Abstract
Background
Accurate assessment of kidney function is important for management of solid organ transplant recipients. In other clinical populations, glomerular filtration rate (GFR) is most commonly estimated using the CKD-EPI (Chronic Kidney Disease–Epidemiology Collaboration) creatinine or the 4-variable MDRD (Modification of Diet in Renal Disease) Study equation. The accuracy of these equations compared to other GFR estimating equations in transplant recipients has not been carefully studied.
Study Design
Diagnostic test study.
Setting & Participants
Solid organ transplant recipients >6 months post-transplantation from 5 clinical populations [N=3,622, including recipients of kidney (53%), liver (35%) and other or multiple organs (12%)]
Index Test
Estimated GFR (eGFR) using creatinine-based GFR estimating equations identified from a systematic review of the literature. Performance of the CKD-EPI creatinine and MDRD Study equations was compared to alternative equations.
Reference Test
Measured GFR (mGFR) from urinary clearance of iothalamate or plasma clearance of iohexol.
Measurements
Error (difference between the mGFR and eGFR) expressed as P30 (proportion of absolute percent error <30%) and mean absolute error.
Results
We identified 26 GFR estimating equations. Mean mGFR was 55.1 ± 22.7 (SD) ml/min/1.73 m2. P30 and mean absolute error for the CKD-EPI and MDRD Study equations were 78.9% (99.6% CI, 76.9%–80.8%) for both and 10.6 (99.6% CI, 10.1–11.1) vs. 11.0 (99.6% CI, 10.5–11.5) ml/min/1.73 m2, respectively; these equations were more accurate than any of the alternative equations (p<0.001 for all pair-wise comparisons for both measures). They performed better than or as well as the alternative equations in most subgroups defined by demographic and clinical characteristics, including the type of transplanted organ.
Limitations
Study population included few non-whites and people with solid organ transplants other than liver and kidneys.
Conclusions
The CKD-EPI creatinine and MDRD Study equations perform better than the alternative creatinine-based estimating equations in solid organ transplant recipients. They can be used for clinical management.
INDEX WORDS: GFR estimation, renal function, kidney transplantation, solid organ transplant recipient, creatinine-based eGFR equation
Accurate assessment of kidney function is important for the management of solid-organ transplant recipients. Glomerular filtration rate (GFR) is most commonly estimated using serum creatinine-based estimating equations. Numerous equations have been developed, but their performance in transplant recipients has not been systematically and comprehensively evaluated. Clinical practice guidelines recommend monitoring kidney function to detect nephrotoxicity of immunosuppressive medications, to identify early signs of rejection in kidney transplant recipients, to adjust doses of drugs that are excreted by the kidneys, to guide testing and treatment for kidney disease complications, and to estimate prognosis.1
Guidelines provide conflicting recommendations about methods for GFR estimation in transplant recipients and there is some concern that currently available equations may be less accurate in transplant recipients than other clinical populations.2 The Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for the care of kidney transplant recipients recommends using any one of several creatinine-based equations to estimate GFR.1 The US Food and Drug Administration (FDA) recommends the use of the Cockcroft-Gault equation for drug development programs and in package inserts.3 Currently, the National Kidney Disease Education Program (NKDEP) recommends using the isotope-dilution mass spectrometry (IDMS)–traceable 4-variable MDRD (Modification of Diet in Renal Disease) Study equation for reporting of estimated GFR (eGFR) by clinical laboratories4,5, but the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation has recently been shown to be more accurate than the MDRD Study equation and has begun to replace it in laboratory reports.6–9 There is a need to determine the accuracy of GFR estimating equations in solid-organ transplant recipients.
We conducted a systematic evaluation of the development methods of all published creatinine-based GFR estimating equations and evaluated their performance in a large and diverse population of solid organ transplant recipients. We compared the performance of the CKD-EPI and the MDRD Study equations with all the other creatinine-based equations.
Methods
Study Overview
We first performed a systematic review of published creatinine-based equations to develop an inventory of eGFR equations and evaluate their development methods. We then performed a study of diagnostic test accuracy to compare the performance of these equations to measured GFR (mGFR) in solid organ transplant recipients.
Systematic Review
We searched MEDLINE for articles that described creatinine-based GFR estimating equations from 1950 until October 2012 with no language restrictions (see Table S1, available as online supplementary material, for search terms). We supplemented this by hand searching the references of relevant articles that reported either a new eGFR equation or compared the performance of existing equations as well as the NKF-KDOQI (National Kidney Foundation–Kidney Disease Outcomes Quality Initiative) guidelines for the laboratory measurements for clinical assessment of kidney disease.10
One investigator (S.K.S.) reviewed the articles for inclusion in the systematic review. Inclusion criteria were studies in which a GFR estimating equation (index text) was developed using either GFR measured with an exogenous filtration marker or measured creatinine clearance as a reference test in a population older than 18 years. We excluded studies that: 1) developed a nomogram rather than an equation; 2) developed equations by modifying an existing equation to improve its performance for use in a different clinical population or racial and ethnic subgroup; 3) developed equations that used variables other than age, sex, race, weight, BMI, body surface area, and serum creatinine and urea (or serum urea nitrogen [SUN]) for GFR estimation, as other variables may not be available in routine clinical practice.
We used criteria for developing and validating GFR estimating equations recommended by Earley et al7 to extract the information on the equation development cohort, index test and the reference test characteristics. Information included the following: year of equation development; development cohort characteristics (overall number, mean GFR, mean age, and type of the population [e.g. inpatient, outpatient or both]); reference test characteristics (filtration marker and clearance method, whether the equation was developed using multiple GFR measurements from the same patients, whether the GFR was scaled to body surface area); index test characteristics (creatinine assay used and its traceability to a standard reference material [SRM]); and whether equations were validated in an external validation cohort.
Comparison of Equation Performance
Data Source
The study population included subjects with one measurement of GFR, using urinary or plasma clearance of an exogenous filtration marker, and serum creatinine using assays that were standardized to the SRM.11 We included a total of 3,622 subjects from five clinical populations (studies).8,12 Data from four studies (Baylor, Groningen, Lund, Cleveland Clinic) comprised all solid organ transplant recipients from the CKD-EPI 2009 creatinine equation external validation cohort (n=1,112).8 Additional subjects from one of the study sites (Groningen; n=586) and data from the Mayo Clinic (n=1,924) were also included.12 Inclusion and exclusion criteria of study populations have been previously described 8,12 and are shown in Item S1 and Figure S1. One of the equations we tested required serum urea. Serum urea or SUN was not available in 764 subjects; therefore, we included 2,858 subjects for GFR estimation using that equation. None of the patients included were receiving trimethoprim/sulfamethoxazole. All studies were approved by the institutional review boards at the participating medical centers and Tufts Medical Center.
Reference and Index Tests
We considered mGFR as the reference test and eGFR computed by the equations identified in the systematic review as the index tests. We compared the performance of the CKD-EPI creatinine and MDRD Study equations to the alternative equations.
Statistical Analysis
We evaluated the distribution of continuous variables by assessing the mean ± standard deviation and categorical variables by number (percentage) in the whole data set as well as in subgroups according to the study population characteristics and transplant organ type. We defined error as mGFR minus eGFR (mGFR – eGFR) for each subject and percent error as this difference relative to mGFR, ie, (mGFR-eGFR)/mGFR. We computed bias as the average error and percent error, with mean or median used as appropriate for the distribution. We computed accuracy as the mean absolute error and proportion of subjects with absolute percent error less than 30% (P30). 13 We used P30 as the primary outcome as it is a commonly used metric of accuracy in GFR estimating equations, and used mean absolute error as the secondary outcome.
