower than the other three groups. There is no significant difference (n.s.) among the GFP control recorded at ZT 16-19, and the nNOS siRNA recorded at either ZT 4-7 or ZT 16-19. *p<0.05.
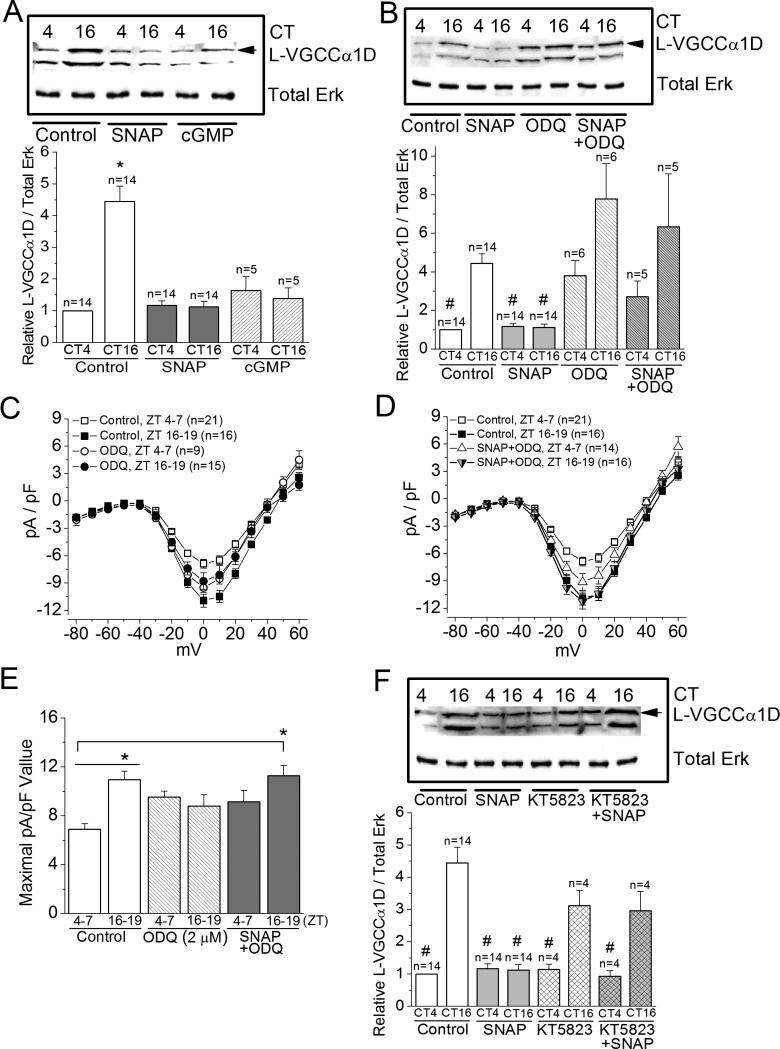
Figure 5. NO decreases the protein expression of L-VGCCα1D subunits at night.
(A), (B), and (F) Cultured retinal cells were treated with various chemicals for 2 hr prior to being processed for Western blotting at CT4 or CT 16 and analyzed for L-VGCCα1D subunit. (C), (D), and (E) Cultured photoreceptors were treated under various conditions for 2 hr prior to patch-clamp recordings at ZT 4-7 and ZT 16-19 for L-VGCC currents. (A) Treatment with 8-Br-cGMP (cGMP) or SNAP at night (CT 16) dampens the protein expression of L-VGCCα1D compared to the control (treated with 0.1% DMSO), while both 8-Br-cGMP (cGMP) and SNAP have no effect on the L-VGCCα1D expression during the day (CT 4). * indicates that the protein level of LVGCCα1D of the control group harvested at CT 16 is significantly higher than the controls harvested at CT4, as well as cells treated with SNAP or 8-Br-cGMP (cGMP). (B) Treatment with ODQ (SNAP+ODQ) reverses the effect of SNAP on the protein levels of L-VGCCα1D. # indicates that the protein levels of the control harvested at CT4 and both SNAP treated groups (CT4 and CT16) are significantly lower than the level of the control harvested at CT16. There is no statistical difference among the control harvested at CT 16, treatments with ODQ alone (both CT 4 and CT 16), and ODQ with SNAP (SNAP+ODQ; both CT 4 and CT 16). (C) and (D) The average current density (pA/pF)-voltage (mV) relationship of cells recorded during the day (ZT 4-7) and at night (ZT 16-19) from controls, cells treated with ODQ, or ODQ+SNAP are shown. (E) Treatment with ODQ reverses the effect of SNAP on LVGCC currents. * indicates that the L-VGCC maximal current density of control cells recorded at ZT 16-19 is significantly higher than control cells recorded at ZT 4-7; the maximal L-VGCC current density of cells treated with SNAP+ODQ and recorded at ZT 16-19 is significantly higher than the control cells recorded at ZT 4-7. (F) Treatment with KT5823 (KT823+SNAP) reverses the SNAP effect on L-VGCCα1D protein levels, while treatment with KT5823 alone at CT 4 or CT 16 does not affect the protein levels of L-VGCCα1D compared to the controls. # indicates that the protein levels of L-VGCCα1D in these groups are significantly lower than the controls harvested at CT 16. *, # p<0.05.
Nitrate/nitrite colorimetric assay
Nitric oxide is quickly oxidized to nitrate or nitrite in biological systems making its direct measurement problematic. However, commercially available kits allow for the quantification of nitrate / nitrite as an indicator of NO. We chose to use a colorimetric kit from Cayman Chemical (Ann Arbor, MI, USA). The procedure was provided by the manufacturer. Whole retinas were collected at six time points (CT 0, 4, 8, 12, 16, and 20) and homogenized in RIPA buffer. Nitrates were converted to nitrite, and Griess reagents convert total nitrites into a purple azo compound measured at 540 nm. Results were analyzed against a nitrate standard curve and normalized to total protein as determined by the Bradford assay (Bio-Rad, Hercules, CA, USA).
cGMP enzyme immunoassay
A commercially available kit (Arbor Assays, Ann Arbor, MI, USA) was used to detect cGMP levels in intact and cultured retinas. The procedure was provided by the manufacturer. Whole retinas were collected at six time points as above and homogenized in the sample diluent provided by the manufacturer. Cultured cells were collected at CT4 and CT16. Results were measured at 450 nm, analyzed against a cGMP standard curve, and normalized to total protein as determined by the Bradford assay.
siRNA transfection
Chick cone photoreceptors were cultured at E12 on coated coverslips and entrained under LD cycles. On the 5th day, cells were transfected with siRNAs specifically targeting nNOS and transferred to DD. Electrophysiological recordings were performed at CT 4-7 or 16-19 on the second day of DD. The sequence of the chicken nNOS gene (Nos1) is not known. However, analysis between mouse Nos1 (Gene ID: 18125) and the predicted chicken Nos1 (gene ID: 427721) shows 79% homology. The siRNA pool (ON-TARGETplus Mouse Nos1 siRNA SMART pool; Thermo Scientific; Lafayette, CO; catalog # L-047847-01-0005) contains 4 individual siRNAs targeting 4 different sites within the mouse Nos1 mRNA. Two of these sites are conserved between the mouse and chicken. Transfections were carried out by the Helios Gene Gun System (Bio-Rad). The procedure for transfection was described previously (Shi et al. 2009a, Shi et al. 2009b). Briefly, 0.8~1μg siRNA (60 pmole) and 0.4 μg plasmid encoding enhanced green fluorescent protein (phrGFP II-1 vector; Strategene, La Jolla, CA) were mixed and co-precipitated onto 1 μm gold microcarriers according to the manufacturer's protocol. The control cells were transfected with GFP only. The particle delivery system generated a helium shock wave with a pressure gradient of 200 p.s.i. to accelerate the coated microcarriers into cultured cells.
