Abstract
Purpose
The antitumor activity of chimeric antigen receptor (CAR)-redirected cytotoxic T lymphocytes (CTLs) should be enhanced if it were possible to increase their proliferation and function after adoptive transfer without concomitantly increasing the proliferation and function of regulatory T cells (Tregs). Here we explored whether the lack of IL-7Rα in Tregs can be exploited by the targeted manipulation of the interleukin-7 (IL-7) cytokine-cytokine receptor axis in CAR-engrafted Epstein Barr Virus-specific CTLs (EBV-CTLs) to selectively augment their growth and anti-tumor activity even in the presence of Tregs.
Experimental Design
We generated a bicistronic retroviral vector encoding a GD2-specific CAR and the IL-7Rα subunit, expressed the genes in EBV-CTLs and assessed their capacity to control tumor growth in the presence of Tregs in vitro and in vivo when exposed to either interleukin-2 (IL-2) or IL-7 in a neuroblastoma xenograft.
Results
We found that IL-7, in sharp contrast to IL-2, supports the proliferation and antitumor activity of IL-7Rα.CAR-GD2+ EBV-CTLs both in vitro and in vivo even in the presence of fully functional Tregs.
Conclusions
IL-7 selectively favors the survival, proliferation, and effector function of IL-7Rα-transgenic/CAR-redirected EBV-CTLs in the presence of Tregs both in vitro and in vivo. Thus, IL-7 can have a significant impact in sustaining expansion and persistence of adoptively CAR-redirected CTLs.
INTRODUCTION
The expression of CARs in T lymphocytes to redirect their antigen specificity has significantly expanded the clinical application of adoptive T-cell immunotherapies against a variety of human malignancies(1, 2). CAR molecules are chimeric proteins in which a single chain antibody binding site is fused with the signaling domain CD3ζ that activates T lymphocytes upon binding to the tumor antigen(3). However, in this form CAR molecules do not provide adequate costimulation to T cells(1, 4, 5). To overcome this limitation, CARs can be expressed by CTLs whose native receptors are specific for virus latency proteins such as those derived from the EBV-CTLs(6, 7). These virus-specific CTLs can receive physiologic costimulation from professional antigen presenting cells processing latent viral antigens and kill tumor cells through their CAR(6, 7). While this approach can produce complete and sustained antitumor responses, for example in some patients with neuroblastoma, in most recipients CAR-engrafted EBV-CTLs have limited in vivo survival and fail to consistently eradicate disease(8, 9). It is likely that the combination of host/tumor associated inhibitory factors and insufficient in vivo immunostimulation limit the expansion and persistence of these cells(10).
Regulatory T cells (Tregs) play a significant role in impairing the antitumor effects of tumor-specific CTLs(11). Tregs are frequently increased in the peripheral blood and in tumor biopsies of cancer patients(12–17), and their presence often correlates with poor clinical outcome(15). Thus, the development of strategies aimed at eliminating Tregs or at selectively favoring the expansion of antitumor CTLs may significantly contribute in enhancing the engraftment and antitumor effects of adoptively transferred CTLs. To date, most efforts to increase in vivo immunostimulation of adoptively transferred T cells have focused on administration of IL-2(18). Although this cytokine is a potent T-cell growth factor, it is not selective for effector T-cell subsets, and can also enhance the growth and inhibitory activity of Tregs(19).
One means by which T lymphocytes can be selectively expanded is by using IL-7, a γchain cytokine that promotes homeostatic expansion of naïve and memory T cells but has no activity on Tregs, which lack the IL-7Rα (the private chain of the IL-7 receptor)(20–23). Administration of recombinant IL-7 was well tolerated in early phase clinical trials, and expanded naive and central-memory T-cell subsets but not Tregs(20, 21). Unfortunately, under physiological conditions, IL-7 cannot support the in vivo expansion of adoptively transferred CAR-redirected CTLs as this is an effector-memory T-cell subset that, like Tregs, also lacks IL-7Rα(24).
Here we developed models in vitro and in vivo to demonstrate that human Tregs clearly inhibit the antitumor effects of CAR-redirected EBV-CTLs. We also show that selective modulation of the IL-7 cytokine-cytokine receptor axis in CAR-engrafted EBV-CTLs augments their antitumor effects in vivo in the presence of Tregs. This strategy should safely enhance the persistence and survival of adoptively transferred CAR-redirected virus-specific CTLs in cancer patients.
MATERIALS AND METHODS
Plasmid construction, retrovirus production and tumor cell lines
The full-length human IL-7Rα linked through the 2A(TAV) sequence to the CAR-GD2 encoding the CD28 endodomain(25) was cloned into the SFG retroviral vector to generate the bicistronic vector SFG.IL-7Rα.CAR-GD2. The retroviral vectors encoding eGFP and Firefly Luciferase (FFLuc) were previously described(26). Retroviral supernatant was prepared using transient transfection of 293T cells (26). The neuroblastoma cell line CHLA-255(27) (kindly provided by Dr Leonid Metelitsa) was derived from a patient, and we verified that this line retains the surface expression of the target antigen GD2.
Generation and transduction of EBV-CTLs
EBV-transformed lymphoblastoid cells (LCLs) and EBV-CTLs were prepared using peripheral blood mononuclear cells (PBMCs), obtained from healthy donors as previously described(28). EBV-CTLs were transduced with retroviral supernatant after three stimulations with autologous LCLs, as previously described(8), and then maintained in culture by weekly stimulation with LCLs and recombinant IL-2 (50 IU/ml) or IL-7 (2.5 ng/ml) (PeproTech; Rocky Hill, NJ).