We performed pair wise comparisons of the CKD-EPI and MDRD Study equations with alternative equations. A priori, we decided to perform pairwise comparisons for only those alternative equations that had a P30 of ≥60% and mean absolute error of ≤20 ml/min/1.73 m2 to ensure that the comparisons are clinically meaningful. To overcome the problem of non-independence of the observations, we used generalized estimating equation models to calculate the point estimates and confidence intervals (CIs) for the difference between the P30 or mean absolute error of the CKD-EPI or the MDRD Study equations and each of the alternative equations (see Item S2 for further details). We obtained the point estimates and CIs for median percent and absolute percent error by the bootstrap method (2000 bootstraps). To circumvent the problem of inflated type I error rate due to 12 pairwise comparisons, we used Bonferroni’s approach to set the α for each comparison at 0.004 to achieve an overall α of 0.05 and reported 99.6% CIs. We also compared the performance (P30 and mean absolute error) of the CKD-EPI equation with the MDRD Study equation. Since both equations were derived on the log scale, in sensitivity analyses, for this comparison, we also computed percent error on the log scale. Finally, we used interaction terms in the model to assess whether the difference in the performance of the CKD-EPI or the MDRD Study equation and the alternative equations was similar across the levels of the following clinical and demographic variables: age (≤55, >55 years), sex, race (non-white, white), weight (<75, 75–100, >100 kg), level of mGFR (< 60, ≥60 ml/min/1.73 m2), study, and transplant organs (kidneys, liver, lungs, heart and pancreas). We had complete data on these variables on all subjects. We based our conclusions on clinical and statistical significance.
Results
Systematic Review
Our search for creatinine-based GFR estimating equation revealed 4,947 articles (Figure 1). After initial screening, we selected 78 articles for full text review, of which 36 articles reported 37 eGFR equations. We identified an additional 14 equations based on hand searches of the articles and the KDOQI clinical practice guideline for chronic kidney disease. Of the 51 equations, a total of 26 equations met our criteria for inclusion in the systematic review.8,14–37 The equations and their characteristics are shown in Tables 1 and S2. For equations not specifically named in the literature, we simply use the first author’s last name. The first equation (Edwards) was reported in 1959 while the most recent equation (Berlin Initiative Study [BIS] 1) was reported in 2012.14,37 Only 2 equations (CKD-EPI and MDRD Study) had a development cohort comprising greater than 1000 subjects.8,28 Five equations had transplant recipients in their development cohort; of these, 2 equations were developed exclusively in organ transplant recipients (Nankivell in kidney transplant recipients and Nankivell-SPK in simultaneous pancreas and kidney transplant recipients),23,24 and three equations were developed using both transplant recipients and the other populations (CKD-EPI, Gates, and Mayo).8,19,32 Twelve equations used creatinine as the filtration marker for the reference test with the remainder using iothalamate, inulin, ethylenediaminetetraacetic acid (EDTA) or diethylenetriaminepentaacetic acid (DTPA). In most of the equations (n=19), GFR was measured by urinary clearance of the filtration marker. The mean mGFR in the development cohort was 57 ml/min/1.73 m2 for the equations that reported GFR scaled to body surface area of 1.73 m2 (n=12), and 62 ml/min for the equations that did not scale eGFR to body size (n=11). A total of 2 equations did not report the mean GFR in the development cohort and 1 equation reported eGFR scaled to body surface area of 3 m2. Five equations used a creatinine assay that was traceable to SRM.5,8,34,36,37 Sixteen equations were developed in a population in which serum creatinine was in a steady state. Age, sex and race were used in 19, 20, and 2 equations, respectively. Fifteen equations were evaluated in a validation cohort in the original publication.
Fig 1.
Search strategy to identify eGFR equations
*KDOQI; Kidney Disease Outcomes Quality Initiative
Table 1.
Characteristics of creatinine-based equations identified by the systematic review according to year of publication
| Equation | Development Population | Reference Test | Index Test | Validation Cohort |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | N | Mean Age (y) |
Population | Transplant Recipients Included |
Clearance | Filtration marker |
Scaled to BSA |
Multiple measurements |
Mean GFR |
Cr assay | SR M |
Cr in Steady State |
Age Variable |
Sex Variable |
Race Variable |
||
| Edwards 14 | 1959 | 44 | U | OP/IP | N | U | Cr | Y | Y | ? | PK | N | B | N | Y | N | N |
| Cockcroft-Gault15 | 1974 | 249 | 55 | OP | N | U | Cr | N | Y | 55* | PK | N | Y | Y | Y | N | Y |
| Rowe 16 | 1976 | 548 | U | OP | N | U | Cr | Y | N | 79* | PK | N | Y | Y | N | N | Y |
| Mogensen17 | 1980 | 9 | U | ? | ? | U | Iothal | ? | ? | ? | PK | N | ? | N | N | N | ? |
| Taylor 18 | 1982 | 169 | U | OP/IP | N | P | Cr | Y | N | 69 | PU | N | ? | Y | N | N | N |
| Gates 19 | 1985 | 90 | 56 | IP | B | U | Cr | Y | Y | 37 | ? | N | Y | Y | Y | N | Y |
| Kaji 20 | 1990 | 18 | 55 | ? | N | U | Cr | N | N | 32* | PK | N | Y | N | N | N | Y |
| Robinson 21 | 1990 | 106 | U | IP | N | U | Cr | N | Y | 102* | PU | N | N | Y | Y | N | Y |
| Walser 22 | 1993 | 85 | U | IP | N | U | DTPA | O | Y | 13† | PK | N | Y | Y | Y | N | N |
| Nankivell-SPK23 | 1995 | 33 | 42 | OP | E | P | DTPA | N | Y | 55* | PK | N | Y | Y | Y | N | Y |
| Nankivell 24 | 1995 | 146 | 43 | OP/IP | E | P | DTPA | N | Y | 52* | PU | N | B | N | Y | N | Y |
| AASK-pilot 25 | 1996 | 193 | 53 | OP | N | U | Iothal | Y | ? | 69 | PK | N | Y | Y | Y | N | N |
| Baracskay 26 | 1997 | 41 | 73 | OP | N | P | Iothal | N | N | 71* | ? | N | Y | Y | N | N | N |
| Martin 27 | 1998 | 80 | 62 | ? | N | P | EDTA | N | N | 66* | EN | N | ? | N | Y | N | Y |
| MDRD Study 5,28 | 1999 | 1070 | 51 | OP | N | U | Iothal | Y | N | 40 | PK | Y | Y | Y | Y | Y | Y |
| Yukawa 29 | 1999 | 179 | 60 | IP | N | U | Cr | N | Y | 44* | PK | N | ? | Y | N | N | N |
| AASK-main 30 | 2001 | 1703 | 54 | IP | N | U | Iothal | Y | N | 57 | PK | N | Y | Y | Y | N | N |
| Wright 31 | 2001 | 62 | 58 | IP | N | U | EDTA | N | N | 73* | EN | N | N | Y | Y | N | Y |
| Mayo32 | 2004 | 900 | 45 | OP | B | U | Iothal | Y | N | 82 | PK | N | Y | Y | Y | N | N |
| Virga 33 | 2005 | 530 | 57 | ? | N | U | Cr | N | N | 55* | PK | N | Y | Y | Y | N | Y |
| Nix-HPLC 34 | 2006 | 27 | 51 | OP | N | U | Cr | N | N | 71* | HPLC | Y | Y | N | Y | N | N |
| Nix-J 34 | 2006 | 27 | 51 | OP | N | U | Cr | N | N | 71* | PK | N | Y | N | Y | N | N |
| CHUQ 35 | 2008 | 773 | 54 | OP/IP | N | U | Cr | Y | N | 67 | PK | N | B | Y | Y | N | Y |
| CKD-EPI 8 | 2009 | 5504 | 47 | OP | B | U | Iothal | Y | N | 68 | EN | Y | Y | Y | Y | Y | Y |
| Matsuo 36 | 2009 | 413 | 51 | IP | ? | U | Inulin | Y | N | 59 | EN | Y | B | Y | Y | N | Y |
| BIS-137 | 2012 | 570 | 78 | OP | N | P | Iohex | Y | N | 60 | EN | Y | Y | Y | Y | N | Y |
Note: N=26 equations. Unless otherwise indicated, GFR is expressed in mL/min/1.73 m2.
