Abstract
Aims
The current challenge in atrial fibrillation (AF) treatment is to develop effective, efficient, and safe ablation strategies. This randomized controlled trial assesses the medium-term efficacy of duty-cycled radiofrequency ablation via the circular pulmonary vein ablation catheter (PVAC) vs. conventional electro-anatomically guided wide-area circumferential ablation (WACA).
Methods and results
One hundred and eighty-eight patients (mean age 62 ± 12 years, 116 M : 72 F) with paroxysmal AF were prospectively randomized to PVAC or WACA strategies and sequentially followed for 12 months. The primary endpoint was freedom from symptomatic or documented >30 s AF off medications for 7 days at 12 months post-procedure. One hundred and eighty-three patients completed 12 m follow-up. Ninety-four patients underwent PVAC PV isolation with 372 of 376 pulmonary veins (PVs) successfully isolated and all PVs isolated in 92 WACA patients. Three WACA and no PVAC patients developed tamponade. Fifty-six percent of WACA and 60% of PVAC patients were free of AF at 12 months post-procedure (P = ns) with a significant attrition rate from 77 to 78%, respectively, at 6 months. The mean procedure (140 ± 43 vs. 167 ± 42 min, P<0.0001), fluoroscopy (35 ± 16 vs. 42 ± 20 min, P<0.05) times were significantly shorter for PVAC than for WACA. Two patients developed strokes within 72 h of the procedure in the PVAC group, one possibly related directly to PVAC ablation in a high-risk patient and none in the WACA group (P = ns). Two of the 47 patients in the PVAC group who underwent repeat ablation had sub-clinical mild PV stenoses of 25–50% and 1 WACA patient developed delayed severe PV stenosis requiring venoplasty.
Conclusion
The pulmonary vein ablation catheter is equivalent in efficacy to WACA with reduced procedural and fluoroscopy times. However, there is a risk of thrombo-embolic and pulmonary stenosis complications which needs to be addressed and prospectively monitored.
ClinicalTrials.gov Identifier
Keywords: Electrophysiology, Ablation, Atrial fibrillation, Duty-cycled bipolar and unipolar radiofrequency
What's new?
This is the only multicentre randomized controlled trial of pulmonary vein ablation catheter (PVAC) vs. standard wide-area encirclement for PAF following HRS/EHRA/ECAS consensus statement recommendations on reporting efficacy at 12 months.
The study confirms equivalent efficacy reported in smaller cohort and shorter duration studies but demonstrates an important tail-off in efficacy between 6 and 12 months.
There was a 2% incidence of stroke in the PVAC ablation arm which although not statistically significant corroborates concerns from meta-analysis that measures to prevent this devastating complication need to be undertaken and evaluated prospectively.
Introduction
Radio-frequency energy (RF) ablation utilizing 3D mapping systems has shown considerable efficacy vs. medical therapy in treating both paroxysmal and persistent atrial fibrillation (AF).1,2 With this has come the requirement to simplify and shorten the procedure to meet the exponential increase in patient demand as demographic trends predict an epidemic of this condition. The advent of fluoroscopically guided ‘one-shot’ ablation technologies, e.g. cryoablation, laser, RF multipolar catheters, offers the opportunity to simplify the procedure and expand the population it is available to. As with any new technology, initial studies tend to be small and are directed to highly selected patient populations. Although there have been a number of studies evaluating duty-cycled unipolar and bipolar RF pulmonary vein (PV) isolation, there have only been two randomized controlled trials and both did not employ the HRS/EHRA/ECAS consensus statement recommendation of a 12-month time point in all cases to evaluate AF burden off drugs and indeed one trial studied a mixed population of paroxysmal atrial fibrillation (PAF) and persistent patients.3 This single-blinded multicentre randomized controlled trial of multipolar ablation technology was designed to evaluate the efficacy of this technology in four UK centres representative of the current paroxysmal AF referral patterns in the UK and compare it with standard 3D mapping-guided wide-area circumferential antral ablation (WACA).
Methods
The study was designed as a prospective, single-blinded, randomized multicentre controlled trial. Ethical approval was granted by the ULCH Ethics Committee. All subjects provided informed consent. Paroxysmal AF was defined in each patient as self-terminating episodes of documented AF (on 72 h—7-day Holter) with episodes lasting between <48 h and 7 days maximum. Patients were randomized and allocated equally between the standard 3D electroanatomically guided WACA ablation and PVAC utilizing duty-cycled RF energy delivery (PVAC, Medtronic Ablation Frontiers). This is a 9F deflectable circular multielectrode catheter that enables mapping and circumferential PV ablation.4 The accompanying GENius multichannel, duty-cycled RF generator (Medtronic Ablation Frontiers) enables the delivery of energy in a unipolar or bipolar configuration to all electrodes simultaneously or individually. During an RF application, energy delivery to individual electrodes is temperature controlled by a software algorithm that modulates power to reach the user-defined target temperature (maximum 8 W per electrode with the PVAC in a 4 : 1 power setting or 10 W in all other settings). The patients were blinded to the catheter used for the duration of the trial.
The endpoints of the trial were:
The differences in procedure times using the circular ablation (PVAC) catheter when compared with WACA and PVI.
The efficacy of the circular ablation catheter vs. WACA and PVI in eliminating paroxysmal AF measured by a 7-day ECG Holter recording off anti-arrhythmic drugs (AADs) at 12 months following AF ablation (adjudicated by each centre).
The safety profile of the techniques for procedural and long-term complications.
The principal inclusion criteria were patients with paroxysmal AF who had failed at least one anti-dysrhythmic drug who had been listed for a planned PVI procedure. Exclusion criteria included patient objection, prior AF ablation, left atrial size >60 mm, mechanical prosthetic mitral valve replacement, hypertrophic cardiomyopathy, contraindications to anticoagulation, pregnancy.
After the informed consent had been obtained, consecutive patients were randomized to one method for PV isolation: (i) the duty-cycled bipolar and unipolar RF ablation (PVAC group) or (ii) a point-by-point antral ablation (WACA group). Anticoagulation with warfarin was stopped 3 days before admission and bridged with heparin or anticoagulation was maintained to achieve an international normalized ratio (INR) of 2–3.5 during the course of the procedure and AADs continued until 5 days pre-procedure. Transoesophageal echocardiography was performed in all patients immediately before ablation to exclude left atrial thrombus. Ablation was performed under conscious sedation or general anaesthetic. The WACA group had three-dimensional reconstructions of the left atrium (LA) and PV ostia performed using the EnSite Verismo software (St. Jude Medical) or CARTO-XP (Biosense Webster).
