Abstract
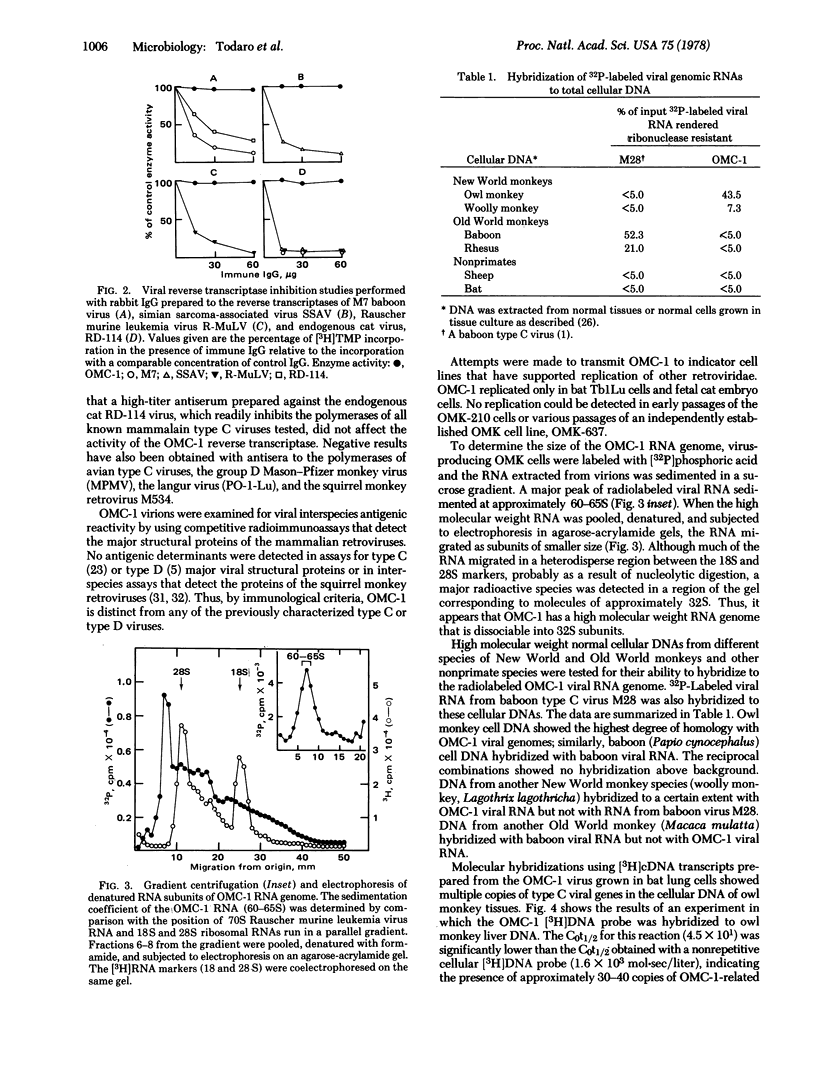
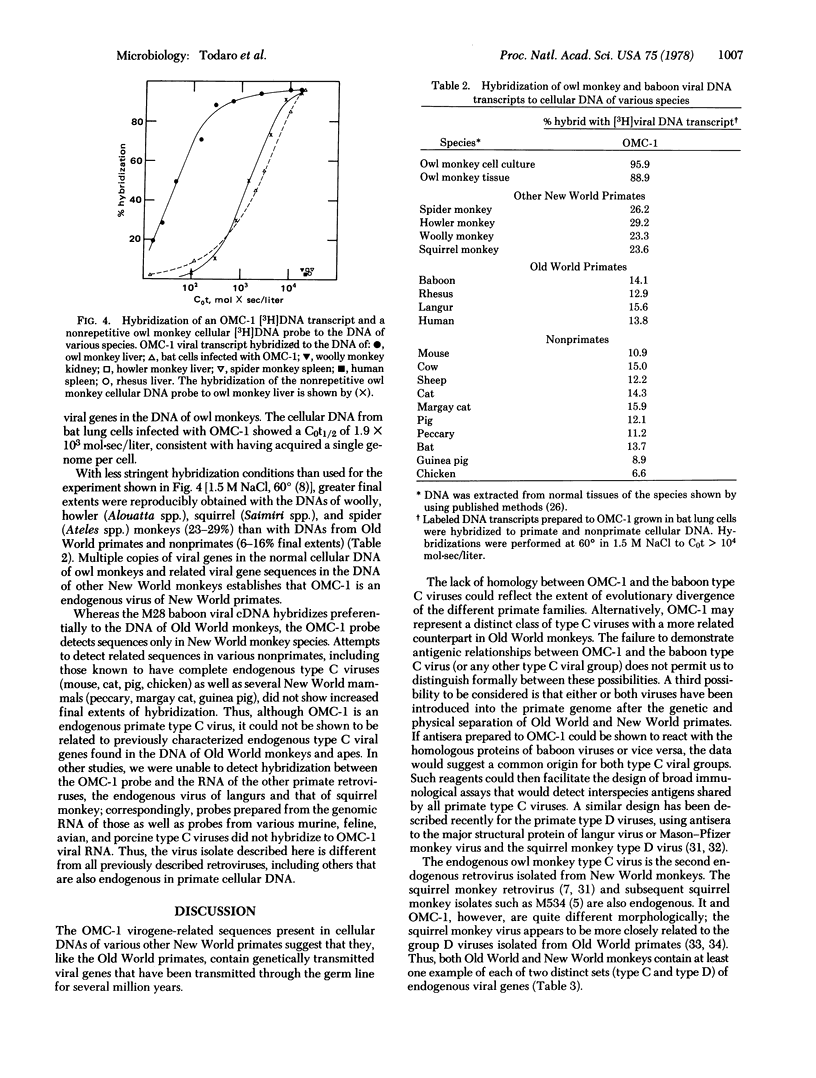
A type C virus (OMC-1) detected in a culture of owl monkey kidney cells resembled typical type C viruses morphologically, but was slightly larger than previously characterized mammalian type C viruses. OMC-1 can be transmitted to bat lung cells and cat embryo fibroblasts. The virions band at a density of 1.16 g/ml in isopycnic sucrose density gradients and contain reverse transcriptase and a 60-65S RNA genome composed of approximately 32S subunits. The reverse transcriptase is immunologically and biochemically distinct from the polymerases of othe retroviruses. Radioimmunoassays directed to the interspecies antigenic determinants of the major structure proteins of other type C viruses do not detect a related antigen in OMC-1. Nucleic acid hybridization experiments using labeled viral genomic RNA or proviral cDNA transcripts to normal cellular DNA of different species show that OMC-1 is an endogenous virus with multiple virogene copies (20-50 per haploid genome) present in normal owl monkey cells and is distinct from previously isolated type C and D viruses. Sequences related to the OMC-1 genome can be detected in other New World monkeys. Thus, similar to the Old World primates (e.g., baboons as a prototype), the New World monkeys contain endogenous type C viral genes that appear to have been transmitted in the primate germ line.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basch R. S. An improved method for counting tritium and carbon-14 in acrylamide gels. Anal Biochem. 1968 Oct 10;26(1):184–188. doi: 10.1016/0003-2697(68)90044-4. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Heinemann R., Wilson G. L., Callahan R., Todaro G. J. Detection of baboon type C viral sequences in various primate tissues by molecular hybridization. J Virol. 1974 Jul;14(1):56–67. doi: 10.1128/jvi.14.1.56-67.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Lieber M. M., Livingston D. M., Sherr C. J., Todaro G. J., Kalter S. S. Infectious C-type virus isolated from a baboon placenta. Nature. 1974 Mar 1;248(5443):17–20. doi: 10.1038/248017a0. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Scolnick E. M. RNA in mammalian sarcoma virus transformed nonproducer cells homologous to murine leukemia virus RNA. Virology. 