Abstract
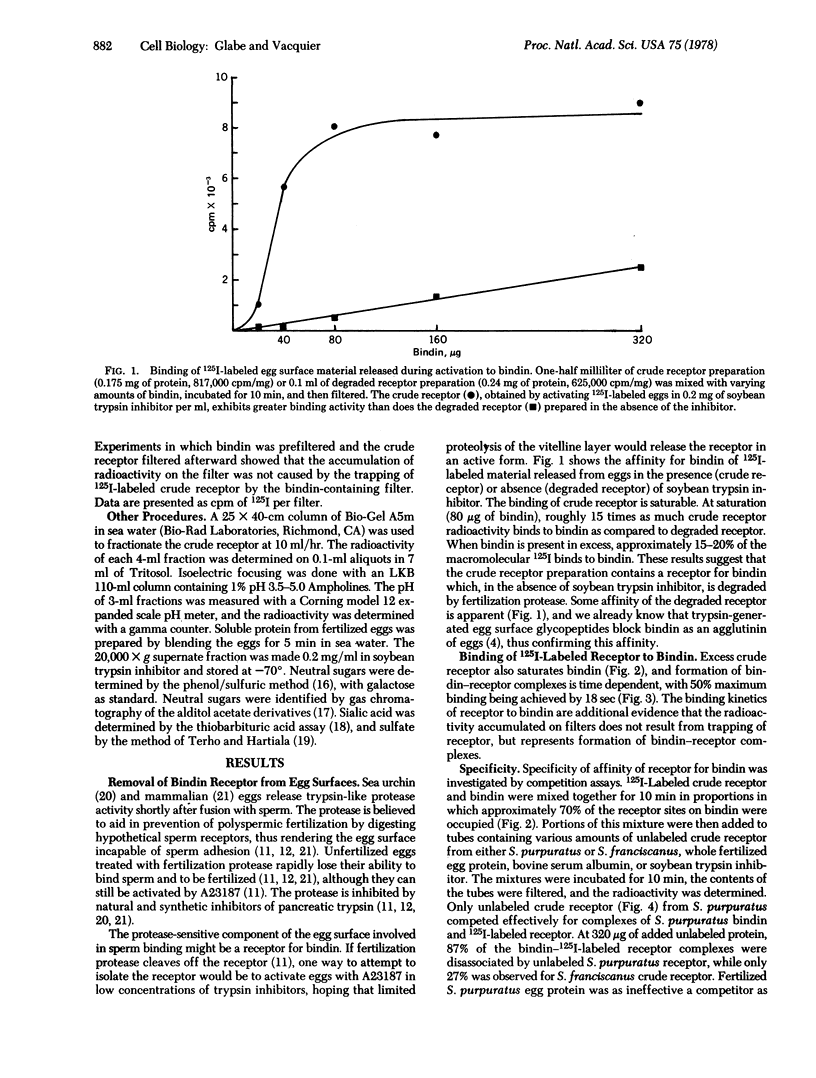
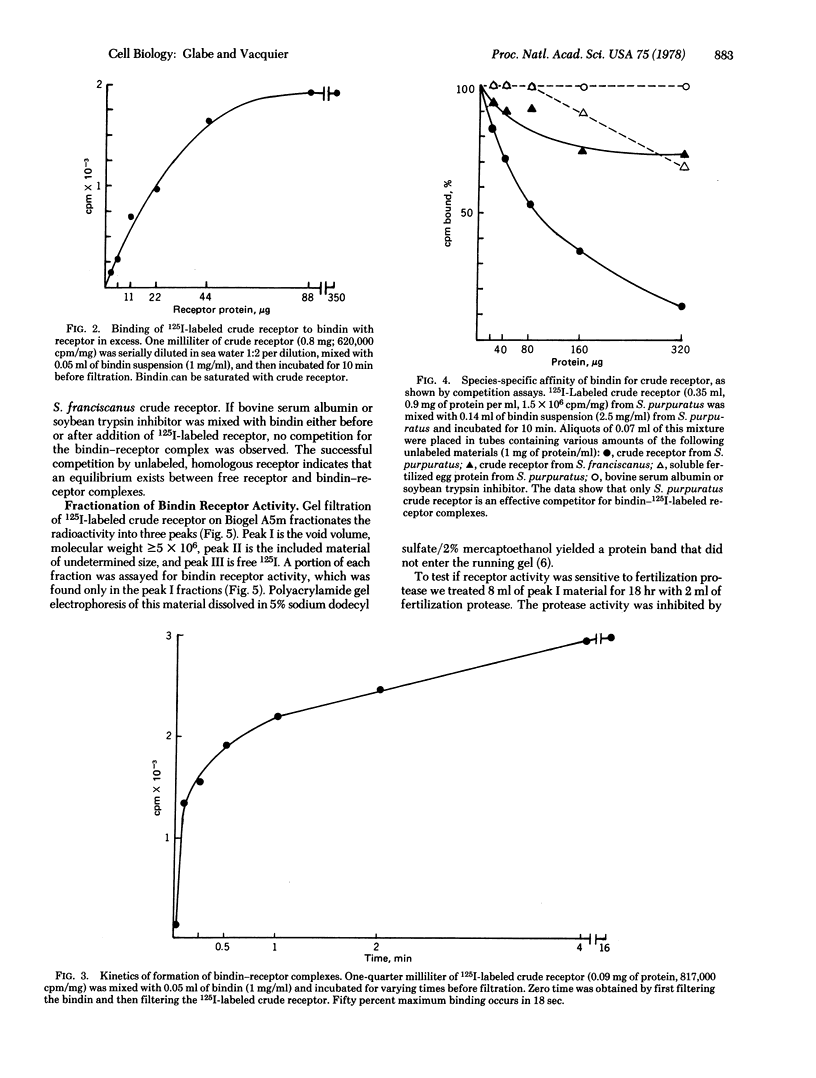
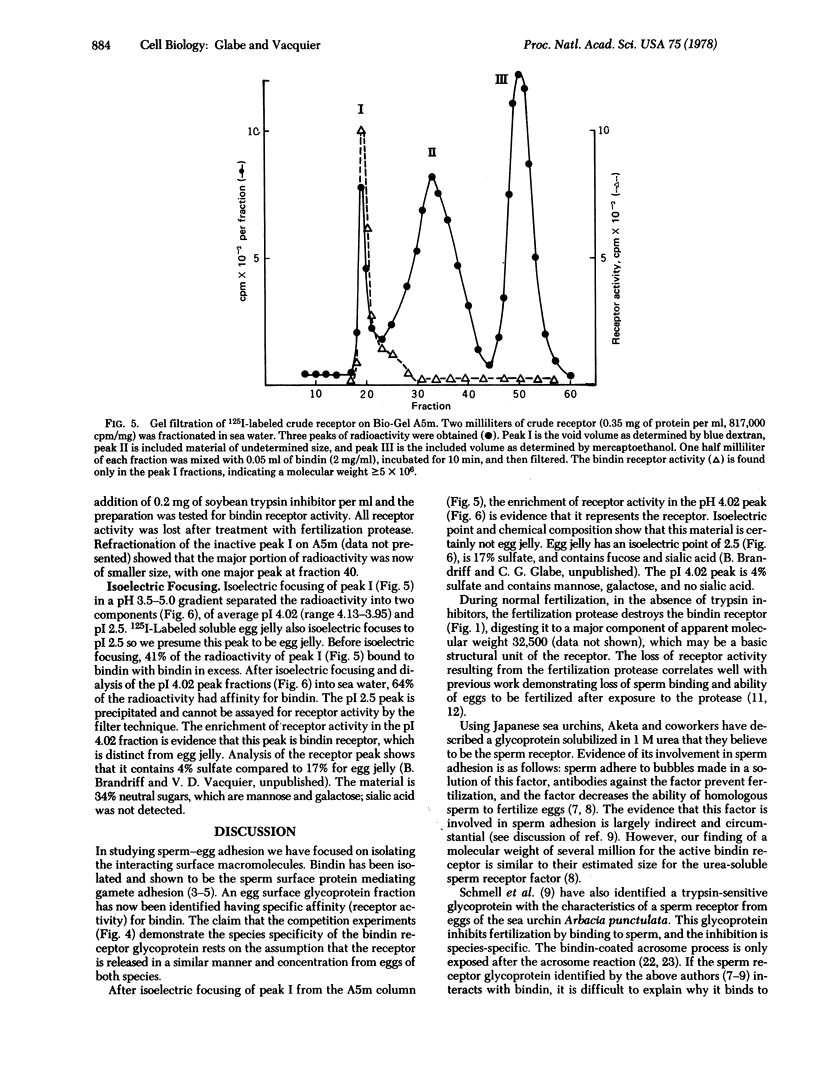
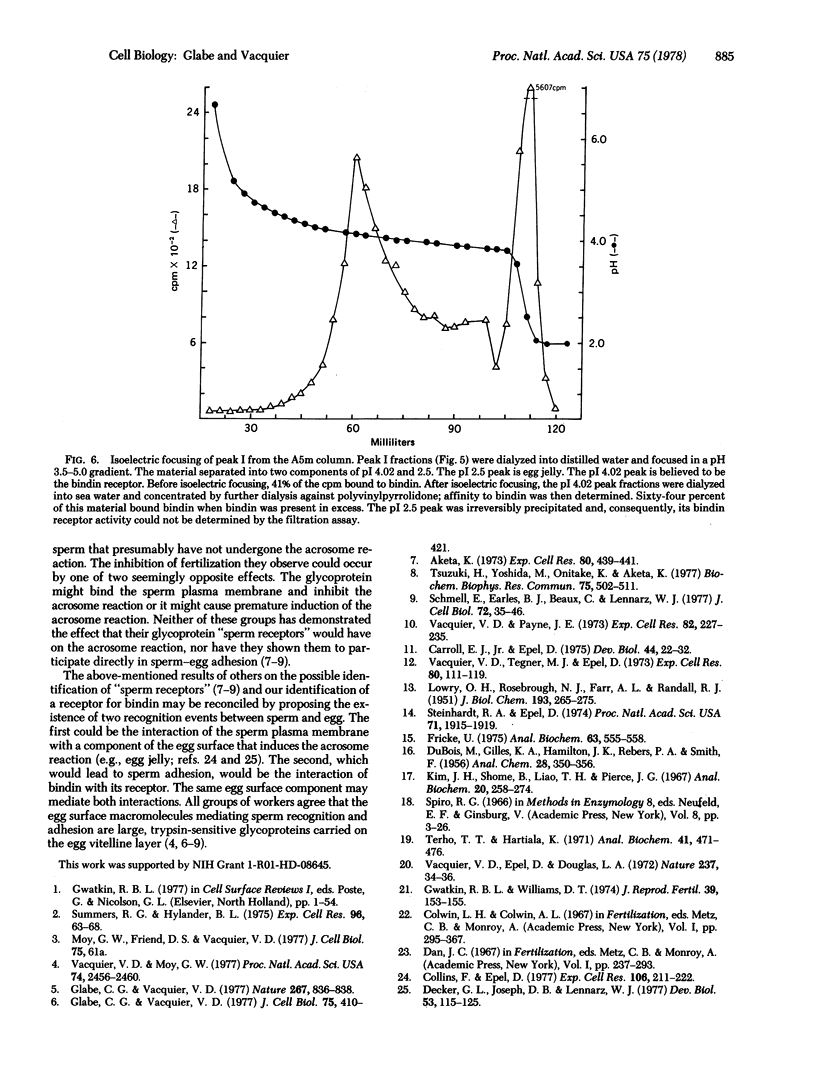
Bindin is an insoluble protein coating the sperm acrosome process and mediating the adhesion of sperm to sea urchin eggs. Milligrams of bindin have been isolated. Here we report the identification, isolation, and partial characterization of a high molecular weight, trypsin-sensitive glycoprotein fraction from the sea urchin egg surface having species-specific affinity for bindin. This glycoprotein may be the egg surface receptor for bindin. The bindin receptor was released from 125-I-labeled eggs by parthenogenetic activation of eggs with ionophore A23187 in the presence of soybean trypsin inhibitor. The receptor has an isoelectric point of 4.02 and a molecular weight in sea water greater than or equal to 5 X 10(6), suggesting that it is an aggregate. It contains 34% neutral sugars, which are galactose and mannose.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aketa K. Physiological studies on the sperm surface component responsible for sperm-egg bonding in sea urchin fertilization. I. Effect of sperm-binding protein on the fertilizing capacity of sperm. Exp Cell Res. 1973 Aug;80(2):439–441. doi: 10.1016/0014-4827(73)90317-0. [DOI] [PubMed] [Google Scholar]