Abbreviations and definitions: Year; Year of publication, SRM; Traceable to standardized reference material, Y; Yes, N; No, ?; Unknown, B; Both, OP; Outpatient, IP; Inpatient, E; Exclusive, U; Urinary, P; Plasma, Cr; Creatinine, Iothal; Iothalamate, Iohex; Iohexol, DTPA; Diethylenetriaminepentaacetic acid, GFR; glomerular filtration rate, PK; Picrate kinetic, PU; Picrate non-modified, EN; Enzymatic, HPLC; High-performance liquid chromatography; MDRD, Modification of Diet in Renal Disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; BIS, Berlin Initiative Study; CHUQ, Le Centre hospitalier universitaire de Québec; AASK, African American Study of Kidney Disease and Hypertension; SPK, simultaneous pancreas and kidney; BSA, body surface area; SRM, standard reference material; J, Jaffe;
Units are ml/min,
Units are ml/min/3 m2
Comparison of Equation Performance
Clinical and Demographic Characteristics
The clinical and demographic characteristics of the 3622 subjects who were used for evaluation of equation performance are shown by study in Table 2. Of the overall study population, the mean age was 54 years, 98% of the subjects were white and 57% were males. The mean mGFR was 55 ml/min/1.73 m2. Kidney (53%) and liver (35%) transplant recipients were most common, with recipients of heart, lung, pancreas or more than one organ constituting the remainder (12%). There was a wide variation in clinical and demographic variables among recipients of different organs (Table S3) and by availability of SUN concentrations (Table S4).
Table 2.
Clinical and demographic variables of the population subdivided according to the study
| Total | Mayo | Groningen | Baylor | Lund | CCFP | |
|---|---|---|---|---|---|---|
| No. (row %) | 3622 (100.0) | 1924 (53.1) | 953 (26.3) | 686 (18.9) | 44 (1.2) | 15 (0.4) |
| Age (y) | 54.0 (13.0) | 55.9 (13.4) | 50.6 (12.9) | 53.9 (10.7) | 51.5 (14.1) | 41.3 (16.0) |
| Female sex | 1541 (42.6) | 812 (42.2) | 397 (41.7) | 308 (44.9) | 20 (45.5) | 4 (26.7) |
| Black race | 72 (2.0) | 23 (1.2) | 3 (0.3) | 44 (6.4) | 0 (0.0) | 2 (13.3) |
| Height (cm) | 172.1 (10.4) | 171.5 (10.4) | 173.8 (9.8) | 171.4 (10.9) | 169.8 (9.9) | 175.9 (9.2) |
| Weight (kg) | 83.7 (20.2) | 85.4 (21.8) | 80.6 (15.8) | 84.2 (20.3) | 73.6 (17.1) | 81.5 (17.0) |
| BSA (m2) | 1.96 (0.25) | 1.97 (0.27) | 1.95 (0.21) | 1.96 (0.26) | 1.84 (0.23) | 1.97 (0.20) |
| BMI (kg/m2) | 28.2 (5.9) | 28.9 (6.4) | 26.6 (4.5) | 28.6 (6.0) | 25.4 (4.9) | 26.4 (5.5) |
| Scr (mg/dl) | 1.49 (0.64) | 1.45 (0.60) | 1.56 (0.61) | 1.47 (0.63) | 1.93 (1.63) | 2.07 (1.19) |
| SUN* (mg/dl) | 27.7 (12.8) | 27.7 (12.8) | 33.5 (25.9) | 28.1 (13.9) | 27.9 (21.9) | 36.3 (27.7) |
| GFR (ml/min/1.73 m2) | 55.1 (22.7) | 54.1 (21.6) | 54.6 (20.4) | 59.3 (27.7) | 43.6 (17.1) | 47.0 (28.4) |
| Diabetes | 733 (20.3) | 419 (21.8) | 127 (13.3) | 173 (25.2) | 12 (27.3) | 2 (13.3) |
| Marker | ||||||
| Iohexol | 44 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 44 (100.0) | 0 (0.0) |
| Iothalamate | 3578 (98.9) | 1924 (100.0) | 953 (100.0) | 686 (100.0) | 0 (0.0) | 15 (100.0) |
| Organ | ||||||
| Kidney | 1905 (52.7) | 1027 (53.4) | 869 (91.2) | 0 (0.0) | 0 (0.0) | 9 (60.0) |
| Liver | 1251 (34.6) | 520 (27.0) | 0 (0.0) | 686 (100.0) | 44 (100.0) | 1 (6.7) |
| Lung | 147 (4.1) | 147 (7.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Heart | 110 (3.0) | 33 (1.7) | 74 (7.8) | 0 (0.0) | 0 (0.0) | 3 (20.0) |
| Pancreas | 26 (0.7) | 26 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Multiple organs | 183 (5.1) | 171 (8.9) | 10 (1.0) | 0 (0.0) | 0 (0.0) | 2 (13.3) |
Note: Unless otherwise indicated, values for categorical variables are given as number (column percentage); values for continuous variables, as mean ± standard deviation.
Abbreviation: Mayo; Mayo Clinic, Rochester, Minnesota: Baylor; Baylor University Medical Center, Dallas, Texas, US: Groningen; Groningen Institute for Kidney Diseases, Groningen, Netherlands: Lund; Division of Nephrology, Lund University, Lund, Sweden: CCFP; Cleveland Clinic Foundation Prospective Cohort, Cleveland, Ohio; SUN; serum urea nitrogen; BSA, body surface area; BMI, body mass index; Scr, serum creatinine; GFR, glomerular filtration rate;
SUN reported on 2,858 patients. See Figure S1 for details of patients missing SUN.
Performance in the Whole Cohort
Table 3 shows the performance of the 26 equations. The CKD-EPI equation had a mean error of 0.4 (99.6% CI, −0.3 to 1.1) ml/min/1.73 m2, P30 of 78.9% (99.6% CI, 76.9%–80.8%) and mean absolute error of 10.6 (99.6% CI, 10.1–11.1) ml/min/1.73 m2. The MDRD Study equation had a mean error of 4 (99.6% CI, 3.3–4.7) ml/min/1.73 m2, P30 of 78.9% (99.6% CI, 76.9%–80.8%), and mean absolute error of 11.0 (99.6% CI, 10.5–11.5) ml/min/1.73 m2. There was a wide range in the performance of the other equations, with mean error ranging between −67.3 and 24.1 ml/min/1.73 m2 and P30 between 4.9% and 78.9%. Only 12 other equations (including the Gates and the Nankivell-SPK equations) met the criteria of a P30 ≥60% and absolute error ≤20 ml/min/1.73 m2 for further comparison to the CKD-EPI and the MDRD Study equations (Figure 1).
Table 3.