Patch-clamp recordings and statistical analysis
Whole-cell patch-clamp recordings of cone photoreceptor L-type Ca2+ channels (L-VGCCs) were carried out using direct rupture whole-cell configuration on the second day of DD. The external solution was (in mM): NaCl 110, BaCl2 10, MgCl2 0.4, KCl 5.3, TEACl 20, HEPES 10, and glucose 5.6, pH 7.4 with NaOH. The pipette solution was (in mM): Cs acetate 135, CsCl 10, MgCl2 2, CaCl2 0.1, EGTA 1.1, and HEPES 10, pH 7.4 adjusted with CsOH (Gleason et al. 1992, Ko et al. 2009b, Ko et al. 2007). Recordings were made from cells with elongated cell bodies with one or more prominent oil droplets. Currents were recorded at room temperature (24°C) using an Axopatch 200B amplifier (Molecular Devices, Union City, CA, USA). Signals were low-pass filtered at 2 kHz and digitized at 5 kHz with Digidata 1440A interface and pCLAMP 10.0 software (Molecular Devices). After the gigaohm (GΩ) seal was formed, the electrode capacitance was compensated. The membrane capacitance, series resistance, and input resistance of the recorded photoreceptors were measured by applying a 5 mV (100 ms) depolarizing voltage step from a holding potential of -65 mV after established electrical contact with the cell. Cells with a series resistance > 10% of the input resistance (series resistance > 100 MΩ or input resistance < 1 GΩ) were discarded. The membrane capacitance reading was used as the value for whole-cell capacitance. The current-voltage relationships were elicited from a holding potential of -65 mV using 200 ms steps (5 s between steps) to test potentials over a range of -80 to +60 mV in 10 mV increments. To calculate current densities, we divided current amplitudes by membrane capacitances. Each group contained 12–17 cells.
Statistics
All data are presented as mean ± standard error of the mean (s.e.m.). The Student's t-test or one-way ANOVA followed by Tukey's post hoc test for unbalanced n was used for statistical analyses. All controls and SNAP-treated cells from various sets of experiments were pooled for various comparisons. Throughout, p < 0.05 was regarded as significant.
Results
Nitric oxide synthase (NOS) and cGMP levels are under circadian control in avian retinas
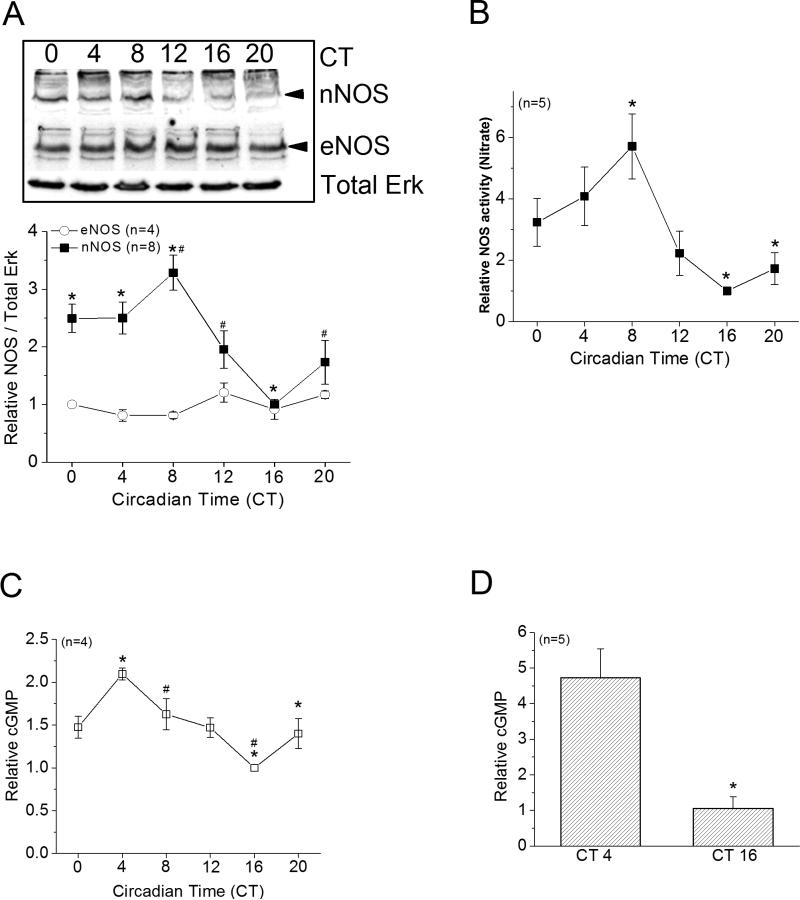
We examined whether the expression and activities of NOS were under circadian control. After circadian entrainment, on the second day of constant darkness (DD), retinas were taken at 6 time points throughout the course of a day. We found that the protein level of nNOS peaked during the day (at CT 8), while eNOS did not display circadian rhythmic expression (Figure 1A). We further analyzed the retinal content of nitrate as an indicator of NOS activity and found that the NOS activity was also highest during the day (at CT 8, Figure 1B), which correlated with the circadian profile of nNOS expression. Since NO can further activate guanylyl cyclase (GC) and lead to cGMP production and activation of protein kinase G (PKG)(Michel & Vanhoutte 2010), we investigated whether retinal cGMP content was under circadian control, which would give an indication of global guanylyl cyclase activity in the retina. The retinal content of cGMP displayed a circadian rhythm with a small amplitude (about 2 folds from the peak to trough) and was high during the day (Figure 1C). The content of cGMP in photoreceptor enriched cultures was also significantly higher in cells harvested during the day (Figure 1D). Hence, in the chick retina, both NO and cGMP were under circadian control.
Figure 1. Neuronal NOS (nNOS) and cGMP content are under circadian regulation in the retina.
(A-C) Embryos were entrained in 12 hr LD cycles for several days and kept in DD. On the second day of DD, retinas were harvested at 6 circadian time points (CT 0, 4, 8, 12, 16, and 20) for Western immunoblotting for protein levels of NOS, assays of nitrate as an indicator for NOS activity, or assays of cGMP content. (A) The protein expression of nNOS, but not eNOS, is under circadian control, which peaked at CT 8. * indicates that the protein levels of nNOS at CT 0, 4, and 8 are significantly higher than CT 16. # indicates that the protein level of nNOS at CT 8 is significantly higher than CT 12 and CT 20. (B) The levels of nitrate are also under circadian control, which peaked at CT 8. * indicates that the nitrate level at CT 8 is significantly higher than CT 16 and CT 20. (C) The cGMP content is rhythmic with a peak at CT 4. * indicates that the cGMP content at CT4 is significantly higher than CT 16 and CT 20. # indicates that the cGMP content at CT8 is significantly higher than CT 16. *, # p<0.05. (D) Photoreceptor enriched cultures were entrained in LD for 5 days and kept in DD. On the second day of DD, cells were harvested at CT 4 and CT 16 for cGMP assays. The cGMP content was significantly higher in cells harvested at CT 4 compared to cells harvested at CT 16. *p<0.05
Exogenous NO donor SNAP affects L-VGCCs at night
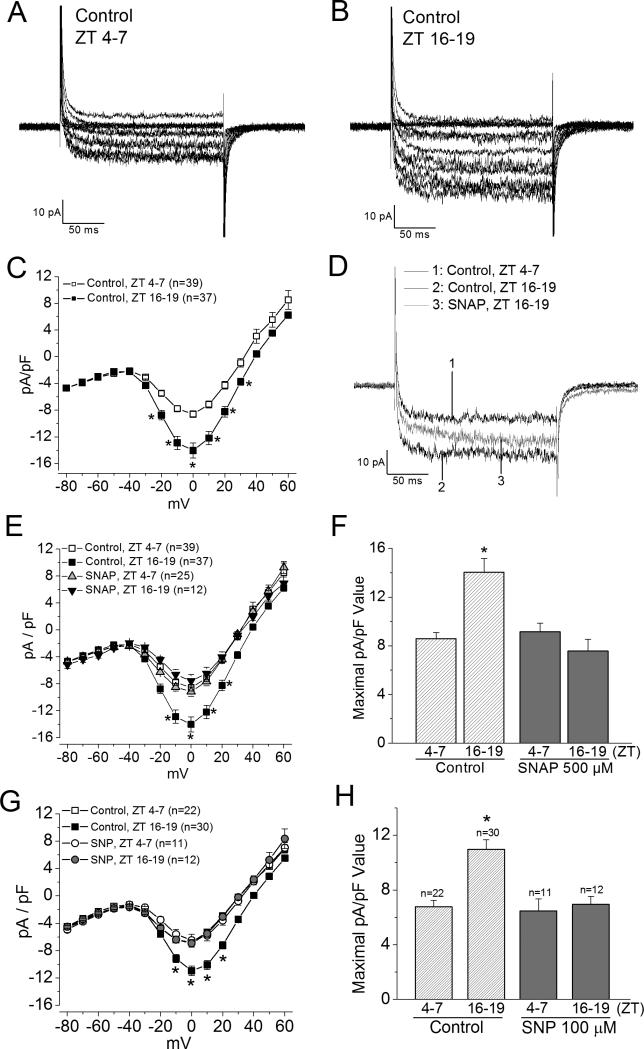
Since NO regulates L-type voltage-gated calcium channels (L-VGCCs) by increasing the L-VGCC currents in rods while decreasing them in cones (Kourennyi et al. 2004), we examined whether NO caused a circadian phase-dependent modulation of cone L-VGCCs. We previously showed that there is a circadian regulation of L-VGCCs from the mRNA levels to the protein expression of the channel pore forming α1 subunit (Ko et al. 2009b, Ko et al. 2007), and the L-VGCC current density is higher when cone photoreceptors are recorded at night than during the day (Figure 2A, B, C; Ko et al. 2009b, Ko et al. 2007). Application of NO donor SNAP (500 μM) for 2 hr prior to recordings decreased L-VGCC currents in cone photoreceptors when cells were recorded at night (Figure 2D, E, F) with no significant effects on these currents during the day. Treatment with another NO donor SNP (100 μM) for 2 hr prior to recordings had a similar effect (Figure 2G, H). Hence, exogenous NO only decreased L-VGCC currents at night without affecting LVGCCs during the day.
