Abstract
Background
Median survival of non-small cell lung cancer (NSCLC) patients with brain metastases is poor. We examined concurrent erlotinib and whole brain radiotherapy (WBRT) followed by maintenance erlotinib in patients with untreated brain metastases, given the potential radiosensitizing properties of erlotinib and its direct effect on brain metastases and systemic activity.
Methods
Eighty NSCLC patients with KPS of 70 and greater and multiple brain metastases were randomly assigned to placebo (n = 40) or erlotinib (100mg, n = 40) given concurrently with WBRT (20 Gy in 5 fractions). Following WBRT, patients continued with placebo or erlotinib (150mg) until disease progression. The primary end point was neurological progression-free survival (nPFS); hazard ratios (HRs) were calculated using Cox regression. All P values were two-sided.
Results
Fifteen patients (37.5%) from each arm were alive and without neurological progression 2 months after WBRT. Median nPFS was 1.6 months in both arms; nPFS HR 0.95 (95% CI = 0.59 to1.54; P = .84). Median overall survival (OS) was 2.9 and 3.4 months in the placebo and erlotinib arms; HR 0.95 (95% CI = 0.58 to 1.55; P = .83). The frequency of epidermal growth factor receptor (EGFR) mutations was low with only 1 of 35 (2.9%) patients with available samples had activating EGFR-mutations. Grade 3/4 adverse event rates were similar between the two groups (70.0% in each arm), except for rash 20.0% (erlotinib) vs 5.0% (placebo), and fatigue 17.5% vs 35.0%. No statistically significant quality of life differences were found.
Conclusions
Our study showed no advantage in nPFS or OS for concurrent erlotinib and WBRT followed by maintenance erlotinib in patients with predominantly EGFR wild-type NSCLC and multiple brain metastases compared to placebo. Future studies should focus on the role of erlotinib with or without WBRT in patients with EGFR mutations.
Up to 40% of patients with non-small cell lung cancer (NSCLC) develop brain metastases (BM), which are associated with poor outcome (median survival <5 months) (1–3). Treatment options include whole-brain radiotherapy (WBRT) with or without corticosteroids. Modifying the radiation dose or fractionation or combining radiotherapy with radiosensitizers have not substantially improved prognosis (4–10). More than half of patients treated with WBRT ultimately die of progressive systemic disease (11–13).
Erlotinib, an epidermal growth factor receptor (EGFR) pathway inhibitor, is currently approved as first-line treatment for advanced NSCLC patients harboring EGFR mutations, and, as maintenance, second-line or third-line treatments following chemotherapy (14–17). Pre-clinical data show that erlotinib enhances the inhibitory effect of ionizing radiation in lung cancer, and it crosses the blood-brain barrier, so it could be used to provide sufficient radiosensitizing and therapeutic level in the brain (18–22).
To exploit the potential radiosensitizing properties, the direct effect on brain metastases, and systemic activity of erlotinib, we examined the role of erlotinib given concurrently with WBRT, then as maintenance.
Methods
Patients
Inclusion criteria were: histologically or cytologically confirmed NSCLC and newly diagnosed multiple BM documented by MRI or contrast CT scan, but did not require immediate chemotherapy for symptom control; aged 18–76 years; no previous cranial radiotherapy; at least 28 days since any chemotherapy; Glasgow Coma Score of 14 and greater; Karnofsky performance status of 70 and greater; 3 or fewer sites of extra-cranial metastases; adequate renal and liver function; negative pregnancy test; and age-modified (age cut-off 76 years instead of 66 years) Radiation Therapy Oncology Group Recursive Partitioning Analysis (RTOG RPA) class I and II (class I is KPS ≥ 70, controlled primary tumor, metastases to brain only, and class II is uncontrolled primary tumor, or primary controlled, but metastases to brain and other sites) (23). Patients with other previous or current malignant disease, solitary brain metastasis suitable for stereotactic radiosurgery or surgical resection, previously treated with any EGFR anti-cancer therapy or currently being treated with Cox II inhibitor were excluded.