1973 Feb;51(2):370–382. doi: 10.1016/0042-6822(73)90436-4. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Evolution of primate oncornaviruses: An endogenous virus from langurs (Presbytis spp.) with related virogene sequences in other Old World monkeys. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4557–4561. doi: 10.1073/pnas.74.10.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Evolution of type C viral genes: evidence for an Asian origin of man. Nature. 1976 May 13;261(5556):101–108. doi: 10.1038/261101a0. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Callahan R., Benveniste R. E., Sherr C. J., Schidlovsky G., Todaro G. J. A new class of genetically transmitted retravirus isolated from Mus cervicolor. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3579–3583. doi: 10.1073/pnas.73.10.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcher D., Teramoto Y. A., Schlom J. Interspecies radioimmunoassay for the major structural proteins of primate type-D retroviruses. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5739–5743. doi: 10.1073/pnas.74.12.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Faras A. J. In vitro transcription of theavian oncornavirus genome by the RNA-directed DNA polymerase: analysis of DNA transcripts synthesized in reconstructed enzymatic reactions. J Virol. 1977 Apr;22(1):86–96. doi: 10.1128/jvi.22.1.86-96.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein B., Wolf H. Purification and properties of Herpesvirus saimiri DNA. Virology. 1974 Mar;58(1):55–64. doi: 10.1016/0042-6822(74)90140-8. [DOI] [PubMed] [Google Scholar]
- Giard D. J., Aaronson S. A., Todaro G. J., Arnstein P., Kersey J. H., Dosik H., Parks W. P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973 Nov;51(5):1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- Goldberg R. J., Scolnick E. M., Parks W. P., Yakovleva L. A., Lapin B. A. Isolation of a primate type-C virus from a lymphomatous baboon. Int J Cancer. 1974 Dec 15;14(6):722–730. doi: 10.1002/ijc.2910140605. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Heberling R. L., Barker S. T., Kalter S. S., Smith G. C., Helmke R. J. Oncornavirus: isolation from a squirrel monkey (Saimiri sciureus) lung culture. Science. 1977 Jan 21;195(4275):289–292. doi: 10.1126/science.63993. [DOI] [PubMed] [Google Scholar]