Figure 2. NO decreases L-VGCC current densities at night in cone photoreceptors.
Retinas were dissociated, cultured, and entrained in LD cycles for 6 days. On the last day of LD, cone photoreceptors were recorded during ZT 4-7 or ZT 16-19. (A) and (B) Two representative voltage step elicited L-VGCC current traces were recorded during the day (ZT 4-7) and at night (ZT 16-19). (C) The average current density (pA/pF)-voltage (mV) relationship of cells recorded during the day (ZT 4-7) and at night (ZT 16-19) are shown. Leak subtraction with a P/4 protocol was used in these recordings. * indicates that the current densities elicited at -20, -10, 0, 10, 20, 30, and 40 mV are significantly different between cells recorded during the day and at night; *p<0.05. (D-F) Treatment with an NO donor, SNAP (500 μM), decreased L-VGCC current densities when photoreceptors were recorded at night (ZT 16-19). (D) Representative traces recorded from three different cells: 1. a control cell recorded at ZT 4-7; 2. a control cell recorded at ZT 16-19; 3. a cell treated with SNAP recorded at ZT 16-19. The step command was from -65 mV to 0 mV for 200 ms. (E) The average current density-voltage relationship of the control and SNAP treated photoreceptors are shown. Cells were treated with SNAP for 2 hr prior to recordings. Recordings for this set of experiments were not leak-subtracted. * indicates that the current densities of the control group recorded at ZT 16-19 are significantly different from the other three groups when L-VGCCs were elicited at -10, 0, 10, and 20 mV. *p<0.05. (F) The average maximal current density (pA/pF) values elicited at 0 mV from the control and SNAP treated groups recorded at ZT 4-7 and ZT 16-19 are shown. * indicates that the maximal current density value of the control group recorded at ZT 16-19 is significantly different from the other three groups. *p<0.05. (G) and (H) Treatment with another NO donor, SNP (100 μM), showed a similar result as with SNAP. * indicates that the maximal current density value of the control group (0.1% DMSO) recorded at ZT 16-19 is significantly different from the other three groups. *p<0.05.
Treatment with a NOS inhibitor, L-NAME (100 μM), for 2 hr to inhibit endogenous NOS had an apparent increase (without statistical significance) in LVGCCs when cells were recorded during the day, but L-NAME appeared to slightly decrease L-VGCCs when cells were recorded at night (Figure 3A, B). Since in chick retina, nNOS is under circadian control (Figure 1A), we further examined whether specific inhibition of nNOS would have circadian phase-dependent effects on cone LVGCCs. At the moment, only a predicted sequence is available for the chicken Nos1 gene. However, after sequence alignment between mouse Nos1 and the predicted chicken Nos1, we found 79% homology (supplementary information). We cotransfected cultured cone photoreceptors with a plasmid encoding green fluorescent protein (GFP) and a pool of four siRNAs that specifically targeted the mouse Nos1 gene at different sites (the controls were transfected with GFP only). Two of these sites are highly conserved. Thus, the mouse siRNAs should also target the chicken Nos1.
We found that photoreceptors transfected with Nos1 siRNA had a significant increase in L-VGCC currents when cells were recorded during the day (CT 4-7) compared to the control (CT 4-7; Figure 3C, D) but did not affect L-VGCC currents in cones recorded at night (CT 16-19). Hence, NO participated in the circadian phase-dependent regulation of L-VGCCs in cone photoreceptors.
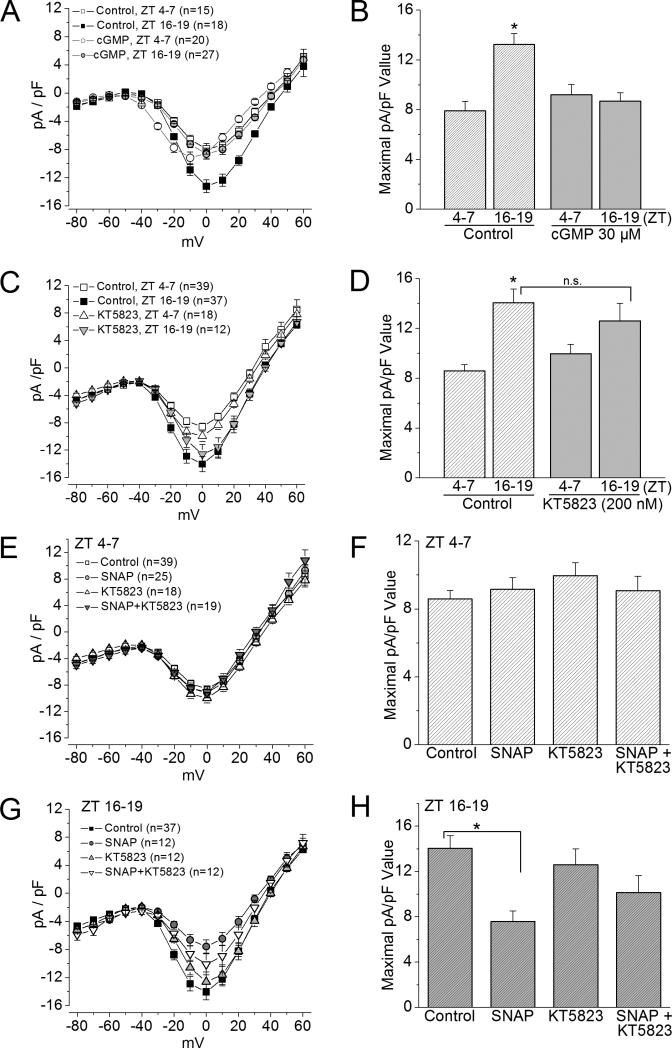
The exogenous NO effect on L-VGCCs is in part through cGMP-PKG signaling
Since NO activates guanylyl cyclase (GC) and leads to cGMP production and subsequent activation of protein kinase G (PKG) (Michel & Vanhoutte 2010), we examined whether the action of exogenous NO on L-VGCCs was through cGMPPKG signaling. Application of 8-Br-cGMP (cGMP; 30 μM) for 2 hr mimicked the action of SNAP on cone L-VGCCs (Figure 4A and B), in which the L-VGCC currents decreased when photoreceptors were recorded at night. 8-Br-cGMP did not have any significant effect on cells recorded during the day. Treatment with the PKG inhibitor KT5823 (200 nM) slightly increased the L-VGCC currents during the day and mildly decreased L-VGCCs at night without statistical significance compared to the controls (cells recorded either during the day or at night; Figure 4C and D), but KT 5823 did reverse the effect of SNAP when cells were recorded at night (Figure 4G and H).
Figure 4. cGMP mimics the effects of NO on L-VGCC currents.