Patients were randomly assigned to receive erlotinib or placebo after telephoning the trials center. Randomization was stratified using: presence/absence of extra-cranial metastases, number of sites of brain metastases, age-modified RTOG RPA score, and center.
Study Design and Treatment
We performed a two-stage randomized, multicenter, phase II double-blind, placebo controlled trial (NCT00554775). Multicenter ethics approvals were obtained, and written informed consent from all patients was obtained.
All patients received standard WBRT administered in 20 Gy in 5 daily fractions, starting within 4 weeks of the baseline CT or MR brain scan. Simulation was mandatory for whole brain irradiation and immobilization was recommended. Treatment was delivered by linear accelerator of energy ranging from 4–8 MV photons.
Erlotinib or matched placebo tablets were taken once daily starting on day 1 of WBRT (continuing through weekends). During WBRT the erlotinib dose was 100mg/day (this dose was chosen because of concerns over possible neurotoxicity when the trial was designed). After completing WBRT the erlotinib dose was increased to the standard 150mg/day, until disease progression with symptomatic deterioration. The dose could be reduced or stopped following grade 3 or 4 adverse events that were not controlled by optimal supportive care.
Steroids were limited to dexamethasone; at least 4mg were prescribed during WBRT and for one week after. If medically feasible, the dose was then reduced according to local policy.
Assessments
Within 4 weeks before starting treatment, patients had a physical examination, full blood count, serum chemistry, chest X-ray, CT or MRI scan of the brain, and CT scan of the body. A clinical examination, the mini mental state examination (MMSE), and assessment of motor strength, visual acuity and gait (MVG) were completed before random assignment, two weekly for the first 8 weeks, then monthly until 12 months, and then two-monthly until death. Biological marker assessments, consisting of blood samples and diagnostic tissue (where available), were collected before random assignment. Quality of life (QoL) assessments, using the EuroQol EQ- 5D questionnaire, were made before random assignment, monthly for the first 12 months, and then at 18 and 24 months after random assignment. Adverse events were recorded up to 28 days after finishing erlotinib treatment, and graded using the National Cancer Institute Common Terminology Criteria of Adverse Events (version 3).
Translational Research
Sufficient diagnostic biopsy material (paraffin blocks) and DNA were available for 35 patients of 36 who provided blocks. The DNA extraction was performed using Qiagen FFPE DNA Extraction Kit. EGFR analysis was performed using Qiagen EGFR RGQ polymerase chain reaction (PCR) Kit for real-time PCR analysis of 29 somatic mutations in the EGFR gene (Qiagen, Hilden, Germany).
Statistical Analysis
The primary endpoint was 2-month neurological progression-free survival (nPFS), defined as evidence of either clinical or radiological progression, ie, any one of the following: a loss of 3 points on the MMSE questionnaire compared to baseline; a loss of 3 points on the central nervous system (CNS) scale for MVG or a loss of 2 points for VG (ie, visual acuity & gait assessed by telephone) when compared to baseline; a deterioration of 10 or more points in Karnofsky performance status, compared to baseline, at the 6 week assessment or later; an increase in dexamethasone dose suggestive of progressive disease at the 6 week assessment or later; an increase of 20% or greater in the sum of the longest diameters (LDs) of all metastases present at a baseline of 0.5cm or greater, taking as reference the smallest sum LD recorded since the start of treatment; or the appearance of a new metastasis. Other end points included overall survival (OS), adverse events, and QoL.
A Simon minimax two-stage design for phase II studies was used, to detect a neurological PFS rate at 2 months of 65% or greater with erlotinib, assuming that neurological PFS is less than 50% among patients treated with WBRT only. Using a one-sided 10% statistical significance level and 90% power, the target accrual was 40 patients in the erlotinib arm in the first stage. If, after 2 months, 20 or more patients were alive and neurological progression-free the trial would continue to the second phase and recruit an additional 32 patients for each arm. The same number of placebo patients was specified (ie, a total target of 80 patients in the first stage). The data was reviewed at the end of the first stage by an independent data monitoring committee (IDMC) and all analyses were performed on an intention-to-treat basis. Hazard ratios (HRs), with 95% confidence intervals (CIs) and two-sided P values, were calculated using the Cox proportional hazards model. Survival curves are presented using the Kaplan-Meier method. Patients were censored using the date they were last seen if no event had occurred. Analysis was performed on an intention-to-treat (ITT) basis and generated using SAS software version 9.3 (SAS Institute, Cary, NC).
