Abstract
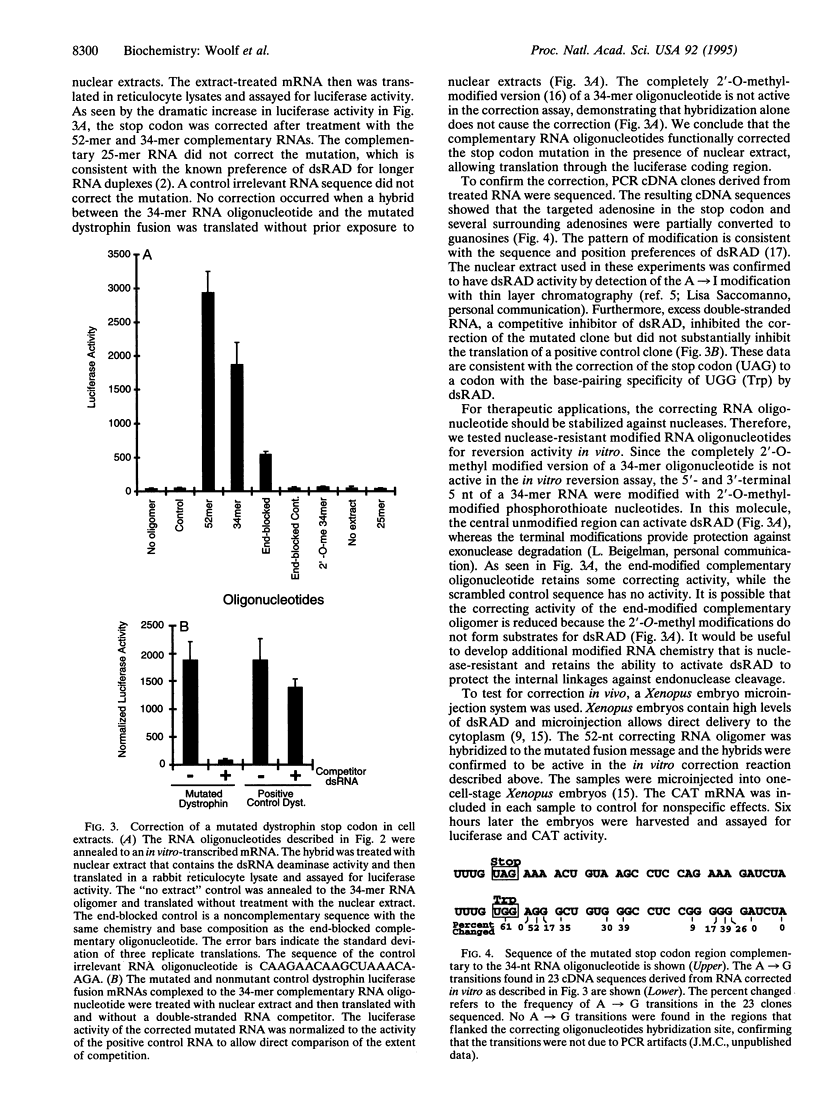
If RNA editing could be rationally directed to mutated RNA sequences, genetic diseases caused by certain base substitutions could be treated. Here we use a synthetic complementary RNA oligonucleotide to direct the correction of a premature stop codon mutation in dystrophin RNA. The complementary RNA oligonucleotide was hybridized to a premature stop codon and the hybrid was treated with nuclear extracts containing the cellular enzyme double-stranded RNA adenosine deaminase. When the treated RNAs were translated in vitro, a dramatic increase in expression of a downstream luciferase coding region was observed. The cDNA sequence data are consistent with deamination of the adenosine in the UAG stop codon to inosine by double-stranded RNA adenosine deaminase. Injection of oligonucleotide-mRNA hybrids into Xenopus embryos also resulted in an increase in luciferase expression. These experiments demonstrate the principle of therapeutic RNA editing.