All surface electrocardiograms and bipolar intracardiac electrograms were registered in a BARD digital recording system. Signals were sampled at 1 kHz, filtered at 0.1–100 Hz for surface electrocardiograms and at 30–250 Hz for intracardiac signals.
Randomization and blinding
Individual patients were randomized to either PVAC or WACA using random permuted blocks with equal numbers of patients allocated to each arm. Treatments were guaranteed to be balanced after a given block size. The block size chosen was not revealed to the investigators. A computer generated list of allocations (‘WACA & PVI’ or ‘circular catheter’) was produced by the Medical Statistics Department at UCLH. These sequential allocations were sealed in envelopes. Each sealed envelope was opened immediately prior to the procedure so patients were randomly allocated in the order they arrive in the catheter laboratory for their procedure. The contents of the envelopes were not known to the investigators.
The 11 attending electrophysiologists performing the procedures were all experienced operators (in both techniques) undertaking at least 100 AF ablations per annum and >5 years experience of AF ablation and there was no significant difference in their experience between the treatment groups. The patients were blinded to the catheter used during the procedure for the duration of the trial. It was not possible to blind the operators. The persons analysing the Holter data were blinded to the catheter used. In total 10 patients were recruited from Southampton, Bournemouth, and Glenfield Heart Centre, respectively, and the remainder from the Heart Hospital, UCLH.
Pulmonary vein ablation catheter ablation
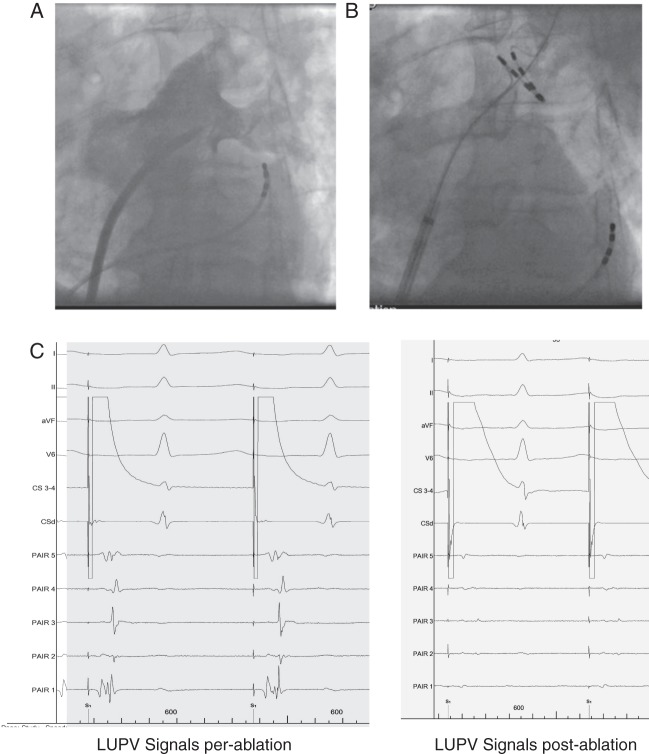
The technique of PVI isolation using this technique has been described in detail elsewhere.5 In brief a single trans-septal puncture was performed using an SL1 sheath and this was exchanged for a Bard 11F channel sheath to pass the PVAC catheter into the LA following initial pulmonary venography to define the PV ostia. A bolus of heparin (50–100 IU/kg) was administered post trans-septal puncture and additional heparin was given throughout the procedure to maintain the activated clotting time (ACT) in a specified therapeutic range. The circular decapolar 9 Fr bidirectional PVAC catheter was advanced over a 0.032 inch wire, which was selectively positioned in each PV or PV branch, using PV angiography to define the PV ostium. Distal ablation in the PV was avoided and lesions were targeted in the antral region or at the ostium. Selectively cannulating specific branches of the veins was especially important to optimize the catheter–atrial wall contact when isolating large common ostia. With this single catheter, it was possible to map local signals, to stimulate and to apply RF over all or selected electrode pairs. Filter settings at the electrophysiological (EP) workstation were 100–500 Hz. The PVAC was connected to the GENius (Medtronic, Inc.) RF generator (Version 11 software Medtronic Ablation Frontiers GENius Multi-Channel RF Generators (Model Number 990018) were employed until May 2011 for the first 74 PVAC patients and then the remaining 20 with subsequent software versions (12.2, 14.0–14.4)). It delivered RF in a combination of one or more of the five bipolar channels. Each electrode was supplied with a thermocouple, which permitted a continuous local temperature monitoring. The target temperature was 60°C with a maximum power of 8 W. Bipolar/unipolar RF delivery was set in a 4 : 1 ratio, creating a 3 to 4 mm-wide and deep lesion.6 Only exceptionally, RF ablation was set in a 2 : 1 bipolar/ unipolar proportion, when the created lesion was estimated to be non-transmural with the 4 : 1 setting. Impulse duration was 60 s. Premature termination of ablation was generally avoided, and only performed when pain occurred, or the catheter was dislocated. After several RF applications over all electrode pairs, individual pairs were activated to ablate any remaining sleeves. In large veins where contact was poor, some poles were initially switched off if no signal was recorded. The endpoint was electrical isolation of the vein (i.e. no PV potentials) confirmed by pacing to demonstrate entrance block (with a steerable quadraploar catheter/decapolar catheter in the CS or LA) placing the PVAC within 1 cm of the ostium to check the level of isolation (Figure 1). If a vein could not be isolated standard catheter ablation was not applied.
Figure 1.
Example of PVAC positioning and signals recorded in LUPV pre and post ablation. (A) Pulmonary venogram (B) PVAC position in the large LUPV branch. (C) Unipolar signals recorded pre and post ablation with distal CS pacing illustrating signal attenuation and PV isolation.
Wide area antral circumferential ablation
The technique for WACA has been described elsewhere with the additional use of a lasso catheter to record PV signals.7 In summary, a double trans-septal puncture was performed using an SL1 and Agilis guide sheath and 3D geometry created using the Carto or NAVX mapping system. Thereafter, an antral point-by-point circumferential ablation around ipsilateral PVs, with a distance of 0.5–1.0 cm from the ostia, using a 4 mm open-tip irrigated catheter (IBI Therapy Coolpath Duo 7 Fr, St Jude Medical Coolflex or Biosense Thermocool) was performed. Maximum power was set to 30–35 W anteriorly going selectively up to 40 W if PV isolation could not be achieved and 25–30 W posteriorly. Temperature was limited to 43°C. Irrigation was adjusted manually between 17 and 30 mL/min. Complete electrical isolation was monitored on the lasso in the PV and confirmed by pacing from the CS decapolar catheter during sinus rhythm and differential pacing manoeuvers. Electrical cardioversion was performed if the patient remained in AF after PV isolation as was the case in the PVAC group.
