Abstract
The prevalence of diabetes increases with age, driven in part by an absolute increase in incidence among adults aged 65 years and older. Individuals with diabetes are at higher risk for cardiovascular disease, and age strongly predicts cardiovascular complications. Inflammation and oxidative stress appear to play some role in the mechanisms underlying aging, diabetes, cardiovascular disease, and other complications of diabetes. However, the mechanisms underlying the age-associated increase in risk for diabetes and diabetes-related cardiovascular disease remain poorly understood. Moreover, because of the heterogeneity of the older population, a lack of understanding of the biology of aging, and inadequate study of the effects of treatments on traditional complications and geriatric conditions associated with diabetes, no consensus exists on the optimal interventions for older diabetic adults. The Association of Specialty Professors, along with the National Institute on Aging, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Heart, Lung, and Blood Institute, and the American Diabetes Association, held a workshop, summarized in this Perspective, to discuss current knowledge regarding diabetes and cardiovascular disease in older adults, identify gaps, and propose questions to guide future research.
Introduction
In the U.S., approximately one in four adults aged 65 years or older has diabetes (1). Patients with diabetes are at very high risk for developing cardiovascular disease (CVD) and associated morbidity and mortality, and this risk increases dramatically with age (2,3). Contributors to CVD risk in diabetes include hyperglycemia, dyslipidemia, obesity, insulin resistance, inflammation, hypertension, autonomic dysfunction, and diminished vascular responsiveness. The Cardiovascular Health Study and others have documented the CVD risk in diabetic patients, as well as relevant associations in elderly populations, in some detail (3,4). Although many interventions targeting hypertension and dyslipidemia have been demonstrated to reduce risk of CVD in individuals with diabetes, only limited data are available from studies directly testing the effectiveness of these interventions in older populations with diabetes (2,3). Thus far, interventions targeting hyperglycemia have had limited or no benefit on cardiovascular risk reduction (reviewed in 3). Interventions to prevent or delay progression from prediabetes to diabetes in high-risk individuals have been effective, especially lifestyle interventions in older individuals (5), but long-term outcomes for cardiovascular events are not known.
This workshop brought together experts in diabetes, diabetes-related CVD, and geriatric conditions (3). The primary goals of the workshop were to review current knowledge and to identify key research areas to be addressed. Discussions focused on clinical epidemiology of diabetes and concomitant CVD in aging populations, including racial and ethnic disparities in diabetes prevalence, metabolic changes with age and their impact on diabetes, vulnerabilities of the heart and blood vessels to aging with diabetes, and end-organ sequelae that lead to disability. The workshop concluded with a thorough discussion of prevention and treatments for CVD in older patients with diabetes or prediabetes and unanswered research questions in this area.
Epidemiology of Diabetes and Concomitant CVD in an Aging Population
Among adults aged 65 years or older in the U.S., the combined prevalence of prediabetes and diabetes (both diagnosed and undiagnosed) ranges from 50 to 80%, depending on which measures of hyperglycemia are used (1). Approximately $300 billion is spent annually on patients with diabetes in the U.S., and the majority of these costs is spent on older adults with long-standing diabetes and severe complications (6). With the baby-boom cohort reaching the geriatric age range, the number of new diabetes cases is expected to increase, even if prevention efforts are somewhat successful.
Prevalence of diagnosed and undiagnosed diabetes is higher among Mexican Americans, African Americans, and Asian Americans than it is among white Americans. Although disparities are apparent in access to care in the U.S., Diabetes Study of Northern California (DISTANCE) data (see Table 1 for study names and descriptions) indicate marked racial and ethnic disparities in the prevalence and incidence of diabetes and cardiovascular outcomes, even in a population with uniform access to care (7). Furthermore, there is substantial heterogeneity within large ethnic groups. For example, incidence and prevalence vary widely in subpopulations of Asian Americans. These disparities are similar between men and women but recent evidence suggests that they are magnified among adults older than 60 years.
Table 1.
Names and characteristics of large clinical studies in diabetes
| Acronym | Study title | Area of interest | Study population | Reference |
|---|---|---|---|---|
| ACCORD | Action to Control Cardiovascular Risk in Diabetes | Outcomes of hyperglycemia treatment and lipid lowering in diabetes | 5,518 patients with type 2 diabetes and receiving open-label simvastatin | 41 |
| ADVANCE | Action in Diabetes and Vascular Disease | Outcomes of hyperglycemia treatment and blood pressure lowering in diabetes | 11,140 patients with type 2 diabetes | 46 |
| BARI 2D | Bypass Angioplasty Revascularization Investigation in 2 Diabetes | CAD treatment | 2,368 patients with type 2 diabetes and heart disease | 44 |
| DISTANCE | Diabetes Study of Northern California | Health disparities | More than 2 million adults enrolled in Kaiser Permanente Northern California in 2010, including more than 210,000 patients with prevalent diabetes and more than 15,000 incident cases in the calendar year | 7 |
| DPP | Diabetes Prevention Program | Effects of lifestyle vs. metformin on progression to diabetes | 3,234 overweight individuals with prediabetes | 5 |
| Look AHEAD | Action for Health in Diabetes | Effects of intervention on cardiovascular morbidity and mortality | 5,145 overweight adults with diabetes | 38 |
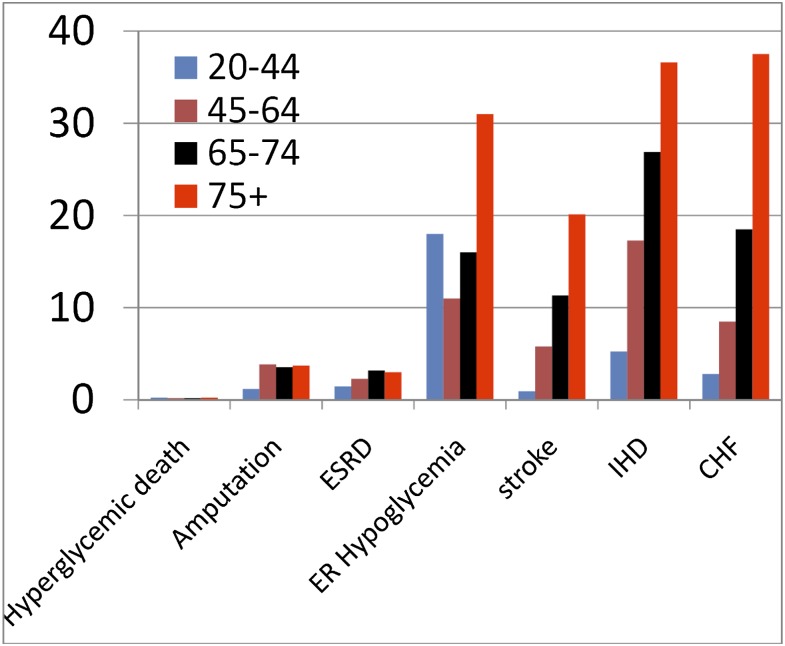
Individuals with diagnosed diabetes are at higher risk for coronary heart disease (CHD) (8), but CHD risk also is elevated modestly among individuals with prediabetes, defined as fasting plasma glucose concentrations of 100–125 mg/dL. Data from the National Diabetes Surveillance System indicate that the incidence of several diabetes-related complications, including hyperglycemic death, amputation, stroke, and ischemic heart disease (IHD), declined substantially between 1989 and 2009, particularly among older patients. Although relative glucose-related risk for CHD appears to decrease with age (9), the overall absolute risk for CVD increases dramatically with age (9), and age strongly predicts diabetes-related congestive heart failure (CHF), IHD, and stroke (Fig. 1).
