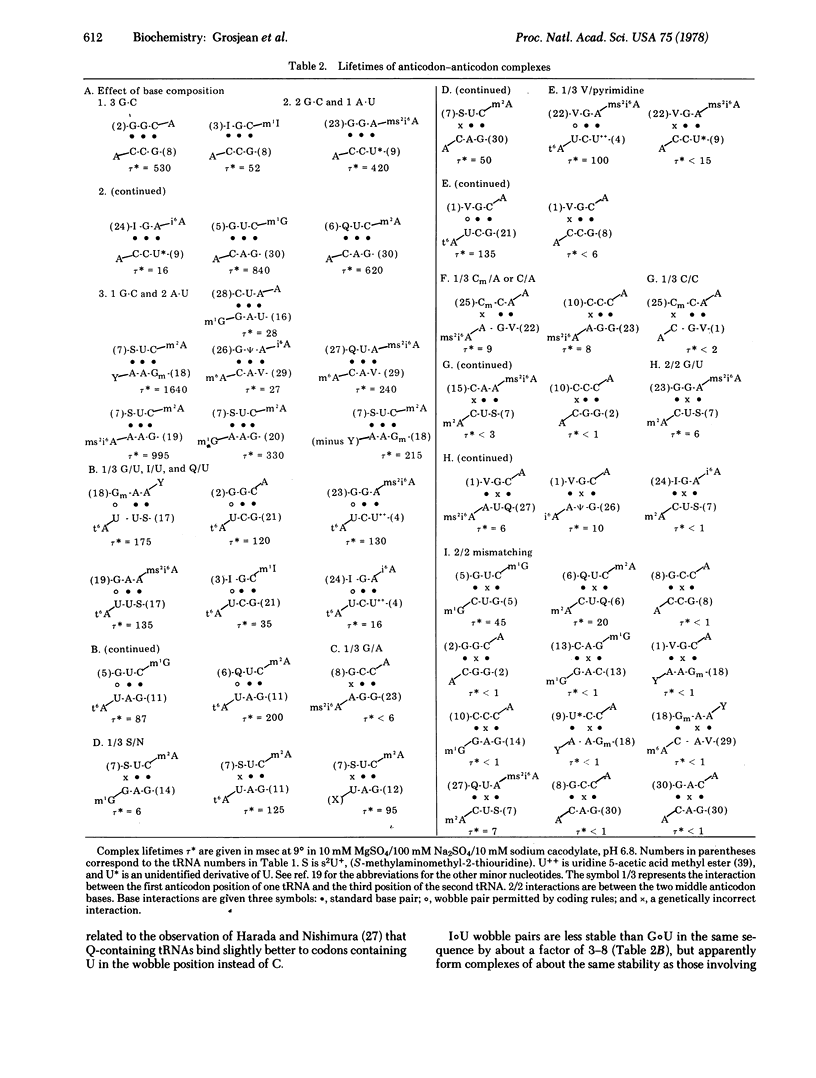
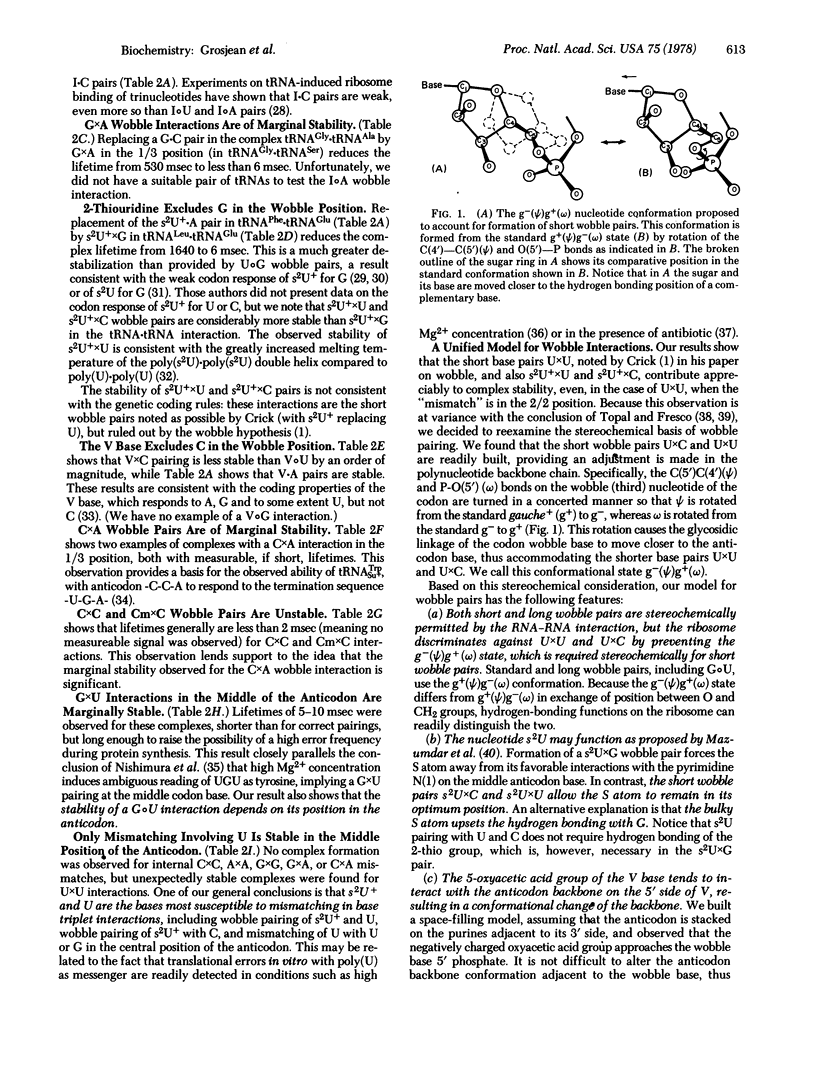
Abstract
We report the relative stabilities, in the form of complex lifetimes, of complexes between the tRNAs complementary, or nearly so, in their anticodons. The results show striking parallels with the genetic coding rules, including the wobble interaction and the role of modified nucleotides S2U and V (a 5-oxyacetic acid derivative of U). One important difference between the genetic code and the pairing rules in the tRNA-tRNA interaction is the stability in the latter of the short wobble pairs, which the wobble hypothesis excludes. We stress the potential of U for translational errors, and suggest a simple stereochemical basis for ribosome-mediated discrimination against short wobble pairs. Surprisingly, the stability of anticodon-anticodon complexes does not vary systematically on base sequence. Because of the close similarity to the genetic coding rules, it is tempting to speculate that the interaction between two RNA loops may have been part of the physical basis for the evolutionary origin of the genetic code, and that this mechanism may still be utilized by folding the mRNA on the ribosome into a loop similar to the anticodon loop.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borer P. N., Dengler B., Tinoco I., Jr, Uhlenbeck O. C. Stability of ribonucleic acid double-stranded helices. J Mol Biol. 1974 Jul 15;86(4):843–853. doi: 10.1016/0022-2836(74)90357-x. [DOI] [PubMed] [Google Scholar]
- Bähr W., Faerber P., Scheit K. H. The effects of thioketo substitution upon uracil-adenine interactions in polyribonucleotides. Synthesis and properties of poly (2-thiouridylic acid) and poly(2,4-dithiouridylic acid). Eur J Biochem. 1973 Mar 15;33(3):535–544. doi: 10.1111/j.1432-1033.1973.tb02713.x. [DOI] [PubMed] [Google Scholar]
- Crick F. H. Codon--anticodon pairing: the wobble hypothesis. J Mol Biol. 1966 Aug;19(2):548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- Eigen M. Selforganization of matter and the evolution of biological macromolecules. Naturwissenschaften. 1971 Oct;58(10):465–523. doi: 10.1007/BF00623322. [DOI] [PubMed] [Google Scholar]
- Eisinger J. Complex formation between transfer RNA'S with complementary anticodons. Biochem Biophys Res Commun. 1971 May 21;43(4):854–861. doi: 10.1016/0006-291x(71)90695-4. [DOI] [PubMed] [Google Scholar]