Anticoagulation
Fourteen patients in the WACA group (mean INR 2.6 ± 0.4) and 19 patients in the PVAC group (mean INR 2.3 ± 0.4) continued warfarin for the duration of the procedure. The remainder stopped warfarin 3 days pre-procedure and was placed on therapeutic fragmin such that their INR on the day of procedure was 1.2–1.5. In the PVAC patients, a bolus of heparin (50–100 IU/kg) was administered post trans-septal puncture and additional heparin was given throughout the procedure to maintain the ACT between 250 and 300 s in patients with an INR of >2 and >300 s in those who were not anticoagulated with INR > 2. ACT was checked initially 20 min after the first bolus and then every 30 min once therapeutic. Ablation was commenced once ACT was >250 s in the warfarinized patients and >300 s in the non-fully warfarinized cases. The mean ACTs after the initial ACT during the case were 291 ± 48 s (range 223–370 s) in the PVAC group and 302 ± 43 s (234–426 s) in the WACA group. For WACA patients, ACTs were maintained with a target of >250 s. Protamine was administered post-procedure and LMW heparin recommenced (when required) 3–6 h post-procedure when haemostasis was confirmed.
Procedural data
The number of RF impulses and duration required for completing isolation were recorded in the PVAC group and the total RF time in the WACA group. Total skin-to-skin procedural time, left atrial dwell time (time from trans-septal puncture to procedure end), and total fluoroscopy time were also collected. In-hospital complications were also recorded.
Follow-up
All patients were maintained on anticoagulants for the first 3 months post-procedure. These were stopped if the patients were free of AF and had a CHADSVASc score of <2.0. Anti-arrhythmics were continued post ablation and stopped routinely at 3 months post-procedure if the patient was asymptomatic and had no recorded AF. Patients were reviewed at 3, 6, and 12 months post-procedure with a 72 h Holter. Holter recordings were also repeated if the patient developed arrhythmia symptoms despite a normal Holter recording at the specified follow-up times. Serial Holter monitoring for the purpose of the trial was stopped if AF was documented on resting ECG or Holter. Any patients still taking AADs for suspected symptomatic but non-documented AF recurrence had them stopped at 12 months for the 7-day Holter.
A blanking period of 3 months was employed. More than 30 s of documented AF or other atrial tachycardia >3 months post-procedure was regarded as a failure of PVI during follow-up. The clinic physicians had access to the patient's procedural details if required. A decision for re-ablation was made if the patient had documented symptomatic recurrences of AF or AT beyond the 3-month blanking period. These patients were treated as ablation failures for the analysis of 12-month success. Patients who failed to complete the PV isolation procedure or were lost to follow-up were censured from the 12-month ablation outcomes analysis. Therefore, outcomes are presented as on treatment analysis as opposed to intention to treat.
Statistical analysis
Continuous variables were expressed as mean ± SD. Categorical variables are expressed as percentage. The χ2 and t tests were used for comparisons between groups, and the Kaplan–Meier curve was used to compare arrhythmia-free survival between groups. A P value of <0.05 was considered significant. Pilot data indicated that the standard deviation of WACA and PVAC procedure time is 30 min. The study required a sample size of 48 patients in each group to detect a significant difference (90% power, P = 0.05) in procedure time between techniques. The trial was designed as a non-inferiority study in terms of comparing outcomes for atrial arrhythmia recurrence. If the PVAC ablation actually has freedom of AF of 70%, it was calculated as 70 patients in each group to prove non-inferiority (80% power). All authors have reviewed the data and accepted responsibility for its integrity. The trial was registered with Clinical Trials.gov-ClinicalTrials.gov Identifier: NCT00678340.
Results
A total of 188 PAF patients were finally recruited into the trial between September 2007 and March 2012. The mean age of participants was 62 ± 12 years (116 M : 72 F). The full demographic profile of the patient treatment arms is shown in Table 1. The patients had well-preserved ventricular function and no significant valvular heart disease.
Table 1.
Demographics
| WACA | PVAC | P value | |
|---|---|---|---|
| N | 94 | 94 | |
| Age, years (mean) | 62 ± 11 | 58 ± 12 | P < 0.05 |
| Male : female (%) | 58 : 36 | 58 : 36 | |
| HT (%) | 28 | 24 | ns |
| Diabetes (%) | 3 | 6 | ns |
| Structural heart disease | 3 | 6 | ns |
| Mean ejection fraction | 62 ± 11 | 64 ± 6 | ns |
| Mean LA size (mm) | 39 ± 5 | 38 ± 7 | ns |
| TIA/CVA | 2 | 3 | ns |
| AF frequency | |||
| Daily | 16 | 17 | – |
| 2–3/week | 24 | 21 | – |
| 1/week | 17 | 20 | – |
| 1/month | 19 | 17 | – |
| 2–3/month | 18 | 19 | – |
| CHADSVASC score | |||
| 0 | 28 | 30 | – |
| 1 | 26 | 24 | – |
| 2 | 25 | 19 | – |
| 3 | 11 | 18 | – |
| 4 | 3 | 3 | – |
| 5 | 1 | 0 | – |
| Anti-dysrhythmic drugs (no patients treated)a | |||
| Class I | 33 | 34 | – |
| Amiodarone | 11 | 15 | – |
| Sotalol | 20 | 21 | – |
| β-blockers | 50 | 54 | – |
TIA/CVA, transient ischaemic attack/cerebrovascular accident; HT, hypertension.
aDescribes all drugs employed at any time in any patient.
There was a clinically insignificant age difference between the PVAC and WACA groups. There were no significant differences in the experience of the physicians performing the procedures in each arm. The CONSORT diagram (Figure 2) illustrates the number of patients recruited and follow-up.
Figure 2.