(A) and (B) Treatment with 8-Br-cGMP (cGMP; 30 μM) for 2 hr prior to recordings mimics the effect of SNAP. * indicates that the maximal current density (pA/pF) value of the control group recorded at ZT 16-19 is significantly different from the other three groups. *p<0.05. (C) and (D) Treatment with a PKG inhibitor KT5823 (200 nM) has no statistically significant effect on L-VGCC currents. * indicates that the maximal current density value of the control group recorded at ZT 16-19 is significantly different from the control group recorded at ZT 4-7. *p<0.05. (E) and (F) All photoreceptors were recorded during the day (ZT 4-7). There is no statistical difference among cells under different treatments, including control, SNAP (500 μM), KT 5823, and SNAP with KT 5823 (SNAP+KT5823). (G) and (H) All photoreceptors were recorded at night (ZT 16-19). KT5823 reverses the inhibitory effect of SNAP (SNAP+KT5823), while KT5823 has no effect on L-VGCCs. * indicates that the maximal current density value of the control group recorded at ZT 16-19 is significantly different from cells treated with SNAP. *p<0.05.
In addition to patch recordings, we examined whether the circadian phase-dependent NO-cGMP-PKG signaling on L-VGCCs was in part through regulating the protein levels of L-VGCCα1D. Treatment with the NO donor SNAP for 2 hr decreased the protein level of the L-VGCCα1D subunit when cultures were treated and harvested at night (Figure 5), and 8-Br-cGMP (cGMP) mimicked the effect of SNAP (Figure 5A). These data echoed the patch-clamp recordings that treatment with SNAP or 8-Br-cGMP decreased L-VGCC current densities when photoreceptors were recorded at night (Figures 2E, 2F, 4A and 4B). The guanylyl cyclase inhibitor ODQ (2 μM) reversed the effect of SNAP on L-VGCCα1D and L-VGCC current densities when cells were treated or recorded at night (Figure 5B-5E). Interestingly, treatment with ODQ alone during the day significantly enhanced the protein level of L-VGCCα1D (Figure 5B), as well as increased the L-VGCC currents in appearance (Figure 5E; not statistically different from the control) but did not significantly enhance L-VGCCα1D or L-VGCC currents when cells were treated at night. Treatment with the PKG inhibitor KT5823 also reversed the effect of SNAP on LVGCCα1D when cells were treated at night (Figure 5F), which matched the patch-clamp recordings of L-VGCC currents (Figure 4G and 4H). Therefore, the circadian phase-dependent modulation of L-VGCCs by NO was in part mediated through cGMP-PKG signaling. It is important to note that inhibition of guanylyl cyclase might have additional effects on L-VGCCs mediated through other signaling pathways.
NO-cGMP-PKG signaling is upstream of MAPK and phosphatidylinositol 3 kinase (PI3K)
We previously demonstrated that both MAPK-Erk and PI3K-protein kinase B (Akt) signaling pathways participate the circadian rhythms of L-VGCCs mainly through regulating the trafficking of L-VGCCα1D (Ko et al. 2009b, Ko et al. 2007). The phosphorylation states of both Erk and Akt are under circadian control, which reach the highest at night (Ko et al. 2009b, Ko et al. 2007). Inhibition of Erk activity does not affect the circadian rhythm of phosphorylated Akt (pAkt), while inhibition of PI3K or Akt does not affect the circadian rhythm of phosphorylated Erk (pErk). Hence, Erk and PI3K-Akt work in parallel to regulate the circadian rhythm of LVGCCs (Ko et al. 2009b, Ko et al. 2007).
We next examined whether the circadian phase-dependent effect of NO-cGMP-PKG signaling on L-VGCCs was upstream or downstream to Erk and/or PI3KAkt. Treatment with SNAP or cGMP dampened the circadian rhythm of pErk (Figure 6A), and both ODQ and KT5823 reversed the effect of SNAP (Figure 6B, C), while treatment with KT5823 alone did not affect the circadian rhythm of pErk. Interestingly, inhibition of guanylyl cyclase with ODQ significantly elevated pErk during the day to night time levels (Figure 6B). Similar effects of SNAP, 8-Br-cGMP, ODQ, and KT5823 were found in the circadian rhythms of pAkt (Figure 7). Hence, NO-cGMP-PKG signaling was upstream of both Erk (Figure 6) and PI3K-Akt (Figure 7).
Figure 7. NO-cGMP-PKG signaling is upstream of Akt.
(A) The level of phosphorylated Akt (pAkt) is significantly higher at night (CT 16) than during the day (CT 4). Treatment with SNAP or 8-Br-cGMP (cGMP) significantly decreases the level of pAkt at night (CT 16). * indicates that the pAkt level of controls at night (CT 16) is significantly higher than all other groups. (B) Treatment with ODQ alone increases pAkt levels at CT 4. Treatment with ODQ reverses the effect of SNAP on pAkt. # indicates that these groups are significantly different from the control at CT 16. There is no difference among control at CT 16, ODQ treated groups at CT 4 and CT 16, and ODQ+SNAP group at CT 4 and CT 16. (C) Treatment with KT5823 shows similar results in pAkt as ODQ. # indicates that these groups are significantly different from the control at CT 16. *, # p<0.05.
Discussion
In this study, we found that NO elicited a circadian phase-dependent modulation of L-VGCCs in the retina. The protein expression and L-VGCC currents are high at night and low during the day (Ko et al. 2009b, Ko et al. 2007), while the protein expression of retinal nNOS and NOS activity were also under circadian control but peaked during the day (Figure 1) in opposite circadian phase from LVGCCs. An exogenous NO donor, SNAP or SNP, decreased L-VGCC currents in cone photoreceptors at night but not during the day, and 8-Br-cGMP mimicked the effect of SNAP on cone L-VGCCs (Figures 2, 3, 4). Applications of the guanylyl cyclase (GC) inhibitor ODQ or PKG inhibitor KT5823, reversed the effect of SNAP on L-VGCCs (Figures 4, 5). Hence, NO, in part through activation of cGMP-PKG signaling, modulated the cone L-VGCCs in a circadian phase-dependent manner.
We previously showed that dissociation of retinal photoreceptors in cultures does not disrupt the circadian rhythm (Ko et al. 2001), so we are able to use both intact retinas and dissociated cultures to provide different lines of evidence in this study. Cell cultures allow for pharmacological electrophysiology studies to monitor L-VGCC activities. On the other hand, since biochemical assays typically require larger quantities of tissues, we chose to use intact retinas for the necessary analyses. After circadian entrainment, the circadian rhythms in photoreceptors (from ion channel activities to activities / phosphorylation states of signaling molecules) remain the same regardless whether the retinas (or whole embryos) are under light-dark (LD) cycles or in constant darkness (DD) (Huang et al. 2012, Ko et al. 2001, Ko et al. 2009b, Ko et al. 2007). More specifically, we demonstrated that the protein expression of L-VGCCs is greater at CT16 compared to CT4 in both intact retina and cultured photoreceptors, and current densities of L-VGCCs from cultured photoreceptors are larger during the subjective night (CT16-19) than during the subjective day (CT4-7) (Ko et al. 2007).
Nitric oxide-dependent signaling is known to participate in mammalian circadian rhythms (Ding et al. 1994, Ferreyra & Golombek 2001, Golombek et al. 2004, Melo et al. 1997, Watanabe et al. 1995). Intracerebroventricular injection of L-NAME blocks light-induced phase shifts (Weber et al. 1995a), as well as the light-induced resetting of circadian wheel-running rhythm (Ding et al. 1994) in hamsters. This NO-dependent circadian phase-shifting could also be cGMP-PKG signaling dependent (Weber et al. 1995b). In the SCN, exogenous NO donors produce light-like phase shifts of circadian rhythms of neuronal firing rate in vitro (Ding et al. 1994), while treatment with L-NAME in cultured SCN 2.2 cells phase-shift the circadian rhythms of glucose metabolism (Menger et al. 2007). Here, we demonstrated that NO elicited circadian phase-dependent modulation in the retina. Thus, in the SCN and retina, NO-dependent signaling is important in the post-translational regulation of circadian oscillators at both circadian inputs and outputs. Moreover, administration of NO scavengers into the SCN blocks the light-induced phase-advance in circadian behavioral rhythms, indicating that NO also serves as an intercellular messenger to communicate the light signal and synchronize individual cellular oscillators within the SCN (Plano et al. 2007). Since there are various cell types in the retina that contain circadian oscillators (Green & Besharse 2004), it is possible that NO could be an intercellular messenger contributing to the overall circadian functional output in the retina and assisting the visual system to adapt to the daily changes in ambient illumination.
