Abstract
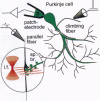
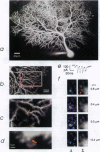
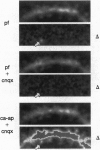
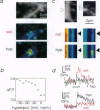
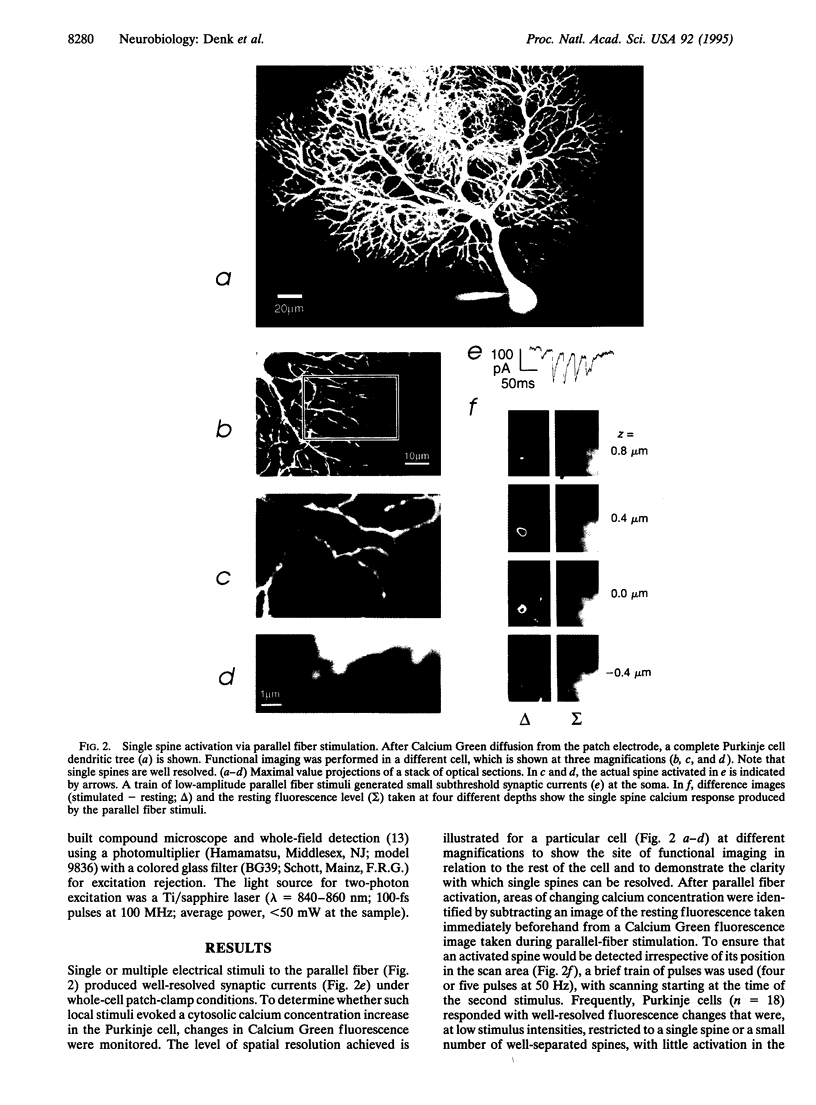
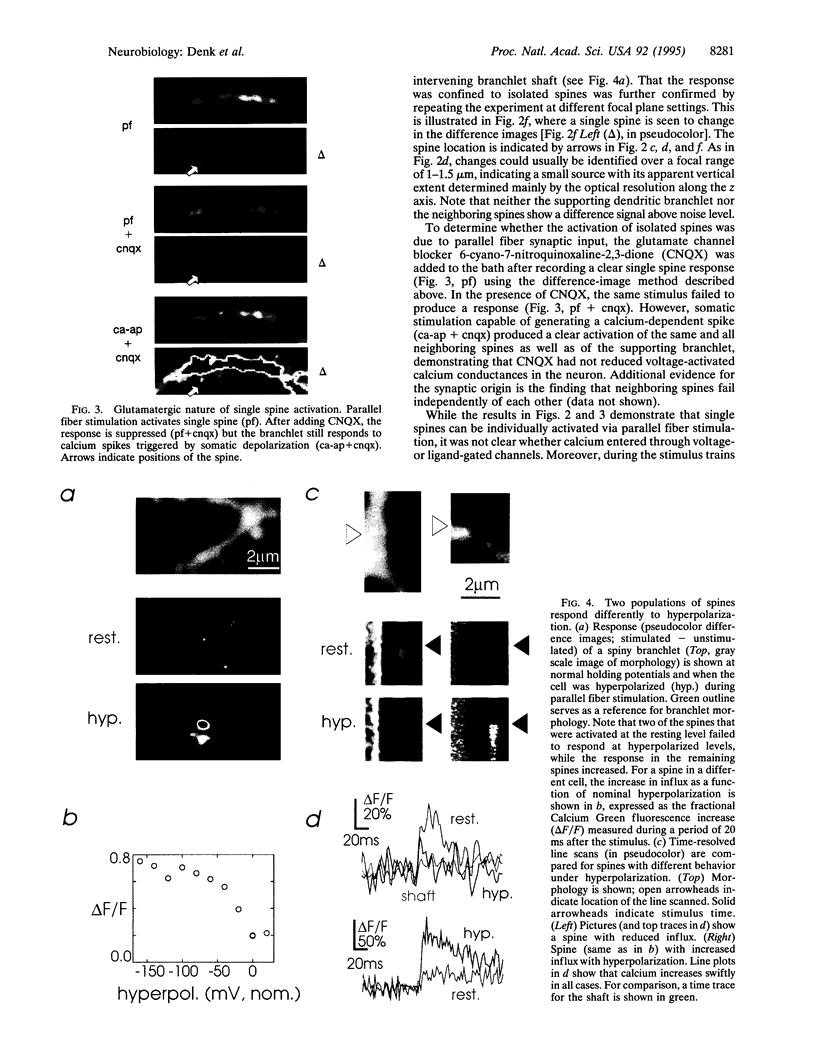
Of fundamental importance in understanding neuronal function is the unambiguous determination of the smallest unit of neuronal integration. It was recently suggested that a whole dendritic branchlet, including tens of spines, acts as the fundamental unit in terms of dendritic calcium dynamics in Purkinje cells. By contrast, we demonstrate that the smallest such unit is the single spine. The results show, by two-photon excited fluorescence laser scanning microscopy, that individual spines are capable of independent calcium activation. Moreover, two distinct spine populations were distinguished by their opposite response to membrane hyperpolarization. Indeed, in a subpopulation of spines calcium entry can also occur through a pathway other than voltage-gated channels. These findings challenge the assumption of a unique parallel fiber activation mode and prompt a reevaluation of the level of functional complexity ascribed to single neurons.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Denk W., Delaney K. R., Gelperin A., Kleinfeld D., Strowbridge B. W., Tank D. W., Yuste R. Anatomical and functional imaging of neurons using 2-photon laser scanning microscopy. J Neurosci Methods. 1994 Oct;54(2):151–162. doi: 10.1016/0165-0270(94)90189-9. [DOI] [PubMed] [Google Scholar]
- Denk W., Strickler J. H., Webb W. W. Two-photon laser scanning fluorescence microscopy. Science. 1990 Apr 6;248(4951):73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- Eilers J., Augustine G. J., Konnerth A. Subthreshold synaptic Ca2+ signalling in fine dendrites and spines of cerebellar Purkinje neurons. Nature. 1995 Jan 12;373(6510):155–158. doi: 10.1038/373155a0. [DOI] [PubMed] [Google Scholar]
- Kashiwabuchi N., Ikeda K., Araki K., Hirano T., Shibuki K., Takayama C., Inoue Y., Kutsuwada T., Yagi T., Kang Y. Impairment of motor coordination, Purkinje cell synapse formation, and cerebellar long-term depression in GluR delta 2 mutant mice. Cell. 1995 Apr 21;81(2):245–252. doi: 10.1016/0092-8674(95)90334-8. [DOI] [PubMed] [Google Scholar]
