Abstract
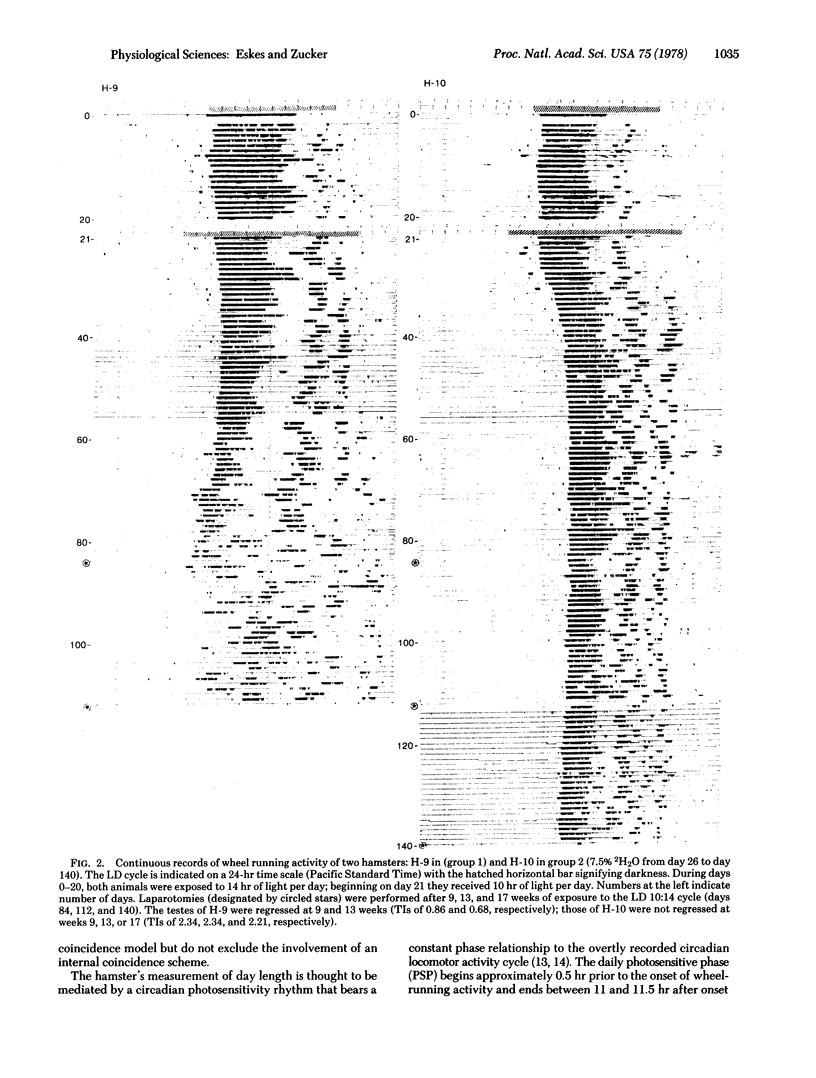
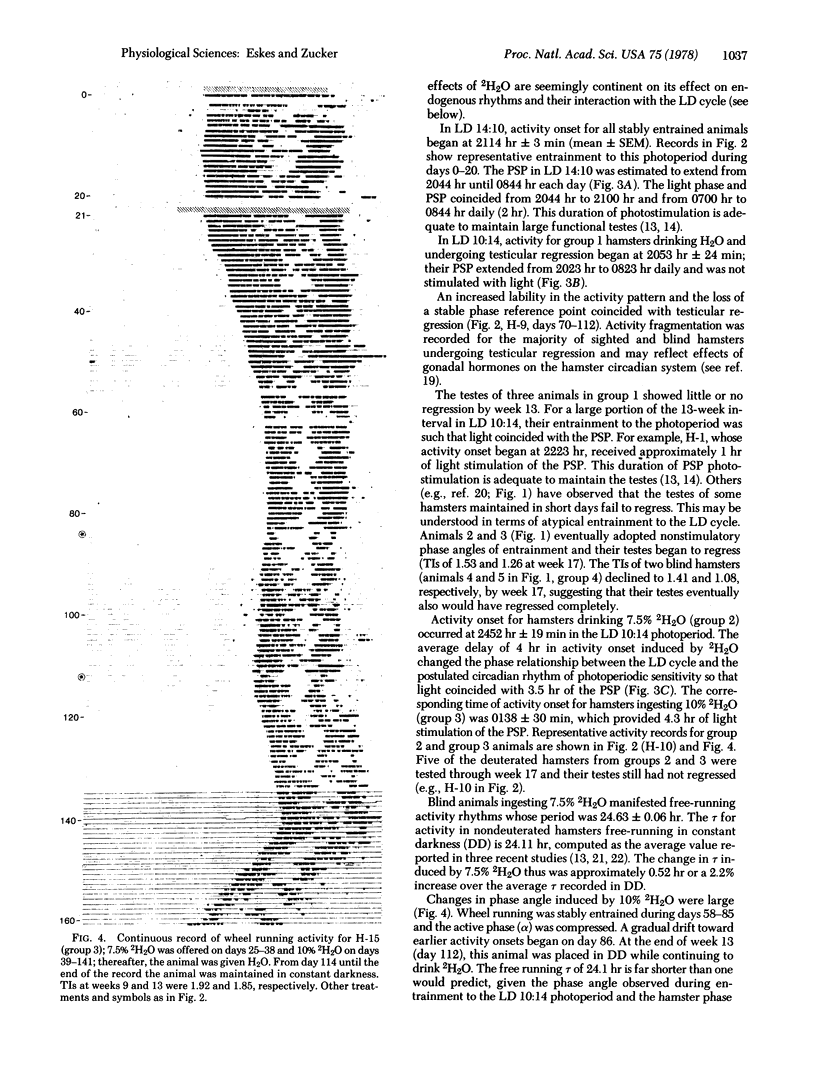
The testes of hamsters exposed to short days (10 hr of light per day) regress within 13 weeks. Administration of 7.5% deuterium oxide to hamsters lengthens the period of free running circadian activity rhythms by 2.2% and prevents testicular regression during short-day exposure. This consistent with predictions derived from an external coincidence model for photoperiodic time measurement: Deuterium oxide changes phase relationships between the light-dark cycle and the circadian system, the hamster's daily photosensitive phase is stimulated with light during short days, and the testes remain large. Conservation of the period of circadian rhythms within narrow limits has adaptive significance for hamster photoperiodism and for the occurrence and phasing of the annual reproductive cycle.
Full text
PDF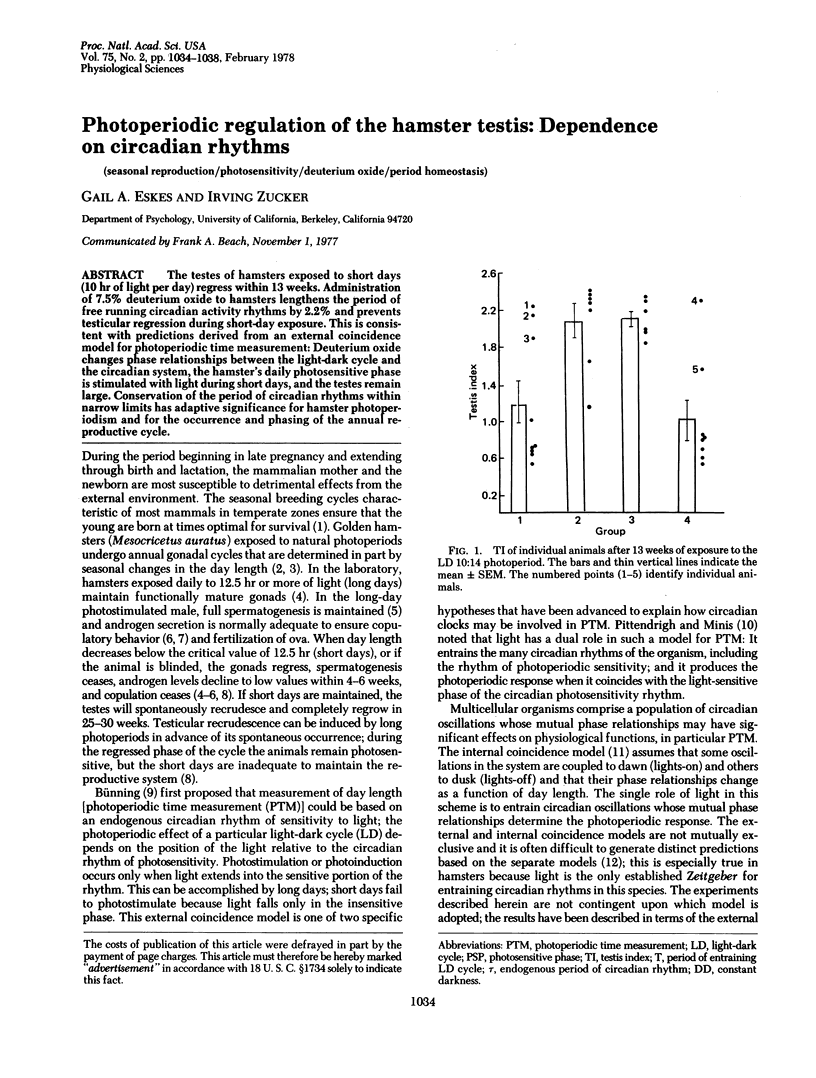


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berndtson W. E., Desjardins C. Circulating LH and FSH levels and testicular function in hamsters during light deprivation and subsequent photoperiodic stimulation. Endocrinology. 1974 Jul;95(1):195–205. doi: 10.1210/endo-95-1-195. [DOI] [PubMed] [Google Scholar]