While the modulatory effect of NO on L-VGCCs could be due direct S-nitrosylation of the channel pore forming α1 subunit (Almanza et al. 2007), our results showed that the circadian phase-dependent modulation of L-VGCCs by NO was in part mediated through cGMP-PKG signaling, since 8-Br-cGMP mimicked the effect of NO on L-VGCCs, and most S-nitrosylation actions by NO are cGMP-PKG independent (Martinez-Ruiz et al. 2011). In the central nervous system, activation of NOS produces sustained potentiation of Ras signaling (Yun et al. 1998), which mediates NO-elicited PKG activation (Chen et al. 2008, Yun et al. 1998). Hence, there is cross-talk between NO-elicited PKG activation and Ras signaling. Both MAPK-Erk and PI3K-Akt are downstream of Ras and serve as part of the circadian output signaling pathways to regulate the trafficking of L-VGCCs (Ko et al. 2009b, Ko et al. 2007), with the phosphorylation states of Erk and Akt (pErk and pAkt), as well as Ras activity, all under circadian control with peaks at night (Ko et al. 2001, Ko et al. 2004b, Ko et al. 2009b, Ko et al. 2007). We found that NO was upstream of both Erk and Akt leading to the circadian regulation of L-VGCCs (Figures 6, 7), which could be due to the cross-talk between NO signaling and Ras. In neurons and endothelial cells, NO-cGMP signaling increases the phosphorylation states / activities of Erk and Akt (Culmsee et al. 2005, Endo & Launey 2003, Parenti et al. 1998, Patel et al. 2010). However, to our surprise, instead of activating Erk and Akt, treatments with SNAP or 8-Br-cGMP decreased pErk and pAkt at night, and ODQ or KT5823 partially reversed the inhibitory effect of SNAP, which indicated that exogenous NO/cGMP dampened the activity of Erk and Akt only at night. If inactivation of Akt is caused by the direct S-nitrosylation from NO (Yasukawa et al. 2005, Slomiany & Slomiany 2011), 8-Br-cGMP should not be able to mimic the effect of SNAP to inhibit pAkt (Martinez-Ruiz et al. 2011).
Another possible explanation for the NO dampening effects on pErk and pAkt is the direct S-nitrosylation of Ras, which can either activate or inactivate (Heo & Campbell 2004, Batista et al. 2013, Raines et al. 2006) Ras-signaling depending on the particular Ras subtype (such as p21Ras or H-Ras), as well as the cellular compartmentalization of Ras. However, most S-nitrosylation-dependent actions on Ras are cGMP/PKG independent, which is different from our observation that the circadian modulation of NO on Erk, Akt, and L-VGCCs was through cGMP/PKG signaling. While the molecular mechanism of regulation of circadian rhythmicity by NO is not clearly understood, presumably through the downstream signaling of NO, it may lead to the activation of the human fos promoter (Gudi et al. 1999) or phosphorylation of cAMP response element (CRE)-binding protein (CREB), which would further result in the upregulation of clock gene expression and induce circadian phase-dependent changes or phase-shift (Colbran et al. 1992, Ferreyra & Golombek 2001, Kunieda et al. 2008, Langmesser et al. 2009). Hence, there will be a need for future investigation as to how exogenous NO/cGMP decreases pErk, pAkt, and L-VGCC currents at night.
One unexpected observation is that L-NAME only slightly increased L-VGCC currents during the day and decreased L-VGCCs at night, but the results were not statistically significant (Figure 3). There was a significant increase of L-VGCCs in cells transfected with Nos1 siRNA to specifically inhibit nNOS. L-NAME is a widely used general NOS inhibitor. One possible explanation is that even though eNOS did not display circadian rhythmicity, inhibition of eNOS by L-NAME might somehow affect L-VGCCs in a circadian-independent manner. Another possible explanation is that L-NAME might have other unknown actions indirectly affecting L-VGCCs (Supplementary Figure 1). In addition, the chicken Nos1 gene is only a predicted sequence, so there is no specific siRNA targeting chicken Nos1 available as stated earlier. Since the mouse Nos1 and predicted chicken Nos1 share79% homology, we used a pool of mouse siRNAs for our transfection studies. Indeed, we observed that transfection with the mouse siRNA pool targeting nNOS increased the L-VGCC currents during the day (Figure 3). However, with limited genomic information in chickens, we cannot exclude the possibility that the mouse siRNA pool might target other chicken proteins and indirectly affect L-VGCCs. Both pharmacological and transfection studies come with technical limitations, but they should not affect our conclusion that NO plays a role in the circadian phase-dependent modulation of LVGCCs in cone photoreceptors.
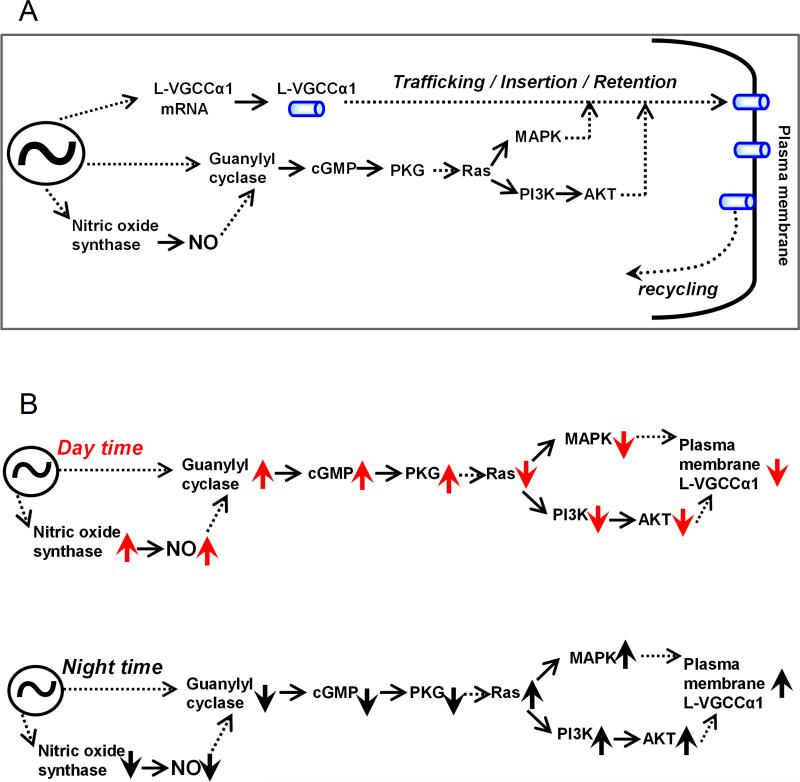
Another interesting observation is that inhibition of guanylyl cyclase (GC) by ODQ apparently enhanced pErk, pAkt, as well as L-VGCCs during the day, while inhibition of NOS did not cause such increase of L-VGCCs. In addition, the cGMP content in both intact retinas and entrained photoreceptor cultures was high during the day, and the peak of cGMP content was ~4 hr advanced of NOS activity (Figure 1). We postulate that in the retina, especially in cone photoreceptors, the activity/expression of GC and the production of cGMP are under circadian control that is independent of the circadian rhythm of NOS activities and endogenous NO production. The action of NO was partially through GC-cGMP-PKG signaling, and both were upstream of MAPK-Erk and PI3K-Akt to modulate the circadian rhythm of L-VGCCs (Figure 8A). We hypothesize that during the day time (Figure 8B), NOS and GC activities are higher (up arrows), which lead to elevated NO and cGMP compared to the night time. Through a complex signaling network with other cellular components yet to be identified, the higher NO and cGMP lead to the lower activities (down arrows) of MAPK-Erk and PI3K-Akt signaling, which ultimately causes LVGCCs to be lower during the day. At night (Figure 8B), when GC and NOS activities are lower, MAPK-Erk and PI3K-Akt signaling activities are higher, which lead to higher L-VGCCs in cone photoreceptors. Overall, the circadian regulation of L-VGCCs in photoreceptors involves the rhythmic expression of mRNA and protein of the pore-forming α1 subunit, as well as the post-translational regulation by the complex signaling network in channel protein trafficking and translocation from cytosol to plasma membrane, and insertion / retention in the cell membrane (Figure 8A)(Ko et al. 2009a, Ko et al. 2009b, Ko et al. 2007, Shi et al. 2009b). Furthermore, we cannot rule out that these signaling molecules could also participate in other post-translational regulation of L-VGCCs, such as recycling or internalization, which will require future investigation.