- Kuno M., Maeda N., Mikoshiba K. IP3-activated calcium-permeable channels in the inside-out patches of cultured cerebellar Purkinje cells. Biochem Biophys Res Commun. 1994 Mar 30;199(3):1128–1135. doi: 10.1006/bbrc.1994.1348. [DOI] [PubMed] [Google Scholar]
- Llano I., DiPolo R., Marty A. Calcium-induced calcium release in cerebellar Purkinje cells. Neuron. 1994 Mar;12(3):663–673. doi: 10.1016/0896-6273(94)90221-6. [DOI] [PubMed] [Google Scholar]
- Llinás R., Hess R. Tetrodotoxin-resistant dendritic spikes in avian Purkinje cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2520–2523. doi: 10.1073/pnas.73.7.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R. Neurobiology. Thorny issues in neurons. Nature. 1995 Jan 12;373(6510):107–108. doi: 10.1038/373107a0. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980 Aug;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W. N., Werman R. Mapping calcium transients in the dendrites of Purkinje cells from the guinea-pig cerebellum in vitro. J Physiol. 1987 Aug;389:319–336. doi: 10.1113/jphysiol.1987.sp016659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G., Häusser M. Initiation and spread of sodium action potentials in cerebellar Purkinje cells. Neuron. 1994 Sep;13(3):703–712. doi: 10.1016/0896-6273(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Sugimori M., Llinás R. R. Real-time imaging of calcium influx in mammalian cerebellar Purkinje cells in vitro. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5084–5088. doi: 10.1073/pnas.87.13.5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tank D. W., Sugimori M., Connor J. A., Llinás R. R. Spatially resolved calcium dynamics of mammalian Purkinje cells in cerebellar slice. Science. 1988 Nov 4;242(4879):773–777. doi: 10.1126/science.2847315. [DOI] [PubMed] [Google Scholar]
- Yuste R., Denk W. Dendritic spines as basic functional units of neuronal integration. Nature. 1995 Jun 22;375(6533):682–684. doi: 10.1038/375682a0. [DOI] [PubMed] [Google Scholar]