Figure 1.
Incidence (per 1,000) of major diabetes complications among adults with diabetes, by age, 2009. Source: National Diabetes Surveillance System, available from http://www.cdc.gov/diabetes. CHF, congestive heart failure; ER, emergency room; ESRD, end-stage renal disease; IHD, ischemic heart disease.
Fasting plasma glucose concentration is a risk factor for CVD. However, the association of fasting glucose with CHD is more moderate than other risk factors such as total cholesterol, non-HDL cholesterol, and particularly systolic blood pressure, which has a relationship with CHD that is nearly log linear (8). The 2-h plasma glucose concentration during an oral glucose tolerance test (OGTT) is a strong determinant of cardiovascular risk. OGTT-defined prediabetes and diabetes predict incidence of CVD and death, even after accounting for corresponding categories based on fasting plasma glucose (4). Duration of diabetes likewise appears to be an important determinant for the development of CVD. Risk for heart failure or stroke is higher among individuals with a history of diabetes than among those newly diagnosed (10), and diagnosed diabetes is an equivalent risk factor to previous myocardial infarction (MI) among men aged 60–79 years with earlier—but not recent—onset diabetes (11).
Common Age-Associated Metabolic Changes and Their Impact on Diabetes
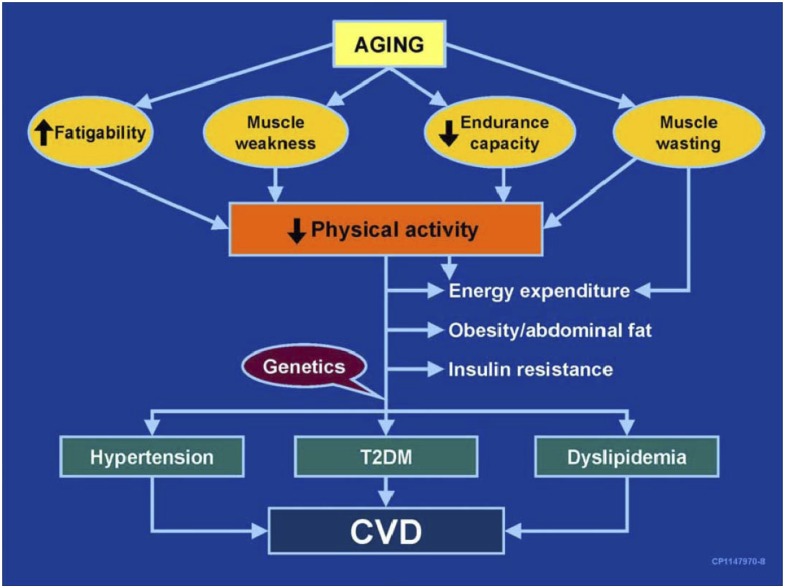
Insulin sensitivity appears to decline with age (12). Skeletal muscle is an important site of age-related insulin resistance. Factors contributing to age-associated insulin resistance include visceral adiposity and associated adipokines and inflammation, oxidative stress, mitochondrial dysfunction, and possibly an intrinsic decline in insulin sensitivity in muscle fibers. One pathway linking aging, muscle, and risks for diabetes and CVD is shown in Fig. 2.
Figure 2.
A proposed pathway linking aging, muscle, and risks for diabetes and CVD. Source: Nair KS. Aging muscle. Am J Clin Nutr 2005;81:953–963. T2DM, type 2 diabetes mellitus.
Age-associated declines in protein synthesis and quality result in accumulations of damaged proteins and impaired muscle strength and quality at a time when accumulating oxidative damage, DNA damage and degradation, and declines in mitochondrial copy number and mitochondrial protein synthesis render muscle mitochondria less able to produce ATP (13). Notably, exercise increases insulin sensitivity and reverses age-related declines in mitochondrial oxidative capacity and ATP production, and resistance training increases the content of a novel PGC-1α splicing isoform associated with hypertrophy and enhanced muscle strength (14).
Another mechanism links aging, pancreatic β-cell impairments, and diabetes risks. Even among people with normal glucose tolerance, insulin secretion is impaired with age, possibly because of a decrease in pancreatic islet mass and β-cell proliferative capacity (reviewed in 15). β-Cell impairments are progressively worse in prediabetes and overt diabetes. Alterations in the expression of cell-cycle proteins that control cellular senescence, such as p16INK4A, might play a role in age-related declines in β-cell function (15). Under conditions of uncomplicated obesity and associated declines in insulin sensitivity, β-cells can adapt by producing more insulin (15). However, metabolic stressors and increasing defects in glucose regulation can overwhelm adaptive mechanisms. Thus, diabetes is accompanied by a loss of functional β-cells and a resulting decline in insulin secretion (16).
Older individuals with type 2 diabetes tend to be overweight and deconditioned with central adiposity and insulin resistance, which promote dyslipidemia and atherogenesis (12) (Fig. 2). The metabolic abnormalities associated with diabetic dyslipidemia include insulin resistance, hypertriglyceridemia, an abnormal distribution of apoB-enriched low-density and remnant lipoprotein particles, and low HDL cholesterol. Excess fat deposition in the liver in type 2 diabetes triggers the overproduction of triglyceride- and apoB-enriched lipoproteins. Hepatic fat accumulation also induces overproduction of inflammatory proteins that alter levels of hepatic lipase, cholesterol ester transfer proteins, and lipoprotein lipase. These alterations lead to the remodeling of large, buoyant LDL into small, dense, apoB-laden remnant lipoproteins and the alteration of HDL composition into particles enriched in triglyceride and deficient in cholesterol ester and apoA-I. This dysfunctional HDL exhibits reduced cholesterol efflux, anti-inflammatory, antioxidative, and antiapoptotic capacity that activate macrophages and endothelial and smooth muscle cells while the abnormal LDL remnants are deposited into the vessel wall, accelerating foam cell formation and atherogenesis (17). Ultimately, insulin resistance and lipotoxicity enhance inflammation, amyloid formation, macrophage proliferation, and smooth and endothelial cell activation and thereby accelerate atherogenesis and plaque formation further. These abnormalities in HDL and LDL function may contribute to the heightened inflammation, amyloid deposition, and increased reactive oxygen species that also contribute to β-cell failure (18). The effects of age on all these risk factors, as well as on the primary and secondary mechanisms underlying dyslipidemia and accelerated atherosclerosis in individuals with type 2 diabetes, is a fertile area for future investigation.