Full text
PDF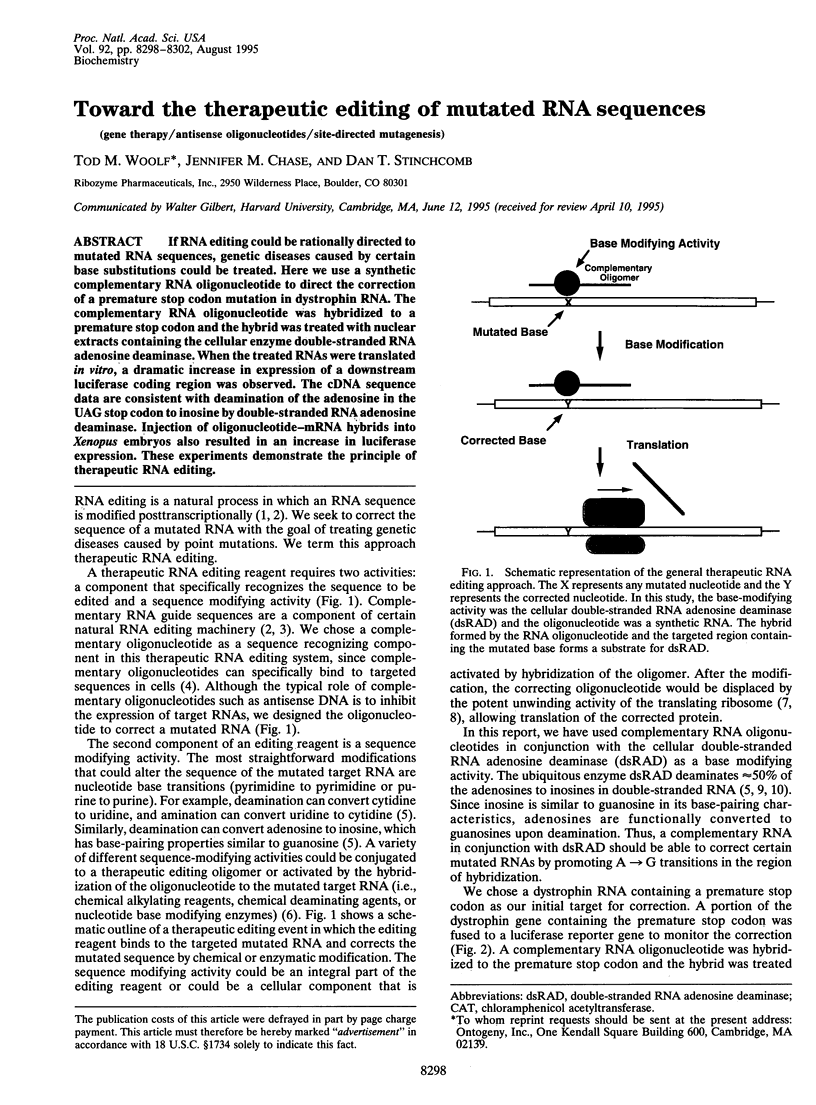



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backus J. W., Schock D., Smith H. C. Only cytidines 5' of the apolipoprotein B mRNA mooring sequence are edited. Biochim Biophys Acta. 1994 Sep 13;1219(1):1–14. doi: 10.1016/0167-4781(94)90240-2. [DOI] [PubMed] [Google Scholar]
- Bartel D. P., Szostak J. W. Isolation of new ribozymes from a large pool of random sequences [see comment]. Science. 1993 Sep 10;261(5127):1411–1418. doi: 10.1126/science.7690155. [DOI] [PubMed] [Google Scholar]
- Bass B. L., Weintraub H. A developmentally regulated activity that unwinds RNA duplexes. Cell. 1987 Feb 27;48(4):607–613. doi: 10.1016/0092-8674(87)90239-x. [DOI] [PubMed] [Google Scholar]
- Bass B. L., Weintraub H. An unwinding activity that covalently modifies its double-stranded RNA substrate. Cell. 1988 Dec 23;55(6):1089–1098. doi: 10.1016/0092-8674(88)90253-x. [DOI] [PubMed] [Google Scholar]
- Blum B., Bakalara N., Simpson L. A model for RNA editing in kinetoplastid mitochondria: "guide" RNA molecules transcribed from maxicircle DNA provide the edited information. Cell. 1990 Jan 26;60(2):189–198. doi: 10.1016/0092-8674(90)90735-w. [DOI] [PubMed] [Google Scholar]
- Bulman D. E., Gangopadhyay S. B., Bebchuck K. G., Worton R. G., Ray P. N. Point mutation in the human dystrophin gene: identification through western blot analysis. Genomics. 1991 Jun;10(2):457–460. doi: 10.1016/0888-7543(91)90332-9. [DOI] [PubMed] [Google Scholar]
- Dai X., De Mesmaeker A., Joyce G. F. Cleavage of an amide bond by a ribozyme. Science. 1995 Jan 13;267(5195):237–240. doi: 10.1126/science.7809628. [DOI] [PubMed] [Google Scholar]
- Dominski Z., Kole R. Restoration of correct splicing in thalassemic pre-mRNA by antisense oligonucleotides. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8673–8677. doi: 10.1073/pnas.90.18.8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M., Single F. N., Köhler M., Sommer B., Sprengel R., Seeburg P. H. RNA editing of AMPA receptor subunit GluR-B: a base-paired intron-exon structure determines position and efficiency. Cell. 1993 Dec 31;75(7):1361–1370. doi: 10.1016/0092-8674(93)90622-w. [DOI] [PubMed] [Google Scholar]
- Lamond A. I. 2'-O-alkyloligoribonucleotides: probes for studying the biochemistry and cell biology of RNA processing. Biochem Soc Trans. 1993 Feb;21(1):1–8. doi: 10.1042/bst0210001. [DOI] [PubMed] [Google Scholar]