- Eisinger J., Feuer B., Yamane T. Codon-anticodon binding in tRNAphe. Nat New Biol. 1971 May 26;231(21):126–128. doi: 10.1038/newbio231126a0. [DOI] [PubMed] [Google Scholar]
- Eisinger J., Gross N. The anticodon-anticodon complex. J Mol Biol. 1974 Sep 5;88(1):165–174. doi: 10.1016/0022-2836(74)90302-7. [DOI] [PubMed] [Google Scholar]
- Garen A. Sense and nonsense in the genetic code. Three exceptional triplets can serve as both chain-terminating signals and amino acid codons. Science. 1968 Apr 12;160(3824):149–159. doi: 10.1126/science.160.3824.149. [DOI] [PubMed] [Google Scholar]
- Gralla J., Crothers D. M. Free energy of imperfect nucleic acid helices. 3. Small internal loops resulting from mismatches. J Mol Biol. 1973 Aug 5;78(2):301–319. doi: 10.1016/0022-2836(73)90118-6. [DOI] [PubMed] [Google Scholar]
- Grosjean H., Söll D. G., Crothers D. M. Studies of the complex between transfer RNAs with complementary anticodons. I. Origins of enhanced affinity between complementary triplets. J Mol Biol. 1976 May 25;103(3):499–519. doi: 10.1016/0022-2836(76)90214-x. [DOI] [PubMed] [Google Scholar]
- Grosjean H., Takada C., Petre J. Complex formation between transfer RNAs with complementary anticodons: use of matrix bound tRNA. Biochem Biophys Res Commun. 1973 Aug 6;53(3):882–893. doi: 10.1016/0006-291x(73)90175-7. [DOI] [PubMed] [Google Scholar]
- Harada F., Nishimura S. Possible anticodon sequences of tRNA His , tRNA Asm , and tRNA Asp from Escherichia coli B. Universal presence of nucleoside Q in the first postion of the anticondons of these transfer ribonucleic acids. Biochemistry. 1972 Jan 18;11(2):301–308. doi: 10.1021/bi00752a024. [DOI] [PubMed] [Google Scholar]
- Hilbers C. W., Shulman R. G., Kim S. H. High resolution NMR study of the melting of yeast tRNA Phe. Biochem Biophys Res Commun. 1973 Dec 10;55(3):953–960. doi: 10.1016/0006-291x(73)91235-7. [DOI] [PubMed] [Google Scholar]
- Hirsh D. Tryptophan transfer RNA as the UGA suppressor. J Mol Biol. 1971 Jun 14;58(2):439–458. doi: 10.1016/0022-2836(71)90362-7. [DOI] [PubMed] [Google Scholar]
- Hopfield J. J. Kinetic proofreading: a new mechanism for reducing errors in biosynthetic processes requiring high specificity. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4135–4139. doi: 10.1073/pnas.71.10.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Högenauer G. Binding of UGA to wild type and suppressor tryptophan tRNA from E. coli. FEBS Lett. 1974 Mar 1;39(3):310–312. doi: 10.1016/0014-5793(74)80137-7. [DOI] [PubMed] [Google Scholar]