Vulnerabilities of the Heart and Blood Vessels to Aging and Diabetes
Even up to age 80, there are no substantial aging-related changes in overall resting cardiac function, but there are notable age-associated changes in specific aspects of cardiac structure and cardiovascular function (Table 2). Lifestyle-associated stressors, such as poor diet, physical inactivity, and smoking, can accelerate these changes.
Table 2.
Age-related changes in the cardiovascular system
| Heart |
| Slowing kinetics of diastolic filling |
| Left ventricular wall thickening |
| Left atrial enlargement |
| Augmentation in the atrial contribution to diastolic |
| ventricular filling |
| Decreased cardiovascular reserve function |
| Increased risk for arrhythmia |
| Altered regulation of cardiomyocyte calcium homeostasis |
| Arterial system |
| Diffuse intimal thickening |
| Stiffening of the aorta and carotid arteries |
| Endothelial dysfunction |
Diabetes also has a major impact on the heart (19,20). Systolic and diastolic dysfunction and changes in coronary blood flow in the presence of diabetes suggest that diabetes impairs the ability of the heart to use free fatty acids as energy substrates. The impact of hyperglycemia on left ventricular function can be influenced by many factors, including sex, endothelial dysfunction, duration of diabetes, alterations in cellular metabolism, and dysfunction in the autonomic nervous system. Diabetes can worsen age-related changes in diastolic dysfunction by as much as fivefold (19,20). It is not clear if microvascular abnormalities contribute to overall cardiac dysfunction or how the presence of diabetes affects responses to cardiovascular injury or infarct with aging.
The mechanisms underlying age- and diabetes-related changes to the vasculature are not understood fully, but inflammation and oxidative stress appear to play a synergistic role. Aging arteries exhibit a chronic inflammatory profile similar to that seen with atherosclerosis, hypertension, and diabetes. Inflammation and oxidative stress together promote extracellular matrix remodeling, increased tone, and intrinsic cell stiffness in the vascular smooth muscle. Oxidative stress resulting from increased superoxide availability in the arteries, uncoupling of nitric oxide synthase, and mitochondrial dysfunction also promotes endothelial dysfunction (21,22). Angiotensin II plays a role in this synergy by mediating interactions among chronic inflammation, mitochondrial dysfunction (21), longevity pathways (22), and diabetes-related pathways. The effects of inhibiting angiotensin II signaling mimic those of caloric restriction (23). While there may be an important role for adult stem cells located in perivascular areas in maintaining endothelial function, this topic was not discussed at the workshop.
Acute hyperglycemia does not appear to affect endothelial function (24), but vessel function appears to be impaired among individuals with type 2 diabetes. Diminished sympathetic nerve activity and insulin resistance are possible factors, as insulin itself can be sympathoexcitatory. These effects might be counteracted by lifestyle changes. Sodium restriction, caloric restriction, and weight loss improve elastic artery compliance and vascular endothelial function (25). Aerobic exercise partially preserves vascular endothelial function and large elastic artery compliance (26).
End-Organ Sequelae That Lead to Disability
Impaired vascular function can interact with diabetic peripheral neuropathy (DPN) to cause foot ulcerations and amputations. Hyperglycemia and advanced glycation end products (AGEs) increase oxidative stress, which activates kinases such as protein kinase C and increases production of proinflammatory mediators, collagen, and fibronectin (27). These pathways also deplete endothelial and neural nitric oxide, leading to endothelial dysfunction and damage to the vasa nervorum, thereby contributing to nerve fiber injury and DPN. However, there are few data on the interaction of DPN with the vasculature in older adults with diabetes.
Although cognitive decline has not been viewed as a traditional diabetes complication, type 2 diabetes increases the risk for both vascular dementia and Alzheimer disease (3,28). Diabetes and Alzheimer disease pathology can exert synergistic effects with age on the brain vasculature, blood flow, and delivery of substrates necessary for cognition (29). The brain can be affected by hyperglycemia, which increases production of AGEs and their receptors, and by hypoglycemia, which creates excitotoxic insults and, in extreme cases, neuron cell death.
Defects in memory and executive function appear in midlife and become progressively worse and disabling with older age, creating a cycle of increasing cognitive impairment and poor self-management. Both type 2 diabetes and Alzheimer disease are associated with reduced cholesterol synthesis in the brain. Among middle-aged adults with prediabetes and diabetes, areas of glucose hypometabolism, gray matter atrophy, and reduced blood flow are apparent in the brain in a pattern similar to that seen with Alzheimer disease (29,30). Obesity, hypertension, and dyslipidemia influence age-related risk for dementias (28,30), particularly in midlife. Around the age of 70 years, however, mild elevations in blood pressure, cholesterol, and weight appear to be associated inversely with dementia, and work in animal models suggests that these abnormalities might even be protective.
Alzheimer disease pathology, and particularly β-amyloid, also may contribute to brain insulin resistance. β-Amyloid induces insulin resistance by downregulating insulin receptors from dendritic membranes, administration of β-amyloid in nonhuman primates induces insulin resistance in the hippocampus (31), and dietary induction of mild insulin resistance in older human adults decreases memory and increases β-amyloid levels and markers of oxidative injury. In contrast, administration of insulin blocks the synapse loss induced by β-amyloid (32), and in an initial study, intranasal administration of insulin improved memory and peripheral insulin sensitivity (33). A large clinical trial of intranasal insulin is now under way to test effects on cognition.
Diabetic kidney disease (defined as the presence of albuminuria, an impaired glomerular filtration rate (GFR), or both) occurs in approximately 35% of patients with diabetes (34). The prevalence of diabetic kidney disease increases with age, exceeding 50% among diabetic patients aged 65 years or older. Diabetic kidney disease is associated with longer duration of diabetes and with diabetes-related complications, such as impaired lower-extremity function, incident disability, and incident dementia. Diabetic kidney disease contributes to the excess cardiovascular mortality risk among patients with type 2 diabetes (35). Mechanisms through which diabetic kidney disease contributes to CVD are a subject of intensive current research.