Results
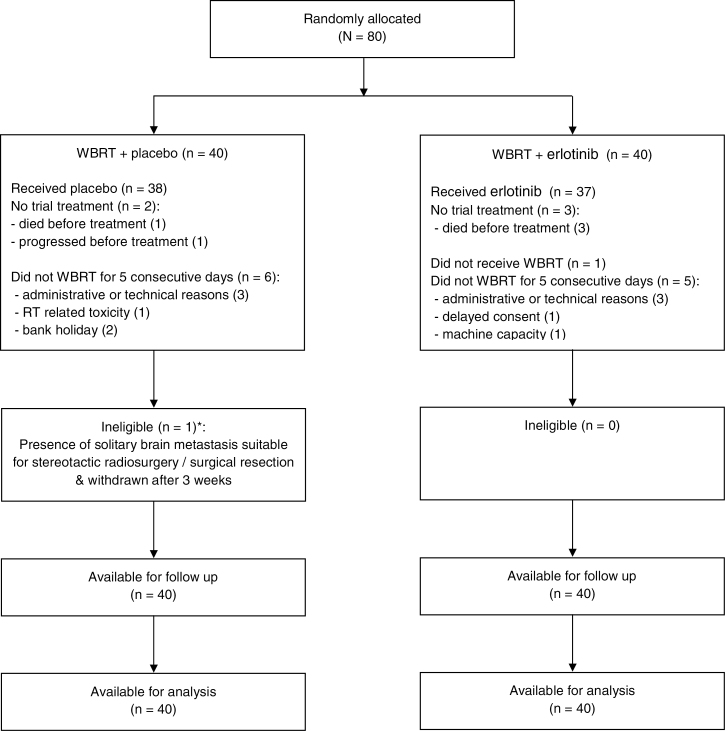
Eighty patients were recruited (June 2009 to June 2010) from 18 centers across the UK National Cancer Research Network: 40 placebo and 40 erlotinib. Table 1 shows the baseline characteristics, and Figure 1 the CONSORT diagram.
Table 1.
Baseline characteristics
| Variable | WBRT+placebo (n = 40)* | WBRT+erlotinib (n = 40)† |
|---|---|---|
| No. (%) | No. (%) | |
| Age at random assignment, y | ||
| Median | 62.2 | 61.3 |
| Range | 41 - 73 | 48 - 75 |
| Sex | ||
| Female | 19 (47.5) | 25 (62.5) |
| Male | 21 (52.5) | 15 (37.5) |
| Age-modified RTOG RPA‡ | ||
| I | 8 (20.0) | 10 (25.0) |
| II | 32 (80.0) | 30 (75.0) |
| Brain metastases, no. | ||
| ≤3 | 26 (65.0) | 23 (57.5) |
| >3 | 14 (35.0) | 17 (42.5) |
| Extra-cranial metastases | ||
| Absent | 16 (40.0) | 15 (37.5) |
| Present | 24 (60.0) | 25 (62.5) |
| Karnofsky performance score | ||
| 70 | 13 (32.5) | 9 (22.5) |
| 80 | 15 (37.5) | 22 (55.0) |
| 90 | 9 (22.5) | 9 (22.5) |
| 100 | 3 (7.5) | 0 (0.0) |
| Histology | ||
| Adenocarcinoma | 20 (50.0) | 23 (57.5) |
| Squamous | 7 (17.5) | 5 (12.5) |
| Other | 6 (15.0) | 5 (12.5) |
| Unspecified | 7 (17.5) | 7 (17.5) |
* One patient died before treatment, and one patient progressed before treatment.
† Three patients died before treatment.
‡ Radiation Therapy Oncology Group Recursive Partitioning Analysis.
Figure 1.
CONSORT diagram, including the number of patients who started and continued trial treatment, and the reasons for stopping early.