Expansion of Tregs
To obtain significant numbers of cells for the in vitro and in vivo experiments, Tregs were isolated and expanded as previously described(29). Briefly, CD25bright T cells were purified from PBMCs by positive selection using immuno-magnetic selection in the presence of non-saturating concentrations (2 μl/1 × 107 PBMCs) of anti-human CD25 magnetic beads (Miltenyi Biotech, Germany). On day 0, the purified CD25+ T cells were activated in 24-well plates coated with OKT3 (1 μg/mL) and anti-CD28 antibody (BD Pharmingen, Franklin Lakes, New Jersey) (1 μg/mL) in RPMI-1640 in the presence of rapamycin (Sigma, St Louis, MO) at a final concentration of 100 nM. On days 7 and 14, cells were restimulated with OKT3/CD28 antibodies, irradiated feeder cells, rapamycin and IL-2 (50 IU/mL) in small bioreactors (G-REX)(29). At the end of the 3 week culture (day 21), cells were used for in vitro and in vivo experiments. The cell fraction obtained from buffy coats after the selection of CD25bright T cells was further enriched for CD4+ cells which were then used as negative control in parallel culture experiments in which we evaluated the immunosuppressive activity of Tregs (29, 30).
Immunophenotyping
Cells were stained with fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, peridinin-chlorophyll-protein complex (PerCP)-, or allophycocyanin (APC)-conjugated monoclonal antibodies (mAbs). We used CD3, CD4, CD8, CD25 and CD127 (IL-7Rα specific) from Becton Dickinson (BD bioscience, Mountain View, CA) and FoxP3 from eBioscience Inc (San Diego, CA, USA). CAR-GD2 expression by transduced EBV-CTLs was detected using the specific anti-idiotype antibody 1A7, followed by staining with the secondary antibody RAM-IgG1-PE (Becton Dickinson)(8). STAT5 phosphorylation in Tregs and EBV-CTLs was assessed after cytokine stimulation for 15 minutes using the anti-phospho-STAT5 (Y694) mAb-Alexa Fluor 647 Conjugate (BD Phosflow Reagents, San Jose, CA). Cells were analyzed using a BD FACScalibur system equipped with the filter set for quadruple fluorescence signals and the CellQuest software (BD Biosciences). For each sample, we analyzed a minimum of 10,000 events.
Carboxyfluorescein diacetate succinimidyl ester (CFSE)-based assays
Proliferation of Tregs or EBV-CTLs or activated PBMC was assessed by CFSE dilution. Briefly, EBV-CTLs were labeled with 1.5 μM CFSE (Invitrogen) and activated with LCLs (ratio 4:1) with or without IL-2 (12.5 IU/ml) or IL-7 (10 ng/ml). CFSE dilution was measured by flow cytometry after 7 days of culture. A similar protocol was used to evaluate the proliferation of CFSE-labeled Tregs post activation with OKT3, irradiated feeders and IL-2 or IL-7. To evaluate the suppressive activity of Tregs, CFSE-labeled EBV-CTLs were stimulated with LCLs (ratio 4:1), in the presence of Tregs or control CD4+CD25− cells (ratio 1:1)(30), and of IL-2 (12.5 IU/ml) or IL-7 (10 ng/ml). Similarly, PBMC depleted of CD25bright cells were stained with CFSE and activated in the presence of irradiated allogeneic feeders (ratio 2:1) and OKT3 (500 ng/ml)(29, 30). After 7 days, cells were stained with CD8-APC and CD4-PerCP, analyzed by FACS and cell division assessed by CFSE dilution.
Evaluation of antitumor activity
EBV-CTLs were cultured in the presence of the neuroblastoma cell line (CHLA-255) genetically modified to stably express GFP in the presence or in the absence of Tregs (at the EBV-CTLs:CHLA-255:Treg ratio of 1:2:1) and of IL-2 (12.5 IU/ml) or IL-7 (5 ng/ml). After 7 days, cells were collected, stained with CD3 to identify T cells, and analyzed by FACS. GFP was used to quantify residual tumor cells in culture.
Xenogenic mouse model
To assess the antitumor effect of EBV-CTLs in vivo in the presence of Tregs, we used the xenograft mouse model and an in vivo imaging system previously described(7, 24). Mouse experiments were performed in accordance with Baylor College of Medicine’s Animal Husbandry guidelines. Briefly, 8 – 10 week old NSG mice were engrafted intraperitoneally (i.p.) with the CHLA-255 cells (1 × 106 cells/mouse) genetically modified with FFluc to monitor tumor growth using the IVIS bioluminescence system (Xenogen IVIS 200 Biophotonic Imaging System). The i.p. model was selected to minimize confounding issues due to suboptimal cell biodistribution and simultaneous co-localization at the tumor site of CAR-modified EBV-CTLs and Tregs. When the signal (measured as p/sec/cm2/sr) was consistently increasing, usually by day 7–10, mice received i.p. EBV-CTLs (10 × 106 T cells/mouse) with or without Tregs (10 × 106 T cells/mouse; two infusions one week apart). IL-2 (500 IU/mouse) or IL-7 (200 ng/mouse) were administered intraperitoneally three times a week.