Performance of eGFR equations (n=26) according to P30
| Equation | Mean GFR (ml/min/1.73 m2) | Mean Error (ml/min/1.73 m2) | Median Percent Error (%) | Mean Absolute Error (ml/min/1.73 m2) | Median Absolute Percent Error (%) | P30 (%) |
|---|---|---|---|---|---|---|
| CKD-EPI | 54.6 (53.6, 55.6) | 0.4 (−0.3, 1.1) | 0.8 (−0.4, 2.0) | 10.6 (10.1, 11.1) | 15.9 (15.0, 16.8) | 78.9 (76.9, 80.8) |
| MDRD Study | 51.1 (50.1, 52.0) | 4.0 (3.3, 4.7) | 6.8 (5.7, 7.9) | 11.0 (10.5, 11.5) | 16.6 (15.7, 17.3) | 78.9 (76.9, 80.8) |
| Virga | 49.5 (48.7, 50.4) | 5.5 (4.8, 6.2) | 8.6 (7.2, 10.0) | 11.7 (11.2, 12.2) | 17.9 (17.1, 18.9) | 76.4 (74.4, 78.4) |
| Walser | 51.2 (50.3, 52.1) | 3.9 (3.1, 4.6) | 5.5 (4.0, 6.4) | 11.8 (11.3, 12.4) | 18.0 (17.1, 18.9) | 74.3 (72.2, 76.3) |
| Nix-J | 49.8 (48.8, 50.8) | 5.3 (4.5, 6.1) | 9.9 (8.6, 11.3) | 12.6 (12.0, 13.2) | 19.4 (18.6, 20.3) | 72.9 (70.7, 75.0) |
| CHUQ | 49.6 (48.8, 50.4) | 5.5 (4.7, 6.3) | 9.4 (8.3, 10.6) | 12.6 (12.0, 13.2) | 19.9 (19.1, 21.1) | 72.5 (70.3, 74.6) |
| Wright | 59.8 (58.8, 60.8) | −4.8 (−5.5, −4.0) | −9.7 (−10.9, −8.5) | 11.6 (11.1, 12.1) | 17.4 (16.4, 18.3) | 72.3 (70.1, 74.4) |
| Gates | 60.8 (59.7, 62.0) | −5.8 (−6.6, −5.0) | −10.5 (−11.9, −9.0) | 12.2 (11.6, 12.8) | 17.7 (16.9, 18.6) | 71.3 (69.1, 73.5) |
| Nankivell-SPK | 46.4 (45.6, 47.2) | 8.6 (7.9, 9.4) | 14.3 (12.9, 15.7) | 13.5 (12.9, 14.1) | 21.4 (20.6, 22.4) | 68.5 (66.2, 70.7) |
| Cockcroft-Gault | 61.8 (60.8, 62.9) | −6.8 (−7.5, −6.1) | −14.1 (−15.7, −12.9) | 12.4 (11.9, 13.0) | 18.9 (17.8, 20.1) | 68.3 (66.0, 70.5) |
| Edwards | 61.6 (60.5, 62.7) | −6.5 (−7.3, −5.7) | −12.1 (−13.6, −10.7) | 13.3 (12.7, 13.9) | 19.4 (18.3, 20.5) | 67.3 (65.0, 69.5) |
| Nix-HPLC | 63.0 (62.0, 64.0) | −8.0 (−8.7, −7.2) | −17.2 (−19.1, −15.5) | 13.3 (12.8, 13.8) | 20.9 (19.8, 22.0) | 65.1 (62.8, 67.3) |
| Yukawa | 62.4 (61.5, 63.2) | −7.3 (−8.1, −6.5) | −15.1 (−16.8, −13.1) | 14.2 (13.6, 14.8) | 21.3 (19.9, 22.6) | 63.7 (61.4, 66.0) |
| BIS1 | 65.2 (63.8, 66.6) | −10.1 (−11.3, −9.0) | −14.9 (−16.8, −13.2) | 16.8 (15.8, 17.7) | 21.7 (20.8, 23.0) | 61.6 (59.3, 63.9) |
| AASK-main | 66.0 (64.7, 67.3) | −10.9 (−11.8, −10.1) | −19.3 (−20.7, −17.8) | 15.7 (15.1, 16.4) | 23.9 (22.8, 25.5) | 59.9 (57.5, 62.2) |
| Mayo | 41.0 (40.2, 41.7) | 14.1 (13.4, 14.8) | 25.0 (24.1, 25.8) | 15.8 (15.1, 16.4) | 26.6 (25.8, 27.4) | 59.0 (56.7, 61.4) |
| Matsuo | 67.4 (66.2, 68.6) | −12.4 (−13.2, −11.6) | −23.5 (−24.9, −22.0) | 15.7 (15.0, 16.3) | 24.7 (23.3, 25.9) | 57.6 (55.3, 60.0) |
| Nankivell* | 66.2 (65.0, 67.4) | −11.2 (−12.1, −10.3) | −23.1 (−24.8, −21.3) | 16.0 (15.3, 16.7) | 25.9 (23.8, 27.2) | 54.8 (52.1, 57.4) |
| Baracskay | 64.9 (64.0, 65.9) | −9.9 (−10.9, −8.8) | −19.6 (−21.8, −16.9) | 18.7 (18.0, 19.5) | 27.8 (26.5, 29.1) | 53.4 (51.0, 55.7) |
| Taylor | 52.1 (50.9, 53.4) | 2.9 (1.8, 4.0) | 3.3 (1.3, 5.5) | 18.0 (17.3, 18.7) | 29.6 (27.9, 30.9) | 50.6 (48.2, 53.0) |
| Robinson | 73.0 (71.4, 74.7) | −18.0 (−19.3, −16.7) | −29.3 (−32.0, −26.3) | 23.7 (22.6, 24.8) | 33.6 (31.5, 35.3) | 46.1 (43.7, 48.5) |
| Mogensen | 71.1 (69.8, 72.4) | −16.0 (−17.0, −15.1) | −30.1 (−32.3, −28.2) | 20.4 (19.7, 21.2) | 33.7 (32.0, 35.5) | 45.5 (43.1, 47.9) |
| AASK-pilot | 76.3 (74.9, 77.6) | −21.2 (−22.2, −20.2) | −38.9 (−41.6, −36.6) | 23.9 (23.0, 24.8) | 40.0 (37.7, 41.9) | 38.9 (36.6, 41.2) |
| Kaji | 31.0 (30.4, 31.6) | 24.1 (23.2, 24.9) | 43.8 (42.7, 45.0) | 24.9 (24.0, 25.8) | 44.4 (43.5, 45.6) | 26.9 (24.9, 29.1) |
| Martin | 98.6 (97.0, 100.2) | −43.5 (−44.7, −42.4) | −80.7 (−83.5, −78.2) | 44.0 (42.8, 45.1) | 80.7 (78.1, 83.5) | 9.6 (8.3, 11.1) |
| Rowe | 122.4 (121.9, 122.9) | −67.3 (−68.4, −66.2) | −129.6 (−134.1, −125.6) | 67.5 (66.5, 68.6) | 129.6 (125.5, 134.1) | 4.9 (4.0, 6.0) |
Note: Data given as value (99.6% confidence interval). All estimates are scaled to 1.73 m2 for validity of comparisons. Equations are ranked by P30 in descending order. The equations that had a P30 ≥60% and mean absolute error ≤20 ml/min/1.73m2 are highlighted in bold and were included in the final analysis where we compared the performance of the CKD-EPI equation or the MDRD Study equations with the alternative equations
P30: Percentage of patients who have absolute percent error ≤30%.; eGFR, estimated glomerular filtration rate; GFR; glomerular filtration rate, MDRD, Modification of Diet in Renal Disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; BIS, Berlin Initiative Study; CHUQ, Le Centre hospitalier universitaire de Québec; AASK, African American Study of Kidney Disease and Hypertension; SPK, simultaneous pancreas and kidney; BSA, body surface area; SRM, standard reference material; J, Jaffe
eGFR estimated on 2858 patients on whom serum urea/serum urea nitrogen was available and was in steady state
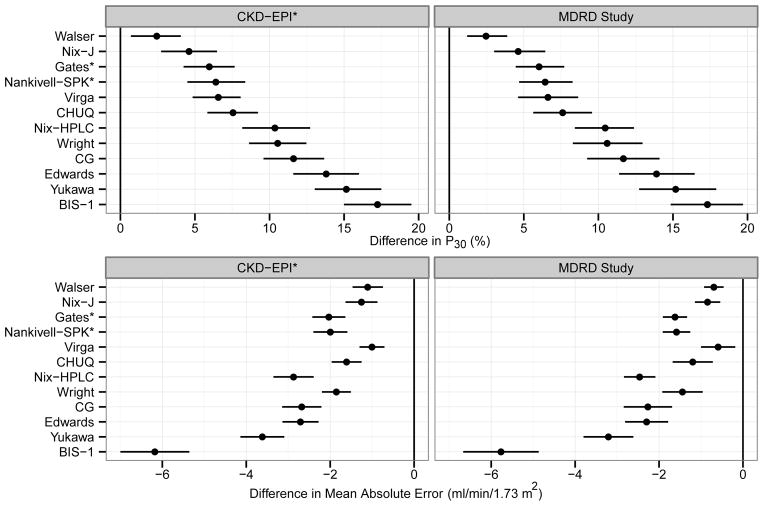
For P30, the CKD-EPI and the MDRD Study equations outperformed all alternative equations (difference in P30 ranged from 2.4%–17.2% [p <0.001 for all pairwise comparisons with the CKD-EPI equation] and 2.5%–17.3% [p <0.001 for all pairwise comparison with the MDRD Study equation]; Figure 2). Similarly, the CKD-EPI and the MDRD Study equations had lower mean absolute errors than all the alternative equations (difference in mean absolute error ranged from −6.2 to −1.0 ml/min/1.73 m2 [p <0.001 for all pairwise comparisons with the CKD-EPI equation] and −5.8 to −0.6 ml/min/1.73 m2 [p <0.001 for all pairwise comparisons with the MDRD Study equation]; Figure 2).