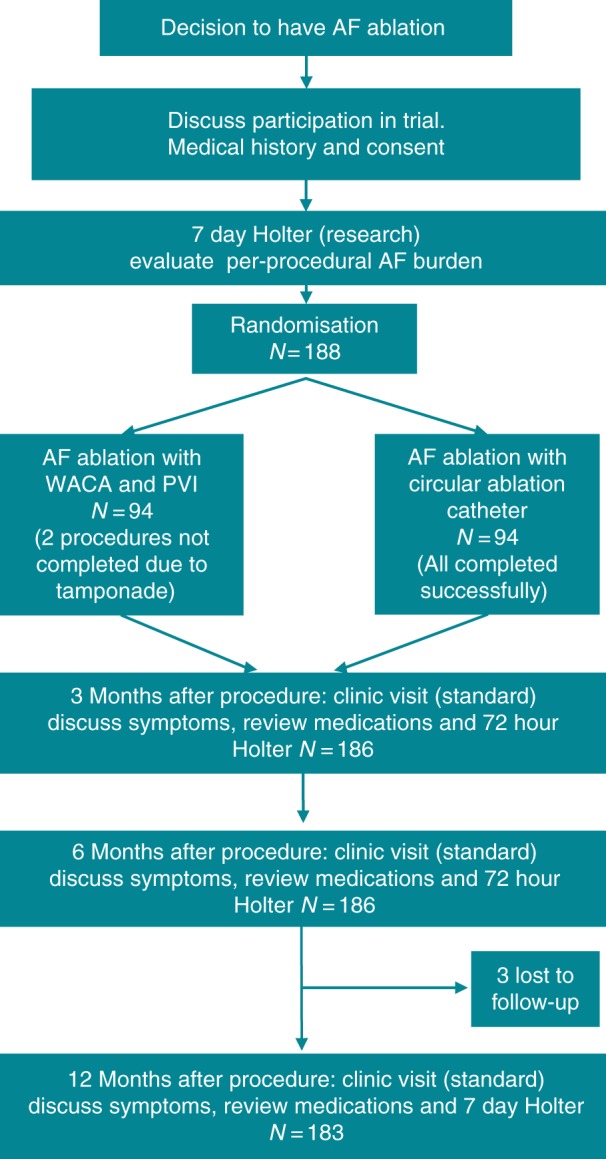
CONSORT diagram illustrating patient recruitment numbers and follow-up.
Immediate procedural outcomes
A total of 94/94 patients underwent successful PVAC PVI. In total 372/376 PVs were successfully isolated with convincing loss of PV potentials (common ostia were counted as two veins). In one patient, it was not possible to isolate a large common left-sided PV and RIPV and in another one RIPV could not be cannulated due to extreme angulation of the vein and its small size. One patient required DC cardioversion to sinus rhythm at the end of the procedure.
In the WACA group, 92/94 patients achieved electrical isolation of the PV's. Three developed cardiac tamponade during ablation and two of these patients declined further AF ablation and the other completed ablation of all four PV's by WACA 3 months later. Therefore, 368/376 PV's were successfully isolated. The former two patients were censured from follow-up in the WACA group as failures to isolate. Two patients required DC cardioversion to sinus rhythm at the end of the procedure.
The mean ACTs after the initial ACT during the procedures were 298 ± 43 s (range 223–426 s) in the PVAC group and 311 ± 43 s (range 234–364 s) in the WACA group.
Overall procedure times were 16% shorter in the PVAC group (P < 0.0001) with a 17% shorter LA dwell time, 17% (P < 0.0001) reduced fluoroscopy time (P < 0.05), and 33% reduced RF time (P < 0.0001) (Table 2). There were no significant differences in the total amount of energy delivered between the two approaches from the data available in 74 PVAC cases and 25 WACA cases (68 609 ± 29 156 J vs. 55 187 + 32 407 J, P = 0.06).
Table 2.
Procedural data
| WACA mean (range) | PVAC mean (range) | P value | |
|---|---|---|---|
| Mean procedure time (min) | 167 ± 42 (95–270) | 140 ± 43 (70–270) | <0.0001 |
| Left atrial dwell time (min) | 133 ± 36 (90–210) | 111 ± 35 (55–220) | <0.0001 |
| Fluoroscopy time (min) | 42 ± 20 (9–86) | 35 ± 16 (4–86) | <0.05 |
| RF application time (min) | 40 ± 14 (8–49) | 27 ± 8 (13–68) | <0.0001 |
| Mean number of RF applications per vein | |||
| PVAC | |||
| L. superior PV | – | 8 | – |
| L. inferior PV | – | 9 | – |
| Left common PV | – | 15 | – |
| R. superior PV | – | 7 | – |
| R. inferior PV | – | 6 | – |
| R. common PV | – | 10 | – |
Complications
In total, there were six complications. There were three cardiac tamponade events during three WACA cases and zero during the PVAC (P = ns). Two occurred soon after trans-septal puncture, one with an NIH catheter manipulation to image the PVs, one during geometry creation with manipulation of the ablation catheter in the left atrial appendage, and one due to steam pop during left-sided WACA applying 30 W near the LA roof.
Two strokes occurred in PVAC subjects and none in the WACA. Both subjects were in sinus rhythm at the start of the case. The first was a subject who had a previous history of transient ischaemic attack (TIA) and treated hypertension (CHADSVASC 3). He had commenced fragmin pre-operatively and stopped warfarin 3 days before, his pre-op INR was 1.5. He had a normal pre-operative transesophageal echocardiogram (TEE) and had therapeutic INR's pre-procedure with heparin bridging and ACT's of 250 s initially to >300 s during the procedure, post-procedural protamine and therapeutic LMW heparin as per usual protocol. The following morning post-procedure, he had a homonymous haemianopia secondary to an occipital infarct which did not recover. It was not possible to know the exact timing of this event as he had returned to the ward sedated that evening and did not notice the field defect until the next morning.
The second patient developed a right eye inferior quadrant field defect 2 days post ablation with PVAC—an magnetic resonance imaging (MRI) confirmed two small infarcts in the fronto-parietal lobes. He had a normal pre-operative TEE and therapeutic INRs having stopped warfarin with heparin cover 3 days pre-op with an INR of 1.2 on the day of procedure. ACT's during the procedure had been between 260 and 320 s. His ablation had been unremarkable but had required DC cardioversion at the end of the case as he developed AF during catheter manipulation. He received post-operative protamine. He had been on therapeutic doses of LMW heparin while recommencing warfarin and had a pre-op CHADSVASC score of 0. This field defect did not recover.