Figure 8. A schematic diagram shows a working model of the circadian regulation of L-VGCCs.
(A) An overview shows how NO participates in the circadian regulation of L-VGCCs. Solid arrows indicate a direct step between two elements, while the dotted arrows indicate an indirect step with unidentified elements in between. (B) The upper and lower panels display the roles of NO in the circadian regulation of L-VGCCs during the day and night, respectively. The upward arrows indicate an increase of quantity or activity of a particular molecule, and the downward arrows indicate a decrease of quantity or activity of a particular molecule. ~ indicates the canonical molecular oscillator.
Even though NO release in the retina is increased upon light stimulation and decreased in the dark-adapted state (Sato et al. 2010), we found that the expression of NOS was under circadian regulation when retinas were maintained in constant darkness in ovo after circadian entrainment. It was the expression of nNOS, but not eNOS, that peaked during the day. NO production was synchronized with the circadian rhythm of nNOS, which indicated that NOS protein level and activity were rhythmic in synchronization. While nNOS is present in photoreceptors (Cao & Eldred 2001, Crousillac et al. 2003, Neufeld et al. 2000, Shin et al. 2000), we cannot rule out the possibility that the source of NO influencing photoreceptor L-VGCCs could be diffusing from amacrine cells, since in mammalian retinas, amacrine nNOS is also under circadian regulation with a peak during the day (Zhang et al. 2005).
The GC-cGMP signaling in the nocturnal hamster retina displays a diurnal rhythm with peaks at night (Weber et al. 1995b). We found that the cGMP content in intact chicken retinas was under circadian regulation with a peak during the day with a rhythmic amplitude ~2 folds across the course of 24 hours. The diurnal/circadian rhythms of cGMP in nocturnal rod-dominant retinas and diurnal cone-dominant retinas are in opposite phases. While cone photoreceptors are predominantly for vision under the daylight, rod photoreceptors are essential for night vision. The opposite diurnal/circadian phases of cGMP contents in nocturnal versus diurnal retinas could be adaptive strategies in the visual functions between nocturnal rod-dominant and diurnal cone-dominant retinas. Further investigation on the role of GC-cGMP signaling in retinal circadian rhythms will be needed.
Interestingly, under light-adapted conditions, while cytosolic Ca2+ concentration decreases, GC activity increases (Takemoto et al. 2009, Koch & Stryer 1988, Dizhoor et al. 1994). Particularly in cone photoreceptors, GC is able to synthesize cGMP at a rate of 250 μM/sec under light-adapted conditions, compared to dark-adapted cones with a rate of 120 μM/sec, so cone photoreceptors show a ~2 folds capacity in GC activation (Takemoto et al. 2009). These results indicate that in cone photoreceptors, GC activity can be regulated on a short-term (light/dark adaptation), as well as long term (across 24 hours) basis with a two-fold capacity. In addition, the circadian rhythmic changes of retinal cGMP content echoes a previous observation, in which the apparent ligand affinity of cGMP-gated cation channels (CNGCs) is under circadian control, with the affinity of CNGCs at night significantly higher than during the day at ~2 folds in chick cone photoreceptors (Ko et al. 2001). Since the overall retinal cGMP content is lower at night, it is reasonable for photoreceptors to develop a mechanism in which the affinity of CNGCs to cGMP is higher during this time in order to compensate for the lower levels of cGMP. The circadian rhythm of CNGC affinity is controlled by its tyrosine phosphorylation on the auxiliary subunit (Chae et al. 2007).
Interestingly, in cone photoreceptors, both CNGCs and L-VGCCs are under circadian control but in different manners (Ko et al. 2001, Ko et al. 2004b, Ko et al. 2009b, Ko et al. 2007). For cone CNGCs, it is their affinity to cGMP that varies throughout the day, and Ras-MAPK-Erk signaling is essential for the circadian regulation of CNGCs. The maximal currents remain relatively constant, and protein synthesis is not required in the circadian regulation of CNGCs (Ko et al. 2004a, Ko et al. 2001, Ko et al. 2004b). As for the circadian profiles of L-VGCCs in cone photoreceptors, both the L-VGCCα1 mRNA and protein expression are under circadian control, and maximal L-VGCC currents are higher at night and lower during the day (Ko et al. 2009b, Ko et al. 2007). The activation of Ras-MAPK-Erk and Ras-PI3K-Akt signaling pathways are also higher at night and promote L-VGCCα1 subunit trafficking from cytoplasm to plasma membrane (Ko et al. 2009b, Ko et al. 2007) . As shown in the present study, nNOS expression, its activity, and NO production were higher during the day, which decreased L-VGCCs in part through inhibiting pErk and pAkt. Since NO was upstream of Ras-MAPK-Erk, it would be of great interest to investigate how NO might modulate CNGCs at different times of a day. Taken together, NO/cGMP/PKG signaling was involved as part of the circadian output pathway to regulate L-VGCCs in photoreceptors.
Supplementary Material
Acknowledgements
We thank Darya Vernikovskaya for her assistance with the cGMP and NO assays. This work was supported by NIHR01EY017452 (National Eye Institute) to G.K.
Abbreviations
- L-VGCC
L-type voltage-gated calcium channel
- Ca2+
calcium
- NO
nitric oxide
- NOS
NO synthase
- nNOS
neural NO synthase
- eNOS
epithelial NO synthase
- iNOS
inducible NO synthase
- CaM
calmodulin
- MAPK
mitogen-activated protein kinase
- Erk
extracellular-signal-regulated kinase
- PI3K
phosphatidylinositol 3 kinase
- Akt
protein kinase B
- PKG
protein kinase G
- SCN
suprachiasmatic nuclei
- LD
light-dark
- DD
constant darkness
- ZT
Zeitgeber time
- CT
circadian time
- SNAP
S-nitroso-N-acetyl-penicillamine
- L-NAME
NG-nitro-L-arginine methyl ester
- ODQ
1H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one
- RIPA buffer
radioimmunoprecipitation assay buffer
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- HRP
horse radish perioxidase
- ECL
enhanced chemiluminescent
Footnotes
The authors have no conflicts of interest to declare.