Prevention and Treatment for Diabetes and CVD
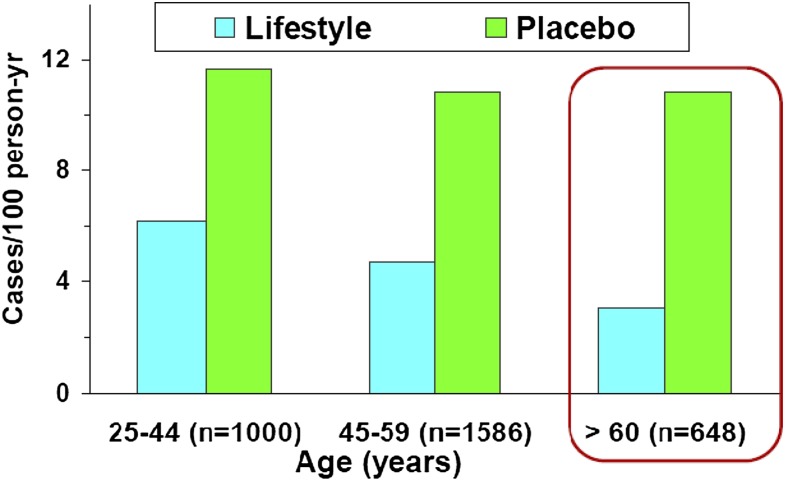
As suggested by the effects of exercise and dietary changes on mitochondrial and vascular function, addressing lifestyle risk factors can have a substantial impact on diabetes and CVD in the older population. In the Diabetes Prevention Program (DPP), a clinical trial with individuals at risk for type 2 diabetes, lifestyle intervention proved especially effective in preventing diabetes among older participants (Fig. 3), with a persistent effect for up to 10 years (5,36). These participants lost more weight, and they showed a lower tendency, compared with middle-aged participants, to regain the weight they had lost (5).
Figure 3.
Diabetes incidence rates by age-group in the DPP (36), demonstrating the effectiveness of the lifestyle intervention vs. placebo, especially in the oldest age-group. Reprinted and modified by permission of Oxford University Press on behalf of the Gerontological Society of America.
Obese, nondiabetic older adults randomized to dietary interventions or exercise maintained weight loss up to 30 months following the study (37). Also, dietary changes and exercise each improved parameters of physical function, but they exerted additive effects when combined. These findings are consistent with those of the Look AHEAD (Action for Health in Diabetes) study in middle-aged and older people with type 2 diabetes, where weight loss and improved fitness lowered the risk for loss of mobility (38). Lifestyle interventions also improve intrahepatic fat content, insulin secretion, insulin sensitivity, and metabolic risk factors for CHD (39), and they increase the likelihood of partial remission of type 2 diabetes (40).
However, weight-loss interventions remain controversial for older adults due to concerns that weight loss will exacerbate sarcopenia and frailty and that attempts to change lifelong habits will cause anxiety and distress. Yet in DPP, otherwise healthy older adults were more active and enthusiastic in adopting lifestyle changes and more successful in achieving their goals (36). Older adults with diabetes and multiple comorbidities can have both sarcopenia and visceral obesity. In such individuals, weight loss could exacerbate age-related declines in physical and metabolic function, leading to development of the frailty syndrome (3).
Treatment strategies used to address CVD risk factors in younger populations with diabetes also apply to older adults with diabetes. Clinical trials of interventions for hypertension and lipid disorders in these populations have been summarized (2,3). Consistent, positive outcomes have been found in older people and people with diabetes, but few studies have included enough older people with diabetes to have adequate statistical power. Older adults may in some cases be more sensitive to therapy than younger adults. For example, in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, older adults responded similarly to protocol-driven therapies as younger participants did, and with some outcomes, such as LDL cholesterol and triglyceride levels, older participants fared better than their younger counterparts. However, in both the intensive and standard glycemia therapy arms of the trial, these same older adults were more prone to severe hypoglycemia than their younger counterparts. Although ACCORD was stopped early because of excessive mortality in the intensive therapy arm, this excessive mortality was confined to participants younger than 65 years (41).
Primary percutaneous intervention (PCI) with drug-eluting stent is the treatment of choice for acute coronary syndromes, regardless of whether diabetes is present. However, mortality and rates of MI and stroke are higher among diabetic patients receiving PCI than among those receiving coronary artery bypass graft (CABG) (42,43). The Bypass Angioplasty Revascularization Investigation in 2 Diabetes (BARI 2D) study of patients with type 2 diabetes and coronary artery disease (CAD) showed no significant difference in the rates of death and major cardiovascular events between patients undergoing prompt revascularization and those undergoing medical therapy (44). Although the majority of participants in these studies were in their 60s and 70s, results were not stratified by age.
Hypoglycemia
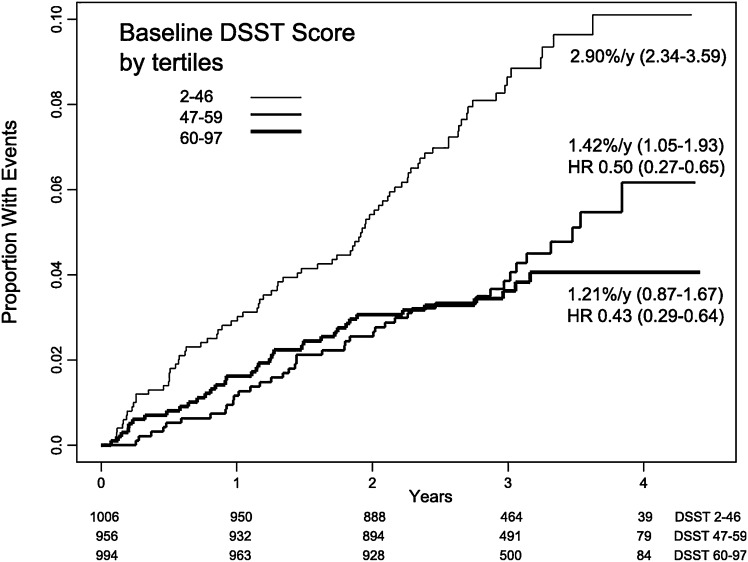
Impairment of protective mechanisms against hypoglycemia (defined as a plasma blood glucose concentration of 70 mg/dL or lower) is a serious issue in individuals with diabetes (45). Such impairments may worsen with duration of diabetes and most likely with age, and data from the ACCORD trial suggest a relationship between poor baseline cognitive function and the risk for severe hypoglycemia, which requires the assistance of others for recognition and treatment (Fig. 4) (41). Indeed, age is a strong predictor of acute hypoglycemia. Concerns about hypoglycemia and its potential impact are important factors in diabetes self-management. For example, patients with diabetic kidney disease, and particularly those who are older and receiving an intensive glucose-lowering regimen, are at increased risk for severe hypoglycemia (3). However, severe hypoglycemia is a difficult outcome to study, and mild hypoglycemia is almost never reported. Thus, the potential impact of hypoglycemia is unclear. Hypoglycemia increases QTc interval, production of proinflammatory markers, platelet activation, and markers of oxidative stress, and it decreases endothelial function and myocardial blood flow (3,45). Although these changes may increase the risk for CVD and death, it is not clear that hypoglycemia is a direct cause of these outcomes. Results from several studies indicate a correlation between severe hypoglycemia and future mortality (3,46). However, correlative findings do not establish causality.