There was no prospective strategy to image the PVs for PV stenosis post-procedure due to cost limitations. Pulmonary vein stenosis was specifically checked for in patients undergoing repeat procedures. One WACA patient developed clinical PV stenosis 12 months post-procedure. This patient had a geometry shift during ablation requiring remapping with NAVX and it is likely that RF was inadvertently delivered too close to the PV common ostium. He had a delayed presentation with increasing exertional dyspnoea 12 months post ablation requiring venoplasty to the left upper and left lower pulmonary vein (LU and LLPV). In 2 of the 47 cases who had a repeat AF ablation procedure with PV angiography, it was noted that the LUPV had a mild 40% stenosis in one PVAC case and 50% in another PVAC case. The latter resulted in a 5 mmHg pressure gradient but since the patient was not symptomatic, it was not angioplastied. No WACA patients developed a PV stenosis.
One PVAC patient developed a pseudo-aneurysm 24 h post-procedure requiring thrombin injection but did not suffer any long-term sequelae.
One year follow-up arrhythmia recurrence
Data are presented according to treatment analysis. In total, 183 patients completed 12 months full follow-up. Three patients were lost to follow-up, one due to death from heart failure 11 months post-procedure, and two who moved away and declined further monitoring. Two of the tamponade patients declined a repeat procedure. This means that there were no 12-month follow-up data in 5/188 randomized patients. Overall, 60% of the PVAC patients were free of AF and atrial arrhythmias off anti-dysrhythmics on 7-day Holter 12 months post ablation. This compares with 56% of the WACA group at 12 months (Figure 3, Kaplan–Meier curves)(P = ns). It is interesting to note that there was a minimal difference in success at 6 months—(77% WACA, 78% PVAC) but a further decline out to 12 months. Twenty-four patients in the PVAC group and 23 in the WACA group required repeat ablation for documented recurrent symptomatic AF. These patients were treated as ablation failures for the analysis of 12-month success.
Figure 3.
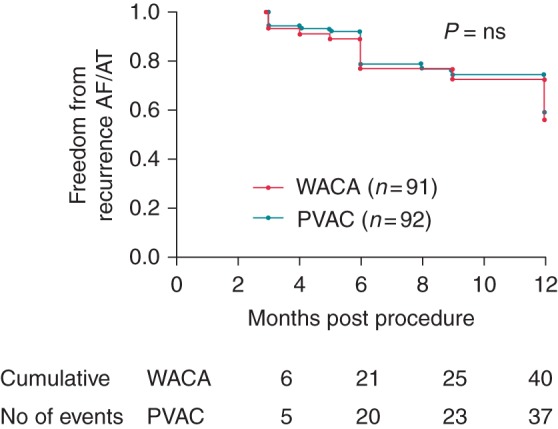
Kaplan–Meier curves demonstrating freedom from AF/AT post ablation.
In the PVAC repeat ablation group, 73 of the 96 ablated PV's had reconnected and 67 of 92 PV's in the WACA group (P = ns). There was no evidence of >50% PV re-stenosis in the PVAC patients on angiography.
Discussion
This is the first single-blinded multicentre randomized controlled trial to directly compare PVAC ablation with conventional WACA in a pure PAF population with a definite endpoint of freedom from AF on 7-day Holter at 12 months as advocated by the HRS/EHRA/ECAS consensus statement.3 Previous studies have either been single-centre, non-randomized, or investigated mixed populations of PAF and persistent patients.5,8–15 This trial demonstrates that there are no significant differences in 1-year long-term outcomes either with PVAC or with WACA. The main differences are borne out by significantly shorter procedure and fluoroscopy times in the PVAC group and lower rates of cardiac tamponade reflecting the different technical aspects of the respective procedures. However, there was a 2% stroke rate in the PVAC arm.
These data mirror the findings from smaller studies with a high success rate 75% at 6 months but then a further 50% attrition rate of recurrence. Furthermore, there is a consistent difference in 37 min reductions in procedure times and shorter fluoroscopy times with PVAC vs. WACA. The WACA fluoroscopy times are ∼20 min longer than currently with the greater stability of and confidence in the geometries of current 3D mapping systems. Therefore, WACA fluoroscopy times would be expected to be shorter if the trial was performed from 2013; however, PVAC fluoroscopy times would remain unchanged. The reported PVAC procedure times and lesion delivery data in this study are equivalent to that described by experienced centres summarized in a recent systematic review—116.9 ± 33.4 min, fluoroscopy time was 26.5 ± 9.6 min, and the number of PVAC applications per patient was 25.1 ± 3.4.16 These time differences clearly reflect the additional time required to create an LA geometry with all its associated potential technical delays that are absent in the case of PVAC where 3D mapping systems are redundant, simply relying on traditional PV angiography for imaging. In both the PVAC and WACA groups, there was no pre-specified waiting time to assess PV reconnection and adenosine testing was not performed since this had not been verified to be of value in reducing recurrences in randomized controlled trials and still remains unresolved.17
Complication rates
There were also clinically significant differences in complication rates between the techniques with importantly a 3% tamponade rate, one delayed presentation of PV stenosis requiring venoplasty in the WACA group, and 0% tamponade rate but two neurological events in the PVAC group. The former tamponade events can most likely be explained by catheter manipulation as opposed to trans-septal puncture not being guided by TEE, since both tamponade events occurred while the NIH/ablation catheter was being manipulated in vulnerable areas of the LA after a >10 min period of unchanged haemodynamic parameters. The steam pop during lesion delivery in the LA most likely relates to increased pressure at the roof site as opposed to high power application as the energy on delivery was limited to 30 W. Although, slightly higher than some series, this event rate falls into the event rates reported in the range of 1–6% (http://guidance.nice.org.uk/CG36/Guidance/pdf/English) from a number of series and is compatible with the worldwide AF survey data of 1.3% taking into account that this was registry-based data which can potentially under-report events.18 Furthermore, some of the AF procedures were performed by experienced clinical fellows under direct Consultant supervision. This may have contributed to the slightly higher tamponade rate in conjunction with the use of the steerable Agilis sheath which could potentially result in higher pressure contact in certain parts of the atrium especially since the contact force-sensing technology was not yet available during the study. None of the tamponades required surgical intervention and promptly responded to direct percutaneous drainage.