Statistical analysis
All in vitro data were summarized by means and standard errors of the mean (SEM). For the bioluminescent experiments, intensity signals were log-transformed and summarized using mean ± SD at baseline and multiple subsequent time points for each group of mice. Changes in intensity of signal from baseline at each time point were calculated and compared using paired t-tests or Wilcoxon signed-ranks test. When the P-value was < 0.05, a mean difference was accepted as statistically significant. For the bioluminescence experiments, intensity signals were log-transformed and summarized using mean and standard deviations (SD) at baseline and multiple subsequent time points for each group of mice. The response profiles over time were analyzed by the generalized estimating equations method for repeated measurements.
RESULTS
Functional IL-7Rα and CAR-GD2 can be coexpressed in EBV-CTLs
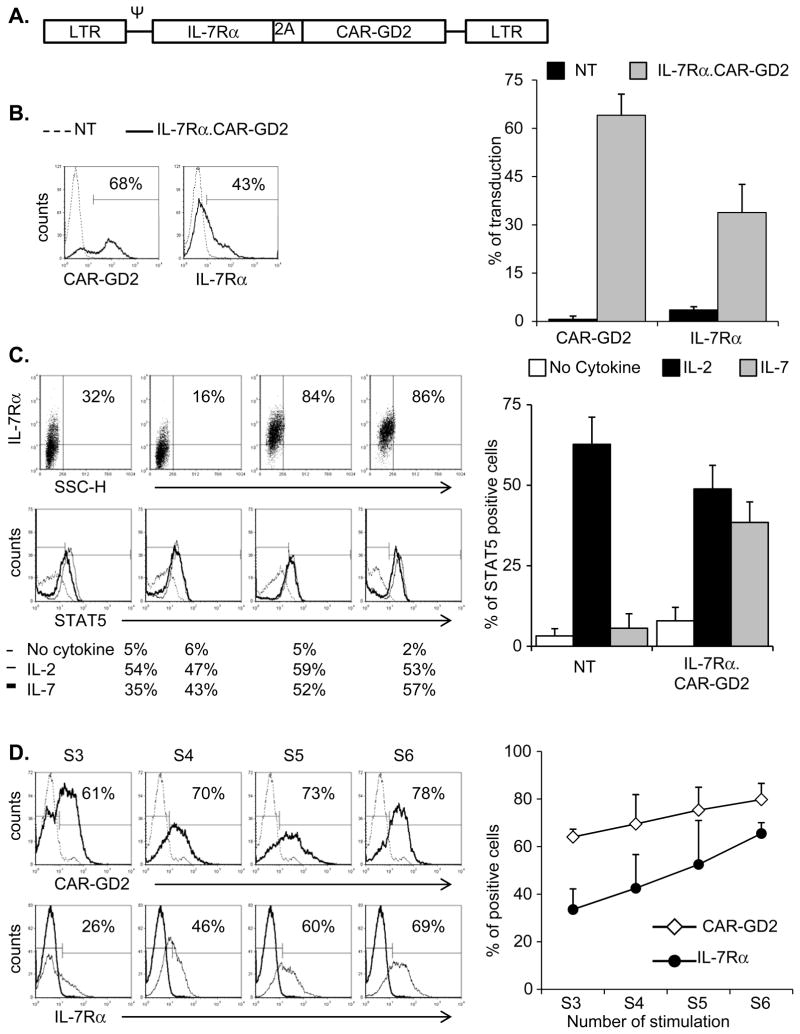
To restore the responsiveness to IL-7 and to redirect the antigen specificity of EBV-CTLs against neuroblastoma, we generated a bicistronic γ-retroviral vector encoding the IL-7Rα and a GD2-specific CAR linked through a 2A (TAV) sequence (SFG.IL-7Rα.2A.CAR-GD2) (Fig. 1A). EBV-CTLs established from 5 healthy EBV-seropositive donors were transduced with the vector, and the expression of both IL-7Rα and CAR-GD2 was measured by FACS analysis. As shown in Fig. 1B, both CAR-GD2 and IL-7Rα were stably expressed (64% ± 3% and 34% ± 9%, respectively) in transduced EBV-CTLs, while the expression of the native IL-7Rα on control cells remained negligible (4% ± 1%). To evaluate the functionality of the transgenic IL-7Rα, we measured the phosphorylation of STAT5 in response to either IL-2 or IL-7. In the absence of cytokines, control and IL-7Rα.CAR-GD2+ EBV-CTLs showed negligible phosphorylation of STAT5 (3% ± 2% and 8% ± 4%, respectively). In IL-7Rα.CAR-GD2+ EBV-CTLs, near equal STAT5 phosphorylation of Tyr-694 was detected in response to IL-2 (49% ± 7%) or IL-7 (38% ± 6%, respectively) (p= NS). By contrast, in control cells, STAT5 was phosphorylated in response to IL-2 (63% ± 8%) but not to IL-7 (6% ± 5%) (p < 0.05) (Fig. 1C). The levels of IL-7Rα-dependent STAT5 phosphorylation in IL-7Rα.CAR-GD2+ EBV-CTLs exposed to IL-7 were very similar to the amount observed in T lymphocytes physiologically expressing the IL-7Rα and exposed to IL-7 (Supplementary Fig. 1A).The functionality of the transgenic IL-7Rα was further supported by progressive selection of transgenic cells if cultures were supplemented with IL-7. As illustrated in Fig. 1D (and Supplementary Fig. 1B), when IL-7Rα.CAR-GD2+ CTLs were stimulated weekly with autologous LCLs and IL-7, the expression of both IL-7Rα and CAR-GD2 progressively increased between the third and sixth antigen-specific stimulation (from 34% ± 9% to 66% ± 5% for IL-7Rα, and from 64% ± 3% to 80% ± 7% for CAR-GD2). By contrast, when CTLs were expanded in the presence of IL-2, no enrichment of either transgenes was observed, since this cytokine equally supports the ex vivo growth of transduced and non transduced CTLs (data not shown).