- Henderson I. C., Lieber M. M., Todaro G. J. Mink cell line Mv 1 Lu (CCL 64). Focus formation and the generation of "nonproducer" transformed cell lines with murine and feline sarcoma viruses. Virology. 1974 Jul;60(1):282–287. doi: 10.1016/0042-6822(74)90386-9. [DOI] [PubMed] [Google Scholar]
- Hino S., Tronick S. R., Heberling R. L., Kalter S. S., Hellman A., Aaronson S. A. Endogenous New World primate retrovirus: interspecies antigenic determinants shared with the major structural protein of type-D RNA viruses of Old World monkeys. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5734–5738. doi: 10.1073/pnas.74.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt R. D., Van Zwieten M. J., Baggs R. B., Sehgal P. K., King N. W., Roach S. M., Blake B. J. Glomerulonephritis in the owl monkey (Aotus trivirgatus). Lab Anim Sci. 1976 Dec;26(6 Pt 2):1088–1092. [PubMed] [Google Scholar]
- Huu Duc-Nguyen, Rosenblum E. N., Zeigel R. F. Persistent infection of a rat kidney cell line with Rauscher murine leukemia virus. J Bacteriol. 1966 Oct;92(4):1133–1140. doi: 10.1128/jb.92.4.1133-1140.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King N. W., Jr, Baggs R. B., Hunt R. D., Van Zwieten M. J., MacKey J. J. Glomerulonephritis in the owl monkey (Aotus trivirgatus): ultrastructural observations. Lab Anim Sci. 1976 Dec;26(6 Pt 2):1093–1103. [PubMed] [Google Scholar]
- Laufs R., Steinke H. No evidence for particles encapsulating RNA-instructed DNA polymerase and high molecular weight virus-related RNA in herpesvirus induced tumours of non-human primates. J Gen Virol. 1975 May;27(2):239–245. doi: 10.1099/0022-1317-27-2-239. [DOI] [PubMed] [Google Scholar]
- Ma N. S., Jones T. C., Miller A. C., Morgan L. M., Adams E. A. Chromosome polymorphism and banding patterns in the owl monkey (Aotus). Lab Anim Sci. 1976 Dec;26(6 Pt 2):1022–1036. [PubMed] [Google Scholar]
- McAllister R. M., Nicolson M., Gardner M. B., Rongey R. W., Rasheed S., Sarma P. S., Huebner R. J., Hatanaka M., Oroszlan S., Gilden R. V. C-type virus released from cultured human rhabdomyosarcoma cells. Nat New Biol. 1972 Jan 5;235(53):3–6. doi: 10.1038/newbio235003a0. [DOI] [PubMed] [Google Scholar]