Fig 2.
The performance of the CKD-EPI or the MDRD Study equation vs. the alternative equations
Difference in P30 (upper panels) and the mean absolute error (lower panels) of the CKD-EPI or the MDRD Study equation and the alternative equations along with their 99.6 % CIs are shown. For the metric of P30, a difference of >0 indicates that CKD-EPI or the MDRD Study equation is superior to the alternative equations. For mean absolute error, a difference of <0 indicates that the CKD-EPI or the MDRD Study equation has a lower mean absolute error than the alternative equations. P values for all pair wise comparisons with the CKD-EPI or the MDRD Study equation are < 0.004.
*Equations that had transplant recipients in the development cohort
The CKD-EPI and MDRD Study equations had similar performance (difference [CKD-EPI – MDRD Study] in P30 of −0.05% [99.6% CI, −1.4% to 1.5] and difference in mean absolute error of −0.4 [99.6% CI, −0.6 to −0.2] ml/min/1.73 m2).
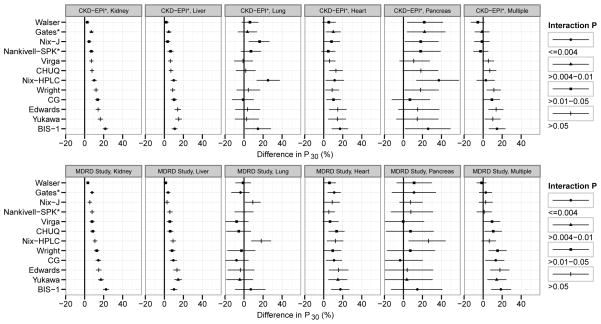
Performance in Subgroups
There were significant pairwise differences in equation performance for many subgroups based on organ, demographic and clinical characteristics, and study (Figure 3 and Figures S2–S7). For all subgroups with significant differences, including organ, the performance of the CKD-EPI equation was either superior or similar to the alternative equations, with the exception of the subgroups with mGFR <60 ml/min/1.73 m2 where the Walser equation performed better (difference in P30 of −3.5% [99.6% CI, −5% to −1.9%]), and with mGFR ≥60 ml/min/1.73 m2 where some other equations performed better (differences in P30 of −5.0% [99.6% CI, −8.1% to −2.0%], −3.7% [99.6% CI, −5.5% to −1.9%], −3.0% [99.6% CI, −5.5% to −0.6%], −4.8% (99.6% CI, −7.4% to −2.2%], and −2.6% [99.6% CI, −4.8% to −0.5%] for Cockcroft-Gault, Virga, Edwards, Yukawa, and Wright equations, respectively (Figure S6). We observed similar findings for interactions of subject level covariates and equations in comparisons with the MDRD Study equation (Figures S2–S7). Comparisons of performance of CKD-EPI and MDRD Study equations by study, organ and level of mGFR are shown in Tables S5 and S6 and Figure S8, respectively. Performance of the CKD-EPI equation was better at higher GFR and performance of the MDRD Study equation was better at lower GFR (Figure S8).
Fig 3.
The difference between the P30 (99.6% CI) of the CKD-EPI or the MDRD Study equation and each of the alternative equations in subgroups by organ [Kidney, Liver, Lung, Heart, Pancreas, and Multiple organs(Heart/Kidney, Heart/Liver, Kidney/Liver, Kidney/Lung, Kidney/Pancreas, Lung/Liver or Lung/Herat)]
A significant global p value (≤0.004) indicates that the difference in the performance of the CKD-EPI (upper panels) or the MDRD Study equation (lower panels) and the alternative equations is different across studies. A difference of >0 indicates that the P30 of the alternative equation is less than the CKD-EPI or the MDRD Study equation. If the lower margin of the 99.6% CI is above 0, then the alternative equation is inferior; if the margin includes 0, then the alternative equation is similar. For the difference in P30, only the values that are >−20% and < 60% are shown for ease of representation.
Interaction P values represent 4 categories; P≤0.004, P>0.004–0.01, P>0.01–0.05, and P>0.05
*Equations that had transplant recipients in the development cohort
Discussion
Clinicians monitor serum creatinine frequently in organ transplant recipients to detect immunologic rejection, infection or toxicity of medications. Most US clinical laboratories report eGFR using the CKD-EPI or MDRD Study equation whenever serum creatinine is ordered.6 However, no single GFR estimating equation is optimal for all populations and GFR ranges,7 and there has been some reluctance by transplant physicians to use GFR estimates in the day-to-day management of solid organ transplant recipients because of the concern that the equations might be less accurate in transplant recipients than in other clinical populations.2 Our systematic review of creatinine-based GFR estimating equations revealed substantial variability in equation development methods and showed that the newer equations were more thorough in their reporting of development methods. Our evaluation of equation performance in a large cohort of solid organ transplant recipients revealed that the CKD-EPI creatinine and IDMS-traceable 4-variable MDRD Study equations were more accurate than the alternative equations (even those equations developed in populations of only transplant recipients) and as accurate as observed in other clinical populations (P30 of approximately 80%). 7 In addition, they performed either better than or as well as any other equation in almost all the subgroups that we examined, including type of organ.