- Maher L. J., 3rd, Dolnick B. J. Comparative hybrid arrest by tandem antisense oligodeoxyribonucleotides or oligodeoxyribonucleoside methylphosphonates in a cell-free system. Nucleic Acids Res. 1988 Apr 25;16(8):3341–3358. doi: 10.1093/nar/16.8.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A. Injected anti-sense RNAs specifically block messenger RNA translation in vivo. Proc Natl Acad Sci U S A. 1985 Jan;82(1):144–148. doi: 10.1073/pnas.82.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monia B. P., Johnston J. F., Ecker D. J., Zounes M. A., Lima W. F., Freier S. M. Selective inhibition of mutant Ha-ras mRNA expression by antisense oligonucleotides. J Biol Chem. 1992 Oct 5;267(28):19954–19962. [PubMed] [Google Scholar]
- Navaratnam N., Morrison J. R., Bhattacharya S., Patel D., Funahashi T., Giannoni F., Teng B. B., Davidson N. O., Scott J. The p27 catalytic subunit of the apolipoprotein B mRNA editing enzyme is a cytidine deaminase. J Biol Chem. 1993 Oct 5;268(28):20709–20712. [PubMed] [Google Scholar]
- Polson A. G., Bass B. L. Preferential selection of adenosines for modification by double-stranded RNA adenosine deaminase. EMBO J. 1994 Dec 1;13(23):5701–5711. doi: 10.1002/j.1460-2075.1994.tb06908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebagliati M. R., Melton D. A. Antisense RNA injections in fertilized frog eggs reveal an RNA duplex unwinding activity. Cell. 1987 Feb 27;48(4):599–605. doi: 10.1016/0092-8674(87)90238-8. [DOI] [PubMed] [Google Scholar]
- Roberts R. G., Gardner R. J., Bobrow M. Searching for the 1 in 2,400,000: a review of dystrophin gene point mutations. Hum Mutat. 1994;4(1):1–11. doi: 10.1002/humu.1380040102. [DOI] [PubMed] [Google Scholar]
- Saccomanno L., Bass B. L. The cytoplasm of Xenopus oocytes contains a factor that protects double-stranded RNA from adenosine-to-inosine modification. Mol Cell Biol. 1994 Aug;14(8):5425–5432. doi: 10.1128/mcb.14.8.5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaringe S. A., Francklyn C., Usman N. Chemical synthesis of biologically active oligoribonucleotides using beta-cyanoethyl protected ribonucleoside phosphoramidites. Nucleic Acids Res. 1990 Sep 25;18(18):5433–5441. doi: 10.1093/nar/18.18.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L. RNA editing--a novel genetic phenomenon? Science. 1990 Oct 26;250(4980):512–513. doi: 10.1126/science.1700474. [DOI] [PubMed] [Google Scholar]
- Sullenger B. A., Cech T. R. Ribozyme-mediated repair of defective mRNA by targeted, trans-splicing. Nature. 1994 Oct 13;371(6498):619–622. doi: 10.1038/371619a0. [DOI] [PubMed] [Google Scholar]
- Wagner R. W., Smith J. E., Cooperman B. S., Nishikura K. A double-stranded RNA unwinding activity introduces structural alterations by means of adenosine to inosine conversions in mammalian cells and Xenopus eggs. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2647–2651. doi: 10.1073/pnas.86.8.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]