However, of more concern are the two strokes which occurred in the peri/post-operative period (2% of cases). The first event could have potentially developed during the procedure or immediately afterwards, while the second occurred 2 days post-op after transition from LMW heparin to warfarin. The former case had a previous history of TIA's, and although had no evidence of LA thrombus pre-operatively on TEE with therapeutic ACT's intra-operatively, it is conceivable the event was either catheter related (char, coagulum or air embolisation) or developed as a result of thrombus formation on the ablation lesions overnight despite post-operative anticoagulation. In a recent meta-analysis of PVAC safety and efficacy, the incidence of thrombo-embolic complications was 0.63%, with 10 embolic events, that is, 2 strokes, 6 TIAs, and 2 myocardial infarctions in 1598 patients (23 studies).16 Similar neurological event rates were reported in a European voluntary survey of 1.1% in 2848 patients from 20 centres and of 1.1% in 634 patients in a large single-centre registry.19,20 Actual neurological events were similar in paroxysmal and persistent patients and unrelated to case load, bridging anticoagulation to low-molecular weight heparin, sheath flushing average procedure times or routine use of TEE pre-procedure but the survey was underpowered to fully examine causes of these events.19 Two randomized trials comparing PVAC with standard ablation had no strokes in their respective arms.5,14 This is despite using lower ACTs of 250–300 s in Bittner et al.'s study. It is possible that tissue overheating recognized as a problem with the GENius 11 generator resulting in its withdrawal in May 2011 could have been an additional factor promoting char/coagulum formation in the two stroke cases. Furthermore, it is now becoming evident that stopping vitamin K antagonists and bridging heparin (undertaken in the 2 PVAC stroke cases) have a higher risk of thrombo-embolic complications than continuing with oral anticoagulation maintaining therapeutic INR's during the procedure.21,22 Though not specifically a cause of stroke, it has been recognized that PVAC technology has been associated with a higher rate of silent cerebral ischaemic lesions on MRI vs. cooled flow RF most probably due to gas bubble formation as opposed to thrombus. In a recent porcine study, microbubble formation was more commonly seen with the PVAC particularly due to overlap between electrodes 1 and 10 23—this has been confirmed clinically.24,25 In a clinical study, diffusion-weighted MR cerebral lesion formation was minimized using a combination of optimal sheath management to prevent the introduction of air into the LA, full heparinization in warfarinized patients, and avoidance of electrode 1–10 contact.26 Avoidance of the latter reduced cerebral lesion formation four-fold. The ERACE trial has confirmed that these interventions significantly reduce the asymptomatic cerebral emboli incidence to 1.7%.27 These factors were not identified during the ablation phase of the trial. However, it is difficult to know whether they would have prevented the peri-procedural stroke and are unlikely to have changed the outcome in the later post-procedural event which is more likely related to thrombus formation in the hypercoaguable state during transition from LMW heparin to warfarin.
There have been concerns regarding the incidence of PV stenosis using the PVAC.28 This study did not search systematically for PV stenosis and only imaged the PV's in patients undergoing repeat ablation or symptomatic PV stenosis causing exertional breathlessness or haemoptysis. Therefore, we may have underestimated its incidence. In a recent prospective study of 100 PVAC cases, cardiac MR/computed tomography (CT) imaging demonstrated a detectable narrowing of the PV diameter in 23 patients and in 28 (7%) PVs.29 Insignificant PV stenosis (<25%) was observed in 12 (2.9%) PVs, mild PV (25–50%) stenosis in 15 PVs (3.7%), and moderate PV stenosis (50–75%) in 1 PV (0.2%). No instances of severe PV stenosis were observed. This suggests that although caution should be applied, the incidence of clinically important PVAC induced PV stenosis is low. The delayed PV stenosis in the WACA patient arose due to inadvertent ablation near the PV ostium due to geometry shift. This highlights the importance of ensuring geometry stability and utilizing fluoroscopy to confirm catheter location.
Recurrence of PAF at 12 months
The lack of a significant difference between recurrence rates in the PVAC and WACA arms despite achieving PV isolation is interesting and reflects the limitations of current ablation strategies for PAF in achieving durable PV isolation. The endpoint of PV isolation was confidently achieved during WACA and tested using differential pacing with PVAC ablation and yet reconnection occurred at the same rate even though RF times were shorter in the PVAC group. Indeed, the WACA RF times are substantially shorter than that reported by Bittner, et al. (35 vs. 65 min) and yet the recurrence rates in this group are equivalent.5 However, there is the caveat that the latter study combined paroxysmal and persistent cases although no additional linear/atrial body ablation was performed. This must reflect the fact that these technologies are unable to deliver adequate transmural permanent lesions or there is a capacity for the PVs to reconnect perhaps by the re-growth of epicardial venous muscle sleeves. Inherent in the process of RF lesion delivery is the development of oedema which may result in transient loss of PV connection.27 Current efforts are focused upon delivering lesions with optimal contact force to minimize this problem and the imaging of lesion formation to ensure trans-murality.30 However, one must bear in mind that although PV reconnection is a very likely the cause of recurrence, up to 30% of PV's reconnections may be clinically silent.31 Inherent in this process is the need to deliver the optimum energy to achieve permanent PV isolation without excessive tissue injury resulting in tamponade, oesophageal injury, or PV stenosis. This problem may be addressed in future by design changes with the PVAC GOLD and new GENius generator which may lead to desired improvements in durable PV lesions. The evolution of irrigated multipolar RF delivery systems (e.g. -n-MARQ, Biosense-Webster) would be expected to also minimize the formation of thrombo-embolic material during RF delivery; however, preliminary reports suggest that there may be an unexpectedly high incidence of silent cerebral emboli and oesophageal injury with this technology.32 All these systems should ideally monitor lesion formation and contact force to maximize the probability of permanent transmural lesion creation.
Comparison with other trials of pulmonary vein ablation catheter in PAF
Six studies have directly compared PVAC with 3D-guided RF ablation. However, only two have done so in a randomized fashion with one examining the importance of PV anatomy.5,8–14 Bulava et al.14 randomized 102 patients. After a mean follow-up of 200 ± 13 days, freedom from recurrent AF in patients off AADs was 71% (36 of 51) vs. 67% (34 of 51), respectively. Bittner et al.5 randomized 80 consecutive patients with PAF (55%) or persistent AF (45%) to PVAC-based or conventional 3D-based RF ablation. After a mean follow-up of 254 ± 99 days, freedom from recurrent AF off AADs was 58% (23 of 40) vs. 43% (17 of 40), respectively. Although well conducted, both studies did not report follow-up at a fixed cut-off of 12 months and in the latter study, a mixed population is described which confounds interpretation of the results to a degree. However, the outcomes at the 9-month time point are equivalent to the data reported in this trial. Overall, our results reflect the pooled estimate of 12 m freedom from AF after PVAC of 52–67%, the wide 95% confidence intervals representing the heterogeneity of the study populations and outcomes, (http://guidance.nice.org.uk/CG36/Guidance/pdf/English) although only three studies have reported 12-month follow-up data.9,10,33 These data are also similar to a European survey in which 20 centres reported outcomes in 1669 PAF cases with a 59% success rate of anti-dysrhythmic medication.19
Study limitations
A limitation of this study was the lack of prospective evaluation for PV stenosis in both the WACA and PVAC groups and so no firm conclusions can be drawn regarding the incidence of subclinical PV stenosis. This would have required routine CT, MRI, or TEE scanning at 6 months post-procedure. Data were only collected in cases undergoing repeat procedures.34 There was no prospective performance of cerebral MRI scans for microemeboli as this issue only came to light in the follow-up phase of the study and so it was not possible to undertake such a prospective analysis.