Several prior studies have compared the performance of creatinine-based GFR estimating equations in kidney, liver and heart transplant recipients and have shown conflicting results.12,38–49 The majority of the studies showed that the CKD-EPI and the MDRD Study equations performed better than other equations, whereas a few studies showed some of the alternative equations performed better. Prior to the publication of the CKD-EPI equation, White et al performed a systematic review of studies to compare the performance of some of the creatinine-based equations and concluded that the MDRD Study equation was the most accurate. 50 The authors pointed out that the studies included in the systematic review were limited by the heterogeneity among test populations, use of non-standardized creatinine assays, incomplete reporting of the equation performance metrics and inclusion of multiple GFR measurements on the same patients. Studies directly comparing the performance of the CKD-EPI and MDRD Study equations have also shown conflicting results. 2,12,47,50 We did not find a difference between these two equations in the overall study population, but showed better performance of the CKD-EPI equation at higher levels of GFR and better performance of the MDRD Study equation at lower levels of GFR, which is consistent with the systematic review by Earley et al 7 based on an analysis of group data.
Creatinine has long been used as a filtration marker, but its serum levels are affected by factors besides GFR, such as creatinine generation by muscle mass and diet.51 GFR estimating equations use readily available clinical and demographic variables as surrogates for this and other non-GFR determinants. It has been suggested that muscle mass in solid organ transplant recipients may differ systematically from that in patients with other clinical conditions because of use of corticosteroids and immunosuppressive medications, periods of prolonged dialysis prior to kidney transplantation, chronic inflammatory state due to prolonged illness, bouts of rejection and infections. Our finding that the CKD-EPI and MDRD Study equations perform better than or as well as they do in non-transplant populations suggests that the non-GFR determinants of serum creatinine may not differ systematically between solid organ transplant recipients and other patients.52 Possibly, there are differences in non-GFR determinants among recipients of types of organs which account for some of the variability in performance of equations across subgroups of types of organs.
The better performance of the CKD-EPI and MDRD Study equations compared to the other equations is likely due to their development in a large study population, use of mGFR instead of creatinine clearance as the reference test in the equation development cohort, use of standardized creatinine and robust statistical methods and inclusion of a variable for race, 7 and, for the CKD-EPI equation, development in a cohort made up of subjects with diverse clinical characteristics and a wide range of GFRs. Use of urinary clearance of iothalamate to measure GFR in the vast majority of our study population (98.8%), as in the development population for the CKD-EPI and MDRD Study equations, may also contribute to the better performance of these equations in our study.
The results of this study have important clinical implications. Our findings that the CKD-EPI and the MDRD Study equations are the two most accurate equations in solid organ transplant recipients as in other clinical populations enables transplant physicians to use the GFR estimates that are routinely reported by the laboratories for clinical purposes. Estimated GFR provides similar information to serum creatinine for monitoring changes in kidney function, as the relative change in eGFR is proportional to the relative change in serum creatinine.1 However, serum creatinine alone cannot be used to determine the level of eGFR, which is essential for detection and staging of chronic kidney disease and for management of its various complications, including anemia, mineral and bone disease 1 and some recent studies suggest worse stage-based management in kidney transplant recipients compared with patients with native kidney diseases. 53,54 Furthermore, transplant recipients require a large number of medications and eGFR is required for accurate dosing. Moreover, the level of eGFR has been shown to be a predictor of patient and allograft survival as well as health care expenditure in several large well-conducted studies. 55,56 Finally, quantification of the change in kidney function may be facilitated by the use of eGFR, since it does not require computation of relative changes, which may be difficult for some physicians to recognize, particularly at the extremes of serum creatinine.13 Nonetheless, as in other clinical populations, GFR estimates based on serum creatinine are limited by imprecision and there is a need for further improvement 12. Recent studies show that the use of cystatin C and creatinine in combination can improve the precision of GFR estimates, both in the general population and kidney transplant recipients. 47,57 Further work is required to evaluate these equations in kidney and other solid organ transplant recipients.
Our study has several strengths. Our study population included recipients of various types of solid organs with a wide range of kidney function from multiple centers, which enhances its generalizability. Our methods were comprehensive and rigorous. We examined all published creatinine-based equations. The GFR was measured using well-accepted methods and serum creatinine assays were standardized to reference methods. We used robust statistical methods to circumvent the problems of non-independence of observations and multiple hypothesis testing. To our knowledge, this is the first instance of the use of generalized estimating equations for assessing the performance of GFR estimating equations and this technique can be used for future studies of diagnostic accuracy.
Our study has some limitations. We pooled data from 5 studies and clinical populations which could have led to heterogeneity of study population, however, the CKD-EPI and the MDRD Study equation performed either similarly to or better than the alternative equations when we assessed the equation performance in each population. Urinary clearance of inulin is considered the gold standard for measuring GFR. However, urinary clearance of iothalamate and inulin demonstrate good co-linearity.58 The GFR was measured by plasma clearance of iohexol in a minority of patients (1.2%), all of whom were in the Lund study, but our results did not differ substantially among studies. We had few non-Caucasians and subjects with solid organ transplants other than liver and kidneys; therefore our assessment of the equation performance in these subgroups is limited. The results may not apply to recipients taking trimethoprim/sulfamethoxazole, which causes systematic underestimation of mGFR by all equations. We did not have information on the immunosuppressive medications. Finally, imprecision in GFR measurements contributes to error between GFR estimates versus measurements, but this should not systematically bias the comparisons between GFR estimating equations.
In conclusion, we showed that the CKD-EPI and the MDRD Study equations are more accurate than any other currently available GFR estimating equations in solid organ transplant recipients, and are as accurate in this population as they are in other clinical populations. These equations can be used for routine monitoring of kidney function in solid organ transplant recipients as they are in other clinical populations. Future studies should focus on developing more accurate GFR estimating equations in this and other populations.
Supplementary Material
Figure S1: Flow diagram of subjects included in study.
Figure S2: Difference between P30 of CKD-EPI or MDRD Study equation and each alternative equation in age subgroups.
Figure S3: Difference between P30 of CKD-EPI or MDRD Study equation and each alternative equation in sex subgroups.
Figure S4: Difference between P30 of CKD-EPI or MDRD Study equation and each alternative equation in race subgroups.
Figure S5: Difference between P30 of CKD-EPI or MDRD Study equation and each alternative equation in weight subgroups.
Figure S6: Difference between P30 of CKD-EPI or MDRD Study equation and each alternative equation in mGFR subgroups.
Figure S7: Difference between P30 of CKD-EPI or MDRD Study equation and each alternative equation in study/center of enrollment subgroups.
Figure S8: P30 of CKD-EPI and MDRD Study equation across levels of mGFR.
Table S1: MEDLINE search terms to identify creatinine-based eGFR equations.
Table S2: Equations included in the systematic review.
Table S3: Clinical and demographic variables of study population by transplanted organ.
Table S4: Clinical and demographic variables of patients by presence of SUN variable.
Table S5: Performance metrics of CKD-EPI and MDRD Study equations by study.
Table S6: Performance metrics of CKD-EPI and MDRD Study equations by transplanted organ.
Item S1: Description of study population.
Item S2: Supplementary methods.
Acknowledgments
We thank all the study patients and staff, whose generous participation and help made this study possible.
Dr Shaffi used a part of this study for fulfillment of the Master’s of Science in Clinical Research degree requirements at Tufts University Sackler School of Graduate Medical Sciences.
Support: This study was funded through the National Institute of Diabetes and Digestive and Kidney Diseases grants T32 DK007777 and U01 DK053869. Ms Ruthazer, who provided statistical support, was funded by the National Center for Advancing Translational Sciences, National Institutes of Health, grant UL1 TR000073.