Figure 1. EBV-CTLs are effectively transduced with the bicistronic vector encoding both the IL-7Rα and the CAR-GD2.
Panel A illustrates the schema of the bicistronic γ-retroviral vector encoding the IL-7Rα and GD2-specific CAR linked through a 2A (TAV) sequence. Panel B shows the expression of CAR-GD2 (top histogram) and IL-7Rα (bottom histogram) evaluated by FACS analysis day 7 after transduction. The dotted line indicates control EBV-CTLs while the bold line indicates the transduced EBV-CTLs. The graph represents mean ± SD of 5 donors. Panel C illustrates IL-7Rα expression in four IL-7Rα.CAR-GD2+ EBV-CTLs generated (upper panels) and STAT5 phosphorylation (lower panels) in the absence of cytokines (thin black line), in response to IL-2 (dotted line) or IL-7 (black bold line). Panel D shows the progressive enrichment in cells expressing the two transgenes IL-7Rα and CAR-GD2 when IL-7Rα.CAR-GD2+ EBV-CTLs were expanded in the presence of IL-7. S3, S4, S5 and S6 indicate the transgene expression detected week 3 (S3), week 4 (S4), week 5 (S5) and week 6 (S6), respectively after transduction. Graph represents mean ± SEM of 4 different EBV-CTL lines.
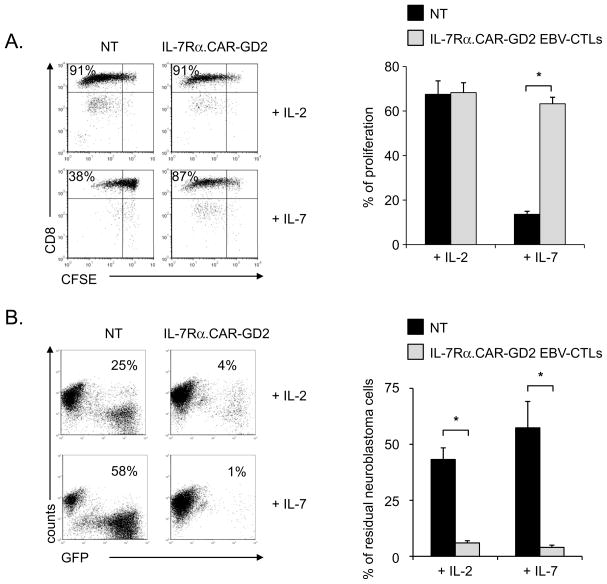
The enrichment of transgenic T cells following exposure to IL-7 was a consequence of the proliferation of IL-7Rα.CAR-GD2+ EBV-CTLs. As illustrated in Fig. 2A, CFSE labeled-control and IL-7Rα.CAR-GD2+ EBV-CTLs divided equally well when stimulated with LCLs (ratio 4:1) in the presence of IL-2 (proliferation: 68% ± 6% and 68% ± 4%, respectively). By contrast, in the presence of IL-7, IL-7Rα.CAR-GD2+ but not control EBV-CTLs had significantly greater proliferation: 63% ± 3% vs. 14% ± 1%, respectively (p<0.001). The number of EBV-CTLs proliferating in response to EBV-LCLs and IL-7 was generally higher than expected based on the ectopic expression of IL-7Rα. This higher level is likely a consequence of the physiological production of IL-2 by EBV-CTLs in response to their cognate EBV antigens (EBV-LCLs) (Supplementary Fig. 2). Finally, exposure of IL-7Rα.CAR-GD2+ EBV-CTLs to IL-7 did not affect their antitumor properties. As shown in Fig. 2B, when EBV-CTLs were cultured with CHLA-255 cells, only IL-7Rα.CAR-GD2+ cells controlled tumor growth in the presence of either IL-2 or IL-7 (6% ± 1% and 4% ± 1%, respectively), while tumor cells outgrew in cultures containing control EBV-CTLs irrespective of the cytokine added (43% ± 5% and 57% ± 12%, respectively) (p<0.001).
Figure 2. IL-7 supports the proliferation and effector function of IL-7Rα.CAR-GD2+ EBV-CTLs.
Panel A shows a representative CFSE-based proliferation assay of control and IL-7Rα.CAR-GD2+ EBV-CTLs. Control and IL-7Rα.CAR-GD2+ EBV-CTLs were activated in the presence of autologous irradiated LCLs and either IL-2 or IL-7. CFSE dilution was evaluated on day 7 using FACS analysis. The graph represents mean ± SD of 5 independent experiments. Panel B illustrates a representative co-culture experiment in which control and IL-7Rα.CAR-GD2+ EBV-CTLs were co-cultured with CHLA-255 GFP-tagged tumor cells (at ratio 1:2) in the presence of IL-2 or IL-7. Residual tumor cells were enumerated by flow cytometry on day 7 of culture. The graph shows mean ± SD of 5 independent experiments. *p<0.001.
