Abstract
Niche construction is the process by which organisms can alter the ecological environment for themselves, their descendants, and other species. As a result of niche construction, differences in selection pressures may be inherited across generations. Homophily, the tendency of like phenotypes to mate or preferentially associate, influences the evolutionary dynamics of these systems. Here we develop a model that includes selection and homophily as independent culturally transmitted traits that influence the fitness and mate choice determined by another focal cultural trait. We study the joint dynamics of a focal set of beliefs, a behavior that can differentially influence the fitness of those with certain beliefs, and a preference for partnering based on similar beliefs. Cultural transmission, selection, and homophily interact to produce complex evolutionary dynamics, including oscillations, stable polymorphisms of all cultural phenotypes, and simultaneous stability of oscillation and fixation, which have not previously been observed in models of cultural evolution or gene–culture interactions. We discuss applications of this model to the interaction of beliefs and behaviors regarding education, contraception, and animal domestication.
Keywords: mathematical model, cultural evolutionary dynamics, human evolution, assortative mating
Throughout history, humans have dramatically modified their ecological niche, for example by constructing densely populated settlements, using agriculture, and domesticating animals. This niche construction, which has involved major cultural changes, has influenced our own evolution and that of other species (1–4) by affecting the selective forces that act on human populations. A classic example of this gene–culture coevolution is the spread of dairy farming through Europe and the corresponding increase in the allele frequency of the lactase persistence gene, which enables humans to digest milk into adulthood (5–9). Less widely studied is the evolutionary pressure that one culturally transmitted behavior can exert on another cultural trait, either by altering the rate of transmission or the fitnesses of variants of the latter; this has been called cultural niche construction (4, 10).
Assortative mating (homophily), where individuals that share a trait are more likely to mate with one another (11–14), and fitness differences may both affect the evolutionary trajectory of a population. In a previous model of cultural niche construction (15), we considered two dichotomous cultural traits, one that determined a cultural phenotype and one niche-constructing trait that controlled the level of selection and assorting on the other trait. In that model, assorting and selection could induce complex evolutionary dynamics, including stable polymorphisms and multiple simultaneously stable equilibria. Polymorphisms were only possible when the three evolutionary forces in the model interacted: fitness differences between phenotypes, assortative mating, and non-Mendelian transmission of at least one trait. The two elements of cultural niche construction in that model—the levels of selection and assorting—were controlled by the same trait. One cultural trait affected both the fitness and mating preferences of individuals with variants of another cultural (or genetic) trait; for example, religious beliefs, intergenerational wealth transfer, number of children desired, and socioeconomic status may be linked to numerous other traits and may affect both mate choice and fertility (12, 16–19).
Here, we use a model of vertical cultural transmission of three dichotomous traits to explore the evolutionary consequences of assortative mating and selection as independent culturally transmitted forces. In this model, separate culturally transmitted traits alter the strength of selection and level of assorting on the focal trait through cultural niche construction. Earlier models of cultural transmission and cultural niche construction have generally incorporated either assortative mating or selection, but not both (10, 20–26). Through niche construction, organisms alter evolutionary pressures that are exerted via ecological, or cultural, changes (1, 2, 4, 27–30). These pressures may be inherited via cultural transmission or ecological inheritance (31). In addition, mating preferences can also influence evolutionary dynamics, and if these two forces are culturally transmitted independently, we can assess the relative contributions of each to cultural evolutionary dynamics. The models of vertical cultural transmission described below include Mendelian genetic transmission as a special case. The three forces in this model—trait transmission, selection pressures, and assortative mating—are relevant to both gene–culture coevolution and human beliefs and behaviors. We find that one of these forces usually dominates the dynamics, and a single phenotype approaches fixation. However, cultural transmission, selection, and homophily can interact to produce complex dynamics, including stable oscillations and polymorphisms of all phenotypes. Furthermore, homophily and selection act in a balance: Increased assortative mating may lower the selective advantage necessary for a trait to spread in the population.
Materials and Methods
We consider a cultural trait (T) that has two possible states, T and t. Two other dichotomous cultural traits influence the evolution of T: S, a trait with variants S and s that determines the selection on T, and M, a trait with variants M and m that determines the rate at which individuals preferentially mate with those of the same T state. There are eight possible three-trait phenotypes, with population frequencies xi: TSM (x1), TSm (x2), TsM (x3), Tsm (x4), tSM (x5), tSm (x6), tsM (x7), and tsm (x8). The relative fitnesses of T and t depend on S, which determines the selection coefficient σi (−1 ≤ σi ≤ 1), where i = 1 represents S and i = 2 represents s. Phenotypes TSM and TSm have fitness 1 + σ1 and phenotypes TsM and Tsm have fitness 1 + σ2, whereas all t phenotypes have fitness 1.
The M trait determines assortative mating (homophily) parameters, namely the probabilities of departure from random mating. We define a choosing parent (the first member of the mating pairs listed in Table S1), whose M state dictates the probability that this phenotype will preferentially mate with an individual of the same T state. A fraction (αi) of individuals mate preferentially with individuals of the same T state (0 ≤ αi ≤ 1, where i = 1 refers to M and i = 2 refers to m), whereas the remaining fraction (1 − αi) mate randomly.
There are 64 possible mating pairs for the eight phenotypes; the frequency of each pair is given in Table S1. For individuals of different T states, the mating frequency is the product of the frequency of each phenotype multiplied by the probability that this phenotype mates at random, (1 − αi). The mating frequency for individuals of the same T state is the sum of the probability that the individuals mate at random [the product of the frequency of each parent’s phenotype multiplied by (1 − αi)] and the probability that the individuals mate assortatively (the product of αi and the frequency of each parent’s phenotype divided by the total frequency of the parents’ T state).
We assume that T, S, and M are transmitted vertically and independently and specify the probabilities that each type of offspring is produced by each mating, expressed in terms of the probabilities bi, ci, and di for i = {0, 1, 2, 3} shown in Table 1, which are assumed to be constant and between 0 and 1. The offspring probabilities from each of the 64 possible matings are obtained by multiplying the corresponding probabilities from each column of Table 1. For example, a TSM individual paired with a TSm individual will produce TSM offspring with probability b3c3d2 and TSm offspring with probability b3c3(1 − d2). If b0 = 0 and b3 = 1, then there is no cultural mutation from one T state to another: Two T parents will always produce T offspring and two t parents will always produce t offspring. These transmission parameters could take special values that represent Mendelian inheritance, for example, if b0 = 0, b1 = b2 = 0.5, and b3 = 1, then the T/t dichotomy can be regarded as genetic. However, if b3 < 1 (or b0 > 0), two T parents can produce t offspring (or vice versa). The corresponding statements are true of ci and di with regard to the S and M traits, respectively.