Footnotes
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9 (Suppl 3):S1–155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 2.White CA, Knoll GA, Poggio ED. Measuring vs estimating glomerular filtration rate in kidney transplantation. Transplant Rev (Orlando) 2010;24(1):18–27. doi: 10.1016/j.trre.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) Guidance for Industry Pharmacokinetics in Patients with Impaired Renal Function — Study Design, Data Analysis, and Impact on Dosing and Labeling. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072127.pdf. Accessed.
- 4. [Accessed September 4, 2013.];Estimating Glomerular Filtration Rate (GFR) - National Kidney Disease Education Program (NKDEP) Available at: http://nkdep.nih.gov/lab-evaluation/gfr/estimating.shtml.
- 5.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 6.Miller WG. Reporting estimated GFR: a laboratory perspective. Am J Kidney Dis. 2008;52(4):645–648. doi: 10.1053/j.ajkd.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 7.Earley A, Miskulin D, Lamb EJ, Levey AS, Uhlig K. Estimating equations for glomerular filtration rate in the era of creatinine standardization: a systematic review. Ann Intern Med. 2012;156(11):785–795. W–270, W–271, W–272, W–273, W–274, W–275, W–276, W–277, W–278. doi: 10.1059/0003-4819-156-6-201203200-00391. [DOI] [PubMed] [Google Scholar]
- 8.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapter 1: Definition and classification of CKD. Kidney inter, Suppl. 2013;3(1):19–62. doi: 10.1038/kisup.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Kidney Foundation. [Accessed February 12, 2013.];KDOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. Available at: http://www.kidney.org/professionals/kdoqi/guidelines_ckd/p5_lab_g4.htm.
- 11.Myers GL, Miller WG, Coresh J, et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52(1):5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 12.Murata K, Baumann NA, Saenger AK, Larson TS, Rule AD, Lieske JC. Relative Performance of the MDRD and CKD-EPI Equations for Estimating Glomerular Filtration Rate among Patients with Varied Clinical Presentations. Clin J Am Soc Nephrol. 2011 doi: 10.2215/CJN.02300311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevens LA, Zhang Y, Schmid CH. Evaluating the performance of equations for estimating glomerular filtration rate. J Nephrol. 2008;21(6):797–807. [PMC free article] [PubMed] [Google Scholar]
- 14.EDWARDS KD, WHYTE HM. Plasma creatinine level and creatinine clearance as tests of renal function. Australas Ann Med. 1959;8:218–224. doi: 10.1111/imj.1959.8.3.218. [DOI] [PubMed] [Google Scholar]
- 15.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 16.Rowe JW, Andres R, Tobin JD, Norris AH, Shock NW. The effect of age on creatinine clearance in men: a cross-sectional and longitudinal study. J Gerontol. 1976;31(2):155–163. doi: 10.1093/geronj/31.2.155. [DOI] [PubMed] [Google Scholar]
- 17.Mogensen CE, Heilskov NS. Prediction of GFR from serum creatinine. Acta Endocrinol Suppl (Copenh) 1980;238:109. [PubMed] [Google Scholar]
- 18.Taylor GO, Bamgboye EA, Oyediran AB, Longe O. Serum creatinine and prediction formulae for creatinine clearance. Afr J Med Med Sci. 1982;11(4):175–181. [PubMed] [Google Scholar]
- 19.Gates GF. Creatinine clearance estimation from serum creatinine values: an analysis of three mathematical models of glomerular function. Am J Kidney Dis. 1985;5(3):199–205. doi: 10.1016/s0272-6386(85)80051-2. [DOI] [PubMed] [Google Scholar]
- 20.Kaji D, Strauss I, Kahn T. Serum creatinine in patients with spinal cord injury. Mt Sinai J Med. 1990;57(3):160–164. [PubMed] [Google Scholar]
- 21.Robinson BA, Frampton CM, Colls BM, Atkinson CH, Fitzharris BM. Comparison of methods of assessment of renal function in patients with cancer treated with cisplatin, carboplatin or methotrexate. Aust N Z J Med. 1990;20(5):657–662. doi: 10.1111/j.1445-5994.1990.tb00395.x. [DOI] [PubMed] [Google Scholar]
- 22.Walser M. Assessing renal function from creatinine measurements in adults with chronic renal failure. Am J Kidney Dis. 1998;32(1):23–31. doi: 10.1053/ajkd.1998.v32.pm9669420. [DOI] [PubMed] [Google Scholar]
- 23.Nankivell BJ, Chapman JR, Allen RD. Predicting glomerular filtration rate after simultaneous pancreas and kidney transplantation. Clin Transplant. 1995;9(2):129–134. [PubMed] [Google Scholar]
- 24.Nankivell BJ, Gruenewald SM, Allen RD, Chapman JR. Predicting glomerular filtration rate after kidney transplantation. Transplantation. 1995;59(12):1683–1689. doi: 10.1097/00007890-199506270-00007. [DOI] [PubMed] [Google Scholar]
- 25.Lewis J, Agodoa L, Cheek D, et al. Comparison of cross-sectional renal function measurements in African Americans with hypertensive nephrosclerosis and of primary formulas to estimate glomerular filtration rate. Am J Kidney Dis. 2001;38(4):744–753. doi: 10.1053/ajkd.2001.27691. [DOI] [PubMed] [Google Scholar]
- 26.Baracskay D, Jarjoura D, Cugino A, Blend D, Rutecki GW, Whittier FC. Geriatric renal function: estimating glomerular filtration in an ambulatory elderly population. Clin Nephrol. 1997;47(4):222–228. [PubMed] [Google Scholar]
- 27.Martin L, Chatelut E, Boneu A, et al. Improvement of the Cockcroft-Gault equation for predicting glomerular filtration in cancer patients. Bull Cancer. 1998;85(7):631–636. [PubMed] [Google Scholar]
- 28.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 29.Yukawa E, Hamachi Y, Higuchi S, Aoyama T. Predictive performance of equations to estimate creatinine clearance from serum creatinine in Japanese patients with congestive heart failure. Am J Ther. 1999;6(2):71–76. doi: 10.1097/00045391-199903000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Toto RD, Kirk KA, Coresh J, et al. Evaluation of serum creatinine for estimating glomerular filtration rate in African Americans with hypertensive nephrosclerosis: results from the African-American Study of Kidney Disease and Hypertension (AASK) Pilot Study. J Am Soc Nephrol. 1997;8(2):279–287. doi: 10.1681/ASN.V82279. [DOI] [PubMed] [Google Scholar]
- 31.Wright JG, Boddy AV, Highley M, Fenwick J, McGill A, Calvert AH. Estimation of glomerular filtration rate in cancer patients. Br J Cancer. 2001;84(4):452–459. doi: 10.1054/bjoc.2000.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141(12):929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 33.Virga G, Gaspari F, Thomaseth K, Cara M, Mastrosimone S, Rossi V. A new equation for estimating renal function using age, body weight and serum creatinine. Nephron Clin Pract. 2007;105(2):c43–53. doi: 10.1159/000097597. [DOI] [PubMed] [Google Scholar]
- 34.Nix DE, Erstad BL, Nakazato PZ, Barletta JF, Matthias KR, Krueger TS. Estimation of creatinine clearance in end-stage liver disease. Ann Pharmacother. 2006;40(5):900–908. doi: 10.1345/aph.1G594. [DOI] [PubMed] [Google Scholar]
- 35.Douville P, Martel AR, Talbot J, Desmeules S, Langlois S, Agharazii M. Impact of age on glomerular filtration estimates. Nephrol Dial Transplant. 2009;24(1):97–103. doi: 10.1093/ndt/gfn473. [DOI] [PubMed] [Google Scholar]