- Högenauer G. The stability of a codon transfer RNA complex. Eur J Biochem. 1970 Feb;12(3):527–532. doi: 10.1111/j.1432-1033.1970.tb00882.x. [DOI] [PubMed] [Google Scholar]
- Ishikura H., Yamada Y., Nishimura S. Structure of serine tRNA from Escherichia coli. I. Purification of serine tRNA's with different codon responses. Biochim Biophys Acta. 1971 Jan 28;228(2):471–481. doi: 10.1016/0005-2787(71)90052-9. [DOI] [PubMed] [Google Scholar]
- Kuntzel B., Weissenbach J., Wolff R. E., Tumaitis-Kennedy T. D., Lane B. G., Dirheimer G. Presence of the methylester of 5-carboxymethyl uridine in the wobble position of the anticodon of tRNAIII Arg from brewer's yeast. Biochimie. 1975;57(1):61–70. doi: 10.1016/s0300-9084(75)80110-6. [DOI] [PubMed] [Google Scholar]
- Mazumdar S. K., Saenger W. Molecular structure of poly-2-thiouridylic acid, a double helix with non-equivalent polynucleotide chains. J Mol Biol. 1974 May 15;85(2):213–219. doi: 10.1016/0022-2836(74)90361-1. [DOI] [PubMed] [Google Scholar]
- Ninio J. A semi-quantitative treatment of missense and nonsense suppression in the strA and ram ribosomal mutants of Escherichia coli. Evaluation of some molecular parameters of translation in vivo. J Mol Biol. 1974 Apr 5;84(2):297–313. doi: 10.1016/0022-2836(74)90586-5. [DOI] [PubMed] [Google Scholar]
- Ninio J. Kinetic amplification of enzyme discrimination. Biochimie. 1975;57(5):587–595. doi: 10.1016/s0300-9084(75)80139-8. [DOI] [PubMed] [Google Scholar]
- Nishimura S., Harada F., Hirabayashi M. Nature of magnesium-induced miscoding. J Mol Biol. 1969 Mar 14;40(2):173–186. doi: 10.1016/0022-2836(69)90467-7. [DOI] [PubMed] [Google Scholar]
- Oashi Z., Saneyoshi M., Harada F., Hara H., Nishimura S. Presumed anticodon structure of glutamic acid tRNA from E. coli: a possible location of a 2-thiouridine derivative in the first position of the anticodon. Biochem Biophys Res Commun. 1970 Aug 24;40(4):866–872. doi: 10.1016/0006-291x(70)90983-6. [DOI] [PubMed] [Google Scholar]
- Pongs O., Bald R., Reinwald E. On the structure of yeast tRNA Phe . Complementary-oligonucleotide binding studies. Eur J Biochem. 1973 Jan 3;32(1):117–125. doi: 10.1111/j.1432-1033.1973.tb02586.x. [DOI] [PubMed] [Google Scholar]