Table 1.
Transmission probabilities from cultural trait pairings
| Offspring | ||
| Parents | T | t |
| T × T | b3 | (1 − b3) |
| T × t | b2 | (1 − b2) |
| t × T | b1 | (1 − b1) |
| t × t | b0 | (1 − b0) |
| S × S | c3 | (1 − c3) |
| S × s | c2 | (1 − c2) |
| s × S | c1 | (1 − c1) |
| s × s | c0 | (1 − c0) |
| M × M | d3 | (1 − d3) |
| M × m | d2 | (1 − d2) |
| m × M | d1 | (1 − d1) |
| m × m | d0 | (1 − d0) |
For mating pairs of each trait, the transmission probabilities are given. For example, when two T individuals reproduce, their pairing results in T offspring with probability b3 and t offspring with probability 1 − b3.
To compute the frequency of a given phenotype in the next generation, each mating frequency is multiplied by the probability that the mating produces offspring of that phenotype and the sum taken over all 64 possible mating combinations. Selection, specified by parameters σ1 and σ2, then operates on these offspring. The relative fitness of T and t individuals depends on S: The relative fitness of TS individuals is represented by 1 + σ1, Ts individuals by 1 + σ2, and t individuals by 1. The full recursions, giving x′i, the phenotype frequencies in the next generation, in terms of xi in the current generation, are given in SI Text, Recursions. Equilibria are solutions of for i = {1, 2, 3, …, 8}, and the number and stability of equilibria depend on the values of the parameters. Notably, isolated equilibria are not the only possible evolutionary outcomes; we also observe apparently stable oscillations in phenotype frequencies.
We used numerical iteration to explore evolutionary dynamics across the parameter space. Multiple initial phenotype frequencies (one near fixation of each of the eight phenotypes and one with all phenotypes present) were tested for a subset of parameter sets. For 85% of these parameter sets, the system approached the same fixed point from all initial values; for most of the remaining parameter sets, multiple stable fixed points were observed. Very rarely, an internal polymorphism or oscillation in phenotype frequency was approached where all phenotypes were initially present. A single set of initial values was tested for the simulations described below, and parameter sets leading to internal polymorphisms or oscillations were subsequently tested from multiple initial phenotype frequencies. For a given set of parameter values, with all phenotypes initially present (x1 = 0.13, x2 = 0.06, x3 = 0.14, x4 = 0.17, x5 = 0.13, x6 = 0.16, x7 = 0.14, and x8 = 0.07) the system in SI Text, Recursions was iterated until convergence. These initial values were chosen to provide opportunities for the stability (or instability) for all of the usual forms of boundary and interior equilibria of the recursions in SI Text, Recursions to be explored. All observed iterations approached either isolated equilibria or apparently stable oscillations. We tested 15.5 million sets of parameters with Mendelian transmission, as defined above, for at least 1 trait and 17 million sets of parameters with non-Mendelian transmission for all traits. In all tested cases, cultural mutation was absent (b0, c0, d0 = 0; b3, c3, d3 = 1); when cultural mutation was introduced (b0, c0, d0 > 0; b3, c3, d3 < 1), the system always approached an internal polymorphism. Parameter sets were chosen such that 0 < α1, α2, b1, b2, c1, c2, d1, d2 < 1 and −1 < σi < 1. Unless one or more traits were Mendelian, no two parameters had the same value. Each set was tested with the initial values of xi listed above and iterated until convergence. For parameter sets that resulted in oscillation or polymorphism, we also iterated the system from nine initial values of the frequency vector = (x1, x2 …, x8)—one near fixation of each of the eight phenotypes and one with all phenotypes present—and examined the equilibrium, if any, approached from each starting vector. We also calculated the three pairwise cultural disequilibria and the third order cultural disequilibrium of the system (SI Text, Cultural Disequilibria, Fig. S1, and Tables S2 and S3).
T and t coexisted at equilibrium for two transmission types, and we chose a set of parameters from each: T, M non-Mendelian, and S Mendelian (Fig. 1); and T, S, and M, all non-Mendelian (Fig. 2). For both types of parameters, we assessed the sensitivity to changes in selection, assorting, and transmission by varying pairs of parameters in turn. For a given parameter set, α1 and α2 were incremented by 0.02 in the interval from 0 to 1, resulting in 51 tested values per parameter and 2,601 possible pairwise combinations of α1 and α2. With α1 and α2 at their values in the original parameter set, this test was repeated for b1 and b2, c1 and c2, and d1 and d2. Because the selection parameters were tested in the range −1 ≤ σi ≤ 1, we incremented σ1 and σ2 by 0.04 to obtain 51 tests. For each combination of parameters, from initial phenotype frequencies near fixation of T: = (0.2, 0.3, 0.19, 0.24, 0.01, 0.015, 0.02, 0.025), the phenotype frequencies after 100,000 generations were recorded. In all cases, the population either approached a stable equilibrium or entered an apparent limit cycle in which the phenotype frequencies oscillated with a stable amplitude and period. To determine whether a stable equilibrium was approached for a given parameter set, we calculated the sum of the absolute values of the differences in phenotype frequencies (x1–x8) between the last iterated generation and those of each of the 50,000 previous generations . The largest of these differences from the endpoint was recorded for each set of parameters. This number was near zero if the system approached equilibrium by 50,000 generations and was positive if the system oscillated. The convergence results for the selected parameter sets are in Figs. 1 and 2, where the colors listed in the legends represent different outcomes for the dynamics.
Fig. 1.