Treatment Compliance
Seventy-five patients (94%) started trial treatment (n = 5 had either died or progressed beforehand). Median time on the study drug was 1.3 (placebo) and 1.8 (erlotinib) months. Thirty-one patients in each group (78%) took their erlotinib/placebo tablets for at least 75% of the time they were in the trial, ie, until they died/stopped treatment early (Supplementary Table 1, available online). Two patients in the placebo arm and 3 in the erlotinib arm temporarily stopped the study drug at 7 or later days. Only one patient in the erlotinib arm experienced toxicity leading to a dose reduction from 150 to 100mg daily (due to skin rash).
One patient (erlotinib) did not receive any WBRT. Six patients (placebo) and 5 patients (erlotinib) did not receive WBRT for 5 consecutive days excluding weekends (Figure 1).
Efficacy
Fifteen patients (37.5%) in each treatment group were alive and free from neurological progression at 2 months, less than the target of 20 or greater, so the IDMC recommended not proceeding to stage 2.
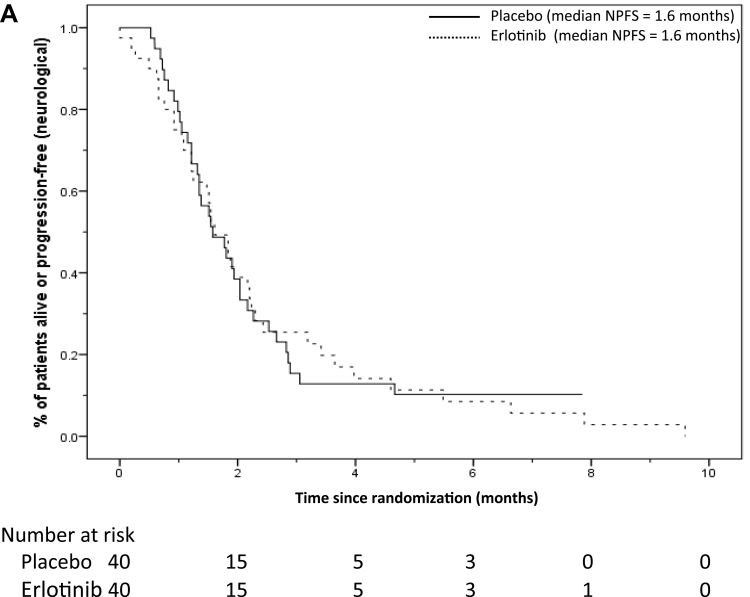
The median follow up was 12.6 months (censoring those who had died). There were 35 placebo and 38 erlotinib patients who had died or had neurological progression, with median neurological PFS of 1.6 months in each group (Figure 2). The 2-month PFS rate was 38.9%, 95% CI = 23.6% to 54.2% (erlotinib), compared to 38.5%, 95% CI = 23.2% to 53.7% (placebo).
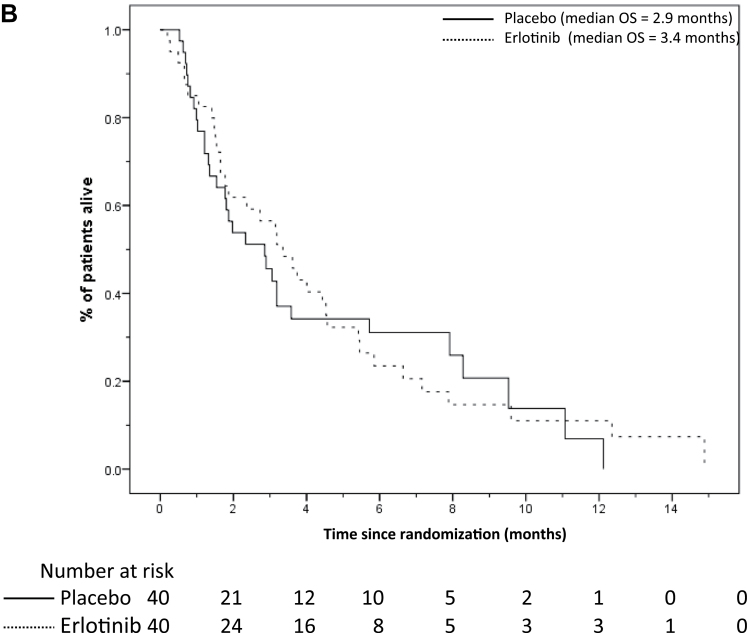
Figure 2.