Ex vivo expanded Tregs do not respond to IL-7
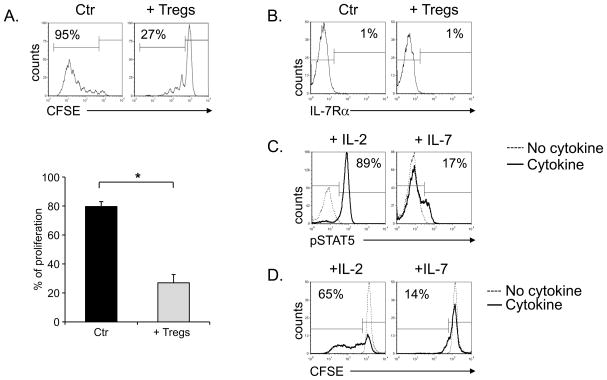
We used ex vivo expanded CD4+CD25+ Tregs isolated from healthy donors rather than freshly isolated Tregs for the following reasons. First, the experiments required a significant number of Tregs that could not be obtained upon fresh isolation even from buffy coat preparations. Second, circulating Tregs obtained after immunomagnetic selection based on CD4 and CD25 selection are frequently contaminated by CD4+CD25+IL7Rα+ cells that lack regulatory activity, but respond to IL-7 (data not shown)(31). We first confirmed that the nominal Treg population retained their inhibitory properties. As shown in Fig. 3A, the proliferation of activated PBMCs (80% ± 3% in the presence of control CD4+CD25− cells) was significantly inhibited in the presence of the expanded Treg population (27% ± 6%) (p< 0.001). We then confirmed that these Tregs, like freshly isolated Tregs(22), lacked expression of IL-7Rα (3% ± 0.4% positive) (Fig. 3B). As a consequence, STAT5 was only phosphorylated in these Tregs in response to IL-2 (MFI = 75 ± 9) (p<0.001) and not in response to IL-7 (MFI = 23 ± 3) (Fig. 3C). Finally, a CFSE-based dilution assay showed that Tregs only proliferated after polyclonal activation in the presence of IL-2 and not on exposure to IL-7 (MFI 1439 ± 207 vs 445 ± 68, respectively; p<0.001) (Fig. 3D).
Figure 3. Ex vivo expanded Tregs do not respond to IL-7.
Panel A shows a CFSE-based assay to illustrate the inhibitory activity of ex vivo expanded Tregs. PBMCs labeled with CFSE were activated in the absence (left histograms) or in the presence of Tregs (right histograms) at a ratio of 1:1. CFSE dilution was measured on day 7 of culture by flow cytometry. The graph represents mean ± SEM of 6 independent experiments. Panel B illustrates the expression of IL-7Rα in ex vivo expanded Tregs in a representative experiment. The plot on the left shows the isotype control, while the plot on the right the IL-7Rα profile. * p< 0.001. Panel C shows the phosphorylation of STAT5 in Tregs not stimulated (dotted lines) or stimulated with IL-2 (left panel) or IL-7 (right panel). Panel D illustrates the proliferative response of Tregs exposed to IL-2 or IL-7. Tregs were labeled with CFSE and stimulated in the presence of IL-2 (left panel) or IL-7 (right panel). CFSE dilution was evaluated on day 7 by flow cytometry. The solid and dotted lines represent the CFSE dilution of Tregs stimulated with or without cytokines, respectively.
IL-7 supports the proliferation and effector function of IL-7Rα.CAR-GD2+ EBV-CTLs in the presence of Tregs
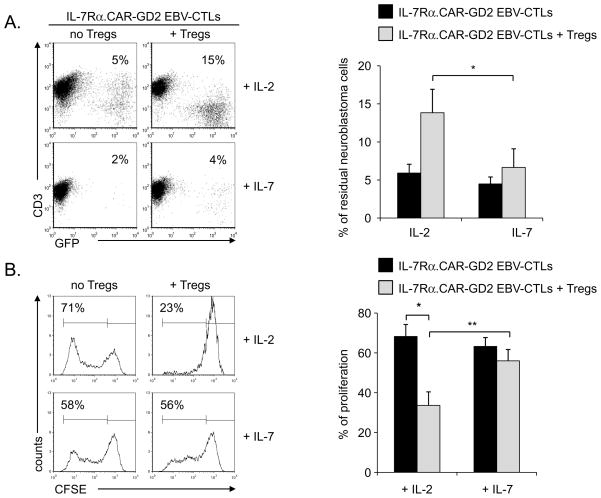
Having demonstrated that IL-7 supports the proliferation and function of IL-7Rα.CAR-GD2+ EBV-CTLs, we then investigated whether the beneficial effects of IL-7 were maintained in the presence of functional Tregs. As illustrated in Fig. 4A, when IL-7Rα.CAR-GD2+ EBV-CTLs were cultured with CHLA-255 cells (effector:target ratio of 1:2) they significantly controlled the growth of these tumor cells by day 7 of culture in the presence of either IL-2 or IL-7 (residual cells were 6% ± 1% and 4% ± 1%, respectively). By contrast, when expanded Tregs were added to the co-culture (ratio CTLs:Tregs 1:1), the antitumor activity of IL-7Rα.CAR-GD2+ EBV-CTLs was significantly inhibited in the presence of IL-2 but not of IL-7 (residual cells in culture: 14% ± 3% vs. 7% ± 2%, respectively; p<0.05). In addition, IL-7 also supported the proliferation of IL7Rα.CAR-GD2+ EBV-CTLs in the presence of Tregs upon physiological costimulation with autologous LCLs. As the CFSE dilution assay shows in Fig. 4B, the proliferation of IL-7Rα.CAR-GD2+ EBV-CTLs in response to IL-2 (68% ± 4%) was significantly compromised in the presence of Tregs (to 34% ± 6%, p<0.01). In contrast, when IL-7 was added to the culture, IL-7Rα.CAR-GD2+ EBV-CTLs divided well even in the presence of Tregs (proliferation was 63% ± 3% without Tregs and 56% ± 2% in the presence of Tregs). The CFSE dilution of IL-7Rα.CAR-GD2+ EBV-CTLs co-cultured with Tregs was significantly increased in the presence of IL-7 as compared to IL-2 (p=0.005).