Parameters that generate stable cycling when S is Mendelian. With α1 = 0.15, α 2 = 0.77, b0 = 0, b1 = 0.14, b2 = 0.16, b3 = 1, c0 = 0, c1 = 0.5, c2 = 0.5, c3 = 1, d0 = 0, d1 = 0.33, d2 = 0.75, d3 = 1, σ1 = 0.39, and σ2 = 0.12, pairs of parameters were varied and the resulting system was iterated. The equilibrium approached after 100,000 generations from each pairwise combination of parameters is presented in a color-coded 51 × 51 matrix. For this and subsequent figures, the initial phenotype frequencies are as follows unless otherwise specified: x1 = 0.2, x2 = 0.3, x3 = 0.19, x4 = 0.24, x5 = 0.01, x6 = 0.015, x7 = 0.02, and x8 = 0.025. If a single phenotype approaches fixation, TSM, TSm, TsM, and Tsm are represented by shades of blue and tSM, tSm, tsM, and tsm by shades of green (both listed from darkest to lightest). If two of the three traits approach fixation but the third trait has both states, TS, Ts, TM, and Tm are represented by shades of red, and tS, ts, tM, and tm by shades of purple. If one trait fixes but the other two are both polymorphic (leading to the coexistence of four phenotypes at equilibrium), the fixation of T and t are represented by shades of orange, S and s by shades of yellow, and M and m by shades of teal. If all eight phenotypes coexist at equilibrium, the polymorphism is shown in dark gray. If multiple phenotypes appear to cycle in frequency without approaching a stable equilibrium, these oscillations are depicted in light gray. (A) Varying the transmission of T results in the stable persistence of T and t in the dark yellow region, which corresponds to the fixation of S but not T or M, and results in oscillation in the light gray region. (B) The transmission of M can lead to stable persistence of T and t in the dark yellow region and to oscillation in the light gray region. (C) With other parameters constant, no tested combinations of assorting levels (αi) generate stable T/t polymorphism, but low values of α1 and high values of α2 can result in oscillation. (D) The vertical stripe of dark yellow corresponds to fixation of S, the horizontal light yellow stripe to fixation of s, and the light gray region corresponds to cycling.
Fig. 2.
Parameters giving polymorphic equilibria and oscillations when all traits are transmitted culturally. With parameters α1 = 0.22, α2 = 0.85, b0 = 0, b1 = 0.35, b2 = 0.18, b3 = 1, c0 = 0, c1 = 0.53, c2 = 0.17, c3 = 1, d0 = 0, d1 = 0.39, d2 = 0.76, d3 = 1, σ1 = 0.32, and σ2 = −0.72, pairs of parameters were varied and the system was iterated for 100,000 generations. (A) In the transmission of T, polymorphisms can exist when ∼0.2 < b1 < ∼0.4 and b2 < ∼0.2 (shown in dark gray), and the system oscillates in the light gray region in the bottom left. (B) In the transmission of S, polymorphisms can exist when c1 and c2 are near the center of their ranges, and oscillation exists in the light gray region c1 < 0.5. (C) In the transmission of M, polymorphisms are so rare as to not be found by this analysis, which tested parameters in increments of 0.02, and oscillations exist in the light gray region when d1 + d2 > 1. (D) For assorting, an isolated polymorphism is observed, and oscillations are observed when α1 < ∼0.3 and α2 > ∼0.8. (E) For selection, a small region of the parameter space produced a polymorphism (dark gray) on the edge of the light gray region that resulted in oscillations. (F) As the level of assorting increases (α1), the selective advantage (σ1) necessary for fixation of TSM decreases.
Results
If selection, assortative mating, and cultural mutation were all absent (σi = 0, αi = 0, b0 = c0 = d0 = 0, b3 = c3 = d3 = 1), the system approached fixation of the phenotype favored by cultural transmission (Table S4). If selection and assortative mating were present, and T, S, and M all conform to Mendelian transmission, one T state approached fixation in all tested cases and selection dictated the phenotype(s) approached at equilibrium; varying the assorting parameters did not alter the equilibrium approached (SI Text, Results in Specific Parameter Regimes).
Case 1. Mendelian and Cultural Transmission, No Cultural Mutation.
We examined 15.5 million parameter sets, sampling each combination of cultural and Mendelian inheritance of traits (Mendelian inheritance of T and S, T and M, S and M, only T, only S, and only M). There were no cases in which all eight phenotypes persisted. When S and M were both Mendelian and T was culturally transmitted, T approached fixation in every case tested. When only S was Mendelian (c1, c2 = 0.5), both T and t (but not all eight possible phenotypes) persisted in 200 cases.
When only S was Mendelian, certain parameter sets led to phenotype frequency oscillations resembling exact cycles. In 392 cases, one S state approached fixation and the remaining four phenotypes appeared to enter a cycle. By varying elements of a parameter set that produced these oscillations, we explored the relative influences of transmission, assorting, and selection (Fig. 1). Oscillation (displayed in light gray in Fig. 1) was approached for more parameter sets than a stable fixed point with T and t present (shown in yellow). However, both outcomes are rare for the range of parameters tested: Stable polymorphism of T occurred in ∼0.01% of the 1,327,104 parameter sets investigated when S was Mendelian but T and M were not, and oscillations occurred in 0.025% of these parameter sets. In what follows, we refer to these oscillations as cycles, even though, as discussed below, they may not be perfect cycles.
Case 2. Cultural Transmission of All Traits, No Cultural Mutation.
With non-Mendelian inheritance of all traits, 17 million combinations of parameters were tested and the system was iterated to 100,000 generations for each. Of these, 1,019 resulted in the persistence of both T and t but not all eight phenotypes, whereas 308 resulted in a stable complete polymorphism. In addition, 3,151 resulted in oscillating phenotype frequencies; in 16% of these, all eight phenotypes were present, and in 84%, only a subset of phenotypes was present. These complex dynamics were not observed when cultural transmission was constrained to be near Mendelian values for all traits (0.3 ≤ b1, b2, c1, c2, d1, d2 ≤ 0.7; e.g., Fig. S2); at least one transmission parameter outside of this range was necessary for internal polymorphisms or oscillations to occur. For example, the parameters α1 = 0.22, α2 = 0.85, b0 = c0 = d0 = 0, b3 = c3 = d3 = 1, b1 = 0.45, b2 = 0.16, c1 = 0.45, c2 = 0.46, d1 = 0.46, d2 = 0.55, σ1 = 0.22, and σ2 = −0.82, with only the b2 transmission parameter deviating substantially from Mendelian transmission (i.e., 0.5), led to cycling.