A) Neurological progression-free survival (NPFS) and B) overall survival (OS) curves according to treatment arms in all patients. The number of patients at risk in each group at various time points is located under each graph.
The unadjusted HR for neurological PFS was 0.99 (95% CI = 0.62 to1.58; P = .97), which became 0.95 (95% CI = 0.59 to 1.54; P = .84) after allowing for the randomization stratification factors. The adjusted HR among the 75 patients who started the study drug was 0.89 (95% CI = 0.54 to 1.46; P = .65).
Sixty-six patients died (31 placebo and 35 erlotinib), with a median OS of 2.9 and 3.4 months, respectively (Figure 2). The unadjusted OS HR was 0.94 (95% CI = 0.58 to 1.54; P = .81), and 0.95 (95% CI = 0.58 to 1.55; P = .83) after allowing for stratification factors. The adjusted HR among the 75 patients who started the study drug was 0.90 (95% CI = 0.54 to1.51; P = .69). The 6-month OS rates for RTOG RPA class I patients were: 56.3% (placebo arm, n = 8 and 3 deaths); and 45.0% (erlotinib arm, n = 10 and 8 deaths). For RTOG class II patients, the rates were: 24.3% (placebo n = 32 and 28 deaths); and 16.6% (erlotinib, n = 30 and 27 deaths) (Supplementary Figure 1, available online).
Toxicity
Twenty-eight (70.0%) patients suffered a grade 3 or 4 adverse event in each treatment group (Table 2). More patients in the erlotinib group experienced rash as expected (20.0% vs 5.0%); and fewer erlotinib patients had grade 3/4 fatigue (17.5% vs 35.0%). No suspected unexpected serious adverse reactions were reported.
Table 2.
Reported grade 3 or 4 toxicities
| Variable | WBRT+placebo (n = 40)* | WBRT+erlotinib (n = 40)† |
|---|---|---|
| No. (%) | No. (%) | |
| Any toxicity (each patient counted once) | 28 (70.0) | 28 (70.0) |
| Dyspnoea | 15 (37.5) | 14 (35.0) |
| Fatigue | 14 (35.0) | 7 (17.5) |
| Rash | 2 (5.0) | 8 (20.0) |
| Infection | 2 (5.0) | 5 (12.5) |
| Myopathy | 4 (10.0) | 2 (5.0) |
| Anorexia | 3 (7.5) | 2 (5.0) |
| Pain | 3 (7.5) | 2 (5.0) |
| Diarrhoea | 2 (5.0) | 2 (5.0) |
| Headache | 4 (10.0) | 0 (0.0) |
| Muscle weakness (myopathy) | 3 (7.5) | 0 (0.0) |
| Anaemia | 2 (5.0) | 0 (0.0) |
| Dehydration | 0 (0.0) | 2 (5.0) |
| Pulmonary embolism | 1 (2.5) | 1 (2.5) |
| Seizure | 2 (5.0) | 0 (0.0) |
| Somnolence | 1 (2.5) | 1 (2.5) |
| Constipation | 0 (0.0) | 1 (2.5) |
| Dry skin | 0 (0.0) | 1 (2.5) |
| Nausea | 0 (0.0) | 1 (2.5) |
| Pneumonitis | 1 (2.5) | 0 (0.0) |
| Other | 9‡ (22.5) | 10║ (25.0) |
* One patient died before treatment, and one patient progressed before treatment.
† Three patients died before treatment.
‡ Ataxia, facial oedema, fainted, fractured humerus, hydrocephalus, low haemoglobin, neuropathy-motor, pleural effusion, urinary retention.
║ Alopecia, aspiration, dysasthria, dyspepsia, expressive dysphasia, hyperglycemia, hypotension, metabolic acidosis, pancreatitis, raised alanine transaminase.