- Neubauer R. H., Wallen W. C., Parks W. P., Rabin H., Cicmanec J. L. Attempts to demonstrate type-C virus in normal and neoplastic tissues of nonhuman primate origin. Lab Anim Sci. 1974 Feb;24(1):235–240. [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Rothenberg E., Baltimore D. Increased length of DNA made by virions of murine leukemia virus at limiting magnesium ion concentration. J Virol. 1977 Jan;21(1):168–178. doi: 10.1128/jvi.21.1.168-178.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schochetman G., Fine D., Arthur L., Gilden R., Heberling R. Characterization of a retravirus isolated from squirrel monkeys. J Virol. 1977 Aug;23(2):384–393. doi: 10.1128/jvi.23.2.384-393.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A., Sherr C. J., Todaro G. J. Specific binding of the type C viral core protein p12 with purified viral RNA. Cell. 1976 Jan;7(1):21–32. doi: 10.1016/0092-8674(76)90251-8. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Fedele L. A., Benveniste R. E., Todaro G. J. Interspecies antigenic determinants of the reverse transcriptases and p30 proteins of mammalian type C viruses. J Virol. 1975 Jun;15(6):1440–1448. doi: 10.1128/jvi.15.6.1440-1448.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilen G. H., Gould D., Fowler M., Dungworth D. L. C-type virus in tumor tissue of a woolly monkey (Lagothrix spp.) with fibrosarcoma. J Natl Cancer Inst. 1971 Oct;47(4):881–889. [PubMed] [Google Scholar]
- Todaro G. J., Benveniste R. E., Sherr C. J., Schlom J., Schidlovsky G., Stephenson J. R. Isolation and characterization of a new type D retrovirus from the asian primate, Presbytis obscurus (spectacled langur). Virology. 1978 Jan;84(1):189–194. doi: 10.1016/0042-6822(78)90231-3. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Sherr C. J., Benveniste R. E. Baboons and their close relatives are unusual among primates in their ability to release nondefective endogenous type C viruses. Virology. 1976 Jul 1;72(1):278–282. doi: 10.1016/0042-6822(76)90331-7. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Tevethia S. S., Melnick J. L. Isolation of an RD-114 related type-C virus from feline sarcoma virus-transformed baboon cells. Intervirology. 1973;1(5):399–404. doi: 10.1159/000148868. [DOI] [PubMed] [Google Scholar]
- Wallace R. E., Vasington P. J., Petricciani J. C., Hopps H. E., Lorenz D. E., Kadanka Z. Development and characterization of cell lines from subhuman primates. In Vitro. 1973 Mar-Apr;8(5):333–341. doi: 10.1007/BF02619057. [DOI] [PubMed] [Google Scholar]