Conclusions
This multicentre randomized controlled trial demonstrates that PVAC PV isolation is equivalent in efficacy to WACA with clinically significantly shorter procedure times for PAF ablation. The challenge still remains to achieve durable efficacy of PAF ablation with the minimum of complications and ensure that new technologies are rigorously evaluated in a randomized fashion with clearly defined long-term clinical endpoints.34 The avoidance of irreversible debilitating complications such as cerebrovascular accident (CVA) is critical in this endeavour with prospective evaluation of preventative interventions in large cohorts to ensure they are effective.
Funding
This work was supported by UCLH Biomedicine NIHR, Glenfield University Hospital, Leicester University NIHR. Funding to pay the Open Access publication charges for this article was provided by UCL.
Acknowledgments
Conflicts of interests: J.M.M., C.M., O.R.S., P.R., P.D.L., and M.D. received an educational grant from Medtronic. J.M. received an educational grant from St Jude Medical.
References
- 1.Parkash R, Tang AS, Sapp JL, Wells G. Approach to the catheter ablation technique of paroxysmal and persistent atrial fibrillation: a meta-analysis of the randomized controlled trials. J Cardiovasc Electrophysiol. 2011;22:729–38. doi: 10.1111/j.1540-8167.2011.02010.x. [DOI] [PubMed] [Google Scholar]
- 2.Chen HS, Wen JM, Wu SN, Liu JP. Catheter ablation for paroxysmal and persistent atrial fibrillation. Cochrane Database Syst Rev. 2012;4 doi: 10.1002/14651858.CD007101.pub2. CD007101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012;14:528–606. doi: 10.1093/europace/eus027. [DOI] [PubMed] [Google Scholar]
- 4.Boersma L, Wijffels M, Oral H, Wever E, Morady F. Pulmonary vein isolation by duty-cycled bipolar and unipolar radiofrequency energy with a multielectrode ablation catheter. Heart Rhythm. 2008;5:1635–42. doi: 10.1016/j.hrthm.2008.08.037. [DOI] [PubMed] [Google Scholar]
- 5.Bittner A, Monnig G, Zellerhoff S, et al. Randomized study comparing duty cycled bipolar and unipolar radiofrequency with point-by-point ablation in pulmonary vein isolation. Heart Rhythm. 2011;8:1383–90. doi: 10.1016/j.hrthm.2011.03.051. [DOI] [PubMed] [Google Scholar]
- 6.Spitzer S, Karolyi L. Catheter ablation of atrial fibrillation with a novel, duty cycled ablation system. Clin Res Cardiol. 2010;5:51–6. [Google Scholar]
- 7.Arentz T, Weber R, Bürkle G, Herrera C, Blum T, Stockinger J, et al. Small or large isolation areas around the pulmonary veins for the treatment of atrial fibrillation? Results from a prospective randomized study. Circulation. 2007;115:3057–63. doi: 10.1161/CIRCULATIONAHA.107.690578. [DOI] [PubMed] [Google Scholar]
- 8.James SA, Shepherd EJ, Gall SA, et al. The impact of novel multi-array catheters on procedure time and outcome for ablation of atrial fibrillation. Europace. 2008;10:II23. [Google Scholar]
- 9.Tivig C, Dang L, Brunner-La Rocca HP, Ozcan S, Duru F, Scharf C. Duty cycled unipolar/bipolar versus conventional radiofrequency ablation in paroxysmal and persistent atrial fibrillation. Int J Cardiol. 2012;157:185–91. doi: 10.1016/j.ijcard.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Mulder AA, Wijffels MC, Wever EF, Boersma LV. Pulmonary vein anatomy and long-term outcome after multi-electrode pulmonary vein isolation with phased radiofrequency energy for paroxysmal atrial fibrillation. Europace. 2011;13:1557–61. doi: 10.1093/europace/eur236. [DOI] [PubMed] [Google Scholar]
- 11.Halimi F, Leclercq JF, Cordoliani Y, Fiorello P, Bertrand C, Attuel P. Increased risk of cerebral microemboli with non-irrigated ablation catheter during pulmonary vein isolation. J Interv Card Electrophysiol. 2011;30:104. [Google Scholar]
- 12.Herrera Siklody C, Deneke T, Hocini M, et al. Incidence of asymptomatic intracranial embolic events after pulmonary vein isolation comparison of different atrial fibrillation ablation technologies in a multicenter study. J Am Coll Cardiol. 2011;58:681–8. doi: 10.1016/j.jacc.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Tuan J, Jeilan M, Kundu S, Osman F, Stafford P, Ng G. Comparison of a new circular ablation catheter with conventional approach for pulmonary vein isolation. Eur Heart J. 2009;30:813. [Google Scholar]
- 14.Bulava A, Hanis J, Sitek D, et al. Catheter ablation for paroxysmal atrial fibrillation: a randomized comparison between multielectrode catheter and point-by-point ablation. Pacing Clin Electrophysiol. 2010;33:1039–46. doi: 10.1111/j.1540-8159.2010.02807.x. [DOI] [PubMed] [Google Scholar]
- 15.De Greef Y, Buysschaert I, Schwagten B, Stockman D, Tavernier R, Duytschaever M. Duty-cycled multi-electrode radiofrequency vs. conventional irrigated point-by-point radiofrequency ablation for recurrent atrial fibrillation: comparative 3-year data. Europace. 2014 doi: 10.1093/europace/eut398. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Andrade JG, Dubuc M, Rivard L, Guerra PG, Mondesert B, Macle L, et al. Efficacy and safety of atrial fibrillation ablation with phased radiofrequency energy and multielectrode catheters. Heart Rhythm. 2012;9:289–96. doi: 10.1016/j.hrthm.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 17.McLellan AJ, Kumar S, Smith C, Morton JB, Kalman JM, Kistler PM. The role of adenosine following pulmonary vein isolation in patients undergoing catheter ablation for atrial fibrillation: a systematic review. J Cardiovasc Electrophysiol. 2013;24:742–51. doi: 10.1111/jce.12121. [DOI] [PubMed] [Google Scholar]