Quality of Life
There was no evidence of differences in the QoL scores between treatment groups at one or two months, adjusting for baseline scores (all P ≥ .40) (Supplementary Table 2, available online).
Biological Substudy
The activating EGFR mutation-positive rate was only 2.9% (1/35). This patient had a neurological PFS time of 1.3 months and survived 1.6 months after random assignment.
Discussion
We showed that concurrent erlotinib with WBRT followed by maintenance erlotinib did not improve neurological PFS or OS in our patients with multiple brain metastases. The median neurological PFS and OS in this group were disappointingly low; 1.6 months and 3.4 months respectively, despite selecting only patients with age-modified RTOG RPA class I and II.
However, a recent single arm phase II trial (40 patients) of erlotinib plus concurrent WBRT reported a median survival of 11.8 months and a high intracranial response rate, but more than 50% of patients had activating EGFR mutations. Similar to our study, they found erlotinib with WBRT was well tolerated with no unexpected neurotoxicity (24). In another phase II study, 28 NSCLC patients harboring EGFR mutations were treated with either gefitinib or erlotinib alone (25). Response rates in the brain were high, with 23 patients (83%) achieving a partial response, with a median progression-free survival of 6.6 months, and an OS of 15.9 months. Both studies showed encouraging results with EGFR tyrosine-kinase inhibitors (TKIs) with or without WBRT in patients with activating EGFR mutation tumors in contrast to our study where patients had predominantly wild-type EGFR tumors. There have been several case reports describing disease response in the brain metastases harboring EGFR mutations with TKI alone, and also that refractory CNS and lepto-meningeal metastases can be improved by escalating the dose of erlotinib, correlated with higher cerebrospinal fluid (CSF) concentrations (19–22, 26, 27).
In contrast to Welsh et al (24), we found the frequency of EGFR mutations in our treated patients was low (3%). This low frequency mutation rate was similar to that reported in another recent study examining the frequency of EGFR mutations in brain metastases among NSCLC patients (28). In 77 brain tumor samples, the rate of EGFR mutations was 3.9% (3/77) in contrast to a KRAS mutation rate of 39% (30/77). The lack of anti-brain metastasis activity in EGFR wild-type tumors is compatible with a reported retrospective study. This study of 69 NSCLC patients with brain metastases found responses were only observed in patients with activating EGFR mutation (82%) but not wild-type tumors (22). Additionally, median time to progression within the brain and OS were 5.8 and 3.1 months for unselected patients, compared to 11.7 and 12.9 months, respectively, for patients harboring EGFR mutations. We hypothesize that for EGFR wild-type tumors, erlotinib concentration achieved in the CSF is sub-therapeutic and also too low to exert radio-sensitizing activity. Further research could explore escalating the erlotinib dose before WBRT or strategies to disrupt the blood-brain barrier in order to increase erlotinib CSF penetration.
We also found that overall median survival for unselected patients with multiple brain metastases treated with WBRT alone was low (2.9 months) despite selecting only patients with Karnofsky performance statuses of 70 and greater (23). Welsh et al (24) used 3000cGy WBRT in 10 fractions, and we used 2000cGy WBRT in five fractions, but this is unlikely to be the main reason why our patients had a poor survival. Previous trials and a systematic review reported no improvement in the frequency and duration of response, survival or QoL for radiation doses ranging from 20 Gy in five fractions to 50 Gy in 20 fractions (5, 29, 30). The RTOG report that patients had a median survival of 7.1, 4.2 and 2.3 months for classes I, II and III, respectively. The sole criterion required to classify a patient in the poorest prognostic group (class III) is a KPS of less than 70. Despite excluding patients with KPS of less than 70, the median OS in our trial was similar to that reported for RPA class III. The three-tier RTOG RPA prognostic classification, based on several cancers (lung, breast, melanoma, colorectal, and others), may not be entirely applicable to NSCLC patients with multiple brain metastases.