Two parameters at a time were varied from a parameter set that produced a stable polymorphism, and the results were consistent with the broader assessment of the parameter space reported above: Boundary equilibria were the most common results of exploring the parameter space, and regions of the parameter space that resulted in cycling were larger than those that produced polymorphisms. In the transmission of the T trait, a polymorphism was approached when ∼0.2 < b1 < ∼0.4 and b2 < ∼0.2 (Fig. 2A). In the transmission of S, polymorphisms were observed when c1 and c2 were near the center of their ranges (Fig. 2B). Only small regions of the (di, αi, and σi) parameter spaces produced a polymorphism (Fig. 2 C–E). For all parameters varied, oscillations (light gray in Fig. 2) were approached for more parameter combinations than polymorphisms. With the cultural transmission held constant, increasing homophily lowered the fitness advantage necessary for T to approach fixation (Fig. 2F), a relationship that was not unique to this set of parameters (SI Text, Results in Specific Parameter Regimes).
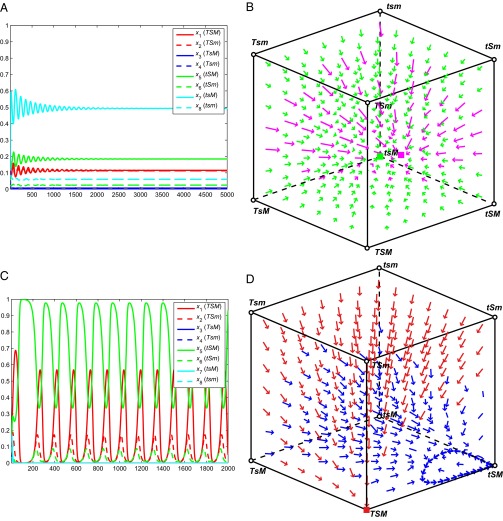
In a third of parameter sets that led to complete polymorphisms (as in Fig. 3A), all tested initial phenotype frequencies led to the same polymorphic equilibrium. In the remaining cases, simultaneous stability of fixation in a single phenotype and a full polymorphism was observed (Fig. 3B). As with polymorphisms, an apparently stable cycle (Fig. 3C) may be approached from all initial phenotype frequencies when all traits are present, or the cycle may have a domain of attraction that only includes a subset of these initial conditions (Fig. 3D). In 70% of parameter sets leading to apparent cycles, all tested initial conditions led to cycling, and in the remaining 30%, a subset of initial conditions led to stable equilibria. In 17% of apparent cycles, all eight phenotypes cycled, and in the remaining 83%, S approached fixation and the four corresponding phenotype frequencies oscillated. The period of these cycles was usually between 30 and 1,000 generations, but a few cases were also observed with periods longer than 10,000 generations (Fig. S3). The amplitude of the cycles varied widely; certain phenotypes approached fixation and then crashed to near extinction, coming to within 10−8 of each (Fig. S3). In these cases, stochastic processes in finite populations would likely lead to extinction of these phenotypes, terminating the cyclic dynamics. To test the robustness of these cycles to stochastic extinction, we iterated each parameter set leading to oscillations with an imposed phenotype frequency threshold, such that any xi < 0.001 was set to zero. For just over half of the parameter sets tested (50.2%), phenotypes that were forced to extinction could be regenerated by mating between existing phenotypes, and the complex dynamics were robust to this perturbation.
Fig. 3.
Polymorphism and oscillation of all phenotypes. (A) With parameters α1 = 0.22, α2 = 0.85, b0 = 0, b1 = 0.35, b2 = 0.18, b3 = 1, c0 = 0, c1 = 0.53, c2 = 0.17, c3 = 1, d0 = 0, d1 = 0.39, d2 = 0.76, d3 = 1, σ1 = 0.32, and σ2 = −0.72, all phenotypes persist in the population, and the equilibrium is stable to perturbation (x1 ≈ 0.1154, x2 ≈ 0.11246, x3 ≈ 0.00716, x4 ≈ 0.00166, x5 ≈ 0.1845, x6 ≈ 0.0248, x7 ≈ 0.4934, and x8 ≈ 0.0606). The number of generations is given on the x axis and the phenotype frequency on the y axis. (B) Each corner of the cube indicates fixation of the phenotype listed, and interior points indicate the presence of multiple phenotypes. With parameters as in A, the system was iterated from various initial frequencies and arrows indicate the equilibrium approached from each. From some starting points within the cube, the system approaches fixation of tsM, indicated by green arrows. From other points, the system approaches a complete polymorphism at the pink square, indicated by pink arrows. (C) With the parameter set α1 = 0.22, α2 = 0.75, b0 = 0, b1 = 0.15, b2 = 0.18, b3 = 1, c0 = 0, c1 = 0.73, c2 = 0.37, c3 = 1, d0 = 0, d1 = 0.39, d2 = 0.76, d3 = 1, σ1 = 0.56, and σ2 = 0.33, S approaches fixation and four phenotypes oscillate in frequency. (D) With parameters as in C, some initial phenotype frequencies lead to fixation of TSM, indicated by red arrows. Other initial conditions lead to a stable cycle of phenotype frequencies with S fixed, represented by dark blue arrows, and the oscillation cycle is illustrated by thicker blue arrows near the tSM vertex.
These cycles persisted for as long as computationally feasible to iterate and were stable to perturbation. We note, however, that the phenotype frequencies in one period of the cycle do not precisely match those in another period. Strictly speaking, these are aperiodic cycles because we do not observe a time ω such that xi(τ + ω) is exactly equal to xi(τ) during these oscillations. To exclude potential numerical artifacts introduced by iterating the recursions in double-precision floating-point format (∼16 significant digits), we evaluated the recursions symbolically for a subset of parameter sets leading to oscillation. With rounding errors eliminated, both the oscillations and the deviations in phenotype frequencies between cycles persisted: Differences in phenotype frequencies between periods cannot be attributed to numerical error.
Discussion
In cultural niche construction, a culturally transmitted trait alters the evolutionary dynamics of another trait, which can be either genetic or cultural (10). Here, the focal trait T can be affected by two other traits: S, which affected the relative fitness of T and t, and M, which influenced the degree to which individuals prefer to mate with others of the same T state. The evolutionary dynamics of T thus resulted from interactions among the rates of transmission of T (bi), S (ci), and M (di); the amount of assortative mating (αi); and the selection pressures (σi).