Figure 4. IL-7, unlike IL-2, supports in vitro the proliferation and function of IL-7Rα.CAR-GD2+ EBV-CTLs in the presence of Tregs.
Panel A. IL-7Rα.CAR-GD2+ EBV-CTLs were co-cultured with CHLA-255 GFP-tagged cells (ratio 1:2) in the presence of IL-2 or IL-7, with or without Tregs. The percentage of residual tumor cells was measured by flow cytometry on day 7 of culture. The plots on the left show a representative experiment, while the graph on the right summarizes mean ± SD of 5 independent experiments. Panel B. IL-7Rα.CAR-GD2+ EBV-CTLs were labeled with CFSE and activated with autologous LCLs in the presence of IL-2 (upper plots) or IL-7 (lower plots) with or without Tregs. CFSE dilution was measured at day 7 of culture by flow cytometry. The plots on the left show a representative experiment, while the graph represents mean ± SD of 5 independent experiments. * p<0.01; ** p=0.005
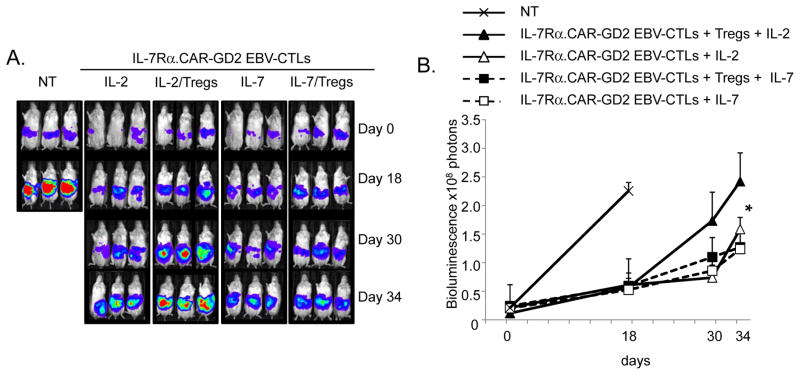
IL-7 supports the in vivo antitumor activity of IL-7Rα.CAR-GD2 EBV-CTLs even in the presence of Tregs
To assess the in vivo capacity of IL-7 to support the antitumor activity of IL-7Rα.CAR-GD2+ EBV-CTLs, we used NSG mice engrafted i.p. with the FFLuc+ cell line CHLA-255. As shown in Fig. 5, control mice that received only tumor cells or control CTLs showed a rapid increase of the bioluminescence signal (2.3 × 108 ± 3 × 107 photons) and were sacrificed by day 18. Mice infused with IL-7Rα.CAR-GD2+ EBV-CTLs and IL-2 had superior tumor control (1.6 x108 ± 2 × 107 photons at day 34), but this effect was abrogated when Tregs were co-infused (2.4 × 108 ± 4 × 107 photons at day 34, p<0.05). In contrast, mice infused with IL-7Rα.CAR-GD2+ EBV-CTLs and IL-7 controlled tumor growth equally well in the absence (1.2 × 108 ± 3 × 107 photons) or in presence of Tregs (1.3 × 108 ± 6 × 106 photons) at day 34.
Figure 5. IL-7, but not IL-2, supports in vivo anti-tumor activity of IL-7Rα.CAR-GD2+ EBV-CTLs in the presence of Tregs.
NSG mice engrafted i.p. with CHLA-255 cells tagged with FFLuc were infused with IL-7Rα.CAR-GD2+ EBV-CTLs and received IL-2 ± Tregs or IL-7 ± Tregs. Tumor growth was monitored using an in vivo imaging system (Xenogen – IVIS imaging system). A group of mice received control EBV-CTLs or tumor cells only (Control). Panel A shows the images of different groups of mice. Panel B illustrates the mean ± SD of photons for 8 mice/group in two independent experiments. * p<0.05.
Discussion
The adoptive transfer of CAR-redirected EBV-CTLs safely induces tumor regression in patients with neuroblastoma and the approach is potentially applicable to other human malignancies(8, 9). To further improve the clinical benefits of this approach we developed a strategy that selectively promotes the in vivo expansion of CAR-redirected CTLs without favoring the proliferation and function of Tregs that may limit long-term persistence and activity of the infused effector cells and thereby compromise antitumor efficacy. Here we demonstrate that CAR-redirected EBV-CTLs engineered to regain responsiveness to IL-7 by restoring their expression of IL-7Rα, proliferate in response to a combination of native TCR receptor and IL-7 stimulation without favoring the expansion and function of Tregs. As a consequence, we observed an increase in their CAR-mediated anti-neuroblastoma activity, even in the presence of Tregs.