- 36.Matsuo S, Imai E, Horio M, et al. Revised Equations for Estimated GFR From Serum Creatinine in Japan. American Journal of Kidney Diseases. 2009;53(6):982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 37.Schaeffner ES, Ebert N, Delanaye P, et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med. 2012;157(7):471–481. doi: 10.7326/0003-4819-157-7-201210020-00003. [DOI] [PubMed] [Google Scholar]
- 38.Zahran A, Qureshi M, Shoker A. Comparison between creatinine and cystatin C-based GFR equations in renal transplantation. Nephrol Dial Transplant. 2007;22(9):2659–2668. doi: 10.1093/ndt/gfm243. [DOI] [PubMed] [Google Scholar]
- 39.Zahran A, Hossain MA, Kora MAE, Galal AZ, Shoker A. Validation of the Virga GFR equation in a renal transplant population. Nephron Clin Pract. 2008;109(3):c140–147. doi: 10.1159/000145457. [DOI] [PubMed] [Google Scholar]
- 40.Gera M, Slezak JM, Rule AD, Larson TS, Stegall MD, Cosio FG. Assessment of changes in kidney allograft function using creatinine-based estimates of glomerular filtration rate. Am J Transplant. 2007;7(4):880–887. doi: 10.1111/j.1600-6143.2006.01690.x. [DOI] [PubMed] [Google Scholar]
- 41.Pöge U, Gerhardt T, Palmedo H, Klehr H-U, Sauerbruch T, Woitas RP. MDRD equations for estimation of GFR in renal transplant recipients. Am J Transplant. 2005;5(6):1306–1311. doi: 10.1111/j.1600-6143.2005.00861.x. [DOI] [PubMed] [Google Scholar]
- 42.Pöge U, Gerhardt T, Stoffel-Wagner B, Sauerbruch T, Woitas RP. Validation of the CKD-EPI formula in patients after renal transplantation. Nephrology, Dialysis, Transplantation: Official Publication of the European Dialysis and Transplant Association - European Renal Association. 2011 doi: 10.1093/ndt/gfr183. [DOI] [PubMed]
- 43.Kukla A, El-Shahawi Y, Leister E, et al. GFR-estimating models in kidney transplant recipients on a steroid-free regimen. Nephrol Dial Transplant. 2010;25(5):1653–1661. doi: 10.1093/ndt/gfp668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gonwa TA, Jennings L, Mai ML, Stark PC, Levey AS, Klintmalm GB. Estimation of glomerular filtration rates before and after orthotopic liver transplantation: evaluation of current equations. Liver Transpl. 2004;10(2):301–309. doi: 10.1002/lt.20017. [DOI] [PubMed] [Google Scholar]
- 45.Tangri N, Alam A, Edwardes MD, Davidson A, Deschênes M, Cantarovich M. Evaluating cimetidine for GFR estimation in liver transplant recipients. Nephrol Dial Transplant. 2010;25(4):1285–1289. doi: 10.1093/ndt/gfp627. [DOI] [PubMed] [Google Scholar]
- 46.Gerhardt T, Pöge U, Stoffel-Wagner B, Palmedo H, Sauerbruch T, Woitas RP. Creatinine-based glomerular filtration rate estimation in patients with liver disease: the new Chronic Kidney Disease Epidemiology Collaboration equation is not better. Eur J Gastroenterol Hepatol. 2011;23(11):969–973. doi: 10.1097/MEG.0b013e32834991f1. [DOI] [PubMed] [Google Scholar]
- 47.Masson I, Maillard N, Tack I, et al. GFR Estimation Using Standardized Cystatin C in Kidney Transplant Recipients. American Journal of Kidney Diseases. 2013;61(2):279–284. doi: 10.1053/j.ajkd.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 48.White CA, Akbari A, Doucette S, Fergusson D, Knoll GA. Estimating glomerular filtration rate in kidney transplantation: is the new chronic kidney disease epidemiology collaboration equation any better? Clin Chem. 2010;56(3):474–477. doi: 10.1373/clinchem.2009.135111. [DOI] [PubMed] [Google Scholar]
- 49.Buron F, Hadj-Aissa A, Dubourg L, et al. Estimating glomerular filtration rate in kidney transplant recipients: performance over time of four creatinine-based formulas. Transplantation. 2011;92(9):1005–1011. doi: 10.1097/TP.0b013e3182301602. [DOI] [PubMed] [Google Scholar]
- 50.White CA, Huang D, Akbari A, Garland J, Knoll GA. Performance of creatinine-based estimates of GFR in kidney transplant recipients: a systematic review. Am J Kidney Dis. 2008;51(6):1005–1015. doi: 10.1053/j.ajkd.2008.02.308. [DOI] [PubMed] [Google Scholar]
- 51.Stevens LA, Coresh J, Greene T, Levey AS. Assessing Kidney Function — Measured and Estimated Glomerular Filtration Rate. New England Journal of Medicine. 2006;354(23):2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 52.Stevens LA, Schmid CH, Zhang YL, et al. Development and validation of GFR-estimating equations using diabetes, transplant and weight. Nephrol Dial Transplant. 2010;25(2):449–457. doi: 10.1093/ndt/gfp510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dawoud D, Harms JC, Williams TA, Kumar V, Allon M. Vascular Access Management in Patients with Failing Kidney Transplants. 2012 doi: 10.1053/j.ajkd.2013.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akbari A, Hussain N, Karpinski J, Knoll GA. Chronic kidney disease management: comparison between renal transplant recipients and nontransplant patients with chronic kidney disease. Nephron Clin Pract. 2007;107(1):c7–13. doi: 10.1159/000105138. [DOI] [PubMed] [Google Scholar]
- 55.Kasiske BL, Israni AK, Snyder JJ, Skeans MA. The relationship between kidney function and long-term graft survival after kidney transplant. Am J Kidney Dis. 2011;57(3):466–475. doi: 10.1053/j.ajkd.2010.10.054. [DOI] [PubMed] [Google Scholar]
- 56.Kasiske BL, Israni AK, Snyder JJ, Skeans MA, Peng Y, Weinhandl ED. A simple tool to predict outcomes after kidney transplant. Am J Kidney Dis. 2010;56(5):947–960. doi: 10.1053/j.ajkd.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 57.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barbour GL, Crumb CK, Boyd CM, Reeves RD, Rastogi SP, Patterson RM. Comparison of inulin, iothalamate, and 99mTc-DTPA for measurement of glomerular filtration rate. J Nucl Med. 1976;17(4):317–320. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Flow diagram of subjects included in study.
Figure S2: Difference between P30 of CKD-EPI or MDRD Study equation and each alternative equation in age subgroups.
Figure S3: Difference between P30 of CKD-EPI or MDRD Study equation and each alternative equation in sex subgroups.
Figure S4: Difference between P30 of CKD-EPI or MDRD Study equation and each alternative equation in race subgroups.
Figure S5: Difference between P30 of CKD-EPI or MDRD Study equation and each alternative equation in weight subgroups.
Figure S6: Difference between P30 of CKD-EPI or MDRD Study equation and each alternative equation in mGFR subgroups.
Figure S7: Difference between P30 of CKD-EPI or MDRD Study equation and each alternative equation in study/center of enrollment subgroups.
Figure S8: P30 of CKD-EPI and MDRD Study equation across levels of mGFR.
Table S1: MEDLINE search terms to identify creatinine-based eGFR equations.
Table S2: Equations included in the systematic review.
Table S3: Clinical and demographic variables of study population by transplanted organ.
Table S4: Clinical and demographic variables of patients by presence of SUN variable.
Table S5: Performance metrics of CKD-EPI and MDRD Study equations by study.
Table S6: Performance metrics of CKD-EPI and MDRD Study equations by transplanted organ.
Item S1: Description of study population.
Item S2: Supplementary methods.