In the model, any of the three traits may be Mendelian, and the resulting gene–culture coevolutionary system can be compared with a fully cultural system. In general, with one or more Mendelian traits, the system always approached fixation in T or t. However, when S was Mendelian but T and M were transmitted culturally, complex dynamics were possible. In some cases, one S state approached fixation but T and M were polymorphic. In other cases, the system entered an apparent limit cycle in which S approached fixation but four phenotype frequencies oscillated in a stable pattern. Only when all transmission was vertical (see SI Text, Horizontal Transmission of T and Table S5 for a discussion of horizontal transmission of T) and non-Mendelian—and selection and assorting were both present—were complete polymorphisms or stable cycles of all eight phenotypes observed.
The homophily parameters (αi), set by M, represented the degrees to which individuals mate assortatively with those of the same T state; the level of homophily could interact with selection and influence the equilibrium frequency of both T and S (Fig. S4). Moreover, for a given set of transmission rates, an increase in homophily could reduce the selective advantage necessary for a trait to approach fixation (Fig. 2 and SI Text, Results in Specific Parameter Regimes). The transmission of M (d1, d2) and the degree of homophily (αi) also interacted in those parameter regimes that produced polymorphic equilibria and oscillations (Figs. 1 and 2). However, when M approached fixation, the identity of the M state in the population at equilibrium was dictated by its transmission (d1 + d2 > 1 for fixation of M, d1 + d2 < 1 for m). In contrast, T and S were more directly affected by selection, and traits were able to approach fixation when they were not directly favored by transmission. In other words, the evolution of T and S could be affected by the interaction with homophily, but the evolution of homophily itself (M) seemed to depend primarily on the transmission rates of its two forms (d1, d2).
In population genetic and ecological models, stable cycles have often been observed; examples include predator–prey dynamics (32), the battle of the sexes (33), Red Queen dynamics (34), genomic imprinting (35), and interactions between recombination and natural selection (36, 37). In two-locus, two-allele symmetric viability models, small parameter ranges were shown by Hastings (36) to give rise to cyclic dynamics rather than isolated fixed points. Cycles have been observed in cultural evolution models, as well. For example, if cultural mutation is high (b0 >> b3), offspring of each generation reject the cultural traits of their parents’ generation and a stable cycle results (7, 38). Limit cycles are also found in models for the evolution of learning in changing environments (39, 40) and models of cultural evolution by sexual selection (41).
The cycles observed in our model were different from those described by Cavalli-Sforza and Feldman (38): Here, cultural mutation is absent and cyclic dynamics were driven by the interaction of cultural transmission, selection, and assorting. The observed phenotype frequencies appeared to oscillate ad infinitum, cycles could be approached from numerous initial conditions, and there was no evidence of bifurcation. However, the phenotype frequencies appeared not to recur exactly in each apparent period of the oscillation, differing by ≤10−5 between adjacent periods. Over long timescales, the period of oscillation did not appear to be a discrete number of generations. The oscillations can be dramatic, covering the space very close to fixation and very close to loss of a phenotype. Unlike the expanding cycles shown by Kesten (42) in a model of extreme segregation distortion, the oscillations here appeared to be extremely close to periodic. The interaction of the three forces presented in this model—cultural transmission, selection pressures, and assortative mating—can give rise to unique and complex evolutionary dynamics.
Our model can represent the dynamics of cultural evolution resulting from the interaction between beliefs and behaviors. Human culture has been defined as ideational, namely as “systems of shared ideas” (ref. 43, p. 68; see also refs. 9 and 44), but behaviors and practices may also be culturally transmitted and hence subject to evolution (38). Here, we can model the interaction of these if we interpret T as a set of beliefs, S as a behavior that can differentially influence the fitness of those with certain beliefs, and M as a preference for partnering based on similar beliefs.
Suppose T represents a set of beliefs about the importance of education: T represents a cultural emphasis on education, including the belief that women should receive secondary education; t represents no such emphasis. Let S represent the practice of delaying childbirth until after schooling is finished; S individuals prefer to wait to complete their education before reproducing and s individuals have no such preference. M indicates the preference for partnering with individuals with similar beliefs about education, whereas m represents no such preference (α2 = 0). We approximate α1, the rate of assortment on beliefs about education, using published homophily estimates for level of education, α1 ≈ 0.3–0.4 (17, 45). Secondary education itself is correlated with lower fertility (46), but the causation is complicated. In one study, childbearing impeded educational attainment more than education reduced fertility (47), but most individuals in that study had completed secondary school. A culturally transmitted preference to delay childbirth until after education is thus considered here as a separate trait. In this application of our model, delaying reproduction until after education (S) will lower the fertility of those who continue schooling into their reproductive years (T), whereas the behavior of delaying fertility until after education might not alter the fitness of those who are unlikely to enter or complete secondary education (t). It follows that TS individuals will have the fewest offspring (0 > σ2 > σ1). Education is correlated with certain fitness benefits, including decreased infant mortality and increased access to health care (48–50), but in several countries fertility is estimated to be lower among women who have completed secondary school (compared with those who complete primary school), with a range of 30–51% fewer children on average (46, 51). Here, individuals who combine a belief in the importance of secondary education (T) and a preference for completing school before childbirth (S) leave fewer offspring. Our model predicts that, to persist in the population, T and S must be culturally transmitted much more often than t and s. When b1 + b2 (transmission of T in mixed T/t matings) and c1 + c2 (transmission of S in mixed S/s matings) are sufficiently large, T and S can approach fixation in the population (Fig. 4). Because homophily lowers the selective advantage necessary for a trait to approach fixation when a different trait is favored by cultural transmission, the human tendency to assort by education level (17, 45) acted to increase the transmission levels of T and S that were necessary for the belief in education or the practice of delaying childbirth to spread in the population. In this framework, we have excluded cultural mutation (b0, c0 > 0, b3, c3 < 1), which would entail a belief in education or the preference for delaying childbirth when one’s parents do not possess these traits and which could also shift the balance in favor of education or delayed childbirth. With or without cultural mutation, strong encouragement that enhances transmission of these cultural beliefs and behaviors is necessary for the persistence of these traits.
Fig. 4.