References
- Adly MA, Spiwoks-Becker I, Vollrath L. Ultrastructural changes of photoreceptor synaptic ribbons in relation to time of day and illumination. Investigative ophthalmology & visual science. 1999;40:2165–2172. [PubMed] [Google Scholar]
- Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. The Biochemical journal. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almanza A, Navarrete F, Vega R, Soto E. Modulation of voltage-gated Ca2+ current in vestibular hair cells by nitric oxide. Journal of neurophysiology. 2007;97:1188–1195. doi: 10.1152/jn.00849.2006. [DOI] [PubMed] [Google Scholar]
- Barnes S, Jacklet JW. Ionic currents of isolated retinal pacemaker neurons: projected daily phase differences and selective enhancement by a phase-shifting neurotransmitter. Journal of neurophysiology. 1997;77:3075–3084. doi: 10.1152/jn.1997.77.6.3075. [DOI] [PubMed] [Google Scholar]
- Barnes S, Kelly ME. Calcium channels at the photoreceptor synapse. Adv Exp Med Biol. 2002;514:465–476. doi: 10.1007/978-1-4615-0121-3_28. [DOI] [PubMed] [Google Scholar]
- Batista WL, Ogata FT, Curcio MF, Miguel RB, Arai RJ, Matsuo AL, Moraes MS, Stern A, Monteiro HP. S-nitrosoglutathione and endothelial nitric oxide synthase-derived nitric oxide regulate compartmentalized ras s-nitrosylation and stimulate cell proliferation. Antioxidants & redox signaling. 2013;18:221–238. doi: 10.1089/ars.2011.4455. [DOI] [PubMed] [Google Scholar]
- Besharse JC, Dunis DA. Methoxyindoles and photoreceptor metabolism: activation of rod shedding. Science. 1983;219:1341–1343. doi: 10.1126/science.6828862. [DOI] [PubMed] [Google Scholar]
- Bredt DS. Nitric oxide signaling specificity--the heart of the problem. J Cell Sci. 2003;116:9–15. doi: 10.1242/jcs.00183. [DOI] [PubMed] [Google Scholar]
- Burnside B. Light and circadian regulation of retinomotor movement. Prog Brain Res. 2001;131:477–485. doi: 10.1016/s0079-6123(01)31038-5. [DOI] [PubMed] [Google Scholar]
- Cahill GM, Besharse JC. Circadian rhythmicity in vertebrate retinas: regulation by a photoreceptor oscillator. Prog. Retinal Eye Res. 1995;14:267–291. [Google Scholar]
- Cao L, Eldred WD. Subcellular localization of neuronal nitric oxide synthase in turtle retina: electron immunocytochemistry. Visual neuroscience. 2001;18:949–960. [PubMed] [Google Scholar]
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- Chae KS, Ko GY, Dryer SE. Tyrosine phosphorylation of cGMP-gated ion channels is under circadian control in chick retina photoreceptors. Investigative ophthalmology & visual science. 2007;48:901–906. doi: 10.1167/iovs.06-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Peng ML, Wang PY, et al. Calcium/calmodulin-dependent kinase II mediates NO-elicited PKG activation to participate in spinal reflex potentiation in anesthetized rats. American journal of physiology. Regulatory, integrative and comparative physiology. 2008;294:R487–493. doi: 10.1152/ajpregu.00600.2007. [DOI] [PubMed] [Google Scholar]
- Colbran JL, Roach PJ, Fiol CJ, Dixon JE, Andrisani OM, Corbin JD. cAMP-dependent protein kinase, but not the cGMP-dependent enzyme, rapidly phosphorylates delta-CREB, and a synthetic delta-CREB peptide. Biochem Cell Biol. 1992;70:1277–1282. doi: 10.1139/o92-174. [DOI] [PubMed] [Google Scholar]
- Crousillac S, LeRouge M, Rankin M, Gleason E. Immunolocalization of TRPC channel subunits 1 and 4 in the chicken retina. Visual neuroscience. 2003;20:453–463. doi: 10.1017/s0952523803204107. [DOI] [PubMed] [Google Scholar]
- Culmsee C, Gerling N, Landshamer S, Rickerts B, Duchstein HJ, Umezawa K, Klumpp S, Krieglstein J. Nitric oxide donors induce neurotrophin-like survival signaling and protect neurons against apoptosis. Molecular pharmacology. 2005;68:1006–1017. doi: 10.1124/mol.105.013086. [DOI] [PubMed] [Google Scholar]
- Ding JM, Chen D, Weber ET, Faiman LE, Rea MA, Gillette MU. Resetting the biological clock: mediation of nocturnal circadian shifts by glutamate and NO. Science. 1994;266:1713–1717. doi: 10.1126/science.7527589. [DOI] [PubMed] [Google Scholar]
- Dizhoor AM, Lowe DG, Olshevskaya EV, Laura RP, Hurley JB. The human photoreceptor membrane guanylyl cyclase, RetGC, is present in outer segments and is regulated by calcium and a soluble activator. Neuron. 1994;12:1345–1352. doi: 10.1016/0896-6273(94)90449-9. [DOI] [PubMed] [Google Scholar]
- Endo S, Launey T. Nitric oxide activates extracellular signal-regulated kinase 1/2 and enhances declustering of ionotropic glutamate receptor subunit 2/3 in rat cerebellar Purkinje cells. Neuroscience letters. 2003;350:122–126. doi: 10.1016/s0304-3940(03)00856-5. [DOI] [PubMed] [Google Scholar]
- Ferreyra GA, Golombek DA. Rhythmicity of the cGMP-related signal transduction pathway in the mammalian circadian system. American journal of physiology. Regulatory, integrative and comparative physiology. 2001;280:R1348–1355. doi: 10.1152/ajpregu.2001.280.5.R1348. [DOI] [PubMed] [Google Scholar]
- Gleason E, Mobbs P, Nuccitelli R, Wilson M. Development of functional calcium channels in cultured avian photoreceptors. Visual neuroscience. 1992;8:315–327. doi: 10.1017/s0952523800005058. [DOI] [PubMed] [Google Scholar]
- Golombek DA, Agostino PV, Plano SA, Ferreyra GA. Signaling in the mammalian circadian clock: the NO/cGMP pathway. Neurochemistry international. 2004;45:929–936. doi: 10.1016/j.neuint.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Green CB, Besharse JC. Retinal circadian clocks and control of retinal physiology. J Biol Rhythms. 2004;19:91–102. doi: 10.1177/0748730404263002. [DOI] [PubMed] [Google Scholar]
- Gudi T, Hong GK, Vaandrager AB, Lohmann SM, Pilz RB. Nitric oxide and cGMP regulate gene expression in neuronal and glial cells by activating type II cGMP-dependent protein kinase. FASEB J. 1999;13:2143–2152. [PubMed] [Google Scholar]
- Haque R, Chaurasia SS, Wessel JH, 3rd, Iuvone PM. Dual regulation of cryptochrome 1 mRNA expression in chicken retina by light and circadian oscillators. Neuroreport. 2002;13:2247–2251. doi: 10.1097/00001756-200212030-00016. [DOI] [PubMed] [Google Scholar]
- Heo J, Campbell SL. Mechanism of p21Ras S-nitrosylation and kinetics of nitric oxide-mediated guanine nucleotide exchange. Biochemistry. 2004;43:2314–2322. doi: 10.1021/bi035275g. [DOI] [PubMed] [Google Scholar]
- Huang CC, Ko ML, Vernikovskaya DI, Ko GY. Calcineurin serves in the circadian output pathway to regulate the daily rhythm of L-type voltage-gated calcium channels in the retina. J Cell Biochem. 2012;113:911–922. doi: 10.1002/jcb.23419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C, Studholme K, Yazulla S, von Gersdorff H. Diurnal changes in exocytosis and the number of synaptic ribbons at active zones of an ON-type bipolar cell terminal. Journal of neurophysiology. 2006;96:2025–2033. doi: 10.1152/jn.00364.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko GY-P, Jian K, Shi L, Ko ML. Circadian regulation of ion channels in photoreceptors. In: Dartt DA, editor. Encyclopedia of the Eye. Vol. 1. Elsevier Ltd.; Oxford: 2010. pp. 296–301. [Google Scholar]
- Ko GY, Ko M, Dryer SE. Circadian and cAMP-dependent modulation of retinal cone cGMP-gated channels does not require protein synthesis or calcium influx through L-type channels. Brain research. 2004a;1021:277–280. doi: 10.1016/j.brainres.2004.05.072. [DOI] [PubMed] [Google Scholar]
- Ko GY, Ko ML, Dryer SE. Circadian regulation of cGMP-gated cationic channels of chick retinal cones. Erk MAP Kinase and Ca2+/calmodulin-dependent protein kinase II. Neuron. 2001;29:255–266. doi: 10.1016/s0896-6273(01)00195-7. [DOI] [PubMed] [Google Scholar]
- Ko GY, Ko ML, Dryer SE. Circadian regulation of cGMP-gated channels of vertebrate cone photoreceptors: role of cAMP and Ras. J Neurosci. 2004b;24:1296–1304. doi: 10.1523/JNEUROSCI.3560-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko GY, Shi L, Ko ML. Circadian regulation of ion channels and their functions. Journal of neurochemistry. 2009a;110:1150–1169. doi: 10.1111/j.1471-4159.2009.06223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko ML, Jian K, Shi L, Ko GY. Phosphatidylinositol 3 kinase-Akt signaling serves as a circadian output in the retina. Journal of neurochemistry. 2009b;108:1607–1620. doi: 10.1111/j.1471-4159.2009.05931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko ML, Liu Y, Dryer SE, Ko GY. The expression of L-type voltage-gated calcium channels in retinal photoreceptors is under circadian control. Journal of neurochemistry. 2007;103:784–792. doi: 10.1111/j.1471-4159.2007.04816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KW, Stryer L. Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature. 1988;334:64–66. doi: 10.1038/334064a0. [DOI] [PubMed] [Google Scholar]