Figure 4.
Relationship between baseline cognitive function and risk for severe hypoglycemia in the ACCORD trial. Kaplan-Meier curves are shown for the proportion of subjects with severe hypoglycemia events according to baseline tertiles of the Digit Symbol Substitution Test (DSST) score. Crude incidence rates and 95% CIs are shown for each group. Log-rank test P = 0.0001. Hazard ratios (HRs) for the middle and highest score groups are with reference to the lowest DSST score group. Patients who scored in the worst tertile on the DSST had the highest rate of severe hypoglycemia, at 2.90%/year or approximately 10% (proportion 0.10) cumulatively over 4 years. The numbers below the x-axis are the actual number of patients in each tertile at each time point (41).
Challenges to Intervention in Older Adults
Predicting Outcomes and Competing Risk
Traditional mathematical models for assessing risk for diabetes complications are based on the epidemiology and natural history of diabetes, as well as on the transitions of patients across their health status. Such models aid in the assessment of competing risk by highlighting the interactions between risks for diabetes complications and nondiabetes events. Clinical trials have demonstrated that the cardiovascular or mortality benefit of glycemic or blood pressure control become apparent only after 5 to 10 years following treatment initiation (2,3). Although most older diabetic patients have 5 or more years of remaining life expectancy to benefit from interventions, remaining life expectancy is important for diabetes care decisions for those older patients near the end of life. A simulation model, based in part on a mortality risk index encompassing age, comorbidities, and functional status, indicates that the expected benefit of glycemic control declines as the levels of morbidity and functional impairment increase (47). Studies of nationally representative samples of older patients have found three naturally occurring clusters of patients based on comorbidity, with significant differences in mortality rates (3,48). Thus, functional status and level of comorbidity are important factors in assessing risk. Models of diabetes complications would benefit from epidemiological data of older patients, including data on geriatric complications, dementia, or other conditions now known to be associated with diabetes.
Heterogeneity
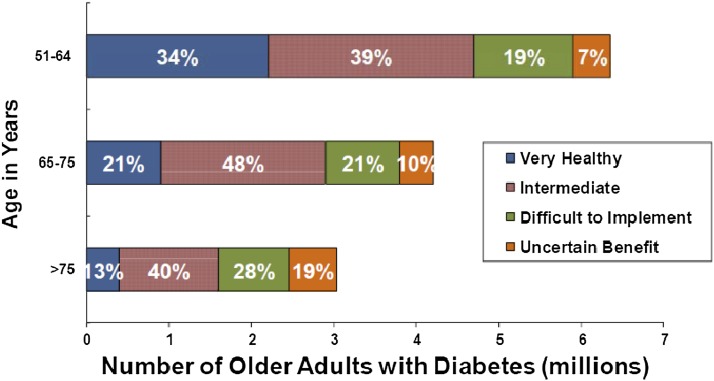
The complexity of the older population with diabetes is increased by several sources of heterogeneity. One is general health status. In one study that stratified participants by health status and age (48), the proportion of those considered healthy was lower among participants aged older than 75 years, compared with those aged 51–64 years (Fig. 5). However, even among participants aged 51–64 years, 19% were expected to have difficulties in self-management and an additional 7% were expected to receive limited benefit from diabetes interventions because of the severity of their comorbidities. Conversely, 53% of diabetic patients aged older than 75 years were relatively healthy. In addition, compared with nondiabetic patients, diabetic participants in the Health and Retirement Study (49) have much higher rates of comorbidities, such as dementia or heart disease, beginning in middle age. However, the relative impact of diabetes decreases with age. How to manage older diabetic patients with severe physical impairments is not clear. Diabetes management overall relies on patient preferences, as it occurs often in the absence of evidence or consensus regarding appropriateness and quality of interventions.
Figure 5.
Heterogeneity in health status among patients with diabetes, based on data from the Health and Retirement Study of people over age 50 (48). People with known diabetes were assigned to one of four mutually exclusive categories: a Very Healthy group with no comorbidities; a healthy Intermediate group with comorbidities constrained to osteoarthritis and hypertension, and with no functional impairments; a group for whom intensive diabetes management would be Difficult to Implement due to multiple comorbidities and/or any one of the following: mild cognitive impairment, poor vision, two or more minor functional impairments; and a group with Uncertain Benefit from intensive diabetes management due to having the poorest health status, with one or more of the following: moderate-to-severe cognitive impairment, two or more major functional dependencies, and/or residence in a long-term nursing facility. As the Health and Retirement Study is a U.S. population-based survey, the y-axis estimates the total number of people in the U.S. over age 50 with diabetes in each category.
Racial and ethnic differences in subclinical disease, disease burden, and access to care also contribute to heterogeneity (50,51). A1C levels are higher among black, Asian, and American Indian patients with diabetes. CVD and CHF incidence are higher among black patients compared with white individuals, but prevalence of coronary calcifications is lower among black, Hispanic, and Asian American individuals, and intima-media thickness is lower among Chinese individuals. CAD burden is lower among black and Hispanic patients, but black patients fare worse after surgery, angioplasty, or coronary revascularizations (50,51). Although all racial groups in the Get With the Guidelines-Stroke program improve in response to interventions such as thrombolysis, deep vein thrombosis prophylaxis, smoking cessation, antithrombotic medications, atrial fibrillation and anticoagulation, and lipid therapy, black patients are less likely than white patients to receive these interventions (52).
Biological differences, such as family history and host genetics, are another source of heterogeneity. The offspring of centenarians have an exceptionally longer health span and are healthier and less likely to have diabetes or CVD than age-matched control subjects (53). Likewise, risk for diabetes and Alzheimer disease is lower for individuals with a parent who lived longer than 80 years. Among individuals with exceptional longevity, enriched genotypes include variants of FOXO3A, insulin growth factor receptor, CETP, and apoC3. Other biological differences may include those in pathways underlying diabetes complications or in age-related changes in fat, muscle, or cognition.
Aging Biology and Pathophysiology: A Possible Paradox
Restoring insulin sensitivity is a valid approach to treating diabetes, but the risks associated with insulin resistance are not always clear. Centenarians appear to have some insulin resistance compared with their offspring and with individuals who do not live as long (53), suggesting that insulin resistance is not necessarily negative. In nematodes, downregulation of the insulin receptor–like daf-2 confers insulin resistance but prolongs life. In rat studies, insulin sensitivity improves, even on ad libitum diets, when visceral fat is removed, and the animals live longer. However, a recent summary of studies of insulin-sensitivity models and longevity suggests that enhanced insulin sensitivity is neither necessary nor sufficient for enhanced longevity in mammals (54). It is possible then that age-related changes in metabolic regulation, vascular function, and other processes are accompanied by protective repair or adaptive responses. In that case, attempts to reverse processes that decline with age, such as insulin resistance, might provide no benefit or even worsen outcomes.
