Abstract
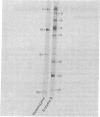
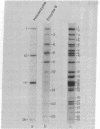
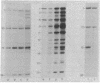
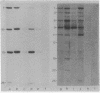
A modified form of Bacillus subtilis RNA polymerase containing a phage SP01-coded regulatory protein (the gene 28 product) selectively transcribes "middle" genes of the phage genome in vitro. In this paper, we identify a subset of restriction endonuclease fragments of SP01 DNA that promote specific transcription by the phage-modified polymerase. In the absence of nucleoside triphosphates, RNA polymerase containing the gene 28 protein selectively binds to these DNA fragments thereby forming stable binary complexes that can be isolated on nitrocellulose filters. In contrast, unmodified RNA polymerase containing sigma factor selectively binds to and transcribes a subset of phage DNA fragments that contain "early" sequences and that are in large part distinct from the fragments recognized by the phage-modified transcriptase. Our results strongly suggest that phage "early" and "middle" genes are transcribed from distinct promoters and that the RNA polymerase containing the gene 28 protein binds to sites that are located at or near promoters for SP01 "middle" genes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Duffy J. J., Geiduschek E. P. Purification of a positive regulatory subunit from phage SP01-modified RNA polymerase. Nature. 1977 Nov 3;270(5632):28–32. doi: 10.1038/270028a0. [DOI] [PubMed] [Google Scholar]
- Duffy J. J., Geiduschek E. P. RNA polymerase from phage SP01-infected and uninfected Bacillus subtilis. J Biol Chem. 1975 Jun 25;250(12):4530–4541. [PubMed] [Google Scholar]
- Duffy J. J., Geiduschek E. P. The virus-specified subunits of a modified B. subtilis RNA polymerase are determinants of DNA binding and RNA chain initiation. Cell. 1976 Aug;8(4):595–604. doi: 10.1016/0092-8674(76)90227-0. [DOI] [PubMed] [Google Scholar]
- Fox T. D. Identification of phage SP01 proteins coded by regulatory genes 33 and 34. Nature. 1976 Aug 26;262(5571):748–753. doi: 10.1038/262748a0. [DOI] [PubMed] [Google Scholar]
- Fox T. D., Losick R., Pero J. Regulatory gene 28 of bacteriophage SPO1 codes for a phage-induced subunit of RNA polymerase. J Mol Biol. 1976 Mar 5;101(3):427–433. doi: 10.1016/0022-2836(76)90157-1. [DOI] [PubMed] [Google Scholar]
- Hinkle D. C., Chamberlin M. J. Studies of the binding of Escherichia coli RNA polymerase to DNA. I. The role of sigma subunit in site selection. J Mol Biol. 1972 Sep 28;70(2):157–185. doi: 10.1016/0022-2836(72)90531-1. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pero J., Nelson J., Fox T. D. Highly asymmetric transcription by RNA polymerase containing phage-SP01-induced polypeptides and a new host protein. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1589–1593. doi: 10.1073/pnas.72.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pero J., Tjian R., Nelson J., Losick R. In vitro transcription of a late class of phage SP01 genes. Nature. 1975 Sep 18;257(5523):248–251. doi: 10.1038/257248a0. [DOI] [PubMed] [Google Scholar]
- Polisky B., Greene P., Garfin D. E., McCarthy B. J., Goodman H. M., Boyer H. W. Specificity of substrate recognition by the EcoRI restriction endonuclease. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3310–3314. doi: 10.1073/pnas.72.9.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. P. Enzymic synthesis of RNA from T7 DNA. J Mol Biol. 1966 Oct 28;21(1):115–127. doi: 10.1016/0022-2836(66)90083-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Air G. M., Barrell B. G., Brown N. L., Coulson A. R., Fiddes C. A., Hutchison C. A., Slocombe P. M., Smith M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977 Feb 24;265(5596):687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H., Schaller H. Mapping and characterization of promoters in bacteriophages fd, f1 and m13. J Mol Biol. 1975 Feb 25;92(2):261–277. doi: 10.1016/0022-2836(75)90226-0. [DOI] [PubMed] [Google Scholar]
- Sonenshein A. L., Roscoe D. H. The course of phage phi-e infection in sporulating cells of Bacillus subtilis strain 3610. Virology. 1969 Oct;39(2):265–275. doi: 10.1016/0042-6822(69)90047-6. [DOI] [PubMed] [Google Scholar]
- Talkington C., Pero J. Restriction fragment analysis of the temporal program of bacteriophage SPO1 transcription and its control by phage-modified RNA polymerases. Virology. 1977 Dec;83(2):365–379. doi: 10.1016/0042-6822(77)90181-7. [DOI] [PubMed] [Google Scholar]
- Tijan R., Pero J. Bacteriophage SP01 regulatory proteins directing late gene transcription in vitro. Nature. 1976 Aug 26;262(5571):753–757. doi: 10.1038/262753a0. [DOI] [PubMed] [Google Scholar]
- Walter G., Zillig W., Palm P., Fuchs E. Initiation of DNA-dependent RNA synthesis and the effect of heparin on RNA polymerase. Eur J Biochem. 1967 Dec;3(2):194–201. doi: 10.1111/j.1432-1033.1967.tb19515.x. [DOI] [PubMed] [Google Scholar]