Successful clinical outcome following adoptive transfer of tumor-specific T cells strongly correlates with the in vivo survival and proliferation of these cells (18, 32, 33). In addition to the intrinsic properties of T lymphocytes, such as central-memory versus effector-memory versus naïve phenotype that directly dictate the self-maintenance capacity of tumor-specific T cells(34), several tumor-associated mechanisms are also pivotal in determining the consequences of administering tumor-specific T cells(10, 35). Tregs in particular are abundant in the tumor microenvironment and are a major factor in impairing T-cell function. Hence, strategies that selectively increase persistence and expansion of adoptively transferred T cells or that eliminate the influence of this cell subset should be as relevant for T-cell therapies as they have proved to be for cancer-vaccine trials(36).
The administration of recombinant cytokines or the use of cytokine-engineered T cells(30, 37, 38) that selectively support T-cell growth without providing functional or proliferative advantages to Tregs represent appealing approaches to overcome the inhibitory function of Tregs within the tumor microenvironment. However, IL-2 that is frequently used to sustain the in vivo proliferation and persistence of adoptively transferred CTLs is non-selective, stimulating both tumor-specific effector T cells and Tregs, as both these cell subsets express the IL-2 high affinity receptor (CD25)(19, 39). Thus, as illustrated in the current and prior studies, the net effect of IL-2 administration is to block the proliferation and antitumor effects of CAR-redirected CTLs both in vitro and in vivo.
While IL-7 shares several functions with IL-2, it also has effects on specific T-cell subsets that depend on their expression of the private IL-7Rα subunit(23). Our experiments demonstrate both in vitro and in vivo that IL-7 can nonetheless support the survival, expansion and effector function of CAR-redirected EBV-CTLs if these cells are engineered to re-express the IL-7Rα, and that it can thereby overcome the inhibitory effects of Tregs. Our approach has significant advantages over the use of IL-2 or cytotoxic drugs to eliminate Tregs in a non-selective manner(40) since it may promote the long-term persistence of CAR-redirected EBV-CTLs both in steady state conditions and in a lymphopenic environment(23). In addition, the administration of recombinant IL-7 unlike recombinant IL-2 appears to be well tolerated even at high doses(20, 21, 41). Finally, since the infusion of virus-specific CTLs after allogeneic stem cell transplant does not induce the occurrence of graft versus host disease(28), our proposed approach of infusing CAR-redirected CTLs with restored responsiveness to the homeostatic cytokine IL-7 may significantly increase the application of CAR technology in the allogeneic setting(42).
One potential concern associated with any genetic manipulation of T cells is that the cells will undergo malignant transformation, or grow in an antigen independent manner. This concern is particularly prominent when the genetic manipulation modifies a growth factor receptor or other portions of a signaling pathway. However, the experience of our own and other groups has been that the genetic manipulation of differentiated T cells to express cytokines or cytokine receptors does not affect the antigen-specificity of these cells and does not elicit uncontrolled proliferation(24, 37, 43). These results were confirmed in the current study even if we can not completely exclude the possibility of secondary paracrine effects due to the ectopic expression of IL-7Rα. If such a concern remains, however, incorporation of suicide or safety switches within the cells may provide a further level of reassurance(37, 44).
Our study suggests that restoring the responsiveness to IL-7 of virus-specific CTLs redirected with a CAR is a strategy that may allow enhanced T-effector cells without concomitant inhibition by Treg and may thereby further improve the clinical outcome of a promising therapeutic approach.
Supplementary Material
TRANSLATIONAL RELEVANCE.
Adoptive transfer of virus-specific CTLs expressing a CAR represents a promising therapy for patients with cancer. However, the in vivo expansion of these cells remains suboptimal so that new strategies are required to selectively expand them without favoring the concomitant proliferation and function of Tregs that are often abundant in cancer patients. Our study provides preclinical data, indicating that the manipulation of the IL-7 cytokine-cytokine receptor axis in CAR-engrafted EBV-CTLs can be used to selectively expand the CTLs whilst avoiding the inhibitory effects of Tregs, which would otherwise be enhanced by use of the more broadly acting T-cell growth factor IL-2.
Acknowledgments
Funding
This work was supported in part by R01 CA142636 NIH-NCI, W81XWH-10-10425 Department of Defense, Technology/Therapeutic Development Award and by P50CA126752 SPORE in Lymphoma from NCI.
Footnotes
Competing Financial Interest
The Center for Cell and Gene Therapy has a research collaboration with Celgene and bluebird bio. All authors reviewed the manuscript and approved the final version of the manuscript.
Authorship
S.K.P., D.P., G.D. and B.S. designed the research, analyzed the data and wrote the manuscript.
S.K.P. and D.P performed the experiments.
A.M. provided technical assistance for some of the experiments.
H.L. performed some of the statistical analysis.
M.K.B provided assistance in the design of the research and critically reviewed the manuscript.