- Carroll E. J., Jr, Epel D. Isolation and biological activity of the proteases released by sea urchin eggs following fertilization. Dev Biol. 1975 May;44(1):22–32. doi: 10.1016/0012-1606(75)90373-5. [DOI] [PubMed] [Google Scholar]
- Collins F., Epel D. The role of calcium ions in the acrosome reaction of sea urchin sperm: regulation of exocytosis. Exp Cell Res. 1977 Apr;106(1):211–222. doi: 10.1016/0014-4827(77)90258-0. [DOI] [PubMed] [Google Scholar]
- Decker G. L., Joseph D. B., Lennarz W. J. A study of factors involved in induction of the acrosomal reaction in sperm of the sea urchin, Arbacia punctulata. Dev Biol. 1976 Oct 1;53(1):115–125. doi: 10.1016/0012-1606(76)90213-x. [DOI] [PubMed] [Google Scholar]
- Fricke U. Tritosol: a new scintillation cocktail based on Triton X-100. Anal Biochem. 1975 Feb;63(2):555–558. doi: 10.1016/0003-2697(75)90379-6. [DOI] [PubMed] [Google Scholar]
- Glabe C. G., Vacquier V. D. Isolation and characterization of the vitelline layer of sea urchin eggs. J Cell Biol. 1977 Nov;75(2 Pt 1):410–421. doi: 10.1083/jcb.75.