- 18.Cappato R, Calkins H, Chen SA, et al. Updated worldwide survey on the methods, efficacy and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:32–8. doi: 10.1161/CIRCEP.109.859116. [DOI] [PubMed] [Google Scholar]
- 19.Scharf C, Ng GA, Wieczorek M, Deneke T, Furniss SS, Murray S, et al. European survey on efficacy and safety of duty-cycled radiofrequency ablation for atrial fibrillation. Europace. 2012;14:1700–7. doi: 10.1093/europace/eus188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulder AA, Balt JC, Wijffels MC, Wever EF, Boersma LV. Safety of pulmonary vein isolation and left atrial complex fractionated atrial electrograms ablation for atrial fibrillation with phased radiofrequency energy and multi-electrode catheters. Europace. 2012;14:1433–40. doi: 10.1093/europace/eus086. [DOI] [PubMed] [Google Scholar]
- 21.Hummel J, Michaud G, Hoyt R, Delurgio D, Rasekh A, Kusumoto F, et al. TTOP-AF Investigators. Phased RF ablation in persistent atrial fibrillation. Heart Rhythm. 2014;11:202–9. doi: 10.1016/j.hrthm.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Natale A, Di Biase L, Burkhardt D, Rutledge N, Santangeli P, Yan R, et al. Does peri-procedural anticoagulation management for AF affect the prevalence of silent thromboembolic lesion detected by diffusion cerebral magnetic resonance imaging (DMRI) in patients undergoing AF ablation with open irrigated radiofrequency energy? Results from a prospective multicentre study. 2013 doi: 10.1016/j.hrthm.2014.03.003. HRS AB 12–01. [DOI] [PubMed] [Google Scholar]
- 23.Haines DE, Stewart MT, Ahlberg S, Barka ND, Condie C, Fiedler GR, et al. Microembolism and catheter ablation I: a comparison of irrigated radiofrequency and multielectrode phased radiofrequency catheter ablation of pulmonary vein ostia. Circ Arrhythm Electrophysiol. 2013;6:16–22. doi: 10.1161/CIRCEP.111.973453. [DOI] [PubMed] [Google Scholar]
- 24.Nagy-Baló E, Tint D, Clemens M, Beke I, Kovács KR, Csiba L, et al. Transcranial measurement of cerebral microembolic signals during pulmonary vein isolation: a comparison of two ablation techniques. Circ Arrhythm Electrophysiol. 2013;6:473–80. doi: 10.1161/CIRCEP.112.971747. [DOI] [PubMed] [Google Scholar]
- 25.Zellerhoff S, Ritter MA, Kochhäuser S, Dittrich R, Köbe J, Milberg P, et al. Modified phased radiofrequency ablation of atrial fibrillation reduces the number of cerebral microembolic signals. Europace. 2014;16:341–6. doi: 10.1093/europace/eut282. [DOI] [PubMed] [Google Scholar]
- 26.Wieczorek M, Lukat M, Hoeltgen R, Condie C, Hilje T, Missler U, et al. Investigation into causes of abnormal cerebral MRI findings following PVAC duty-cycled, phased RF ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2013;24:121–8. doi: 10.1111/jce.12006. [DOI] [PubMed] [Google Scholar]
- 27.Verma A, Debruyne P, Nardi S, Deneke T, DeGreef Y, Spitzer S, et al. ERACE Investigators. Evaluation and reduction of asymptomatic cerebral embolism in ablation of atrial fibrillation, but high prevalence of chronic silent infarction: results of the evaluation of reduction of asymptomatic cerebral embolism trial. Circ Arrhythm Electrophysiol. 2013;6:835–42. doi: 10.1161/CIRCEP.113.000612. [DOI] [PubMed] [Google Scholar]
- 28.Compier MG, Leong DP, Marsan NA, Delgado V, Zeppenfeld K, Schalij MJ, et al. Duty-cycled bipolar/unipolar radiofrequency ablation for symptomatic atrial fibrillation induces significant pulmonary vein narrowing at long-term follow-up. Europace. 2013;15:690–6. doi: 10.1093/europace/eus420. [DOI] [PubMed] [Google Scholar]
- 29.von Bary C, Weber S, Dornia C, Eissnert C, Fellner C, Latzin P, et al. Evaluation of pulmonary vein stenosis after pulmonary vein isolation using a novel circular mapping and ablation catheter (PVAC) Circ Arrhythm Electrophysiol. 2011;4:630–6. doi: 10.1161/CIRCEP.111.963397. [DOI] [PubMed] [Google Scholar]
- 30.Reddy VY, Shah D, Kautzner J, Schmidt B, Saoudi N, Herrera C, et al. The relationship between contact force and clinical outcome during radiofrequency catheter ablation of atrial fibrillation in the TOCCATA study. Heart Rhythm. 2012;9:1789–95. doi: 10.1016/j.hrthm.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 31.Pratola C, Baldo E, Notarstefano P, Toselli T, Ferrari R. Radiofrequency ablation of atrial fibrillation: is the persistence of all intraprocedural targets necessary for long-term maintenance of sinus rhythm? Circulation. 2008;117:136–43. doi: 10.1161/CIRCULATIONAHA.106.678789. [DOI] [PubMed] [Google Scholar]
- 32.Deneke T, Schade A, Müller P, Schmitt R, Christopoulos G, Krug J, et al. Acute safety and efficacy of a novel multipolar irrigated radiofrequency ablation catheter for pulmonary vein isolation. J Cardiovasc Electrophysiol. doi: 10.1111/jce.12316. doi:10.1111/jce.12316. [DOI] [PubMed] [Google Scholar]
- 33.Wieczorek M, Hoeltgen R, Akin E, Salili AR, Oral H, Morady F. Results of short-term and long-term pulmonary vein isolation for paroxysmal atrial fibrillation using duty-cycled bipolar and unipolar radiofrequency energy. J Cardiovasc Electrophysiol. 2010;21:399–405. doi: 10.1111/j.1540-8167.2009.01640.x. [DOI] [PubMed] [Google Scholar]
- 34.Arujuna A, Karim R, Caulfield D, Knowles B, Rhode K, Schaeffter T, et al. Acute pulmonary vein isolation is achieved by a combination of reversible and irreversible atrial injury after catheter ablation: evidence from magnetic resonance imaging. Circ Arrhythm Electrophysiol. 2012;5:691–700. doi: 10.1161/CIRCEP.111.966523. [DOI] [PubMed] [Google Scholar]