- Korenbrot JI, Fernald RD. Circadian rhythm and light regulate opsin mRNA in rod photoreceptors. Nature. 1989;337:454–457. doi: 10.1038/337454a0. [DOI] [PubMed] [Google Scholar]
- Kourennyi DE, Liu XD, Hart J, Mahmud F, Baldridge WH, Barnes S. Reciprocal modulation of calcium dynamics at rod and cone photoreceptor synapses by nitric oxide. Journal of neurophysiology. 2004;92:477–483. doi: 10.1152/jn.00606.2003. [DOI] [PubMed] [Google Scholar]
- Kunieda T, Minamino T, Miura K, et al. Reduced nitric oxide causes age-associated impairment of circadian rhythmicity. Circ Res. 2008;102:607–614. doi: 10.1161/CIRCRESAHA.107.162230. [DOI] [PubMed] [Google Scholar]
- Langmesser S, Franken P, Feil S, Emmenegger Y, Albrecht U, Feil R. cGMP-dependent protein kinase type I is implicated in the regulation of the timing and quality of sleep and wakefulness. PloS one. 2009;4:e4238. doi: 10.1371/journal.pone.0004238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail MM. Circadian nature of rod outer segment disc shedding in the rat. Investigative ophthalmology & visual science. 1980;19:407–411. [PubMed] [Google Scholar]
- Liu X, Zhang Z, Ribelayga CP. Heterogeneous Expression of the Core Circadian Clock Proteins among Neuronal Cell Types in Mouse Retina. PloS one. 2012;7:e50602. doi: 10.1371/journal.pone.0050602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llomovatte DW, Lacoste FF, Zotter C, Sarmiento MI, Rosenstein RE. Photic control of nitric oxide synthase activity in golden hamster retina. Neuroreport. 1997;8:3763–3766. doi: 10.1097/00001756-199712010-00021. [DOI] [PubMed] [Google Scholar]
- Martinez-Ruiz A, Cadenas S, Lamas S. Nitric oxide signaling: classical, less classical, and nonclassical mechanisms. Free radical biology & medicine. 2011;51:17–29. doi: 10.1016/j.freeradbiomed.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Melo L, Golombek DA, Ralph MR. Regulation of circadian photic responses by nitric oxide. J Biol Rhythms. 1997;12:319–326. doi: 10.1177/074873049701200404. [DOI] [PubMed] [Google Scholar]
- Menger GJ, Allen GC, Neuendorff N, Nahm SS, Thomas TL, Cassone VM, Earnest DJ. Circadian profiling of the transcriptome in NIH/3T3 fibroblasts: comparison with rhythmic gene expression in SCN2.2 cells and the rat SCN. Physiol Genomics. 2007;29:280–289. doi: 10.1152/physiolgenomics.00199.2006. [DOI] [PubMed] [Google Scholar]
- Michel T, Vanhoutte PM. Cellular signaling and NO production. Pflugers Arch. 2010;459:807–816. doi: 10.1007/s00424-009-0765-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld AH, Shareef S, Pena J. Cellular localization of neuronal nitric oxide synthase (NOS-1) in the human and rat retina. The Journal of comparative neurology. 2000;416:269–275. doi: 10.1002/(sici)1096-9861(20000110)416:2<269::aid-cne11>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Parenti A, Morbidelli L, Cui XL, Douglas JG, Hood JD, Granger HJ, Ledda F, Ziche M. Nitric oxide is an upstream signal of vascular endothelial growth factor-induced extracellular signal-regulated kinase1/2 activation in postcapillary endothelium. The Journal of biological chemistry. 1998;273:4220–4226. doi: 10.1074/jbc.273.7.4220. [DOI] [PubMed] [Google Scholar]
- Patel NJ, Chen MJ, Russo-Neustadt AA. Norepinephrine and nitric oxide promote cell survival signaling in hippocampal neurons. European journal of pharmacology. 2010;633:1–9. doi: 10.1016/j.ejphar.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Pierce ME, Besharse JC. Circadian regulation of retinomotor movements. I. Interaction of melatonin and dopamine in the control of cone length. J Gen Physiol. 1985;86:671–689. doi: 10.1085/jgp.86.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce ME, Sheshberadaran H, Zhang Z, Fox LE, Applebury ML, Takahashi JS. Circadian regulation of iodopsin gene expression in embryonic photoreceptors in retinal cell culture. Neuron. 1993;10:579–584. doi: 10.1016/0896-6273(93)90161-j. [DOI] [PubMed] [Google Scholar]
- Plano SA, Agostino PV, Golombek DA. Extracellular nitric oxide signaling in the hamster biological clock. FEBS letters. 2007;581:5500–5504. doi: 10.1016/j.febslet.2007.10.058. [DOI] [PubMed] [Google Scholar]
- Raines KW, Cao GL, Lee EK, Rosen GM, Shapiro P. Neuronal nitric oxide synthase-induced S-nitrosylation of H-Ras inhibits calcium ionophore-mediated extracellular-signal-regulated kinase activity. The Biochemical journal. 2006;397:329–336. doi: 10.1042/BJ20052002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Ohtsuka T, Stell WK. Endogenous nitric oxide enhances the light-response of cones during light-adaptation in the rat retina. Vision research. 2010 doi: 10.1016/j.visres.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Shi L, Jian K, Ko ML, Trump D, Ko GY. Retinoschisin, a new binding partner for L-type voltage-gated calcium channels in the retina. The Journal of biological chemistry. 2009a;284:3966–3975. doi: 10.1074/jbc.M806333200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Ko ML, Ko GY. Rhythmic expression of microRNA-26a regulates the L-type voltage-gated calcium channel alpha1C subunit in chicken cone photoreceptors. The Journal of biological chemistry. 2009b;284:25791–25803. doi: 10.1074/jbc.M109.033993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DH, Lim HS, Cho SK, et al. Immunocytochemical localization of neuronal and inducible nitric oxide synthase in the retina of zebrafish, Brachydanio rerio. Neuroscience letters. 2000;292:220–222. doi: 10.1016/s0304-3940(00)01407-5. [DOI] [PubMed] [Google Scholar]
- Slomiany BL, Slomiany A. Ghrelin suppression of Helicobacter pylori-induced S-nitrosylation-dependent Akt inactivation exerts modulatory influence on gastric mucin synthesis. Inflammopharmacology. 2011;19:89–97. doi: 10.1007/s10787-011-0078-4. [DOI] [PubMed] [Google Scholar]
- Takemoto N, Tachibanaki S, Kawamura S. High cGMP synthetic activity in carp cones. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:11788–11793. doi: 10.1073/pnas.0812781106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A, Ono M, Shibata S, Watanabe S. Effect of a nitric oxide synthase inhibitor, N-nitro-L-arginine methylester, on light-induced phase delay of circadian rhythm of wheel-running activity in golden hamsters. Neuroscience letters. 1995;192:25–28. doi: 10.1016/0304-3940(95)11599-r. [DOI] [PubMed] [Google Scholar]
- Weber ET, Gannon RL, Michel AM, Gillette MU, Rea MA. Nitric oxide synthase inhibitor blocks light-induced phase shifts of the circadian activity rhythm, but not c-fos expression in the suprachiasmatic nucleus of the Syrian hamster. Brain research. 1995a;692:137–142. doi: 10.1016/0006-8993(95)00685-j. [DOI] [PubMed] [Google Scholar]
- Weber ET, Gannon RL, Rea MA. cGMP-dependent protein kinase inhibitor blocks light-induced phase advances of circadian rhythms in vivo. Neuroscience letters. 1995b;197:227–230. doi: 10.1016/0304-3940(95)11961-u. [DOI] [PubMed] [Google Scholar]
- Yasukawa T, Tokunaga E, Ota H, Sugita H, Martyn JA, Kaneki M. S-nitrosylation-dependent inactivation of Akt/protein kinase B in insulin resistance. The Journal of biological chemistry. 2005;280:7511–7518. doi: 10.1074/jbc.M411871200. [DOI] [PubMed] [Google Scholar]
- Yun HY, Gonzalez-Zulueta M, Dawson VL, Dawson TM. Nitric oxide mediates N-methyl-D-aspartate receptor-induced activation of p21ras. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:5773–5778. doi: 10.1073/pnas.95.10.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DQ, Zhou T, Ruan GX, McMahon DG. Circadian rhythm of Period1 clock gene expression in NOS amacrine cells of the mouse retina. Brain research. 2005;1050:101–109. doi: 10.1016/j.brainres.2005.05.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.