- SZER W., OCHOA S. COMPLEXING ABILITY AND CODING PROPERTIES OF SYNTHETIC POLYNUCLEOTIDES. J Mol Biol. 1964 Jun;8:823–834. doi: 10.1016/s0022-2836(64)80163-7. [DOI] [PubMed] [Google Scholar]
- Tazawa I., Tazawa S., Ts'o P. O. Studies on oligonucleotides: thermodynamic and optical properties of the oligoinosinate-polycytidylate complexes. J Mol Biol. 1972 Apr 28;66(1):115–130. doi: 10.1016/s0022-2836(72)80010-x. [DOI] [PubMed] [Google Scholar]
- Topal M. D., Fresco J. R. Base pairing and fidelity in codon-anticodon interaction. Nature. 1976 Sep 23;263(5575):289–293. doi: 10.1038/263289a0. [DOI] [PubMed] [Google Scholar]
- Topal M. D., Fresco J. R. Complementary base pairing and the origin of substitution mutations. Nature. 1976 Sep 23;263(5575):285–289. doi: 10.1038/263285a0. [DOI] [PubMed] [Google Scholar]
- Uhlenbeck O. C., Baller J., Doty P. Complementary oligonucleotide binding to the anticodon loop of fMet-transfer RNA. Nature. 1970 Feb 7;225(5232):508–510. doi: 10.1038/225508a0. [DOI] [PubMed] [Google Scholar]
- Uhlenbeck O. C. Complementary oligonucleotide binding to transfer RNA. J Mol Biol. 1972 Mar 14;65(1):25–41. doi: 10.1016/0022-2836(72)90489-5. [DOI] [PubMed] [Google Scholar]
- Unger F. M., Takemura S. A comparison between inosine- and guanosine-containing anticodons in ribosome-free codon-anticodon binding. Biochem Biophys Res Commun. 1973 Jun 19;52(4):1141–1147. doi: 10.1016/0006-291x(73)90619-0. [DOI] [PubMed] [Google Scholar]
- Vormbrock R., Morawietz R., Gassen H. G. Codon-anticodon interaction studies with trinucleoside diphosphates containing 2-thiouridine, 4-thiouridine, 2,4-diethiouridine, or 2-thiocytidine. Biochim Biophys Acta. 1974 Mar 27;340(3):348–358. [PubMed] [Google Scholar]
- Weiss G. B. Translational control of protein synthesis by tRNA unrelated to changes in tRNA concentration. J Mol Evol. 1973;2(2-3):199–204. doi: 10.1007/BF01654000. [DOI] [PubMed] [Google Scholar]
- Yoon K., Turner D. H., Tinoco I., Jr The kinetics of codon-anticodon interaction in yeast phenylalanine transfer RNA. J Mol Biol. 1975 Dec 25;99(4):507–518. doi: 10.1016/s0022-2836(75)80169-0. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Takeishi K., Ukita T. Structural studies on a yeast glutamic acid tRNA specific to GAA codon. Biochim Biophys Acta. 1971 Jan 1;228(1):153–166. doi: 10.1016/0005-2787(71)90555-7. [DOI] [PubMed] [Google Scholar]