Future Research Directions
The convergence of pathways underlying diabetes and CVD on inflammation and oxidative stress suggests that these age-related diseases might arise from a common foundation. An overarching research question then is how various mechanisms contributing to diabetes and CVD affect and are affected by advancing age. Many other potential research directions, including specific questions listed in Table 3, also should be explored. In the past, research has often examined pathways, organs, and systems independently. However, optimal health relies on a balance of interrelated systems, and age or diabetes may shift that balance. The mechanisms underlying the cardiovascular, renal, nerve, and cognitive complications of diabetes suggest a common microvascular pathway. More research is needed on interactions between vascular and cognitive abnormalities and on signaling between the cardiovascular system and the brain. Increased understanding of the pathways common to diabetes, CVD, and other morbidities might yield optimal treatments for diabetes and its complications. More multidisciplinary or interdisciplinary studies are needed.
Table 3.
Questions for future research
| Epidemiology | 1. What is the extent to which associations between glycemia and CVD events apply to the oldest adults? |
| 2. In light of the high prevalence of undiagnosed diabetes and dysglycemia/prediabetes in older adults, do heterogeneous glycemic subgroups matter in terms of future risk, and what are their implications for screening? | |
| 3. Which subgroups are the best candidates for intervention? | |
| 4. Are the diabetes risk factors or their magnitudes of association different for older adults than young and middle-aged adults? | |
| 5. What additional information is needed to improve risk stratification for intervention? | |
| 6. What are significant racial/ethnic disparities in older adults with diabetes, and what are the best approaches to address them? | |
| 7. To what extent are late-life health inequalities a legacy of early-life factors, such as reduced access to care? | |
| 8. How will quality metrics distinguish between true racial/ethnic inequalities in access to or quality of care vs. appropriate compliance with geriatric recommendations for individualized care? | |
| Pathophysiology | 1. Are mechanisms of aging- and diabetes-related impairments of pancreatic β-cell function similar or different? Additive or multiplicative? |
| 2. Are any of these mechanisms reversible or preventable? | |
| 3. Would prevention of aging effects slow the progression to diabetes in at-risk individuals? | |
| 4. How can insulin resistance be prevented? | |
| 5. What causes age-related declines in mitochondrial function, and how do they and declines in insulin sensitivity and β-cell function contribute to the age-related increase in risk for diabetes? | |
| 6. What causes age-related sarcopenia, and what is its impact on diabetes progression and complications? | |
| 7. What are the independent and common mechanisms underlying the interaction between advancing age and diabetes on arterial stiffness and endothelial dysfunction, and how are they modulated by insulin resistance, elevated glucose levels, and other common risk factors, such as hyperlipidemia, abdominal obesity, or hypertension? | |
| 8. How does the resultant vascular dysfunction relate to CVD risk? | |
| 9. How does the myocardium change with age? | |
| 10. What is the time course of processes underlying diabetes and CVD, and what is the trajectory of potential compensatory mechanisms? | |
| 11. Can medications associated with comorbidities contribute to the pathophysiology of diabetes and risk for CVD complications? | |
| 12. What are the glucose-sensing mechanisms in the brain, and how are they affected by age? | |
| 13. What changes are apparent in the prediabetic brain? | |
| Complications and CVD risk | 1. Do mild episodes of hypoglycemia affect CVD risk? |
| 2. Does severe hypoglycemia affect nonfatal CVD outcomes in type 2 diabetes? | |
| 3. What mediates the relationship between severe hypoglycemia and mortality risk? If this relationship is a marker of “vulnerability,” what factors underlie such vulnerability? | |
| 4. What is the significance of hypoglycemia-induced changes in oxidative stress, clotting, and inflammation? | |
| 5. Why do older patients with type 2 diabetes have a blunted counterregulatory response and reduced symptoms of hypoglycemia? | |
| 6. What is the true relationship between mortality risk for older patients with diabetes and the A1C level? | |
| 7. What are the mechanisms underlying non-CVD mortality in patients with diabetes, and how do they relate to mechanisms underlying other complications? How does the pathologic basis of diabetic kidney disease compare with that for other complications? | |
| 8. How does the brain drive insulin and glucose metabolism in the periphery? | |
| Screening, diagnosis, and intervention | 1. Should postload hyperglycemia be used as a screening tool to aid early intervention? |
| 2. What intervention measures can prevent age-related declines in mitochondrial number/function? | |
| 3. Does the failure of glucose-lowering interventions to reduce CVD outcomes arise from suboptimal targeting of therapies, particularly in older adults? | |
| 4. Is β-cell replacement therapy feasible in older patients? | |
| 5. What are the most effective preventive and treatment strategies for CVD? Does prevention of or improvement in vascular dysfunction reduce risk of CVD? | |
| 6. Can targeted therapies and prevention approaches be developed to address diabetic dyslipidemia and other risk factors for CVD? | |
| 7. What level of blood pressure elevation should be treated in patients with diabetes, what drugs should be used, and by how much should blood pressure be lowered? | |
| 8. How important is an aggressive lifestyle intervention in addressing dyslipidemia in patients with diabetes? | |
| 9. What are potential beneficial pleiotropic effects of statin therapy in older patients with diabetes, and should statins be used in patients aged older than 80 years with diabetes? | |
| 10. Is there age-related cognitive impairment associated with statin therapy? | |
| 11. Should triglyceride and HDL cholesterol levels be addressed in older patients with diabetes? | |
| 12. What are the best treatment options for diabetic patients with left main or diffuse CAD? | |
| 13. What are the best treatment options for diabetic patients who have received PCI and need repeat revascularization? | |
| 14. What are the best treatment options for diabetic patients who are at higher risk for adverse outcomes associated with PCI or CABG? | |
| 15. How should the presence of diabetic kidney disease affect current clinical management of diabetes? | |
| 16. Do novel kidney disease CVD pathways offer new opportunities for intervention? | |
| 17. Can diabetic kidney disease be prevented or reversed, and does that alter CVD risk? | |
| 18. How clinically effective and safe are lifestyle interventions in older adults? | |
| 19. What is the feasibility of lifestyle interventions in real-world settings? | |
| 20. Are the effects of lifestyle interventions maintained in the long term, and what mechanisms and behaviors underlie such maintenance? | |
| 21. What are the mechanisms of action underlying the effects of weight loss, and how do they differ from those underlying the effects of unintended weight loss and frailty? | |
| 22. What are the mechanisms of action for nutraceutical or pharmacological strategies, such as antioxidants, nitric oxide boosters, anti-inflammatory agents, glucose/insulin-regulating or CVD risk-reducing drugs, and modulators of energy-sensing pathways and mitochondrial function? | |
| 23. Are there racial and ethnic differences in screening for diabetes and prediabetes? | |
| 24. Do differences in cultural beliefs and/or acculturation across minority subgroups have an impact on lifestyle, medication adherence, and outcomes? | |
| 25. What targeted interventions could further reduce health inequalities in older patients after broadly effective preventive measures, such as risk factor control and lifestyle changes, already have been applied population-wide? | |
| 26. What is the impact of quality-improvement programs and culturally tailored interventions in older adults with diabetes? | |
| 27. What other sources of heterogeneity are most important clinically, and how can improved understanding of these sources be used to define and choose new management strategies and set appropriate glucose and cardiovascular targets? | |
| 28. Despite the presence of heterogeneity, can crosscutting, appropriate, and high-quality diabetes care be defined? | |
| 29. Does the cost-effectiveness of prevention vs. treatment for type 2 diabetes differ by age? |
Animal models of aging are needed to explore many of these questions, keeping in mind that differences between organisms can hamper the translation of observations. Likewise, clinical trial populations are highly selected and have largely excluded older individuals with diabetes so that results are not generalizable to patients in everyday practice. Improved predictive models, including those that account for patient heterogeneity and can be used in real practice, are needed for clinical trial participant selection. Finally, intervention development has suffered because epidemiologically powerful associations have not necessarily been borne out in intervention trials. While clinical end points and mortality remain important, future studies also should include end points, such as functional outcomes, that are of particular interest to older adults. Thus, geriatricians should be involved in clinical trial design and in achieving consensus on the best measures and scales for assessment.