Our finding does not preclude the possibility that erlotinib is effective for EGFR mutation positive tumors as only 2.9% of assessed patients manifested EGFR mutations. Future studies should focus on the role of erlotinib with or without WBRT in NSCLC patients harboring EGFR mutations. This includes comparing erlotinib alone with WBRT plus erlotinib, erlotinib alone with stereotactic radiotherapy or surgery for patients with 3 or fewer brain metastases, escalating the dose of erlotinib in patients with CNS relapse after TKI treatment, or strategies to disrupt blood brain barrier in order to increase erlotinib delivery to the brain.
In conclusion, we showed no statistically significant improvement in neurological PFS or OS in unselected patients treated with concurrent erlotinib and WBRT followed by maintenance erlotinib compared to placebo.
Funding
Cancer Research UK (C1438/A6406 and C1438/A10010) and an educational grant from Roche for the translational studies were awarded to SML. We also thank the support of University College London and University College London Hospital Comprehensive Biomedical Research Centre.
The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
We are most grateful to all the participating patients, clinicians (see below), and local research staff. We would also like to express our gratitude to the independent Data Monitoring Committee for their advice and comments throughout the trial.
Participating clinicians and centers: Christie Hospital (Dr Corrine Faivre-Finn); Charing Cross (Dr Conrad Lewanski); UCLH (Prof SM Lee); Southampton (Dr Andrew Bates); Aberdeen Royal Infirmary (Dr Donald Bissett); Scunthorpe General Hospital (Dr Sunil Upadhyay); Torbay Hospital (Dr Geoffrey Cogill); Velindre Hospital (Dr Jason Lester); Diana, Princess of Wales Hospital (Dr Sunil Upadhyay); Liverpool Heart and Chest (Dr Joseph Maguire); North Wales Cancer Treatment Centre, Glan Clwyd Hospital (Dr Angel Garcia-Alonso); Countess of Chester Hospital (Dr Joseph Maguire); Dorset Cancer Centre (Dr Virginia Laurence); Furness General Hospital (Dr Geraldine Skailes); North Middlesex University Hospital (Dr Julian Singer); Royal Lancaster Infirmary (Dr Geraldine Skailes); St Mary’s Hospital, Isle of Wight (Dr Judith Cave); Wrexham Maelor Hospital (Dr Angel Garcia-Alonso)
References
- 1. Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973–2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865–2872 [DOI] [PubMed] [Google Scholar]
- 2. Mamon HJ, Yeap BY, Janne PA, et al. High risk of brain metastases in surgically staged IIIA non-small-cell lung cancer patients treated with surgery, chemotherapy, and radiation. J Clin Oncol. 2005;23(7):1530–1537 [DOI] [PubMed] [Google Scholar]
- 3. Mujoomdar A, Austin JHM, Malhotra R, et al. Clinical predictors of metastatic disease to the brain from non-small-cell lung carcinoma: primary tumor size, cell type, and lymph node metastasis. Radiology. 2007;242(3):882–888 [DOI] [PubMed] [Google Scholar]
- 4. Borgelt BN, Gelber R, Kramer S, et al. The palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys.1980;6(1):1–9 [DOI] [PubMed] [Google Scholar]
- 5. Gelber RD, Lorson M, Borgelt B, et al. Equivalence of radiation schedules for the palliative treatment of brain metastases in patients with favourable prognosis. Cancer. 1981;48(8):1749–1753 [DOI] [PubMed] [Google Scholar]
- 6. Kurtz JM, Gelber R, Brady LW, et al. The palliation of brain metastases in a favourable patient population: a randomised clinical trial by the radiation therapy oncology group. Int J Radiat Oncol Biol Phys. 1981;7(7):891–895 [DOI] [PubMed] [Google Scholar]
- 7. Komarnicky LT, Phillips TL, Martz K, et al. A randomized phase III protocol for the evaluation of misonidazole combined with radiation in the treatment of patients with brain metastases (RTOG-7916). Int J Radiat Oncol Biol Phys. 1991;20(1):53–58 [DOI] [PubMed] [Google Scholar]
- 8. Sause WT, Scott C, Krisch R., et al. Phase I/II trial of accelerated fractionation in brain metastases RTOG 85-28. Int J Radiat Oncol Biol Phys. 1993;26(4):653–657 [DOI] [PubMed] [Google Scholar]