Transmission of cultural traits relevant to education and fertility. In the education application, the phenotype that approaches fixation depends on the relationship between the transmission of T and the transmission of S. Here, α1 = 0.35, α2 = 0, b0 = c0 = d0 = 0, b3 = c3 = d3 = 1, d1 = 0.49, d2 = 0.56, σ1 = −0.43, and σ2 = −0.22. The fixation of both the belief in the importance of secondary education and the preference for completing education before childbirth (TS, shown in dark blue) is only approached when transmission is high for both traits. This parameter regime can also be applied to the belief that contraception is an acceptable practice (T) and the practice of using contraception (S).
The interaction between cultural beliefs and behaviors regarding contraception also has evolutionary implications. Consider T to be the belief that contraception is an acceptable practice and t to be the belief that it is not. Let S represent the practice of using contraception and s represent natural control of fertility. Belief and practice are separated here to reflect the fact that cultural taboos, as well as misinformation, high costs, and limited access to medical care, may inhibit the practice of contraception even among those who believe it is acceptable; in addition, individuals may use contraception altough they have a cultural or religious belief opposing its use. In this framework, TS individuals exhibit both the belief in and use of contraception, so fertility should be lowest in this group. As with education, the use of (and access to) contraception may be correlated with certain fitness and health benefits, but the net effect is a reduction in the number of children. Compared with countries with restricted access, fertility is 61% lower in countries with unrestricted access to contraception (52). Finally, let M represent the preference for partnering with individuals with similar beliefs about contraception and m represent no such preference. If beliefs regarding contraception’s acceptability align with other cultural beliefs, concerning, for example, the roles of community or religion, then α1 could be quite high (53). As with education, cultural transmission promoting the use of and access to contraception is necessary for the persistence of these traits in the population (Fig. 4).
The model presented here may also be applied to the spread of cattle domestication and adult milk consumption, which has been described as a gene–culture coevolutionary process whereby the domestication of cattle changed the relative fitness of individuals with the lactase persistence gene (LCT), which enables the digestion of milk in adulthood (6, 8, 9, 30, 54). As these two traits coevolve, the genetic trait of lactase persistence can also alter the fitness advantage of raising cattle because dairying individuals with the ability to digest lactose in adulthood will have access to a reliable nutrition source without digestive side effects. T is the culturally transmitted practice of dairying; T individuals raise cattle and t individuals do not. The S allele confers lactase persistence, whereas s individuals lack the ability to digest lactose after childhood. The S allele confers a fitness advantage on individuals who raise dairy cattle (T), but gives little or no advantage to individuals without access to milk (t). Raising cattle confers a fitness advantage to T individuals over t, and the S state should provide T individuals with an additional fitness benefit due to increased access to a consistent food source. M represents a preference for pairing with an individual of the same T state. M indicates homophily based on cattle domestication or herding in general, and m represents no such preference. If the combination of dairying (T) and lactase persistence (S) has the highest relative fitness (σ1 > σ2), the assorting preference is stronger for individuals who raise cattle than those who do not (α1 > α2), and the S trait has Mendelian transmission, the model predicts that TSM will approach fixation if the transmission of M is greater than the transmission of m (d1 + d2 > 1) and TSm will approach fixation if the reverse is true (d1 + d2 < 1), unless the transmission of T is very weak. With these constraints (σ1 > σ2, α1 > α2, c1 = c2 = 0.5), we observed cyclic dynamics only when assortative mating was high (α1 > 0.64) the transmission of dairying (T) was exceedingly low in the offspring of T and t individuals (b1 + b2 < 0.35). Thus, when dairying and the lactase persistence allele are both present, the combination can spread through the population if the offspring of a dairying individual practice dairying even 18% of the time. However, when dairying individuals have higher fitness but overwhelmingly produce nondairying offspring, the dynamics become complex, leading to fluctuating levels of dairying in the population.
It should be noted that in our T S M model, Mendelian transmission refers to haploid genetics, and evolution of haploids generally does not result in maintenance of polymorphism in the absence of mutation. Thus, a more accurate depiction of the dairying–lactose absorption example would include diploid rather than haploid genetics. Diploidy introduces several complications (7). With diploid genetics, a recent analysis of correlations between relatives for a dichotomous phenotype with a genetic component can be placed in the framework of the model studied here (26). Again consider a dichotomous trait T/t whose transmission to offspring depends on the phenotypes of the parental couple and the diploid genotype of the offspring. There are then six phenogenotypes TAA, TAa, Taa, tAA, tAa, taa. The transmission rule is shown in Table 2, where the parameter δ is the effect of substituting A for a in the genotype, η is the cultural contribution from the combination of parental phenotypes, and β is a baseline value. Assortative mating on the basis of T/t occurs at rate α, which, in terms of our model above, is equivalent to assuming α1 = α2 = α. There is no selection, which is equivalent to σ1 = σ2 in the model above.
Table 2.
Bilinear transmission scheme
| Offspring genotypes | |||
| Parents | AA | Aa | aa |
| T × T | 2η + 2δ + β | 2η + δ + β | 2η + β |
| T × t | η + 2δ + β | η + δ + β | η + β |
| t × T | η + 2δ + β | η + δ + β | η + β |
| t × t | 2δ + β | δ + β | β |
For each mating pair, the probability that an offspring is phenotype T is given for each genotype. All transmission probabilities are nonnegative, e.g., 0 ≤ 2η + 2δ + β ≤ 1.
The frequency of T changes over time under the transmission rule of Table 2 and reaches an equilibrium that depends on the frequency p of allele A. At this equilibrium, exact narrow-sense heritability of the phenotypic dichotomy can be computed and compared, for example, to heritability computed from monozygotic and dizygotic twins, or from parent–offspring covariances. For example, if δ = η = 0.2 and α = 0, the true heritability is 0.094, but the twin estimate is 0.035 and the parent-offspring estimate is 0.475. With α = 0.5, these three estimates become 0.127, 0.037, and 0.489, respectively. Thus, in the presence of gene–culture codetermination of trait variation, commonly used heritability estimates can be very far from the true fraction of the total phenotypic variance that is additive and genetic. An often overlooked fact is that these estimates of heriability are sensitive to the allele frequencies (26). In fact, for the same values of δ and η, with α = 0.5, the frequency of 0.2 of allele A gives a true heritability of T/t of almost 0.4, but if the frequency of A is 0.8 the heritability is close to 0.25.