Article Information
Acknowledgments. The authors are grateful to Nancy Woolard at Wake Forest School of Medicine for her assistance with organizing the workshop. To see the agenda, a list of workshop moderators and attendees, and workshop presentations, please visit http://www.im.org/AcademicAffairs/Aging/IGP/ExpandingResearchEfforts/Pages/ASPWorkshoponDiabetesMellitus%20and%20CardiovascularDiseaseinOlderAdults.aspx. The authors recognize early leaders who linked diabetes, CVD, and aging, including W.R.H. and Daniel Porte Jr., who also was a workshop attendee and active contributor to the discussion there. The authors also acknowledge the contributions from planning committee members who reported conflicts of interest with authorship of this Perspective: Basil Eldadah, Judith Fradkin, and K. Sreekumaran Nair.
Funding. This workshop was supported by generous grants to Association of Specialty Professors from the National Institute on Aging (1-U13-AG-040938 01) and the John A. Hartford Foundation (J.B.H., K.E.S., K.P.H.). J.B.H. reports grants from the John A. Hartford Foundation, National Institutes of Health’s National Institute on Aging, and Alliance for Academic Internal Medicine during the conduct of the project, and from the American Diabetes Association outside the submitted work. F.M.H. reports grants from the John A. Hartford Foundation during the conduct of the project.
Duality of Interest. J.B.H. reports grants from Janssen Pharmaceuticals, Takeda Global Research and Development, Sanofi, and Boehringer Ingelheim outside the submitted work. L.H. reports relationships or activities with Spiracur, Organogenesis, Anacor, and Valient. M.S.K. reports grants from Novo Nordisk outside the submitted work. J.P. reports personal fees from Janssen Pharmaceuticals, Novo Nordisk, Bristol-Myers Squibb, AstraZeneca, and Roche/Genentech outside the submitted work; all work is related to consulting. S.Z. is a U.S. government employee. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. J.B.H. and N.M. were coleaders of the workshop and led the planning committee activities, served as presenters and moderators, and played leading roles in the review and editing of the manuscript. F.M.H. wrote the initial draft of the manuscript, based on the material presented at the workshop. J.P.C., A.G., L.H., W.R.H., E.S.H., M.S.K., J.P., and S.Z. were members of the planning committee, which organized the workshop, served as presenters or moderators, and reviewed and edited the manuscript. K.E.S. and K.P.H. helped to organize and moderate the workshop and reviewed and edited the manuscript.
Appendix
Workshop speakers were as follows: Nir Barzilai, MD, Albert Einstein College of Medicine; Caroline Blaum, MD, New York University School of Medicine; Suzanne Craft, PhD, Wake Forest University School of Medicine; J.P.C.; Ian de Boer, MD, University of Washington; Robert H. Eckel, MD, University of Colorado Denver; Hermes Florez, MD, PhD, University of Miami Miller School of Medicine; A.G.; Edward Gregg, PhD, Centers for Disease Control and Prevention; J.B.H.; L.H.; E.S.H.; Michael Joyner, MD, Mayo Clinic, Rochester; Masoor Kamalesh, Indiana University School of Medicine; Andrew Karter, PhD, Kaiser Permanente; Jorge Kizer, MD, Albert Einstein College of Medicine; Edward Lakatta, MD, National Institute on Aging; N.M.; K. Sreekumaran Nair, MD, PhD, Mayo Clinic, Rochester; J.P.; Douglas Seals, PhD, University of Colorado Boulder; Elizabeth Seaquist, MD, University of Minnesota; Dennis T. Villareal, MD, New Mexico VA Health Care System and University of New Mexico School of Medicine; and Jeff Williamson, MD, Wake Forest School of Medicine.
Footnotes
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of National Institute on Aging or the National Institutes of Health. In addition, the views expressed in written conference materials or publications and by speakers or moderators do not necessarily reflect the official policies of the Department of Health and Human Services, nor does mention by trade names, commercial practices, or organizations imply endorsement by the U.S. government.
