Abstract
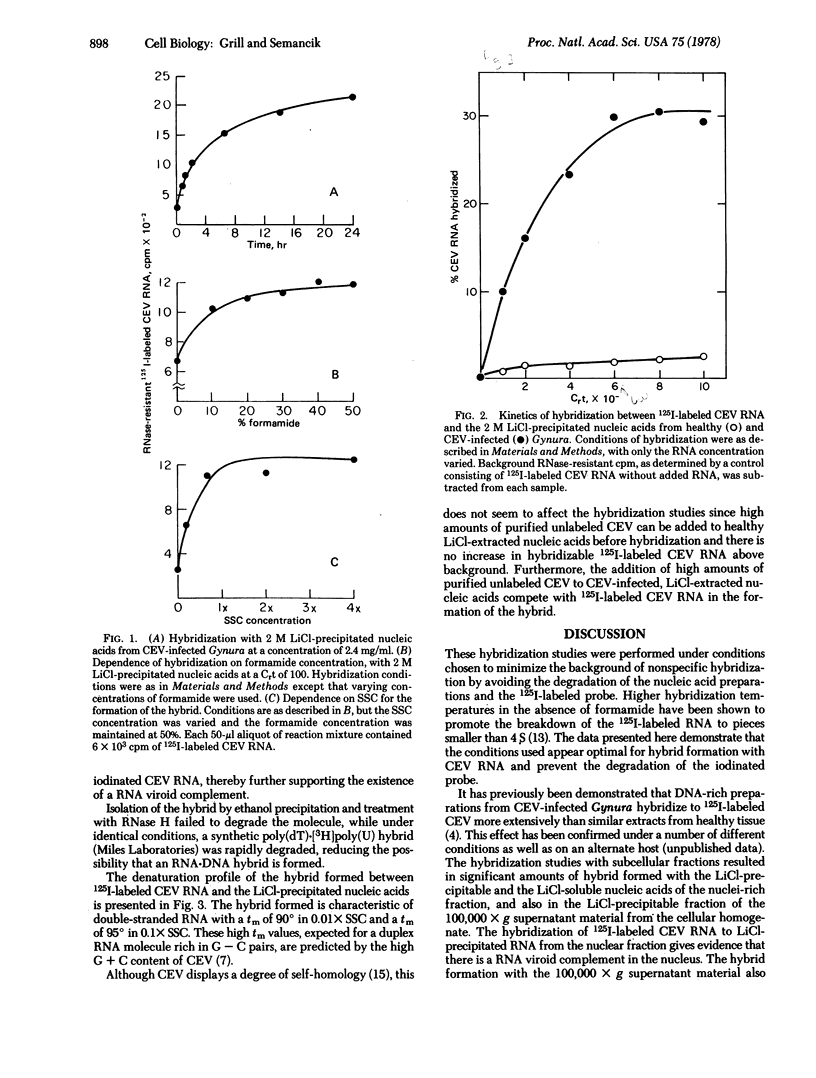
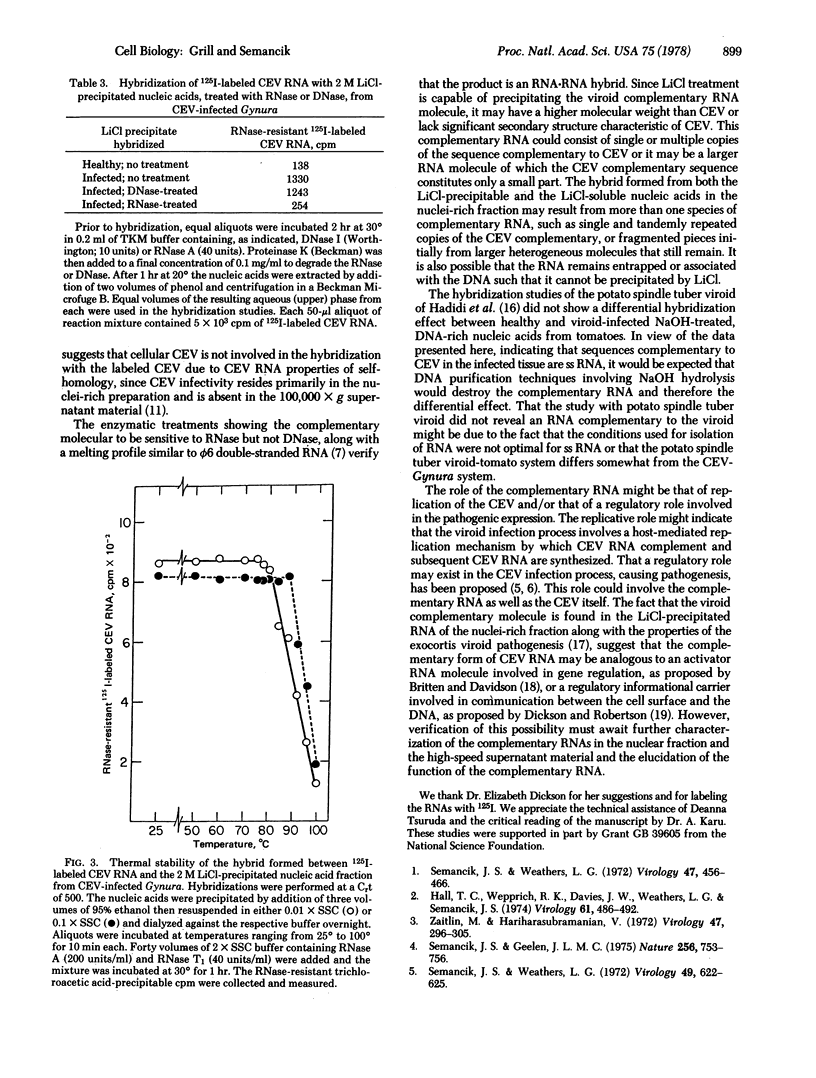
Molecular hybridization with 125I-labeled citrus exocortis viroid RNA has been used to survey nucleic acid preparations from Gynura aurantiaca for viroid complementary molecules. A differential hybridization effect was detected between nucleic acid extracts from healthy and infected tissue in which significant RNase-resistant 125I-labeled citrus exocortis viroid resulted in hybridization studies with the infected tissue extracts. Subsequent characterization indicated that RNA from infected tissue was involved in the formation of a duplex molecule with citrus exocortis viroid RNA and had properties of an RNA·RNA hybrid. Subcellular fractionation of infected tissue indicates that the complementary RNA is present in nuclear and soluble RNA fractions. This RNA may represent an intermediate molecule in the replication of the viroid or a pathogenic expression and may have a regulatory role in the host cell.