In our model of cultural niche construction, in which one cultural trait can alter the selection pressures on another, we examined the interactions between fitness, assortative mating, and cultural transmission even though these are independently culturally transmitted. By treating a trait that alters selection separately from a trait that modifies assortative mating, we were able to assess the sensitivity of the evolutionary dynamics to changes in each of these two forces separately. We observed complex dynamics, including oscillations, internal polymorphisms, and simultaneous stability of oscillation and fixation that have not previously been seen in models of cultural evolution or gene–culture coevolution.
Supplementary Material
Acknowledgments
We thank K. Aoki, L. Altenberg, L. Fogarty, and O. Carja for helpful comments and discussions. This research was supported in part by the Morrison Institute for Population and Resource Studies at Stanford.
Footnotes
The authors declare no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “In the Light of Evolution VIII: Darwinian Thinking in the Social Sciences,” held January 10–11, 2014, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS website at www.nasonline.org/ILE-Darwinian-Thinking.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1400824111/-/DCSupplemental.
References
- 1.Lewontin RC. Organism and environment. In: Plotkin HC, editor. Learning, Development and Culture. New York: Wiley; 1982. pp. 151–170. [Google Scholar]
- 2.Lewontin RC. Gene, Organism and Environment. In: Bendall DS, editor. Evolution from Molecules to Men. Cambridge, UK: Cambridge Univ Press; 1983. pp. 273–285. [Google Scholar]
- 3.Laland KN, Odling-Smee FJ, Feldman MW. Evolutionary consequences of niche construction and their implications for ecology. Proc Natl Acad Sci USA. 1999;96(18):10242–10247. doi: 10.1073/pnas.96.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Odling-Smee FJ, Laland KN, Feldman MW. Niche Construction: The Neglected Process in Evolution. Princeton: Princeton Univ Press; 2003. [Google Scholar]
- 5.Simoons FJ. Primary adult lactose intolerance and the milking habit: A problem in biologic and cultural interrelations. II. A culture historical hypothesis. Am J Dig Dis. 1970;15(8):695–710. doi: 10.1007/BF02235991. [DOI] [PubMed] [Google Scholar]
- 6.Aoki K. A stochastic model of gene-culture coevolution suggested by the “culture historical hypothesis” for the evolution of adult lactose absorption in humans. Proc Natl Acad Sci USA. 1986;83(9):2929–2933. doi: 10.1073/pnas.83.9.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavalli-Sforza LL, Feldman MW. Evolution of continuous variation: Direct approach through joint distribution of genotypes and phenotypes. Proc Natl Acad Sci USA. 1976;73(5):1689–1692. doi: 10.1073/pnas.73.5.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldman MW, Cavalli-Sforza LL. On the theory of evolution under genetic and cultural transmission with application to the lactose absorption problem. In: Feldman MW, editor. Mathematical Evolutionary Theory. Princeton: Princeton Univ Press; 1989. [Google Scholar]
- 9.Durham WH. Coevolution: Genes, Culture and Human Diversity. Stanford, CA: Stanford University Press; 1991. [Google Scholar]
- 10.Ihara Y, Feldman MW. Cultural niche construction and the evolution of small family size. Theor Popul Biol. 2004;65(1):105–111. doi: 10.1016/j.tpb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Burley N. The meaning of assortative mating. Ethol Sociobiol. 1983;4(4):191–203. [Google Scholar]
- 12.Buss DM. Human mate selection. Am Sci. 1985;73(1):47–51. [Google Scholar]
- 13.Gimelfarb A. Processes of pair formation leading to assortative mating in biological populations: Encounter-mating model. Am Nat. 1988;131(6):865–884. doi: 10.1016/0040-5809(88)90032-9. [DOI] [PubMed] [Google Scholar]
- 14.Geary DC, Vigil J, Byrd-Craven J. Evolution of human mate choice. J Sex Res. 2004;41(1):27–42. doi: 10.1080/00224490409552211. [DOI] [PubMed] [Google Scholar]
- 15.Creanza N, Fogarty L, Feldman MW. Models of cultural niche construction with selection and assortative mating. PLoS ONE. 2012;7(8):e42744. doi: 10.1371/journal.pone.0042744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill CT, Rubin Z, Peplau LA. Breakups before marriage: The end of 103 affairs. J Soc Issues. 1976;32(1):147–168. [Google Scholar]
- 17.Theissen D, Gregg B. Human assortative mating and genetic equilibrium: An evolutionary perspective. Ethol Sociobiol. 1980;1(2):111–140. [Google Scholar]
- 18.Smith EA, Borgerhoff-Mulder M, Bowles S, Gurven M, Shenk M. Wealth inequality and intergenerational transmission in pre-modern societies: Conclusions. Curr Anth. 2010;51(1):85–94. [Google Scholar]
- 19.Shennan S. Property and wealth inequality as cultural niche construction. Philos Trans R Soc Lond B Biol Sci. 2011;366(1566):918–926. doi: 10.1098/rstb.2010.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rice J, Cloninger CR, Reich T. Multifactorial inheritance with cultural transmission and assortative mating. I. Description and basic properties of the unitary models. Am J Hum Genet. 1978;30(6):618–643. [PMC free article] [PubMed] [Google Scholar]
- 21.Cloninger CR, Rice J, Reich T. Multifactorial inheritance with cultural transmission and assortative mating. II. A general model of combined polygenic and cultural inheritance. Am J Hum Genet. 1979;31(2):176–198. [PMC free article] [PubMed] [Google Scholar]
- 22.Aoki K, Feldman MW. Recessive hereditary deafness, assortative mating, and persistence of a sign language. Theor Popul Biol. 1991;39(3):358–372. doi: 10.1016/0040-5809(91)90029-f. [DOI] [PubMed] [Google Scholar]