References
- 1.Centers for Disease Control and Prevention National diabetes fact sheet 2011, 2012. Available from http://www.cdc.gov/diabetes/pubs/factsheet11.htm Accessed 28 August 2013
- 2.Cigolle CT, Blaum CS, Halter JB. Diabetes and cardiovascular disease prevention in older adults. Clin Geriatr Med 2009;25:607–641, vii–viii [DOI] [PubMed] [Google Scholar]
- 3.Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care 2012;35:2650–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuller LH, Arnold AM, Psaty BM, et al. 10-year follow-up of subclinical cardiovascular disease and risk of coronary heart disease in the Cardiovascular Health Study. Arch Intern Med 2006;166:71–78 [DOI] [PubMed] [Google Scholar]
- 5.Knowler WC, Fowler SE, Hamman RF, et al. Diabetes Prevention Program Research Group 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herman WH. The economic costs of diabetes: is it time for a new treatment paradigm? Diabetes Care 2013;36:775–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karter AJ, Schillinger D, Adams AS, et al. Elevated rates of diabetes in Pacific Islanders and Asian subgroups: The Diabetes Study of Northern California (DISTANCE). Diabetes Care 2013;36:574–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarwar N, Gao P, Seshasai SR, et al. Emerging Risk Factors Collaboration Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 2013;127:e6–e245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avery CL, Loehr LR, Baggett C, et al. The population burden of heart failure attributable to modifiable risk factors: the ARIC (Atherosclerosis Risk in Communities) study. J Am Coll Cardiol 2012;60:1640–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wannamethee SG, Shaper AG, Whincup PH, Lennon L, Sattar N. Impact of diabetes on cardiovascular disease risk and all-cause mortality in older men: influence of age at onset, diabetes duration, and established and novel risk factors. Arch Intern Med 2011;171:404–410 [DOI] [PubMed] [Google Scholar]
- 12.DeFronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh S, Lertwattanarak R, Lefort N, et al. Reduction in reactive oxygen species production by mitochondria from elderly subjects with normal and impaired glucose tolerance. Diabetes 2011;60:2051–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruas JL, White JP, Rao RR, et al. A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell 2012;151:1319–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halter JB. Aging and Insulin Secretion. In Handbook of the Biology of Aging. 7th ed. Masoro EJ, Austad SN, Eds. Burlington, MA, Elsevier Inc., 2011, p. 373–384 [Google Scholar]
- 16.Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 2012;150:1223–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab 2011;14:575–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kruit JK, Brunham LR, Verchere CB, Hayden MR. HDL and LDL cholesterol significantly influence beta-cell function in type 2 diabetes mellitus. Curr Opin Lipidol 2010;21:178–185 [DOI] [PubMed] [Google Scholar]
- 19.From AM, Scott CG, Chen HH. Changes in diastolic dysfunction in diabetes mellitus over time. Am J Cardiol 2009;103:1463–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huebschmann AG, Kohrt WM, Regensteiner JG. Exercise attenuates the premature cardiovascular aging effects of type 2 diabetes mellitus. Vasc Med 2011;16:378–390 [DOI] [PubMed] [Google Scholar]
- 21.Dai DF, Rabinovitch PS, Ungvari Z. Mitochondria and cardiovascular aging. Circ Res 2012;110:1109–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res 2012;110:1097–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Cavanagh EM, Inserra F, Ferder L. Angiotensin II blockade: a strategy to slow ageing by protecting mitochondria? Cardiovasc Res 2011;89:31–40 [DOI] [PubMed] [Google Scholar]
- 24.Reed AS, Charkoudian N, Vella A, Shah P, Rizza RA, Joyner MJ. Forearm vascular control during acute hyperglycemia in healthy humans. Am J Physiol Endocrinol Metab 2004;286:E472–E480 [DOI] [PubMed] [Google Scholar]
- 25.Pierce GL, Beske SD, Lawson BR, et al. Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults. Hypertension 2008;52:72–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeVan AE, Seals DR. Vascular health in the ageing athlete. Exp Physiol 2012;97:305–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005;54:1615–1625 [DOI] [PubMed] [Google Scholar]
- 28.Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol 2009;66:300–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeda S, Sato N, Uchio-Yamada K, et al. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci USA 2010;107:7036–7041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol 2011;68:51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bomfim TR, Forny-Germano L, Sathler LB, et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease-associated Aβ oligomers. J Clin Invest 2012;122:1339–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Felice FG, Vieira MN, Bomfim TR, et al. Protection of synapses against Alzheimer’s-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc Natl Acad Sci USA 2009;106:1971–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Craft S, Baker LD, Montine TJ, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol 2012;69:29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011;305:2532–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol 2013;24:302–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crandall J, Schade D, Ma Y, et al. Diabetes Prevention Program Research Group The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. J Gerontol A Biol Sci Med Sci 2006;61:1075–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med 2011;364:1218–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rejeski WJ, Ip EH, Bertoni AG, et al. Look AHEAD Research Group Lifestyle change and mobility in obese adults with type 2 diabetes. N Engl J Med 2012;366:1209–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bouchonville M, Armamento-Villareal R, Shah K, et al. Weight loss, exercise or both and cardiometabolic risk factors in obese older adults: results of a randomized controlled trial. Int J Obes (Lond) 2014;38:423–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gregg EW, Chen H, Wagenknecht LE, et al. Look AHEAD Research Group Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA 2012;308:2489–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Punthakee Z, Miller ME, Launer LJ, et al. ACCORD Group of Investigators; ACCORD-MIND Investigators Poor cognitive function and risk of severe hypoglycemia in type 2 diabetes: post hoc epidemiologic analysis of the ACCORD trial. Diabetes Care 2012;35:787–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamalesh M, Sharp TG, Tang XC, et al. VA CARDS Investigators Percutaneous coronary intervention versus coronary bypass surgery in United States veterans with diabetes. J Am Coll Cardiol 2013;61:808–816 [DOI] [PubMed] [Google Scholar]
- 43.Farkouh ME, Domanski M, Sleeper LA, et al. FREEDOM Trial Investigators Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012;367:2375–2384 [DOI] [PubMed] [Google Scholar]
- 44.Bari 2D Study Group. Frye RL, August P, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009;360(24):2503–2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cryer PE. Mechanisms of sympathoadrenal failure and hypoglycemia in diabetes. J Clin Invest 2006;116:1470–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zoungas S, Patel A, Chalmers J, et al. ADVANCE Collaborative Group Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010;363:1410–1418 [DOI] [PubMed] [Google Scholar]
- 47.Huang ES, Zhang Q, Gandra N, Chin MH, Meltzer DO. The effect of comorbid illness and functional status on the expected benefits of intensive glucose control in older patients with type 2 diabetes: a decision analysis. Ann Intern Med 2008;149:11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blaum C, Cigolle CT, Boyd C, et al. Clinical complexity in middle-aged and older adults with diabetes: the Health and Retirement Study. Med Care 2010;48:327–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cigolle CT, Lee PG, Langa KM, Lee YY, Tian Z, Blaum CS. Geriatric conditions develop in middle-aged adults with diabetes. J Gen Intern Med 2011;26:272–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feinstein M, Ning H, Kang J, Bertoni A, Carnethon M, Lloyd-Jones DM. Racial differences in risks for first cardiovascular events and noncardiovascular death: the Atherosclerosis Risk in Communities study, the Cardiovascular Health Study, and the Multi-Ethnic Study of Atherosclerosis. Circulation 2012;126:50–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eaton CB, Abdulbaki AM, Margolis KL, et al. Racial and ethnic differences in incident hospitalized heart failure in postmenopausal women: the Women’s Health Initiative. Circulation 2012;126:688–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwamm LH, Reeves MJ, Pan W, et al. Race/ethnicity, quality of care, and outcomes in ischemic stroke. Circulation 2010;121:1492–1501 [DOI] [PubMed] [Google Scholar]
- 53.Andersen SL, Sebastiani P, Dworkis DA, Feldman L, Perls TT. Health span approximates life span among many supercentenarians: compression of morbidity at the approximate limit of life span. J Gerontol A Biol Sci Med Sci 2012;67:395–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barzilai N, Ferrucci L. Insulin resistance and aging: a cause or a protective response? J Gerontol A Biol Sci Med Sci 2012;67:1329–1331 [DOI] [PubMed] [Google Scholar]