Keywords: molecular hybridization, RNA·RNA hybrid
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. Purification and properties of poliovirus double-stranded ribonucleic acid. J Mol Biol. 1966 Jul;18(3):421–428. doi: 10.1016/s0022-2836(66)80034-7. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Davidson E. H. Gene regulation for higher cells: a theory. Science. 1969 Jul 25;165(3891):349–357. doi: 10.1126/science.165.3891.349. [DOI] [PubMed] [Google Scholar]
- Conejero V., Semancik J. S. Exocortis viroid: alteration in the proteins of Gynura aurantiaca accompanying viroid infection. Virology. 1977 Mar;77(1):221–232. doi: 10.1016/0042-6822(77)90420-2. [DOI] [PubMed] [Google Scholar]
- Dickson E., Robertson H. D. Potential regulatory roles for RNA in cellular development. Cancer Res. 1976 Sep;36(9 Pt 2):3387–3393. [PubMed] [Google Scholar]
- Farace M. G., Aellen M. F., Briand P. A., Faust C. H., Vassalli P., Mach B. No detectable reiteration of genes coding for mouse MOPC 41 immunoglobulin light-chain mRNA. Proc Natl Acad Sci U S A. 1976 Mar;73(3):727–731. doi: 10.1073/pnas.73.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadidi A., Jones D. M., Gillespie D. H., Wong-Staal F., Diener T. O. Hybridization of potato spindle tuber viroid to cellular DNA of normal plants. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2453–2457. doi: 10.1073/pnas.73.7.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T. C., Wepprich R. K., Davies J. W., Weathers L. G., Semancik J. S. Functional distinctions between the ribonucleic acids from citrus exocortis viroid and plant viruses: cell-free translation and aminoacylation reactions. Virology. 1974 Oct;61(2):486–492. doi: 10.1016/0042-6822(74)90284-0. [DOI] [PubMed] [Google Scholar]
- Prensky W., Steffensen D. M., Hughes W. L. The use of iodinated RNA for gene localization. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1860–1864. doi: 10.1073/pnas.70.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semancik J. S., Conejero V., Gerhart J. Citrus exocortis viroid: survey of protein synthesis in Xenopus laevis oocytes following addition of viroid RNA. Virology. 1977 Jul 1;80(1):218–221. doi: 10.1016/0042-6822(77)90395-6. [DOI] [PubMed] [Google Scholar]
- Semancik J. S., Geelen J. L. Detection of DNA complementary to pathogenic viroid RNA in exocortis disease. Nature. 1975 Aug 28;256(5520):753–756. doi: 10.1038/256753a0. [DOI] [PubMed] [Google Scholar]
- Semancik J. S., Morris T. J., Weathers L. G., Rodorf B. F., Kearns D. R. Physical properties of a minimal infectious RNA(viroik) associated with the exocortis disease. Virology. 1975 Jan;63(1):160–167. doi: 10.1016/0042-6822(75)90381-5. [DOI] [PubMed] [Google Scholar]
- Semancik J. S., Morris T. J., Weathers L. G. Structure and conformation of low molecular weight pathogenic RNA from exocortis disease. Virology. 1973 Jun;53(2):448–456. doi: 10.1016/0042-6822(73)90224-9. [DOI] [PubMed] [Google Scholar]
- Semancik J. S., Tsuruda D., Zaner L., Geelen J. L., Weathers J. G. Exocortis disease: subcellular distribution of pathogenic (viroid) RNA. Virology. 1976 Feb;69(2):669–676. doi: 10.1016/0042-6822(76)90495-5. [DOI] [PubMed] [Google Scholar]
- Semancik J. S., Weathers L. G. Exocortis disease: evidence for a new species of "infectious" low molecular weight RNA in plants. Nat New Biol. 1972 Jun 21;237(77):242–244. doi: 10.1038/newbio237242a0. [DOI] [PubMed] [Google Scholar]
- Semancik J. S., Weathers L. G. Exocortis virus: an infectious free-nucleic acid plant virus with unusual properties. Virology. 1972 Feb;47(2):456–466. doi: 10.1016/0042-6822(72)90281-4. [DOI] [PubMed] [Google Scholar]
- Semancik J. S., Weathers L. G. Pathogenic 10 S RNA from exocortis disease recovered from tomato bunchy-top plants similar to potato spindle tuber virus infection. Virology. 1972 Aug;49(2):622–625. doi: 10.1016/0042-6822(72)90518-1. [DOI] [PubMed] [Google Scholar]
- Zaitlin M., Hariharasubramanian V. A gel electrophoretic analysis of proteins from plants infected with tobacco mosaic and potato spindle tuber viruses. Virology. 1972 Feb;47(2):296–305. doi: 10.1016/0042-6822(72)90265-6. [DOI] [PubMed] [Google Scholar]