- 23.Laland KN, Odling-Smee J, Feldman MW. Cultural niche construction and human evolution. J Evol Biol. 2001;14(1):22–33. doi: 10.1046/j.1420-9101.2001.00262.x. [DOI] [PubMed] [Google Scholar]
- 24.Borenstein E, Kendal J, Feldman M. Cultural niche construction in a metapopulation. Theor Popul Biol. 2006;70(1):92–104. doi: 10.1016/j.tpb.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Rendell L, Fogarty L, Laland KN. Runaway cultural niche construction. Philos Trans R Soc Lond B Biol Sci. 2011;366(1566):823–835. doi: 10.1098/rstb.2010.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feldman MW, Christiansen FB, Otto SP. Gene-culture co-evolution: Teaching, learning, and correlations between relatives. Israel J Ecol Evol. 2013;59(2):72–91. [Google Scholar]
- 27.Odling-Smee FJ, Laland KN, Feldman MW. Niche construction. Am Nat. 1996;147(4):641–648. [Google Scholar]
- 28.Laland KN, Odling-Smee J, Feldman MW. Niche construction, biological evolution, and cultural change. Behav Brain Sci. 2000;23(1):131–146. doi: 10.1017/s0140525x00002417. discussion 146–175. [DOI] [PubMed] [Google Scholar]
- 29.Kylafis G, Loreau M. Ecological and evolutionary consequences of niche construction for its agent. Ecol Lett. 2008;11(10):1072–1081. doi: 10.1111/j.1461-0248.2008.01220.x. [DOI] [PubMed] [Google Scholar]
- 30.Laland KN, Odling-Smee J, Myles S. How culture shaped the human genome: Bringing genetics and the human sciences together. Nat Rev Genet. 2010;11(2):137–148. doi: 10.1038/nrg2734. [DOI] [PubMed] [Google Scholar]
- 31.Jones CG, Lawton JH, Shachak M. Organisms as ecosystem engineers. Oikos. 1994;69(3):373–386. [Google Scholar]
- 32.Mougi A, Iwasa Y. Evolution towards oscillation or stability in a predator-prey system. Proc Biol Sci. 2010;277(1697):3163–3171. doi: 10.1098/rspb.2010.0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith JM, Hofbauer J. The “battle of the sexes”: A genetic model with limit cycle behavior. Theor Popul Biol. 1987;32(1):1–14. doi: 10.1016/0040-5809(87)90035-9. [DOI] [PubMed] [Google Scholar]
- 34.Khibnik AI, Kondrashov AS. Three mechanisms of Red Queen dynamics. Proc R Soc B. 1997;264(1384):1049–1056. [Google Scholar]
- 35.Van Cleve J, Feldman MW. Stable long-period cycling and complex dynamics in a single-locus fertility model with genomic imprinting. J Math Biol. 2008;57(2):243–264. doi: 10.1007/s00285-008-0156-4. [DOI] [PubMed] [Google Scholar]
- 36.Hastings A. Stable cycling in discrete-time genetic models. Proc Natl Acad Sci USA. 1981;78(11):7224–7225. doi: 10.1073/pnas.78.11.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akin E. Cycling in simple genetic systems. J Math Biol. 1982;13(3):305–324. [Google Scholar]
- 38.Cavalli-Sforza LL, Feldman MW. Cultural Transmission and Evolution: A Quantitative Approach. Princeton: Princeton Univ Press; 1981. [PubMed] [Google Scholar]
- 39.Feldman MW, Aoki K, Kumm J. Individual versus social learning: Evolutionary analysis in a fluctuating environment. Anthropol Sci. 1996;104(3):209–213. [Google Scholar]
- 40.Wakano JY, Aoki K, Feldman MW. Evolution of social learning: A mathematical analysis. Theor Popul Biol. 2004;66(3):249–258. doi: 10.1016/j.tpb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 41.Nakajima A, Aoki K. On Richerson and Boyd’s model of cultural evolution by sexual selection. Theor Popul Biol. 2002;61(1):73–81. doi: 10.1006/tpbi.2001.1556. [DOI] [PubMed] [Google Scholar]
- 42.Kesten H. Quadratic transformations: A model for population growth I. Adv Appl Probab. 1970;2(2):1–82. [Google Scholar]
- 43.Keesing RM. Cultural Anthropology, A Contemporary Perspective. London: Holt, Rinehart and Winston; 1981. [Google Scholar]
- 44.Keesing RM. Theories of culture. Annu Rev Anthropol. 1974;3(1):73–97. [Google Scholar]
- 45.Botwin MD, Buss DM, Shackelford TK. Personality and mate preferences: Five factors in mate selection and marital satisfaction. J Pers. 1997;65(1):107–136. doi: 10.1111/j.1467-6494.1997.tb00531.x. [DOI] [PubMed] [Google Scholar]
- 46.Cohen JE. Make secondary education universal. Nature. 2008;456(7222):572–573. doi: 10.1038/456572a. [DOI] [PubMed] [Google Scholar]
- 47.Cohen JE, Kravdal Ø, Keilman N. Childbearing impeded education more than education impeded childbearing among Norwegian women. Proc Natl Acad Sci USA. 2011;108(29):11830–11835. doi: 10.1073/pnas.1107993108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cochrane SH, Leslie J, O’Hara DJ. Parental education and child health: Intracountry evidence. Health Policy Educ. 1982;2(3):213–250. doi: 10.1016/0165-2281(82)90011-x. [DOI] [PubMed] [Google Scholar]
- 49.Mosley WH, Chen LC. An analytical framework for the study of child survival in developing countries. Popul Dev Rev. 1984;10(Supp):25–45. [PMC free article] [PubMed] [Google Scholar]
- 50.Macassa G, Ghilagaber G, Bernhardt E, Diderichsen F, Burström B. Inequalities in child mortality in Mozambique: Differentials by parental socio-economic position. Soc Sci Med. 2003;57(12):2255–2264. doi: 10.1016/s0277-9536(02)00545-2. [DOI] [PubMed] [Google Scholar]
- 51.Murphy E, Carr D. Powerful Partners: Adolescent Girls’ Education and Delayed Childbearing. Washington, DC: Population Reference Bureau; 2007. [Google Scholar]
- 52.Potts M. Sex and the birth rate: Human biology, demographic change, and access to fertility regulation methods. Popul Dev Rev. 1997;23(1):1–39. [Google Scholar]
- 53.Heaton TB. Religious group characteristics, endogamy, and interfaith marriages. Sociol Anal. 1990;51(4):363–376. [Google Scholar]
- 54.Tishkoff SA, et al. Convergent adaptation of human lactase persistence in Africa and Europe. Nat Genet. 2007;39(1):31–40. doi: 10.1038/ng1946. [DOI] [PMC free article] [PubMed] [Google Scholar]