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glabe C. G., Vacquier V. D. Species specific agglutination of eggs by bindin isolated from sea urchin sperm. Nature. 1977 Jun 30;267(5614):836–838. doi: 10.1038/267836a0. [DOI] [PubMed] [Google Scholar]
- Gwatkin R. B., Williams D. T. Heat sensitivity of the cortical granule protease from hamster eggs. J Reprod Fertil. 1974 Jul;39(1):153–155. doi: 10.1530/jrf.0.0390153. [DOI] [PubMed] [Google Scholar]
- Kim J. H., Shome B., Liao T. H., Pierce J. G. Analysis of neutral sugars by gas-liquid chromatography of alditol acetates: application to thyrotropic hormone and other glycoproteins. Anal Biochem. 1967 Aug;20(2):258–274. doi: 10.1016/0003-2697(67)90031-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Schmell E., Earles B. J., Breaux C., Lennarz W. J. Identification of a sperm receptor on the surface of the eggs of the sea urchin Arbacia punctulata. J Cell Biol. 1977 Jan;72(1):35–46. doi: 10.1083/jcb.72.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt R. A., Epel D. Activation of sea-urchin eggs by a calcium ionophore. Proc Natl Acad Sci U S A. 1974 May;71(5):1915–1919. doi: 10.1073/pnas.71.5.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers R. G., Hylander B. L. Species-specificity of acrosome reaction and primary gamete binding in echinoids. Exp Cell Res. 1975 Nov;96(1):63–68. doi: 10.1016/s0014-4827(75)80037-1. [DOI] [PubMed] [Google Scholar]
- Terho T. T., Hartiala K. Method for determination of the sulfate content of glycosaminoglycans. Anal Biochem. 1971 Jun;41(2):471–476. doi: 10.1016/0003-2697(71)90167-9. [DOI] [PubMed] [Google Scholar]
- Vacquier V. D., Epel D., Douglas L. A. Sea urchin eggs release protease activity at fertilization. Nature. 1972 May 5;237(5349):34–36. doi: 10.1038/237034a0. [DOI] [PubMed] [Google Scholar]
- Vacquier V. D., Moy G. W. Isolation of bindin: the protein responsible for adhesion of sperm to sea urchin eggs. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2456–2460. doi: 10.1073/pnas.74.6.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacquier V. D., Payne J. E. Methods for quantitating sea urchin sperm-egg binding. Exp Cell Res. 1973 Nov;82(1):227–235. doi: 10.1016/0014-4827(73)90265-6. [DOI] [PubMed] [Google Scholar]
- Vacquier V. D., Tegner M. J., Epel D. Protease released from sea urchin eggs at fertilization alters the vitelline layer and aids in preventing polyspermy. Exp Cell Res. 1973 Jul;80(1):111–119. doi: 10.1016/0014-4827(73)90281-4. [DOI] [PubMed] [Google Scholar]