- Czyba J. C. Les fluctuations de la fécondité chez le hamster doré (Mesocricetus auratus Waterhouse) au cours de l'année. C R Seances Soc Biol Fil. 1968 Jul;162(1):113–116. [PubMed] [Google Scholar]
- Elliott J. A. Circadian rhythms and photoperiodic time measurement in mammals. Fed Proc. 1976 Oct;35(12):2339–2346. [PubMed] [Google Scholar]
- Gaston S., Menaker M. Photoperiodic control of hamster testis. Science. 1967 Nov 17;158(3803):925–928. doi: 10.1126/science.158.3803.925. [DOI] [PubMed] [Google Scholar]
- Morin L. P., Fitzgerald K. M., Rusak B., Zucker I. Circadian organization and neural mediation of hamster reproductive rhythms. Psychoneuroendocrinology. 1977;2(1):73–98. doi: 10.1016/0306-4530(77)90035-x. [DOI] [PubMed] [Google Scholar]
- Morin L. P., Fitzgerald K. M., Zucker I. Estradiol shortens the period of hamster circadian rhythms. Science. 1977 Apr 15;196(4287):305–307. doi: 10.1126/science.557840. [DOI] [PubMed] [Google Scholar]
- Pittendrigh C. S. Circadian surfaces and the diversity of possible roles of circadian organization in photoperiodic induction. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2734–2737. doi: 10.1073/pnas.69.9.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter R. J. Comparative physiology: pineal gland. Annu Rev Physiol. 1973;35:305–328. doi: 10.1146/annurev.ph.35.030173.001513. [DOI] [PubMed] [Google Scholar]