- 9. Murray KJ, Scott C, Greenberg HM. A randomized phase III study of accelerated hyperfractionation versus standard in patients with unresected brain metastases. A report of the Radiation Therapy Oncology Group (RTOG) 9104. Int J Radiat Oncol Biol Phys. 1997;39(3):571–574 [DOI] [PubMed] [Google Scholar]
- 10. Verger E, Gil M, Yaya R, et al. Temozolomide and concomitant whole brain radiotherapy in patients with brain metastases: a phase II randomized trial. Int J Radiat Oncol Biol Phys. 2005;61(1):185–191 [DOI] [PubMed] [Google Scholar]
- 11. Caincross JG, Kim JH, Posner JB. Radiation therapy for brain metastases. Ann Neurol. 1980;7(6):529–541 [DOI] [PubMed] [Google Scholar]
- 12. Zimm S, Wampler GL, Stablein D, et al. Intracerebral metastases in solid-tumor patients: natural history and results of treatment. Cancer. 1981;48(2):384–394 [DOI] [PubMed] [Google Scholar]
- 13. Caincross JG, Posner JB. The management of brain metastases. In: Walker MD. (ed). Oncology of the Nervous System. Boston, MA: Martinus Nijhoff; 1983:341 [Google Scholar]
- 14. Shepherd FA, Rodrigues-Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123–132 [DOI] [PubMed] [Google Scholar]
- 15. Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11(6):521–529 [DOI] [PubMed] [Google Scholar]
- 16. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742 [DOI] [PubMed] [Google Scholar]
- 17. Rosell R, Gervais R, Vergnenegre A, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246 [DOI] [PubMed] [Google Scholar]
- 18. Chinnaiyan P, Huang S, Vallabhaneni G, et al. Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva). Cancer Res. 2005;65(8):3328–3335 [DOI] [PubMed] [Google Scholar]
- 19. Lai CS, Boshoff C, Falzon M, Lee SM. Complete response to erlotinib treatment in brain metastases from recurrent NSCLC. Thorax. 2006;61(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weber B, Winterdahl M, Memon A, et al. Erlotinib accumulation in brain metastases from non-small cell lung cancer: visualization by positron emission tomography in a patient harboring a mutation in the epidermal growth factor receptor. J Thoracic Oncol. 2011;6(7):1287–1289 [DOI] [PubMed] [Google Scholar]
- 21. Hata A, Kaji R, Fujita S, Katakami N. High-dose erlotinib for refractory brain metastases in a patient with relapsed non-small cell lung cancer. J Thoracic Oncol. 2011;6(3):653–654 [DOI] [PubMed] [Google Scholar]
- 22. Porta R, Sánchez-Torres JM, Paz-Ares L, et al. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur Respir J. 2011;37(3):624–631 [DOI] [PubMed] [Google Scholar]
- 23. Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three radiation therapy oncology group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745–751 [DOI] [PubMed] [Google Scholar]
- 24. Welsh JW, Komaki R, Amini A, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol. 2013;31(7):895–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Park SJ, Kim HT, Lee DH, et al. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer. 2012;77(3):556–560 [DOI] [PubMed] [Google Scholar]
- 26. Togashi Y, Masago K, Masuda S, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol. 2012;70(3):399–405 [DOI] [PubMed] [Google Scholar]
- 27. Katayama T, Shimizu J, Suda K, et al. Efficacy of erlotinib for brain and leptomeningeal metastases in patients with lung adenocarcinoma who showed initial good response to gefitinib. J Thorac Oncol. 2009;4(11):1415–1419 [DOI] [PubMed] [Google Scholar]
- 28. Villalva C, Duranton-Tanneur V, Guilloteau K. EGFR, KRAS, BRAF, and HER-2 molecular status in brain metastases from 77 NSCLC patients. Cancer Med. 2013;2(3):296–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Borgelt B, Gelber R, Kramer S, et al. The palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1980;6(1):1–9 [DOI] [PubMed] [Google Scholar]
- 30. Tsao MN, Lloyd N, Wong RK, et al. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst Rev. 2012;4:CD003869. [DOI] [PMC free article] [PubMed] [Google Scholar]