Reference List
- 1.Sadelain M, Brentjens R, Riviere I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21:215–23. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jena B, Dotti G, Cooper LJ. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood. 2010;116:1035–44. doi: 10.1182/blood-2010-01-043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90:720–4. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18:676–84. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 5.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822–6. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossig C, Bollard CM, Nuchtern JG, Rooney CM, Brenner MK. Epstein-Barr virus-specific human T lymphocytes expressing antitumor chimeric T-cell receptors: potential for improved immunotherapy. Blood. 2002;99:2009–16. doi: 10.1182/blood.v99.6.2009. [DOI] [PubMed] [Google Scholar]
- 7.Savoldo B, Rooney CM, Di Stasi A, Abken H, Hombach A, Foster AE, et al. Epstein Barr virus specific cytotoxic T lymphocytes expressing the anti-CD30{zeta} artificial chimeric T-cell receptor for immunotherapy of Hodgkin disease. Blood. 2007;110:2620–30. doi: 10.1182/blood-2006-11-059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–70. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–74. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 11.Baecher-Allan C, Hafler DA. Suppressor T cells in human diseases. J Exp Med. 2004;200:273–6. doi: 10.1084/jem.20040812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson BD, Jing W, Orentas RJ. CD25+ regulatory T cell inhibition enhances vaccine-induced immunity to neuroblastoma. J Immunother. 2007;30:203–14. doi: 10.1097/01.cji.0000211336.91513.dd. [DOI] [PubMed] [Google Scholar]
- 13.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–12. [PubMed] [Google Scholar]
- 14.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, et al. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–72. [PubMed] [Google Scholar]
- 15.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 16.Marshall NA, Christie LE, Munro LR, Culligan DJ, Johnston PW, Barker RN, et al. Immunosuppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood. 2004;103:1755–62. doi: 10.1182/blood-2003-07-2594. [DOI] [PubMed] [Google Scholar]
- 17.Jing W, Yan X, Hallett WH, Gershan JA, Johnson BD. Depletion of CD25(+) T cells from hematopoietic stem cell grafts increases posttransplantation vaccine-induced immunity to neuroblastoma. Blood. 2011;117:6952–62. doi: 10.1182/blood-2010-12-326108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–14. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sportes C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008;205:1701–14. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenberg SA, Sportes C, Ahmadzadeh M, Fry TJ, Ngo LT, Schwarz SL, et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother (1997) 2006;29:313–9. doi: 10.1097/01.cji.0000210386.55951.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–79. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 24.Vera JF, Hoyos V, Savoldo B, Quintarelli C, Giordano Attianese GM, Leen AM, et al. Genetic manipulation of tumor-specific cytotoxic T lymphocytes to restore responsiveness to IL-7. Mol Ther. 2009;17:880–8. doi: 10.1038/mt.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pule MA, Straathof KC, Dotti G, Heslop HE, Rooney CM, Brenner MK. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005;12:933–41. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Vera J, Savoldo B, Vigouroux S, Biagi E, Pule M, Rossig C, et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 2006;108:3890–7. doi: 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song L, Ara T, Wu HW, Woo CW, Reynolds CP, Seeger RC, et al. Oncogene MYCN regulates localization of NKT cells to the site of disease in neuroblastoma. J Clin Invest. 2007;117:2702–12. doi: 10.1172/JCI30751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rooney CM, Smith CA, Ng CY, Loftin SK, Sixbey JW, Gan Y, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–55. [PubMed] [Google Scholar]
- 29.Chakraborty R, Mahendravada A, Perna SK, Rooney CM, Heslop HE, Vera JF, et al. Robust and cost effective expansion of human regulatory T cells highly functional in a xenograft model of graft-versus-host disease. Haematologica. 2013;98:533–7. doi: 10.3324/haematol.2012.076430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perna SK, De AB, Pagliara D, Hasan ST, Zhang L, Mahendravada A, et al. Interleukin 15 Provides Relief to CTLs from Regulatory T Cell-Mediated Inhibition: Implications for Adoptive T Cell-Based Therapies for Lymphoma. Clin Cancer Res. 2013;19:106–17. doi: 10.1158/1078-0432.CCR-12-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177:8338–47. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 32.Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115:925–35. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–26. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoyos V, Savoldo B, Dotti G. Genetic modification of human T lymphocytes for the treatment of hematologic malignancies. Haematologica. 2012;97:1622–31. doi: 10.3324/haematol.2012.064303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–33. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoyos V, Savoldo B, Quintarelli C, Mahendravada A, Zhang M, Vera J, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24:1160–70. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu K, Rosenberg SA. Transduction of an IL-2 gene into human melanoma-reactive lymphocytes results in their continued growth in the absence of exogenous IL-2 and maintenance of specific antitumor activity. J Immunol. 2001;167:6356–65. doi: 10.4049/jimmunol.167.11.6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Terabe M, Berzofsky JA. Immunoregulatory T cells in tumor immunity. Curr Opin Immunol. 2004;16:157–62. doi: 10.1016/j.coi.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 40.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–9. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, et al. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994;86:1159–66. doi: 10.1093/jnci/86.15.1159. [DOI] [PubMed] [Google Scholar]
- 42.Micklethwaite KP, Savoldo B, Hanley PJ, Leen AM, mmler-Harrison GJ, Cooper LJ, et al. Derivation of human T lymphocytes from cord blood and peripheral blood with antiviral and antileukemic specificity from a single culture as protection against infection and relapse after stem cell transplantation. Blood. 2010;115:2695–703. doi: 10.1182/blood-2009-09-242263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quintarelli C, Vera JF, Savoldo B, Giordano Attianese GM, Pule M, Foster AE, et al. Co-expression of cytokine and suicide genes to enhance the activity and safety of tumor-specific cytotoxic T lymphocytes. Blood. 2007;110:2793–802. doi: 10.1182/blood-2007-02-072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673–83. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.