- Reiter R. J. Exogenous and endogenous control of the annual reproductive cycle in the male golden hamster: participation of the pineal gland. J Exp Zool. 1975 Jan;191(1):111–120. doi: 10.1002/jez.1401910111. [DOI] [PubMed] [Google Scholar]
- Reiter R. J., Sorrentino S., Jr Prevention of pineal-mediated reproductive responses in light-deprived hamsters by partial or total isolation of the medial basal hypothalamus. J Neurovisc Relat. 1972;32(14):355–367. doi: 10.1007/BF02327930. [DOI] [PubMed] [Google Scholar]
- Richter C. P. Heavy water as a tool for study of the forces that control length of period of the 24-hour clock of the hamster. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1295–1299. doi: 10.1073/pnas.74.3.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusak B., Morin L. P. Testicular responses to photoperiod are blocked by lesions of the suprachiasmatic nuclei in golden hamsters. Biol Reprod. 1976 Oct;15(3):366–374. doi: 10.1095/biolreprod15.3.366. [DOI] [PubMed] [Google Scholar]
- Zucker I., Morin L. P. Photoperiodic influences on testicular regression, recrudescence and the induction of scotorefractoriness in male golden hamsters. Biol Reprod. 1977 Nov;17(4):493–498. doi: 10.1095/biolreprod17.4.493. [DOI] [PubMed] [Google Scholar]